Abstract
Context:
Phosphodiesterases (PDEs) are key regulatory enzymes of intracellular cAMP levels. PDE11A function has been linked to predisposition to adrenocortical tumors.
Objective:
The aim of the study was to study the PDE11A gene in a large cohort of patients with ACTH-independent macronodular adrenal hyperplasia (AIMAH) and in control subjects.
Design:
The PDE11A entire coding region was sequenced in 46 patients with AIMAH and 192 controls. Two variants found in AIMAH patients were transiently expressed in HEK 293 and adrenocortical H295R cells for further functional studies.
Results:
The frequency of all PDE11A variants was significantly higher among patients with AIMAH (28%) compared to controls (7.2%) (P = 5 × 10−5). Transfection of the two PDE11A variants found in AIMAH patients only (D609N or M878V) showed that cAMP levels were higher, after forskolin stimulation, in cells transfected with the PDE11A mutants, compared to cells transfected with the wild-type PDE11A in HEK 293 cells (P < 0.05). Moreover, transfection with mutants PDE11A increased transcriptional activity of a cAMP-response element reporter construct compared to wild-type PDE11A in HEK 293 cells (P < 0.0004 for D609N and P < 0.003 for M878V) and in the adrenocortical H295R cells (P < 0.05 for D609N and M878V). In addition, analysis of cAMP levels in intact living culture cells by fluorescence resonance energy transfer probes showed increased cAMP in forskolin-treated cells transfected with PDE11A variants compared with wild-type PDE11A (P < 0.05).
Conclusion:
We conclude that PDE11A genetic variants may increase predisposition to AIMAH.
The cAMP pathway plays an important role in endocrine tissues (1). Various alterations of the cAMP signaling have been observed in endocrine tumors. Tumors of the adrenal cortex and various forms of adrenal hyperplasia causing steroid excess illustrate alterations of the cAMP/protein kinase A (PKA) signaling pathway (2–6). For instance, aberrant expression of G protein-coupled receptors is observed in the majority of ACTH-independent macronodular adrenal hyperplasia (AIMAH) (7, 8), somatic activating mutations of the stimulatory α-subunit of the G protein (GNAS) in McCune-Albright syndrome (9), germline-inactivating mutations of the PKA regulatory subunit type 1 (PRKAR1A) in primary pigmented nodular adrenocortical diseases (PPNADs), and Carney complex (10–13).
The intracellular levels of cAMP are tightly regulated. Phosphodiesterases (PDEs) are key regulatory enzymes of intracellular cAMP levels because they hydrolyze cAMP into 5′-AMP (14). Two PDEs, PDE8B and PDE11A, have been shown recently to regulate cortisol secretion (15, 16). Furthermore, a whole-genome association study led to the discovery that these two PDEs are involved in predisposition to adrenal hyperplasia (17–19). Germline PDE11A non-sense mutations were identified initially in micronodular adrenal hyperplasia causing adrenal Cushing syndrome (17). Later, it was shown that various PDE11A missense substitutions could be involved in the development of various forms of adrenal tumors (20, 21). These missense substitutions of PDE11A that are rare in the general population were found with increased frequency among patients with AIMAH, adrenocortical adenomas, and adrenal cancer (21). Moreover, consistent with the hypothesis that PDE11A may play a role as a tumor suppressor gene, it has been reported that adrenal tumors expressing PDE11A variants present a loss of the wild-type allele, thus resulting in a significant reduction of enzyme levels in the affected tissue (21). This association of PDE11A variants and adrenocortical tumors suggests a role in the genetic susceptibility to develop these tumors.
AIMAH is a rare cause of Cushing syndrome, accounting for less than 1% of all cases. AIMAHs are bilateral tumors that are often apparently sporadic. However, familial forms have been reported (5), and the bilateral nature of these benign tumors suggests genetic factors. The genetics of AIMAH is largely unknown. Association of PDE11A variants with AIMAH has been reported to date in a limited series of 20 patients with this rare disease (21). The aim of the present work is to study in a large cohort of patients with AIMAH (and in control subjects) the PDE11A gene. Because demonstration of the alterations of enzymatic activity by these variants is important, functional studies were performed for two missense substitution variants found in the AIMAH patients investigated (M878V and D609N). This showed alterations of PKA-dependent transcription by these mutants. Alterations of the control of cAMP levels by these variants was also for the first time shown in living cells using an in vivo fluorescence resonance energy transfer (FRET)-reporter probe.
Patients and Methods
Patients and controls
Leukocyte samples from 46 patients with AIMAH and from 192 control subjects were collected. These patients and controls had never been analyzed previously.
All patients and controls signed an informed consent for the analysis of leukocyte DNA and for access to their clinical data. The study was approved by an institutional review board (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale, Hôpital Cochin, Paris).
Briefly, controls were collected as part of a program dedicated to the genetic predisposition to endocrine tumors (21). All volunteers were examined by a senior endocrinologist to exclude personal or family history or clinical signs suggestive of functional adrenal tumor.
Patients with AIMAH were identified as such by computed tomographic appearance of their adrenal glands and the biochemical evidence of ACTH-independent cortisol dysregulation (22, 23).
DNA extraction and PDE11A sequencing
Leukocyte DNA was extracted from total blood as previously reported (21). The 21 coding exons (exons 3–23) and the flanking intronic sequences of the PDE11A gene (Ensembl protein coding gene: ENSG00000128655) were amplified by PCR using specific primers as described previously (21). All amplified samples were examined by agarose gel electrophoresis to confirm successful amplification of each exon. Direct sequencing of the purified fragments was then done using the Genetic Sequencer ABI3100 apparatus (Applied Biosystems, Foster City, CA).
Reagents
DMEM, fetal bovine serum, L-glutamine, penicillin, streptomycin, opti-MEM, trypsin, and PBS were obtained from Invitrogen (Invitrogen Life Technologies, Cergy Pontoise, France). Forskolin was obtained from Sigma (St. Louis, MO).
Transfection experiments
The human embryonic kidney (HEK) 293 and H295R (derived from adrenocortical carcinoma) cell lines were cultured as previously reported (24). Transient transfections were done in HEK 293 with Lipofectamine 2000 reagent (Lipo2000; Invitrogen Life Technologies) and in H295R with Effectene Transfection Reagent (QIAGEN, Courtaboeuf, France) following the manufacturer's instructions. Experiments were performed 24 or 48 h after transfection.
The PDE11A open reading frame was cloned into pCR3.1, and the missense mutations (D609N and M878V) were introduced by overlapping PCR, as previously described (17).
Western blot
For Western blots, cells were seeded on six-well plates at a density of 500 × 103 cells per well and transfected with 1 μg of plasmid DNA expressing either the wild-type or the variant form of PDE11A. Cells were lysed in buffer containing (in millimoles) Tris HCl 50 (pH 7.4), NaCl 150, EDTA 5, EGTA 1, Triton X-100 1%, protease and phosphatase inhibitor cocktails (Roche Diagnostics, Meylan, France) and centrifuged 5 min at 800 × g. Proteins in the supernatant were quantified using a BCA assay (Sigma). Similar amounts of proteins were separated on a 10% SDS-PAGE, then electrotransferred to polyvinylidene difluoride membrane, and analyzed by immunoblotting. The polyclonal antibody specific for PDE11A was used as directed by the manufacturer (ab14624; Abcam Inc., Paris, France) at a dilution of 1:500.
Luciferase reporter assays
A cAMP/PKA pathway reporter construct driving the expression of luciferase gene was used for expression studies: 4x(CRE)-Luc (Stratagene, La Jolla, CA) with a basic promoter element (TATA box) joined to four cAMP-responsive element (CRE) repeats.
A cytochrome P450 side chain cleavage reporter driving the expression of luciferase gene was used to evaluate the steroidogenesis in H295 cells (gift from Dr. Antoine Martinez, Clermont Ferrand, France).
The Rous sarcoma virus (RSV)-Renilla construct, containing the RSV promoter-enhancer inserted upstream of the coding sequence of the Renilla luciferase (Promega Corp., Charbonnière, France), was used as a control for transfection efficiency.
Cells were cotransfected with 1 μg of plasmid DNA expressing either the wild-type or the mutated form of PDE11A, 10 ng of the RSV-Renilla, and 250 ng of the luciferase reporter construct and lysed. Both firefly and Renilla luciferase activities were sequentially measured with the Dual Luciferase Reporter Assay System (Promega Corp.).
Results are expressed as firefly luciferase activity normalized to Renilla luciferase activity of the same sample.
cAMP assay
Basal and forskolin-induced (3 min with 1 μm forskolin) levels of cAMP concentration were determined in cell lysates from cell culture or from tissue samples using commercially available assay (Direct cAMP EIA kit; Enzo Life Sciences, Villeurbanne, France) following the manufacturer's instructions. The assay is based on competitive binding, in which endogenous cAMP levels compete with a fixed amount of alkaline phosphate-labeled cyclic nucleotides. The assay is colorimetric, and absorbance is read at 405 nm.
FRET experiments
A total of 200,000 cells were plated on 2-cm diameter glass coverslips. For FRET experiments, cells were cotransfected with 0.4 μg of FRET sensor DNA and 24 pmol of plasmid DNA expressing either the wild-type or the variant forms of PDE11A. All experiments were performed 48 h after transfection at least in duplicate.
The cAMP sensor (Epac1-camps) was described previously (25). This sensor uses variants of cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) as FRET donor and acceptor, respectively. cAMP elevation is reported by an increase of the CFP/YFP emission ratio of Epac1-camps.
Cells were maintained in a control Ringer solution containing (in millimoles) NaCl 121.6, KCl 5.4, MgCl2 1.8, CaCl2 1.8, NaHCO3 4, NaH2PO4 0.8, d-glucose 5, sodium pyruvate 5, and HEPES 10, adjusted to pH 7.4. Control or drug-containing solutions were applied by placing the cell at the opening of a 250-μm (inner diameter) capillary tube. Images were captured every 5 sec using the 40× objective of a Nikon TE 300 inverted microscope connected to a software-controlled (Metafluor; Molecular Devices, Sunnyvale, CA) cooled charge coupled camera (Sensicam PE; PCO, Kelheim, Germany). CFP was excited during 300 msec by a Xenon lamp (Nikon, Champigny-sur-Marne, France) using a 440/20BP filter and a 455LP dichroic mirror. Dual emission imaging of CFP and YFP was performed using an Optosplit II emission splitter (Cairn Research, Faversham, UK) equipped with a 495LP dichroic mirror and BP filters 470/30 and 535/30, respectively. All experiments were performed at room temperature (21–25 C).
Average fluorescence intensity of the entire cell was measured. Background was subtracted, and YFP intensity was corrected for CFP spillover before calculating the ratio. The data were normalized to the ratio measured before the stimulus and expressed as ratio variations, in percentage.
Statistical analysis
All functional studies were done in triplicate, and an average was calculated for each value; each experiment was repeated at least twice. Quantitative data are expressed as means ± sem. Statistical analysis was performed using the Student's t test for expression studies and for FRET measurement and using the nonparametric Mann-Whitney test for cAMP studies. The maximal increase of CFP/YFP ratio was calculated compared with baseline. Then, the comparisons in time courses were performed compared with the wild-type using ANOVA.
A difference was considered significant when P was <0.05.
Results
PDE11A missense variants in AIMAH and controls
By sequencing the entire PDE11A coding sequence in the germline DNA of 46 AIMAH patients, a total of 13 heterozygous missense variants (28%) were found in 13 different patients. Missense variants in AIMAH patients were: R52T (n = 1), Y658C (n = 1), Y727C (n = 6), R804H (n = 3), R867G (n = 1), and M878V (n = 1).
By analyzing the entire PDE11A coding sequence in the germline DNA of 192 controls, a total of 14 heterozygous missense variants (7.2%) were found. Missense variants in control subjects were: A349T (n = 2), Y727C (n = 2), R804H (n = 6), and R867G (n = 4).
In total, we found a significantly higher number of sequence alterations in the 46 patients with AIMAH compared with those in the 192 controls (28 vs. 7.2%; P = 5 × 10−5; odds ratio, 5.01; 95% confidence interval, 1.96–12.58.
Functional studies
Functional studies were performed with two variants (D609N and M878V) that were identified in patients with AIMAH in this study for M878V and in a previous study for D609N (21).
cAMP levels
Wild-type and variant PDE11A (isoform 4, or PDE11A4) expression vectors were transfected in both H295R and HEK 293 cells. The expression of the two PDE11A variants (D609N and M878V) was similar to the wild-type form as shown by Western blot analysis (Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The results of the cAMP-level assay on whole cell extracts are summarized in Fig. 1.
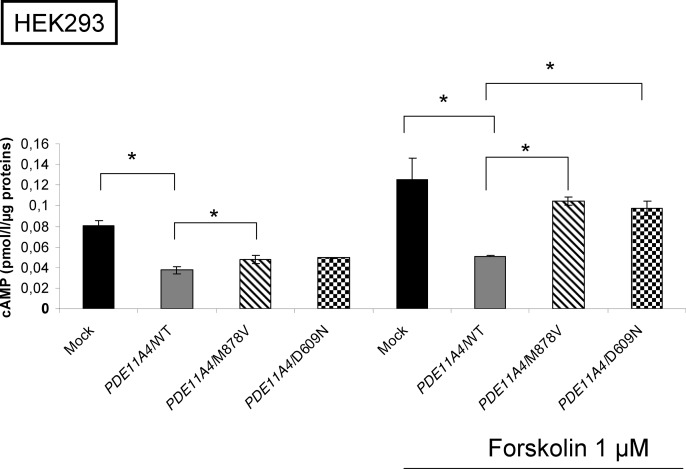
Fig. 1.
cAMP levels in HEK 293 cells transfected with wild-type (WT) or PDE11A4 variants expression vectors. The figure shows the levels of cAMP assayed as described in Patients and Methods in cells transfected with the wild-type PDE11A expression vector or the PDE11A4/M878V and PDE11A4/D609N variants. On the right, the cells were treated for 3 min with 1 μm forskolin. *, P < 0.05.
In basal conditions, cAMP levels were statistically different in cells transfected with the M878V-PDE11A4 expression vectors (P < 0.02) and tended to be statistically different in cells transfected with the D609N-PDE11A4 (P = 0.08) compared with the wild-type. Moreover, the wild-type PDE11A4 blocked the increase of cAMP levels after 3 min of stimulation by forskolin (P < 0.05), whereas cAMP levels increased with the two disease-associated PDE11A variants, indicating a possible lower catalytic function for both of these variants (P < 0.05).
cAMP dynamics in intact cells
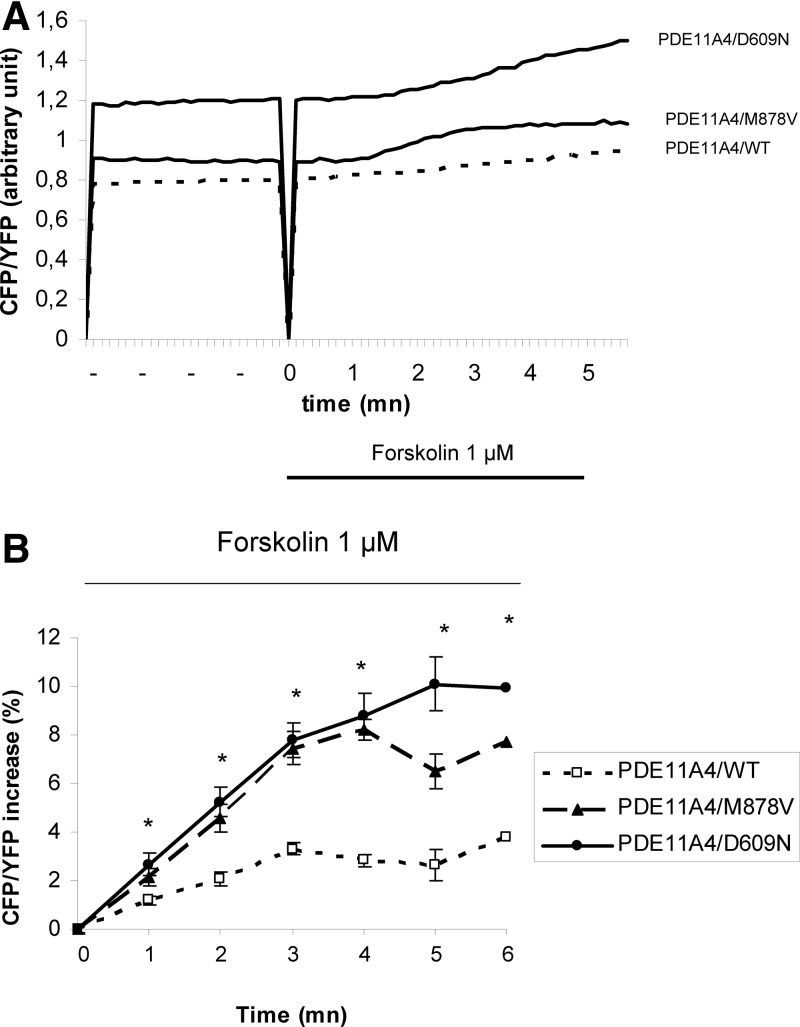
The analysis of cAMP levels in intact living cells by the ratiometric FRET probe Epac1-camps (25) are summarized in Fig. 2. The response to 1 μm forskolin was higher in cells transfected with the two PDE11A4 variants compared with cells transfected with the wild-type PDE11A (M878V = 7.75 ± 0.75, D609N = 9.93 ± 1.11, WT = 3.77 ± 0.62 after 5 mn, P < 0.0001). Furthermore, the maximal increase of CFP/YFP ratio was earlier in the cells transfected with the variants compared with the wild-type PDE11A4 (M878V = 4.3 ± 0.6 mn; D609N = 4.7 ± 1 mn; wild-type = 5.7 ± 0.7 mn; P < 0.0001).
Fig. 2.
Effects of PDE11A4 variants expression on the dynamics of intracellular cAMP analyzed in living cells with FRET measurement. A, Representative traces of CFP/YFP ratio upon forskolin (1 μm) in HEK 293 cells transfected with the wild-type PDE11A (WT), and with the PDE11A variants (PDE11A4/M878V and PDE11A4/D609N). Forskolin application started at time zero and lasted 5 min. B, Average time course of the normalized CFP/YFP ratio upon forskolin (1 μm) stimulation in HEK293 transfected with the wild-type PDE11A cells (WT, n = 88), and in cells transfected with the PDE11A variants (PDE11A4/M878V, n = 73; and PDE11A4/D609N, n = 81). Forskolin application started at time zero and lasted 5 min. *, P < 0.0001.
cAMP-regulated transcription
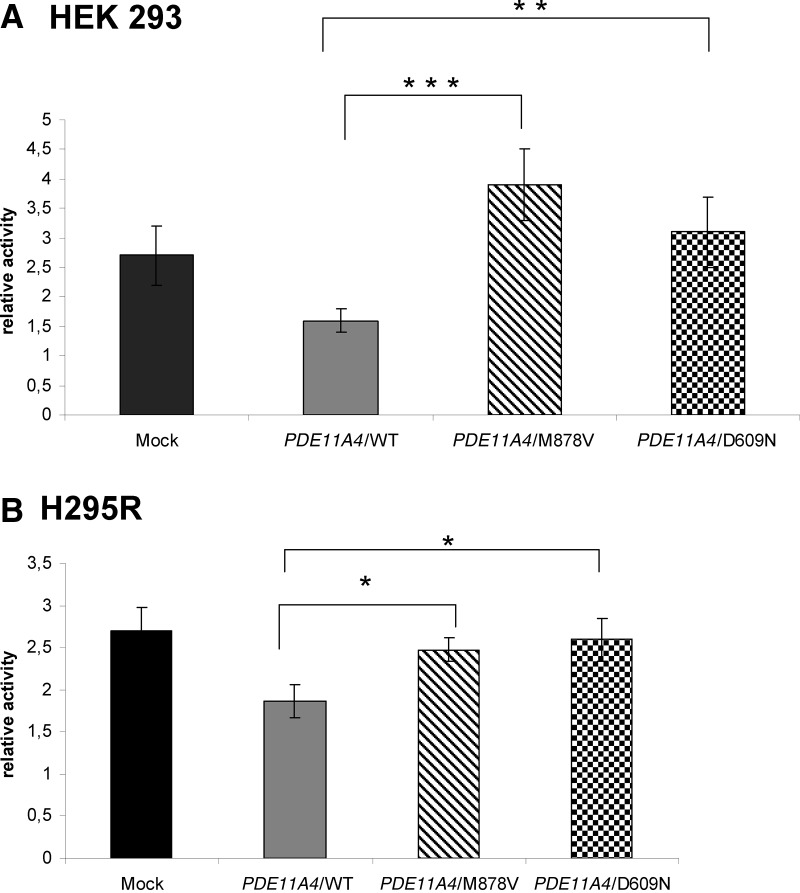
As expected, wild-type PDE11A4 decreased transcriptional activity of the cAMP reporter construct, whereas D609N and M878V failed to do so in HEK 293 cells (P < 0.0004 for M878V and P < 0.003 for D609N) (Fig. 3A), as well as in H295R cells (P < 0.05 for D609N or M878V compared with wild-type) (Fig. 3B).
Fig. 3.
Effects of the PDE11A variants on cAMP-dependent transcription. The figure shows the activity of the CRE reporter construct 4x(CRE)-Luc in cells transfected with the PDE11A wild-type (WT) and the PDE11A4/M878V and PDE11A4/D609N expression vectors. A, Results obtained in HEK 293 cells; B, results obtained in H295R cells. *, P < 0.05; **, P < 0.003; ***, P < 0.0004.
In addition, when the P450 side chain cleavage Luc promoter was transfected, relative P450 activity decreased in wild-type compared with mock (P < 0.003 at basal, P < 0.001 after forskolin), whereas it increased in M878V compared with wild-type (P = 0.006 at basal, P < 0.006 after forskolin). Relative P450 activity tended to increase without achieving significance in D609N after forskolin stimulation (Supplemental Data).
Discussion
It has been demonstrated by various association studies that genetic factors play a role in the susceptibility to develop cancer. The rarity of endocrine tumors limits the possibility to perform genome-wide studies. However, such approaches have been used for thyroid tumors that are the more frequent endocrine tumors (26). For adrenal lesions, limited studies have been performed. It is tempting to speculate that bilateral adrenocortical tumors are more likely to be favored by genetic factors. Among the rare studies performed, variants of the glucocorticoid receptor have been reported to be more frequent in patients with bilateral adrenal incidentalomas than in those with unilateral masses (27). In a previous study, we have reported that PDE11A variants are more frequent in patients with various types of unilateral and bilateral adrenocortical tumors (21). In that study, the frequency seemed especially higher in patients with bilateral tumors classified as AIMAH, but it only included 20 patients. The present study clearly demonstrates a higher frequency of PDE11A variants in a new cohort of patients by comparison with a new cohort of control subjects negative for endocrine tumors. The frequency of PDE11A variants in the present control group (7.2%) is similar to the previous control cohort (5.7%). In AIMAH, the present larger cohort of new patients presents a similar percentage of PDE11A variants (28%) than in our initial study (24%). This replication of the results in a series of patients and control subjects different from the first analysis strongly supports the value of the association of PDE11A variants with the genetic susceptibility to development of AIMAH.
Other PDEs have been implicated in various disorders. Single-nucleotide polymorphisms had been identified in PDE family members and suggested that defects in these genes may contribute to clinical disorders such as schizophrenia (PDE4B) (28), stroke (PDE4D) (29), retinitis pigmentosa (PDE6A and PDE6B) (30), chronic lymphocytic leukemia (PDE7B) (31), major depression (PDE1A and PDE11A) (32, 33), chronic obstructive pulmonary disease (PDE4D) (34), asthma (PDE11A) (35), carotid atherosclerosis (PDE4D) (33), testicular germ cell tumors (PDE11A) (36), or prostate cancer (PDE11A) (37). Variants of PDE11A have also been be found to be more frequent in patients with Carney complex due to PRKAR1A mutations that develop testicular tumors and adrenal Cushing syndrome due to PPNAD (38). It was also shown that two PDEs, PDE8B and PDE11A, were involved in inherited predisposition to adrenocortical hyperplasia, known as isolated micronodular adrenocortical disease, a nonpigmented variant of PPNAD first described by Stratakis and colleagues (17, 20, 39).
The PDE11A variants found in this study have been previously demonstrated to be responsible for reduced enzymatic activity (20). However, the cellular consequences in terms of transcriptional regulation and cAMP dynamics in living cells have not been investigated so far. The human PDE11 family is composed of four splice variants or isoforms, namely PDE11A1, PDE11A2, PDE11A3, and PDE11A4. PDE11A4, which is the longest form among PDE11A variants, is predicted to encode a protein of 934 amino acids with two complete GAF (cGMP-phosphodiesterases, Anabaena adenylyl cyclases and Escherichia coli Fh1A) domain sequences (GAF A and GAF B) and an N-terminal flanking sequence that contains two consensus sequences for PKA and protein kinase G phosphorylation (40). All PDE11A functional variants in AIMAH patients were found in the catalytic domain, except for one variant that was localized in the exon that contains the start codon. The two variants investigated in the present study (D609N and M878V) are located in exons 14 and 22, respectively, of PDE11A. They are both in the catalytic domains of PDE11A4, located on both far sides of this domain. When expressed transiently in HEK 293 and H295R cells, they both induced similar alterations by comparison with the wild-type PDE11A.
The present study shows that expression of the two missense PDE11A variants led to increased cAMP-dependent transcription. Transfection with variants of the PDE11A4 enzyme increased transcriptional activity of the reporter construct compared with transfection with wild-type PDE11A in HEK 293 and in adrenocortical H295R cell lines.
Thus, the presence of these variants leads to increased cAMP levels, which in turn activate cAMP signaling. Indeed, cAMP levels were higher in the extracts prepared from cells transfected with the PDE11A4 variants compared with those with the wild-type PDE11A4 sequence. In addition, FRET-reporter studies demonstrated that cAMP dynamics are modified in cells transfected with PDE11A4 variants. Indeed, with both variants, the cAMP increase in response to forskolin was both faster and higher. This alteration of cAMP dynamics is likely to modulate the tone of the cAMP pathway in adrenocortical cells leading to increased activity, especially in response to extracellular stimuli.
In conclusion, this study supports the role of PDE11A4 variants that stimulate the cAMP pathway as a genetic predisposing factor in AIMAH development. These findings are important to unravel the genetic basis of adrenal tumor development and the design of targeted therapies for the treatment of adrenal Cushing's syndrome.
Acknowledgments
We thank Franck Letourneur from the Genomic Platform of Institut Cochin for his technical support and Viacheslav Nikolaev for providing us the cAMP sensor (Epac1-camps).
This work was supported by the Intramural Program (to A.H., I.L., C.S.) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health; and by the Agence Nationale de la Recherche (ANR08-GENOPAT-002; and ANR-10-Blan-1136). D.V. is the recipient of a fellowship from the Institut National du Cancer.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AIMAH
- ACTH-independent macronodular adrenal hyperplasia
- CFP
- cyan fluorescent protein
- CRE
- cAMP-responsive element
- FRET
- fluorescence resonance energy transfer
- GAF
- cGMP-phosphodiesterases, Anabaena adenylyl cyclases and Escherichia coli Fh1A
- HEK
- human embryonic kidney
- PDE
- phosphodiesterase
- PKA
- protein kinase A
- PPNAD
- primary pigmented nodular adrenocortical disease
- RSV
- Rous sarcoma virus
- YFP
- yellow fluorescent protein.
References
- 1. Roger PP, Dumont JE. 1995. Thyrotropin-dependent insulin-like growth factor I mRNA expression in thyroid cells. Eur J Endocrinol 132:601–602 [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg D, Groussin L, Bertagna X, Bertherat J. 2002. cAMP pathway alterations from the cell surface to the nucleus in adrenocortical tumors. Endocr Res 28:765–775 [DOI] [PubMed] [Google Scholar]
- 3. Groussin L, Massias JF, Bertagna X, Bertherat J. 2000. Loss of expression of the ubiquitous transcription factor cAMP response element-binding protein (CREB) and compensatory overexpression of the activator CREMtau in the human adrenocortical cancer cell line H295R. J Clin Endocrinol Metab 85:345–354 [DOI] [PubMed] [Google Scholar]
- 4. Lacroix A, Ndiaye N, Tremblay J, Hamet P. 2001. Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr Rev 22:75–110 [DOI] [PubMed] [Google Scholar]
- 5. Vezzosi D, Cartier D, Régnier C, Otal P, Bennet A, Parmentier F, Plantavid M, Lacroix A, Lefebvre H, Caron P. 2007. Familial adrenocorticotropin-independent macronodular adrenal hyperplasia with aberrant serotonin and vasopressin adrenal receptors. Eur J Endocrinol 156:21–31 [DOI] [PubMed] [Google Scholar]
- 6. Groussin L, Perlemoine K, Contesse V, Lefebvre H, Tabarin A, Thieblot P, Schlienger JL, Luton JP, Bertagna X, Bertherat J. 2002. The ectopic expression of the gastric inhibitory polypeptide receptor is frequent in adrenocorticotropin-independent bilateral macronodular adrenal hyperplasia, but rare in unilateral tumors. J Clin Endocrinol Metab 87:1980–1985 [DOI] [PubMed] [Google Scholar]
- 7. Bertherat J, Contesse V, Louiset E, Barrande G, Duparc C, Groussin L, Emy P, Bertagna X, Kuhn JM, Vaudry H, Lefebvre H. 2005. In vivo and in vitro screening for illegitimate receptors in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing's syndrome: identification of two cases of gonadotropin/gastric inhibitory polypeptide-dependent hypercortisolism. J Clin Endocrinol Metab 90:1302–1310 [DOI] [PubMed] [Google Scholar]
- 8. Cartier D, Lihrmann I, Parmentier F, Bastard C, Bertherat J, Caron P, Kuhn JM, Lacroix A, Tabarin A, Young J, Vaudry H, Lefebvre H. 2003. Overexpression of serotonin 4 receptors in cisapride-responsive adrenocorticotropin-independent bilateral macronodular adrenal hyperplasia causing Cushing's syndrome. J Clin Endocrinol Metab 88:248–254 [DOI] [PubMed] [Google Scholar]
- 9. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. 1991. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- 10. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. 2000. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- 11. Groussin L, Jullian E, Perlemoine K, Louvel A, Leheup B, Luton JP, Bertagna X, Bertherat J. 2002. Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab 87:4324–4329 [DOI] [PubMed] [Google Scholar]
- 12. Groussin L, Horvath A, Jullian E, Boikos S, Rene-Corail F, Lefebvre H, Cephise-Velayoudom FL, Vantyghem MC, Chanson P, Conte-Devolx B, Lucas M, Gentil A, Malchoff CD, Tissier F, Carney JA, Bertagna X, Stratakis CA, Bertherat J. 2006. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab 91:1943–1949 [DOI] [PubMed] [Google Scholar]
- 13. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vezzosi D, Bertherat J. 2011. Phosphodiesterases in endocrine physiology and disease. Eur J Endocrinol 165:177–188 [DOI] [PubMed] [Google Scholar]
- 15. Tsai LC, Shimizu-Albergine M, Beavo JA. 2011. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol 79:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ceyhan O, Birsoy K, Hoffman CS. 2012. Identification of biologically active PDE11-selective inhibitors using a yeast-based high-throughput screen. Chem Biol 19:155–163 [DOI] [PubMed] [Google Scholar]
- 17. Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. 2006. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- 18. Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libè R, Patronas Y, Robinson-White A, Remmers E, Bertherat J, Nesterova M, Stratakis CA. 2008. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet 16:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horvath A, Mericq V, Stratakis CA. 2008. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med 358:750–752 [DOI] [PubMed] [Google Scholar]
- 20. Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, de Herder W, Carney JA, Bertherat J, Gregersen PK, Remmers EF, Stratakis CA. 2006. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res 66:11571–11575 [DOI] [PubMed] [Google Scholar]
- 21. Libé R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, Stratakis CA, Bertherat J. 2008. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res 14:4016–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. 2009. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab 94:2930–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Libé R, Coste J, Guignat L, Tissier F, Lefebvre H, Barrande G, Ajzenberg C, Tauveron I, Clauser E, Dousset B, Bertagna X, Bertherat J, Groussin L. 2010. Aberrant cortisol regulations in bilateral macronodular adrenal hyperplasia: a frequent finding in a prospective study of 32 patients with overt or subclinical Cushing's syndrome. Eur J Endocrinol 163:129–138 [DOI] [PubMed] [Google Scholar]
- 24. Ragazzon B, Cazabat L, Rizk-Rabin M, Assie G, Groussin L, Fierrard H, Perlemoine K, Martinez A, Bertherat J. 2009. Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF β-stimulated apoptosis in adrenocortical cells. Cancer Res 69:7278–7284 [DOI] [PubMed] [Google Scholar]
- 25. Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. 2004. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279:37215–37218 [DOI] [PubMed] [Google Scholar]
- 26. Landa I, Robledo M. 2011. Association studies in thyroid cancer susceptibility: are we on the right track? J Mol Endocrinol 47:R43–R58 [DOI] [PubMed] [Google Scholar]
- 27. Majnik J, Patocs A, Balogh K, Toth M, Gergics P, Szappanos A, Mondok A, Borgulya G, Panczel P, Prohaszka Z, Racz K. 2006. Overrepresentation of the N363S variant of the glucocorticoid receptor gene in patients with bilateral adrenal incidentalomas. J Clin Endocrinol Metab 91:2796–2799 [DOI] [PubMed] [Google Scholar]
- 28. Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS. 2008. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res 101:36–49 [DOI] [PubMed] [Google Scholar]
- 29. Nakayama T, Asai S, Sato N, Soma M. 2007. PDE4D gene in the STRK1 region on 5q12: susceptibility gene for ischemic stroke. Curr Med Chem 14:3171–3178 [DOI] [PubMed] [Google Scholar]
- 30. Hartong DT, Berson EL, Dryja TP. 2006. Retinitis pigmentosa. Lancet 368:1795–1809 [DOI] [PubMed] [Google Scholar]
- 31. Peiró AM, Tang CM, Murray F, Zhang L, Brown LM, Chou D, Rassenti L, Kipps TA, Insel PA. 2011. Genetic variation in phosphodiesterase (PDE) 7B in chronic lymphocytic leukemia: overview of genetic variants of cyclic nucleotide PDEs in human disease. J Hum Genet 56:676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM, McCann SM, Licinio J. 2006. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci USA 103:15124–15129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao YC, Lin HF, Guo YC, Yu ML, Liu CK, Juo SH. 2010. Sex-differential genetic effect of phosphodiesterase 4D (PDE4D) on carotid atherosclerosis. BMC Med Genet 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homma S, Sakamoto T, Hegab AE, Saitoh W, Nomura A, Ishii Y, Morishima Y, Iizuka T, Kiwamoto T, Matsuno Y, Massoud HH, Massoud HM, Hassanein KM, Sekizawa K. 2006. Association of phosphodiesterase 4D gene polymorphisms with chronic obstructive pulmonary disease: relationship to interleukin 13 gene polymorphism. Int J Mol Med 18:933–939 [PubMed] [Google Scholar]
- 35. DeWan AT, Triche EW, Xu X, Hsu LI, Zhao C, Belanger K, Hellenbrand K, Willis-Owen SA, Moffatt M, Cookson WO, Himes BE, Weiss ST, Gauderman WJ, Baurley JW, Gilliland F, Wilk JB, O'Connor GT, Strachan DP, Hoh J, Bracken MB. 2010. PDE11A associations with asthma: results of a genome-wide association scan. J Allergy Clin Immunol 126:871–873.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horvath A, Korde L, Greene MH, Libe R, Osorio P, Faucz FR, Raffin-Sanson ML, Tsang KM, Drori-Herishanu L, Patronas Y, Remmers EF, Nikita ME, Moran J, Greene J, Nesterova M, Merino M, Bertherat J, Stratakis CA. 2009. Functional phosphodiesterase 11A mutations may modify the risk of familial and bilateral testicular germ cell tumors. Cancer Res 69:5301–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faucz FR, Horvath A, Rothenbuhler A, Almeida MQ, Libé R, Raffin-Sanson ML, Bertherat J, Carraro DM, Soares FA, Molina Gde C, Campos AH, Alexandre RB, Bendhack ML, Nesterova M, Stratakis CA. 2011. Phosphodiesterase 11A (PDE11A) genetic variants may increase susceptibility to prostatic cancer. J Clin Endocrinol Metab 96:E135–E140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Libé R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, Ragazzon B, Guillaud-Bataille M, Groussin L, Clauser E, Raffin-Sanson ML, Siegel J, Moran J, Drori-Herishanu L, Faucz FR, Lodish M, Nesterova M, Bertagna X, Bertherat J, Stratakis CA. 2011. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab 96:E208–E214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rothenbuhler A, Horvath A, Libé R, Faucz FR, Fratticci A, Raffin Sanson ML, Vezzosi D, Azevedo M, Levy I, Almeida MQ, Lodish M, Nesterova M, Bertherat J, Stratakis CA. 2012. Identification of novel genetic variants in phosphodiesterase 8B (PDE8B), a cAMP specific phosphodiesterase highly expressed in the adrenal cortex, in a cohort of patients with adrenal tumors. Clin Endocrinol (Oxf) 77:195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuasa K, Omori K, Yanaka N. 2000. Binding and phosphorylation of a novel male germ cell-specific cGMP-dependent protein kinase-anchoring protein by cGMP-dependent protein kinase Iα. J Biol Chem 275:4897–4905 [DOI] [PubMed] [Google Scholar]