Abstract
Context:
Studies in rodents have demonstrated that sympathetic activity reduces bone formation and bone mass; these effects are mediated by the noncollagenous matrix protein, osteopontin.
Objective:
The objective of the study was to relate sympathetic activity (measured using microneurography at the peroneal nerve) to bone microstructure (assessed by high resolution peripheral quantitative computed tomography), bone turnover, and plasma osteopontin levels.
Design, Setting, and Patients:
Twenty-three women aged 20–72 yr (10 premenopausal and 13 postmenopausal) were studied in the Clinical Research Unit.
Results:
Sympathetic activity (bursts per 100 heart beats) was 2.4-fold higher in postmenopausal as compared with premenopausal women (P < 0.001). In the two groups combined and after age adjustment, sympathetic activity was inversely correlated with trabecular bone volume fraction (r = −0.55, P < 0.01) and thickness (r = −0.59, P < 0.01) and positively correlated with trabecular separation (r = 0.45, P < 0.05). Sympathetic activity was negatively correlated with serum amino-terminal propeptide of type I collagen in postmenopausal women (r = −0.65, P = 0.015), with a similar trend in premenopausal women (r = −0.58, P = 0.082). Sympathetic activity was also negatively correlated with plasma osteopontin levels (r = −0.43, P = 0.045), driven mainly by the correlation in postmenopausal women (r = −0.76, P = 0.002).
Conclusion:
These findings represent the first demonstration in humans of a relationship between sympathetic activity and bone microstructure and circulating levels of amino-terminal propeptide of type I collagen and osteopontin. Given the critical role of osteopontin in mediating the effects of β-adrenergic signaling on bone, the inverse association between sympathetic activity and plasma osteopontin levels may reflect a negative feedback loop to limit the deleterious effects of sympathetic activity on bone metabolism. Based on the higher sympathetic activity observed in postmenopausal women, additional human studies are needed to define the role of increased sympathetic activity in mediating postmenopausal bone loss.
There is considerable evidence from mouse models that the sympathetic nervous system (SNS) communicates peripherally with osteoblasts to regulate bone mass and metabolism (1–3). The SNS targets osteoblasts primarily via β2-adrenergic receptors (4), although both β1- and β3-adrenergic receptors have also been identified in some cell preparations (5). Deletion of β2-adrenergic receptors in mice results in increased bone mass and higher trabecular bone volume fraction (2, 6), whereas mice treated with β-adrenergic receptor agonists (1, 3) experience trabecular bone loss. Recent studies have also shown that the bone matrix protein, osteopontin, is required for the effects of β-adrenergic signaling in bone because β-adrenergic stimulation has no effect on bone mass in osteopontin knockout mice (3). Acting via intracellular osteopontin, which modulates cAMP levels, β-adrenergic signaling regulates protein products, reflecting increased bone resorption [e.g. cross-linked C-telopeptide of type I collagen (CTX) and receptor activator of nuclear factor-κB ligand (RANKL) (2)] and decreased bone formation [e.g. aminoterminal propeptide of type I collagen (PINP)] and, collectively, contribute to a deficient trabecular bone phenotype (3). Despite these known effects from rodent studies, however, the role of sympathetic activity in regulating bone metabolism in humans is not well characterized.
β-Blockers have been used widely clinically to suppress effects of the SNS, but evidence that they prevent bone loss or fractures has not been consistent (7, 8). The inconsistent findings may reflect the fact that β1- and β2-adrenergic receptor subtypes exert opposite effects on bone remodeling (9) and that lower doses of nonselective (10) and selective (11) β-blockers have been shown to be more effective in preventing bone loss in animals than higher doses. Nonetheless, given the widespread use of β-blockers and their therapeutic potential in the prevention of osteoporosis, it is important, based on the rodent studies, to better understand the relationship between sympathetic activity and bone metabolism in humans. Thus, the purposes of our study were to determine: 1) whether sympathetic neural activity is associated with altered microstructure and biomechanical properties of bone in women; and 2) whether circulating levels of markers of bone turnover (PINP, CTX), regulators of bone turnover [RANKL and its decoy receptor, osteoprotegrin (OPG)] (12), or a mediator of the effects of sympathetic outflow on bone (osteopontin) (3) are associated with sympathetic activity. In addition, given the importance of sclerostin in regulating bone turnover (13, 14), we also measured circulating sclerostin levels in the study subjects and tested for any association with sympathetic activity.
Subjects and Methods
Study subjects
The study was approved by the Mayo Clinic Institutional Review Board, and informed written consent was obtained from all 23 healthy participants. The sample included 10 pre- and 13 postmenopausal women between the ages of 20 and 72 yr. We defined menopause as no menses for 1 yr or more. Subjects were rigorously screened for coexisting disease, and candidates were excluded if they had any acute or chronic disorders associated with alterations in skeletal or cardiovascular structure or function. Anthropometric data were collected on all women, and the subjects were nonobese [body mass index (BMI) <30 kg/m2]; were not smokers; were not diabetic; were normally active (i.e. neither sedentary nor highly exercise trained); were normotensive (systolic blood pressure <130 mm Hg, diastolic blood pressure <90 mm Hg); and were not currently taking hormone replacement therapy, antihypertensive drugs, or other medications, except for oral contraceptives. No subject had ever used any therapies likely to affect the bone such as sodium fluoride, calcitonin, bisphosphonates, or antiepileptic drugs.
Sympathetic activity measurements
The sympathetic activity measurements for this study were performed by N.C. and colleagues, who have extensive experience using microneurography (15–17). Details regarding the sympathetic activity measurements have been described previously (17) and are summarized here briefly. After local anesthesia (2% lidocaine), a 20-gauge, 5-cm catheter was placed in the brachial artery of the nondominant arm using ultrasound guidance under aseptic conditions for the continuous measurement of mean arterial pressure (millimeters of mercury). The catheter was connected to a pressure transducer, which was positioned at heart level and connected to a computer to monitor mean arterial pressure. Microneurographic recordings of sympathetic nerve activity (bursts per 100 heart beats) were recorded from the peroneal nerve posterior to the fibular head using insulated tungsten microelectrodes (18). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses, and no afferent neural response was evoked by skin stimuli (15, 16). The recorded signal was amplified 80,000-fold, band-pass filtered (700–2000 Hz), rectified, and integrated (resistance-capacitance integrator circuit, time constant 0.1 sec) by a nerve-traffic analyzer. This technique has been in use worldwide since the early 1970's and is well validated (18). Note that the sympathetic nerve activity measurements were done as part of another protocol (17), and 23 of these subjects were reenrolled within 6–24 months of those measurements for skeletal phenotyping and blood draws (see below). All of the procedures were performed in the Clinical Research Unit at the Mayo Clinic.
High-resolution peripheral quantitative computed tomography (HRpQCT) measurements
As described previously (19–23), the Xtreme CT (Scanco Medical AG, Brüttisellen, Switzerland) was used to assess cortical and trabecular bone microstructure and volumetric bone mineral density (vBMD) at distal sites of the nondominant radius and tibia of all subjects. In addition to the standard trabecular parameters [bone volume/tissue volume (BV/TV; percentage), trabecular number (TbN; 1/millimeter), trabecular thickness (TbTh; millimeters), and trabecular separation (TbSp; millimeters], additional parameters obtained included connectivity density (Conn Dens; 1/cubic millimeter) and structure model index (SMI), which indicates whether the trabeculae are more platelike (lower values) or rodlike (higher values).
For the cortical parameters, the cortex was segmented from the grayscale image with a Gaussian filter and threshold (21). Recognizing that the default cortical bone analysis performs poorly for subjects with thin or porous cortices (24, 25), we used the extended cortical analysis available from the manufacturer to obtain cortical vBMD (Ct vBMD; milligrams per cubic centimeter), cortical thickness (CtTh; millimeters), endocortical circumference (EC; millimeters), and periosteal circumference (PC; millimeters). Furthermore, we derived cortical pore volume (CtPoV; cubic millimeters) and cortical porosity index (CtPo; percentage) using a validated approach described in detail by Burghardt et al. (26) that has been used by several groups (27, 28), including our own (23). CtPoV is a direct voxel-based measure of the volume of the intracortical pore space, whereas CtPo is a measure of the volume of the intracortical pore space normalized by the sum of the pore and cortical bone volumes. A single operator performed all HRpQCT scans and analyses. Short-term precision (coefficients of variation) of the HRpQCT device in our laboratory has been reported previously (19), based on repeat measures on 20 volunteers on the same day after repositioning.
Microfinite element (μFE) analysis
Linear μFE models of the distal radius and tibia were created directly from the HRpQCT images using software provided by the manufacturer (μFE element analysis solver version 1.15; Scanco Medical AG). Failure loads calculated from such μFE models have been shown to correlate highly (r = 0.87) with compressive loads producing Colles' fractures in cadaveric forearms (29).
Dual-energy x-ray absorptiometry (DXA) measurements
The areal BMD (aBMD, grams per square centimeter) was assessed from DXA scans (Lunar Prodigy System; GE Healthcare, Madison, WI) performed on the nondominant radius (total radius), the nondominant hip [femoral neck (FN)], and the lumbar spine (L1-L4).
Biochemical and bone turnover measurements
Fasting blood samples were obtained on all subjects at the time of the HRpQCT measurements. Assays were performed in serum/plasma that had been stored at −80 C. Bone formation was assessed by serum PINP as measured by a double-antibody RIA [within assay coefficient of variation (CV) of 7.5% and between assay CV of 6.5%; DiaSorin, Stillwater, MN], whereas bone resorption was evaluated by serum CTX as measured by an ELISA (within assay CV of 4.6% and between-assay CV of 8.0%; Roche Diagnostics, Indianapolis, IN).
Plasma osteopontin (OPN) concentrations were measured by ELISA (within assay CV of 3.2%, between assay CV of 5.9%; R&D Systems Inc., Minneapolis, MN). Serum total soluble RANKL (sRANKL) (within assay CV of 3.2%, between assay CV of 8.2%; ALPCO Diagnostics, Salem, NH), serum OPG (within assay CV of 7.0%, between assay CV of 7.5%; ALPCO Diagnostics), and serum sclerostin (within assay CV of 5.0%, between assay CV of 4.0%; ALPCO Diagnostics) were measured by ELISA. All assays were performed according to the manufacturers' instructions. The 25-hydroxyvitamin D [25(OH)D] (within assay CV of 2.4%, between assay CV of 6.8%) was measured using liquid chromatography-tandem mass spectrometry (API 5000; Applied Biosystems-MDS Sciex, Foster City, CA).
Statistical analyses
Because some data were not normally distributed, descriptive characteristics, bone parameters, and serum/plasma markers were summarized using medians and interquartile ranges (IQR). Comparisons between pre- and postmenopausal women were made using the Wilcoxon rank-sum test. Unadjusted and age-adjusted Spearman's correlations were used to describe the relationships between sympathetic activity and bone variables and serum/plasma markers. Scatter plots were fitted with robust regression lines to show the relationships between sympathetic activity and the serum markers (i.e. PINP and OPN). Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). A P-value <0.05 was considered significant.
Results
Clinical characteristics of the subjects are shown in Table 1. The pre- and postmenopausal women were similar in height, weight, and BMI. Sympathetic activity levels were significantly higher in post- compared with premenopausal women (Table 1). Consequently, we examined the relationships between sympathetic activity and bone parameters and serum/plasma markers using both unadjusted and age-adjusted analyses.
Table 1.
Clinical characteristics and biochemical markers of premenopausal (PRE) and postmenopausal (POST) women and all women combined
| All (n = 23) | PRE (n = 10) | POST (n = 13) | P value | |
|---|---|---|---|---|
| Variables | ||||
| Age (yr) | 55.1 (29.3–60.1) | 28.8 (24.3–31.0) | 57.3 (55.4–62.8) | <0.001 |
| Height (cm) | 1.66 (1.62–1.73) | 1.72 (1.62–1.74) | 1.65 (1.62–1.70) | 0.306 |
| Weight (kg) | 66.9 (62.1–72.3) | 70.1 (62.1–73.3) | 66.0 (62.4–70.0) | 0.438 |
| BMI (kg/m2) | 24.2 (22.4–25.5) | 24.7 (22.9–25.0) | 23.8 (22.4–26.0) | 0.926 |
| Sympathetic activity (bursts per 100 heart beats) | 48.9 (29.5–64.1) | 24.8 (11.1–34.3) | 59.1 (50.4–68.1) | <0.001 |
| Bone turnover markers | ||||
| PINP (μg/liter) | 49.5 (36.9–81.6) | 39.7 (35.0–82.7) | 52.0 (44.8–77.1) | 0.556 |
| CTX (ng/ml) | 0.46 (0.33–0.69) | 0.34 (0.29–0.47) | 0.55 (0.36–0.69) | 0.182 |
| Regulatory markers | ||||
| OPN (ng/ml) | 67.9 (58.9–80.7) | 64.8 (59.0–73.5) | 73.8 (58.2–84.4) | 0.556 |
| Total sRANKL (pmol/liter) | 844 (183–7330) | 539 (220–7330) | 1562 (100–5866) | 0.975 |
| OPG (pmol/liter) | 4.4 (3.2–5.4) | 3.4 (2.8–5.8) | 4.6 (3.9–5.4) | 0.278 |
| Sclerostin (pmol/liter) | 32.3 (24.9–40.1) | 26.6 (14.2–29.0) | 36.7 (34.8–40.5) | 0.006 |
| Total 25(OH)D (ng/ml) | 36.0 (31.0–43.0) | 34.5 (30.0–42.0) | 39.0 (34.0–43.0) | 0.514 |
Data are shown as median (IQR).
Table 2 summarizes regional aBMD and the HRpQCT-derived cross-sectional geometry, microarchitectural, and biomechanical indices of the distal radius and tibia in all subjects combined as well as in pre- and postmenopausal women separately. The postmenopausal women had lower regional aBMD by DXA and tended to have deficits in bone microstructure at the distal radius and tibia compared with the premenopausal women, owing mainly to loss of trabeculae, higher trabecular separation, and higher cortical porosity. In addition, indices of bone strength (stiffness, apparent modulus, and failure load) determined by μFE modeling of an axial compressive load were significantly higher in pre- as compared with postmenopausal women (+17 to 28%; P < 0.01).
Table 2.
DXA regional aBMD and HRpQCT trabecular and cortical bone parameters of premenopausal (PRE) and postmenopausal (POST) women and all women combined
| All (n = 23) | PRE (n = 10) | POST (n = 13) | P value | |
|---|---|---|---|---|
| DXA regional aBMD | ||||
| FN (g/cm2) | 0.94 (0.80–1.06) | 1.07 (1.02–1.13) | 0.84 (0.77–0.94) | <0.001 |
| Spine L1-L4 (g/cm2) | 1.17 (1.00–1.29) | 1.26 (1.17–1.44) | 1.04 (0.94–1.07) | 0.004 |
| Total radius (g/cm2) | 0.62 (0.55–0.67) | 0.68 (0.65–0.72) | 0.58 (0.50–0.62) | <0.001 |
| Radius HRpQCT | ||||
| BV/TV | 0.122 (0.107–0.158) | 0.149 (0.127–0.165) | 0.108 (0.094–0.114) | 0.001 |
| TbN (1/mm) | 1.79 (1.53–2.03) | 1.97 (1.83–2.13) | 1.72 (1.43–1.79) | 0.005 |
| TbTh (mm) | 0.069 (0.063–0.076) | 0.074 (0.069–0.078) | 0.063 (0.060–0.069) | 0.011 |
| TbSp (mm) | 0.494 (0.416–0.585) | 0.434 (0.391–0.477) | 0.511 (0.499–0.646) | 0.003 |
| Conn Dens (1/mm3) | 2.64 (2.22–3.31) | 3.02 (2.57–3.60) | 2.44 (1.45–2.78) | 0.012 |
| SMI | 2.78 (2.10–2.98) | 2.55 (2.08–2.82) | 2.87 (2.68–3.22) | 0.088 |
| Ct vBMD (mg/cm3) | 964 (923–983) | 976 (930–984) | 952 (870–967) | 0.100 |
| CtTh (mm) | 0.94 (0.79–1.06) | 0.97 (0.93–1.12) | 0.89 (0.76–1.03) | 0.100 |
| EC (mm) | 49 (44–53) | 48 (44–53) | 49 (48–53) | 0.687 |
| PC (mm) | 67 (63–71) | 67 (63–71) | 67 (64–69) | 0.951 |
| CtPoV (mm3) | 7.1 (5.2–9.9) | 5.6 (3.3–7.9) | 9.6 (6.7–12.7) | 0.010 |
| CtPo (%) | 1.40 (0.89–2.26) | 0.89 (0.71–1.50) | 2.17 (1.34–2.45) | 0.005 |
| Stiffness, K (kN/mm) | 76 (59–86) | 78 (77–93) | 61 (59–75) | 0.007 |
| E (MPa) | 1,888 (1700–2076) | 2,055 (1,965–2,312) | 1,741 (1551–1888) | 0.008 |
| Failure load (n) | 3,771 (2,990–4,380) | 4,008 (3,935–4,689) | 3,020 (2,867–3,721) | 0.010 |
| Tibia HRpQCT | ||||
| BV/TV | 0.14 (0.121–0.157) | 0.154 (0.144–0.168) | 0.132 (0.108–0.140) | 0.011 |
| TbN (1/mm) | 1.82 (1.67–2.00) | 1.89 (1.81–2.17) | 1.78 (1.64–1.95) | 0.182 |
| TbTh (mm) | 0.081 (0.066–0.086) | 0.083 (0.077–0.093) | 0.070 (0.065–0.084) | 0.107 |
| TbSp (mm) | 0.474 (0.419–0.524) | 0.443 (0.381–0.474) | 0.497 (0.442–0.534) | 0.067 |
| Conn Dens (1/mm3) | 3.19 (2.82–4.03) | 3.42 (3.12–4.15) | 2.99 (2.82–3.37) | 0.182 |
| SMI | 1.55 (1.25–2.22) | 1.34 (1.18–1.89) | 1.72 (1.35–2.27) | 0.145 |
| Ct vBMD (mg/cm3) | 878 (823–945) | 945 (902–965) | 829 (811–866) | <0.001 |
| CtTh (mm) | 1.15 (0.99–1.34) | 1.31 (1.04–1.54) | 1.03 (0.99–1.20) | 0.077 |
| EC (mm) | 87 (84–93) | 88 (75–94) | 87 (85–92) | 0.733 |
| PC (mm) | 106 (101–112) | 107 (96–112) | 106 (103–111) | 0.852 |
| CtPoV (mm3) | 46.9 (35.1–62.1) | 35.9 (29.6–42.4) | 56.6 (52.8–66.8) | 0.007 |
| CtPo (%) | 5.15 (2.97–6.84) | 2.98 (2.48–4.32) | 6.46 (6.14–7.55) | 0.001 |
| Stiffness (kn/mm) | 191 (152–217) | 218 (207–248) | 172 (142–178) | 0.001 |
| E (MPa) | 2,042 (1,762–2359) | 2,366 (2,089–2,671) | 1,775 (1,727–2,033) | 0.001 |
| Failure load (n) | 9,575 (7,773–11,213) | 11,245 (10,164–12,376) | 8,598 (7,099–9,132) | 0.001 |
Data are shown as median (IQR).
At the distal radius, in both unadjusted and age-adjusted analyses, significant negative correlations were observed between sympathetic activity and trabecular BV/TV as well as trabecular thickness, whereas significant positive correlations were observed between sympathetic activity and trabecular separation and SMI (Table 3; for plots of BV/TV and trabecular thickness vs. sympathetic activity, see Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Inverse correlations were also observed between sympathetic activity and trabecular number and connectivity density at the distal radius: these correlations were attenuated after adjustment for age but remained marginally significant. No significant relationships were found between cortical vBMD, cortical thickness, endocortical circumference, periosteal circumference, and cortical pore volume at the radius and sympathetic activity. In contrast, the cortical porosity index at the radius was positively correlated with sympathetic activity, although this relationship was no longer significant after adjustment for age.
Table 3.
Spearman's ρ (r) correlation coefficients for relationships between sympathetic activity and bone parameters in premenopausal (PRE) and postmenopausal (POST) women and all women combined
| All unadjusted (n = 23) |
All age-adjusted (n = 23) |
PRE (n = 10) |
POST (n = 13) |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | r | P value | |
| DXA regional aBMD | ||||||||
| FN (g/cm2) | −0.67 | <0.001 | −0.28 | 0.201 | −0.43 | 0.215 | 0.10 | 0.734 |
| Spine L1-L4 (g/cm2) | −0.45 | 0.030 | 0.01 | 0.967 | −0.27 | 0.446 | 0.26 | 0.384 |
| Total radius (g/cm2) | −0.75 | <0.001 | −0.49 | 0.022 | −0.55 | 0.098 | −0.10 | 0.748 |
| Radius HRpQCT | ||||||||
| BV/TV | −0.73 | <0.001 | −0.55 | 0.009 | −0.30 | 0.403 | −0.37 | 0.219 |
| TbN (1/mm) | −0.63 | 0.001 | −0.38 | 0.077 | −0.30 | 0.405 | −0.31 | 0.306 |
| TbTh (mm) | −0.66 | <0.001 | −0.59 | 0.004 | −0.69 | 0.027 | −0.33 | 0.266 |
| TbSp (mm) | 0.67 | <0.001 | 0.45 | 0.036 | 0.30 | 0.405 | 0.42 | 0.156 |
| Conn Dens (1/mm3) | −0.59 | 0.003 | −0.40 | 0.062 | −0.16 | 0.652 | −0.31 | 0.297 |
| SMI | 0.48 | 0.022 | 0.46 | 0.030 | 0.44 | 0.200 | 0.23 | 0.448 |
| Ct vBMD (mg/cm3) | −0.28 | 0.190 | −0.16 | 0.481 | −0.20 | 0.580 | 0.09 | 0.762 |
| CtTh (mm) | −0.30 | 0.165 | −0.05 | 0.840 | −0.41 | 0.244 | 0.12 | 0.707 |
| EC (mm) | 0.12 | 0.575 | 0.16 | 0.487 | 0.21 | 0.556 | 0.05 | 0.873 |
| PC (mm) | 0.02 | 0.913 | 0.16 | 0.478 | 0.27 | 0.455 | −0.10 | 0.748 |
| CtPoV (mm3) | 0.37 | 0.084 | −0.18 | 0.425 | −0.43 | 0.215 | 0.00 | 1.000 |
| CtPo (%) | 0.45 | 0.030 | −0.11 | 0.612 | −0.14 | 0.701 | −0.05 | 0.859 |
| Stiffness (kn/mm) | −0.51 | 0.012 | −0.24 | 0.276 | −0.54 | 0.108 | 0.01 | 0.986 |
| E (MPa) | −0.55 | 0.007 | −0.45 | 0.035 | −0.24 | 0.511 | −0.23 | 0.448 |
| Failure load (n) | −0.50 | 0.016 | −0.22 | 0.318 | −0.50 | 0.138 | −0.05 | 0.873 |
| Tibia HRpQCT | ||||||||
| BV/TV | −0.43 | 0.040 | −0.24 | 0.289 | 0.04 | 0.907 | 0.18 | 0.566 |
| TbN (1/mm) | −0.16 | 0.460 | 0.17 | 0.449 | 0.22 | 0.532 | 0.18 | 0.553 |
| TbTh (mm) | −0.34 | 0.112 | −0.34 | 0.117 | −0.41 | 0.244 | −0.01 | 0.964 |
| TbSp (mm) | 0.25 | 0.241 | −0.08 | 0.719 | −0.10 | 0.777 | −0.22 | 0.464 |
| Conn Dens (1/mm3) | −0.23 | 0.297 | −0.05 | 0.810 | 0.03 | 0.934 | 0.18 | 0.553 |
| SMI | 0.37 | 0.080 | 0.38 | 0.077 | 0.33 | 0.347 | 0.15 | 0.616 |
| Ct vBMD (mg/cm3) | −0.62 | 0.002 | −0.12 | 0.601 | −0.05 | 0.881 | 0.09 | 0.762 |
| CtTh (mm) | −0.35 | 0.104 | −0.08 | 0.720 | −0.31 | 0.385 | 0.07 | 0.831 |
| EC (mm) | 0.08 | 0.733 | 0.07 | 0.742 | 0.21 | 0.556 | 0.07 | 0.817 |
| PC (mm) | 0.02 | 0.916 | 0.04 | 0.848 | 0.09 | 0.803 | 0.13 | 0.674 |
| CtPoV (mm3) | 0.29 | 0.174 | −0.36 | 0.096 | −0.36 | 0.310 | −0.43 | 0.144 |
| CtPo (%) | 0.45 | 0.030 | −0.34 | 0.119 | −0.32 | 0.366 | −0.27 | 0.364 |
| Stiffness (kn/mm) | −0.61 | 0.002 | −0.28 | 0.208 | −0.36 | 0.310 | −0.09 | 0.775 |
| E (MPa) | −0.62 | 0.002 | −0.33 | 0.133 | −0.16 | 0.651 | −0.10 | 0.748 |
| Failure load (n) | −0.59 | 0.003 | −0.25 | 0.265 | −0.19 | 0.603 | −0.09 | 0.775 |
Values are presented as r and P values. Statistically significant correlations are shown in bold.
Sympathetic activity was also inversely correlated with the indices of bone strength at the distal radius, although these associations were attenuated after adjustment for age (Table 3). Nonetheless, even after adjusting for age, the negative correlation between sympathetic activity and apparent modulus (E; stiffness corrected for cross-sectional area) remained statistically significant (r = −0.45; P = 0.035).
At the distal tibia, the correlations between sympathetic activity and bone parameters were similar to those observed at the distal radius, but the magnitude of these correlations tended to be lower and not significant following adjustment for age (Table 3). Significant inverse correlations were observed between sympathetic activity and regional aBMD of the FN, total femur, spine, total radius, and total body (Table 3), but only the correlation between sympathetic activity and aBMD of the total radius remained significant following adjustment for age. Separate analyses within pre- and postmenopausal groups resulted in correlation coefficients between sympathetic activity and bone parameters that were similar in magnitude and direction to those observed in all women combined (Table 3).
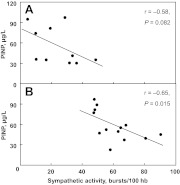
We next examined the relationships between sympathetic activity and serum/plasma markers in all women combined and separately in pre- and postmenopausal women. As shown in Table 4, sympathetic activity was inversely correlated with PINP, although in all women combined, these correlations did not reach statistical significance. After separating the women based on menopausal status, a significant inverse correlation was observed between sympathetic activity and serum PINP in postmenopausal women, with a similar trend in premenopausal women (Fig. 1). Sympathetic activity was not significantly correlated with bone resorption as measured by serum CTX levels.
Table 4.
Spearman's ρ (r) correlation coefficients for relationships between sympathetic activity and biochemical markers in premenopausal (PRE) and postmenopausal (POST) women and all women combined
| All unadjusted (n = 23) |
All age-adjusted (n = 23) |
PRE (n = 10) |
POST (n = 13) |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | r | P value | |
| Bone turnover markers | ||||||||
| PINP (μg/liter) | −0.21 | 0.340 | −0.32 | 0.147 | −0.58 | 0.082 | −0.65 | 0.015 |
| CTX (ng/ml) | −0.02 | 0.929 | −0.19 | 0.399 | −0.39 | 0.260 | −0.49 | 0.086 |
| Regulatory markers | ||||||||
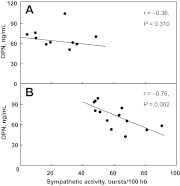
| OPN (ng/ml) | −0.21 | 0.326 | −0.43 | 0.045 | −0.36 | 0.310 | −0.76 | 0.002 |
| Total sRANKL (pmol/liter) | −0.15 | 0.485 | −0.34 | 0.121 | −0.10 | 0.777 | −0.28 | 0.353 |
| OPG (pmol/liter) | 0.08 | 0.710 | −0.06 | 0.789 | −0.35 | 0.328 | −0.12 | 0.707 |
| Sclerostin (pmol/liter) | 0.49 | 0.018 | 0.15 | 0.507 | 0.54 | 0.108 | −0.26 | 0.384 |
| Total 25(OH)D (ng/ml) | 0.24 | 0.276 | 0.28 | 0.208 | 0.79 | 0.006 | −0.22 | 0.462 |
Values are presented as r and P values. Statistically significant correlations are shown in bold.
Fig. 1.
Scatter plots fitted with robust regression lines showing the relationships between sympathetic activity and PINP in the pre- (A) and postmenopausal (B) women. The Spearman's rho (r) correlation coefficients and P values are shown for each group.
After adjusting for age in all women, sympathetic activity was inversely correlated with plasma OPN levels (Table 4). This relationship was driven mainly by the correlation in postmenopausal women (Fig. 2). Correlations between sympathetic activity and total sRANKL and OPG were not statistically significant. In all women combined, sympathetic activity was positively correlated with serum sclerostin levels, although this relationship was not statistically significant following adjustment for age. In all women combined, sympathetic activity was not significantly correlated with serum 25(OH)D levels, unadjusted or after adjusting for age (Table 4). After separating the women based on menopausal status, sympathetic activity was positively correlated with serum 25(OH)D levels in premenopausal women, whereas the correlation between sympathetic activity and serum 25(OH)D levels in postmenopausal women was not statistically significant.
Fig. 2.
Scatter plots fitted with robust regression lines showing the relationships between sympathetic activity and OPN in the pre- (A) and postmenopausal (B) women. The Spearman's rho (r) correlation coefficients and P values are shown for each group.
After adjusting for age in all women, plasma OPN levels were significantly correlated with serum PINP levels (r = 0.76, P < 0.001). In addition, after adjusting for age, serum sclerostin levels were not significantly correlated with serum PINP (r = 0.26, P = 0.236) or plasma OPN levels (r = 0.37, P = 0.088).
Discussion
We report, for the first time in humans, that sympathetic activity is inversely associated with trabecular microstructure, compressive bone strength, serum PINP, and plasma osteopontin levels. Our findings in humans are consistent with studies in mice (1–3) demonstrating that excessive sympathetic activity inhibits bone formation and has detrimental effects on trabecular bone microstructure. In addition, given the critical role of osteopontin in mediating effects of β-adrenergic signaling in bone cells (3), the inverse association between sympathetic outflow and plasma osteopontin levels that we observed may reflect a negative feedback loop to limit the deleterious effects of sympathetic activity on bone metabolism.
The previous data in mice, in combination with our current data in humans, may have important therapeutic implications for osteoporosis. Indeed, β-blockers have been shown to increase bone formation and mass in rodents (1, 10, 11) and could represent potential anabolic drugs for safely stimulating bone formation in humans. However, whether the effects of β-blockers observed in animals are also present in humans remains to be seen. As noted earlier, Reid et al. (30) compared 160 mg/d of propranolol to placebo over 3 months and found that this dose of a nonselective β-blocker did not stimulate bone formation. In addition, whereas some epidemiological studies support a protective effect of β-blockers on fractures (7), others suggest that the evidence is lacking (8). The inconsistent findings may be explained by pharmacological studies in rodents that have demonstrated that lower doses of β-blockers are more effective in preventing bone loss than higher doses (10, 11). The precise reasons for this are unclear, but it may have to do with the fact that at lower doses, these nonselective β-blockers may principally antagonize the β2-receptors, whereas other β-adrenergic receptor subtypes may be affected at the higher doses. Clearly, further studies are needed to test the impact of different doses of β-blockers on osteopontin and other mediators/outcome parameters related to the effects of sympathetic outflow on bone and to further define mechanism for the possible dose-related effects of β-blockers on bone.
One notable finding of the present study was the stronger correlations observed between sympathetic activity and bone parameters at the radius as compared with the tibia, which suggests that mechanical loading may suppress the deleterious effects of the SNS on bone. This explanation is supported by a recent study in mice demonstrating that β-adrenergic receptor inhibition exerts a predominant anabolic stimulus in response to mechanical stimulation (9). Another notable finding was the stronger correlations we observed between sympathetic activity and trabecular as compared with cortical bone parameters. The reason for this is not clear, although Reid (31) has hypothesized that the SNS may have dual actions on different skeletal compartments due to differential innervation of these skeletal regions by sympathetic nerve fibers. This hypothesis is supported by the present study in humans and by studies in mice treated with β-adrenergic agonists (isoproterenol) that exhibit similar skeletal heterogeneity (1, 3). Importantly, the results from the μFE models, which estimate bone biomechanical properties (i.e. bone strength) in response to a simulated axial compression test (29), support the deleterious impact of excessive sympathetic outflow on bone strength. Indeed, sympathetic activity was inversely associated with apparent modulus (stiffness corrected for cross-sectional area) of the distal radius.
Using cell-based and murine genetics approaches, a recent study demonstrated that the administration of a β2-adrenergic agonist (isoproterenol) increased the expression of osteopontin in plasma and bone and that osteopontin was necessary for the suppression of bone mass by the SNS (3). In contrast to that study in mice, the present study showed that sympathetic activity was inversely associated with plasma osteopontin levels, driven mainly by the strong negative correlation in postmenopausal women. The reason for this difference between mice and humans is not apparent but could reflect a negative feedback loop to limit the deleterious effects of sympathetic activity on bone metabolism. Further studies are needed to test this hypothesis.
We have previously described the relationship between serum sclerostin levels and bone parameters and turnover markers in men and women (14). In the present study, we examined the relationship between serum sclerostin levels and sympathetic activity. Our data suggest that the effects of the SNS on bone are not mediated by sclerostin. Indeed, the association between sclerostin levels and sympathetic activity was not significant after adjusting for age. Nonetheless, the data from the present study are consistent with our previous work (14) demonstrating that serum sclerostin levels increase with age in women.
In mice, sympathetic signaling through β2-adrenergic receptors on osteoblasts has been shown to trigger an increase in the circulating levels of RANKL (2). However, in the present study, we did not detect a relationship between sympathetic activity and the RANKL/OPG cytokine system in women. There may be several reasons for this occurrence. First, the nonosseous sources of RANKL and OPG may account for a significant proportion of these cytokines in the circulation (32). Second, the levels of these cytokines in serum may not mirror their levels or activity in the bone microenvironment (32). Third, the commercially available assays for OPG detect all forms of OPG, rather than the biologically active dimeric form (33). Lastly, serum RANKL does not include the majority of RANKL, which is cell bound and can be measured only after a bone biopsy (34).
The impact of hormonal fluctuations during the menstrual cycle and oral contraceptive (OC) use on sympathetic activity and the regulation of the cardiovascular system have been areas of recent investigation. Within the premenopausal group of the present study, OC use was not associated with differences in sympathetic activity, bone parameters or markers of bone turnover (PINP and CTX) (data not shown). This finding is consistent with a previous study (35) that showed a minimal effect of OC use on sympathetic activity. In addition, another study (36) showed no difference in sympathetic activity between the early follicular and midluteal phases of the menstrual cycle in young women, suggesting that hormonal fluctuations that occur during the normal menstrual cycle have a minimal effect on sympathetic activity.
Our study has a number of strengths and limitations. The primary strengths include the use of HRpQCT, inclusion of μFE modeling, and the sophisticated microneurography recordings of sympathetic activity. However, because of the invasive nature of performing microneurography in humans, our sample size was relatively small. We sought to minimize this limitation by analyzing the data using Spearman's correlations, which are appropriate for smaller sample sizes and more robust to potential outliers. To increase power, for certain analyses we combined the pre- and postmenopausal groups but based conclusions only on age-adjusted analyses of the combined groups. We recognize that additional studies involving larger numbers of pre- and postmenopausal women are needed to further validate our findings. In addition, our data are cross-sectional and therefore need to be confirmed by longitudinal studies. Furthermore, we acknowledge that the microneurography recordings were performed some time before the skeletal measurements and blood collection. Given the invasive nature of performing microneurography in humans, it was not possible to repeat these measurements. However, it should be noted that sympathetic activity is not expected to change in a significant manner over this period of time (37). Finally, it is important to note that the microneurography recordings from the peroneal were used as a surrogate for whole-body sympathetic activity, and we acknowledge that these recordings are not necessarily equivalent to the sympathetic outflow encountered by bone. Nonetheless, these recordings have been shown to be highly related to whole-body sympathetic activity and to the renal and cardiac sympathetic activity in resting humans, as assessed by norepinephrine spillover (37). Thus, microneurography is still considered the gold standard for directly assessing sympathetic activity in humans.
These limitations notwithstanding, our study does support the hypothesis that sympathetic neural activity regulates bone microstructure and metabolism in humans. When combined with the data demonstrating that mice treated with β-adrenergic receptor agonists (1, 3) experience trabecular bone loss, our study using direct measurements of sympathetic activity in humans suggests that excessive sympathetic outflow may have detrimental consequences for trabecular microstructure and bone strength. Based on the higher sympathetic activity observed in postmenopausal women, our findings point to the need for additional human studies to define the role of increased sympathetic activity in mediating postmenopausal bone loss as well as the potential utility of β-adrenergic blockers (selectivity and dosage) in the treatment of osteoporosis.
Acknowledgments
We thank Sara Achenbach for help with the statistical analyses, Brenda Coates for sample processing, James Peterson for data management, Margaret Holets for performing the HRpQCT scans, and the Mayo Immunochemical Core Laboratory for performance of the biochemical and hormonal assays.
This work was supported by National Institutes of Health Grants AG004875, AR027065, HL083947, T32 DK007352, and UL1 RR024150 (to the Mayo Center for Translational Science Activities).
Disclosure Summary: None of the authors has a conflict to disclose.
Footnotes
- aBMD
- Areal BMD
- BMI
- body mass index
- BV/TV
- bone volume/tissue volume
- Conn Dens
- connectivity density
- CtPo
- cortical porosity index
- CtPoV
- cortical pore volume
- CtTh
- cortical thickness
- Ct vBMD
- cortical vBMD
- CTX
- cross-linked C-telopeptide of type I collagen
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- E
- modulus
- EC
- endocortical circumference
- μFE
- microfinite element
- FN
- femoral neck
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- IQR
- interquartile range
- OC
- oral contraceptive
- 25(OH)D
- 25-hydroxyvitamin D
- OPG
- osteoprotegrin
- OPN
- osteopontin
- PC
- periosteal circumference
- PINP
- aminoterminal propeptide of type I collagen
- RANKL
- receptor activator of nuclear factor-κB ligand
- SMI
- structure model index
- SNS
- sympathetic nervous system
- sRANKL
- soluble RANKL
- TbN
- trabecular number
- TbSp
- trabecular separation
- TbTh
- trabecular thickness
- vBMD
- volumetric bone mineral density.
References
- 1. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- 2. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 3. Nagao M, Feinstein TN, Ezura Y, Hayata T, Notomi T, Saita Y, Hanyu R, Hemmi H, Izu Y, Takeda S, Wang K, Rittling S, Nakamoto T, Kaneko K, Kurosawa H, Karsenty G, Denhardt DT, Vilardaga JP, Noda M. 2011. Sympathetic control of bone mass regulated by osteopontin. Proc Natl Acad Sci USA 108:17767–17772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore RE, Smith CK, 2nd, Bailey CS, Voelkel EF, Tashjian AH., Jr 1993. Characterization of β-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23:301–315 [DOI] [PubMed] [Google Scholar]
- 5. Kellenberger S, Muller K, Richener H, Bilbe G. 1998. Formoterol and isoproterenol induce c-fos gene expression in osteoblast-like cells by activating β2-adrenergic receptors. Bone 22:471–478 [DOI] [PubMed] [Google Scholar]
- 6. Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. 2009. Mice lacking β-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 150:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiens M, Etminan M, Gill SS, Takkouche B. 2006. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260:350–362 [DOI] [PubMed] [Google Scholar]
- 8. Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Bréart G. 2005. β-Blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l'Osteoporose prospective study. J Am Geriatr Soc 53:550–552 [DOI] [PubMed] [Google Scholar]
- 9. Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL. 2012. Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res 27:1252–1262 [DOI] [PubMed] [Google Scholar]
- 10. Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D. 2006. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther 318:1118–1127 [DOI] [PubMed] [Google Scholar]
- 11. Sato T, Arai M, Goto S, Togari A. 2010. Effects of propranolol on bone metabolism in spontaneously hypertensive rats. J Pharmacol Exp Ther 334:99–105 [DOI] [PubMed] [Google Scholar]
- 12. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319 [DOI] [PubMed] [Google Scholar]
- 13. Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869 [DOI] [PubMed] [Google Scholar]
- 14. Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S. 2011. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. 2005. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. 2006. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. 2011. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589:5285–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundlöf G, Wallin BG. 1977. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272:383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., 3rd 2006. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laib A, Hildebrand T, Häuselmann HJ, Rüegsegger P. 1997. Ridge number density: a new parameter for in vivo bone structure analysis. Bone 21:541–546 [DOI] [PubMed] [Google Scholar]
- 21. Laib A, Häuselmann HJ, Rüegsegger P. 1998. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6:329–337 [PubMed] [Google Scholar]
- 22. Laib A, Rüegsegger P. 1999. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24:35–39 [DOI] [PubMed] [Google Scholar]
- 23. Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, 3rd, Khosla S. 2012. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res 27:637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kazakia GJ, Hyun B, Burghardt AJ, Krug R, Newitt DC, de Papp AE, Link TM, Majumdar S. 2008. In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res 23:463–474 [DOI] [PubMed] [Google Scholar]
- 25. Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. 2007. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41:505–515 [DOI] [PubMed] [Google Scholar]
- 26. Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. 2010. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 47:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. 2010. Age- and gender-related differences in the geometric properties and biomechanical significance of intra-cortical porosity in the distal radius and tibia. J Bone Miner Res 25:983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. 2010. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res 25:882–890 [DOI] [PubMed] [Google Scholar]
- 29. Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. 2002. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 30:842–848 [DOI] [PubMed] [Google Scholar]
- 30. Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB. 2005. Effects of a β-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216 [DOI] [PubMed] [Google Scholar]
- 31. Reid IR. 2004. Leptin deficiency: lessons in regional differences in the regulation of bone mass. Bone 34:369–371 [DOI] [PubMed] [Google Scholar]
- 32. Rogers A, Eastell R. 2005. Review: circulating osteoprotegerin and receptor activator for nuclear factor κB ligand: clinical utility in metabolic bone disease assessment. J Clin Endocrinol Metab 90:6323–6331 [DOI] [PubMed] [Google Scholar]
- 33. Chen D, Sarikaya NA, Gunn H, Martin SW, Young JD. 2001. ELISA methodology for detection of modified osteoprotegerin in clinical studies. Clin Chem 47:747–749 [PubMed] [Google Scholar]
- 34. Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. 2003. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111:1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Minson CT, Halliwill JR, Young TM, Joyner MJ. 2000. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102:1473–1476 [DOI] [PubMed] [Google Scholar]
- 36. Minson CT, Halliwill JR, Young TM, Joyner MJ. 2000. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101:862–868 [DOI] [PubMed] [Google Scholar]
- 37. Wallin BG, Charkoudian N. 2007. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve 36:595–614 [DOI] [PubMed] [Google Scholar]