Abstract
Context:
Vitamin D deficiency during pregnancy may be associated with suboptimal fetal growth, but direct evidence is lacking.
Objectives:
The aim of the study was to validate a method for fetal femur volume (FV) measurement using three-dimensional ultrasound and to detect correlations between FV and maternal vitamin D concentration.
Design, Setting, and Participants:
A novel method for assessing FV consists of three ultrasound measurements—femur length, proximal metaphyseal diameter (PMD), and midshaft diameter—and a volume equation; this was validated by comparing ultrasound to computed tomography measurements in six pregnancies after mid-trimester termination. This method was then applied in a cohort of healthy pregnant women participating in the Southampton Women Survey. Fetal three-dimensional ultrasound and maternal 25-hydroxyvitamin D [25(OH)D] levels were performed at 34 wk; dual-energy x-ray absorptiometry of the newborn was performed shortly after birth. Univariate and multiple linear regression analyses were performed between maternal characteristics and fetal outcomes.
Main Outcome Measures:
We performed ultrasound measurements of the fetal femur.
Results:
In 357 pregnant participants, serum 25(OH)D correlated significantly with FV (P = 0.006; r = 0.147) and PMD (P = 0.001; r = 0.176); FV also demonstrated positive univariate correlations with maternal height (P < 0.001; r = 0.246), weight (P = 0.003; r = 0.160), triceps skinfold thickness (P = 0.013; r = 0.134), and a borderline negative effect from smoking (P = 0.061). On multiple regression, independent predictors of FV were the maternal height and triceps skinfold thickness; the effect of 25(OH)D on FV was attenuated, but it remained significant for PMD.
Conclusion:
Using a novel method for assessing FV, independent predictors of femoral size were maternal height, adiposity, and serum vitamin D. Future trials should establish whether pregnancy supplementation with vitamin D is beneficial for the fetal skeleton, using FV and PMD as fetal outcome measures.
Maternal vitamin D deficiency during pregnancy is common in developed countries (1); however its impact on the developing fetus is not clear. If there is a link to suboptimal skeletal growth during intrauterine life, this may be associated with a raised lifetime risk of osteoporosis, as a result of fetal programming (2). Assessment of fetal skeletal size and growth are therefore important, yet relevant fetal biometric markers are lacking. Bone mineral content (BMC) or bone mineral density (BMD) of the newborn can be assessed using dual-energy x-ray absorptiometry (DEXA) (3), but this technique is rather impractical, involves a small amount of radiation, and is only feasible after birth. Birth weight has a strong correlation with neonatal BMC (3, 4) and is often used as a surrogate marker; however, it is a crude measure that is affected not only by bone mass, but also by soft tissue growth. Prenatally, assessment of femur length (FL) using ultrasound is the most commonly used marker of skeletal biometry; it is an integral component of fetal size assessment (5) and weight estimation (6); a short FL is also a possible marker for chromosomal anomalies (7) and fetal growth restriction (8). No significant correlation has been found between FL and maternal vitamin D concentration in adult pregnant women (9); however a positive correlation has been demonstrated in pregnant adolescents (10).
Although not widely used, femur volume (FV) may represent an important bone marker, not only in the context of fetal growth restriction but also as a measure of skeletal health. Volume measurement using three-dimensional (3D) ultrasound can be achieved with multiplanar segmentation, a method requiring manual tracing of the femoral contour on parallel cross-sectional slices (11). However, this technique is time-consuming and prone to interobserver error (12). One way to make volume measurements more reproducible is to use a model that can achieve accurate volume estimation with fewer manual steps. This is conceptually similar to two-dimensional ultrasound where, for example, manual tracing of the head circumference has been abandoned in favor of a much simpler calculation using two intersecting diameters (13); this is based on the assumption that the head has the approximate shape of an ellipse.
The aims of this study were: 1) to describe and validate a simple method for fetal FV measurement using 3D ultrasound; and 2) to detect possible correlations between FV and maternal vitamin D concentration or other maternal predictors of bone size.
Subjects and Methods
This study consisted of two parts: 1) description of a new method for FV measurement in 3D ultrasound and validation using postmortem computed tomograms of fetal femurs after pregnancy termination; and 2) correlation between maternal characteristics, fetal ultrasound measurements at 34 wk gestation, and neonatal DEXA indices shortly after delivery in an observational cohort of healthy pregnant women.
FV method and validation
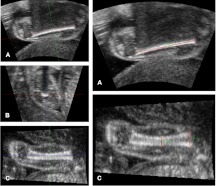
When the femur is visualized in 3D ultrasound from a longitudinal coronal view, both the reference coronal plane (A) and the transverse plane (B) suffer from acoustic shadow (Fig. 1). On the contrary, the reconstructed sagittal plane (C) allows visualization of the femur without shadowing, where both the superior and inferior boundaries are visible (Fig. 1). The A plane can be used for FL measurement according to the principles of two-dimensional ultrasound. The C plane is then selected for measurement of the proximal metaphyseal diameter (PMD) and midshaft diameter (MSD). The distinction between proximal and distal metaphysis is possible on the C plane, because the fetal buttock and knee are easily discernible. These three linear measurements—namely FL, PMD, and MSD (expressed in centimeters)—are then used to estimate FV (expressed in milliliters), on the assumption that the femur is a modified cylinder, narrower at the midshaft and wider at the metaphyses; and that every cross-section is circular. Such a three-dimensional shape is called hyperboloid, and its volume can be calculated using a simple mathematical formula (14):
Fig. 1.
Femur measurement technique using 3D ultrasound and the MITK software (near field at the bottom of the image). Before measurement, the orthogonal orientation axes are aligned with the longitudinal axis of the femur in planes A and C and their intersection positioned at the midshaft (left, A, B, C). The coronal A plane is then used for FL measurement (right A), and the sagittal C plane is used for PMD and MSD measurement (right C).
The validity of this model was tested by comparing the FV measured from 3D ultrasound to the volume calculated from computed tomography (CT). CT permits excellent bone visualization without acoustic artifact (15), and this represented the “gold standard” measurement; however, because it requires ionizing radiation, it cannot be used safely in normal pregnancy. Women undergoing planned medical termination beyond 16 wk gestation for fetal abnormality (but in the absence of skeletal dysplasia) gave their written informed consent to participate in this validation study, which was approved by a regional research ethics committee.
A 3D ultrasound scan of the fetus was performed immediately before the termination by one operator, using the Philips HD-9 ultrasound machine (Philips Ultrasound, Bothell, WA) with a mechanical 3D curvilinear transducer (Philips V7–2). Routine care for pregnancy termination included transabdominal fetocide for gestations over 21 wk plus 6 d, administration of antiprogestogens and oxytocic agents to induce labor, and vaginal delivery of the fetus.
After delivery, a whole-body postmortem CT was performed on a GE Lightspeed scanner (GE Healthcare, Milwaukee, WI) using a 0.625-mm-slice thickness and a fixed settings protocol. All CT scans were performed within 72 h of the ultrasound examination. Offline measurements were carried out by one operator, using the open-source image analysis software program MITK (Medical Imaging Interaction Toolkit MITK, version 0.12.2; German Cancer Research Centre, Division of Medical and Biological Informatics, Heidelberg, Germany; www.mitk.org). Volume CT rendered images were created using the GE Advantage Workstation 4.4 (GE Medical Systems, San Francisco, CA). Ultrasound measurements were performed blinded to the CT measurements, and results were compared with the t test for paired samples.
Observational study of maternal vitamin D, fetal FV, and neonatal DEXA
This was a longitudinal, observational study of maternal 25-hydroxyvitamin D [25(OH)D] concentration, fetal ultrasound measurements, and neonatal bone mineral indices using DEXA. We used a cohort of healthy pregnant women from Southampton United Kingdom, taking part in the Southampton Women's Survey, an observational study of maternal nutrition and fetal growth in pregnancy, which has been described elsewhere (16). Women aged 20 to 34 were invited to take part between 2002 and 2005; they were interviewed by research staff at three appointments—preconception, early pregnancy (11 wk), and late pregnancy (34 wk)—where anthropometric data and nutritional and lifestyle information were collected using questionnaires. This study was approved by a regional research ethics committee, and all women participated after informed consent.
Fetal ultrasound scans were performed at 19 and 34 wk by one operator, using the KretzGE Voluson 730 system (GE Healthcare) with a curvilinear 3D transducer; and a blood sample was obtained at 34 wk for serum 25(OH)D concentration by the Diasorin RIA (Diasorin, Stillwater, MN). The FVs from the 34-week scan were analyzed offline by one operator, using the FV method described above and blinded to all maternal and neonatal characteristics, including the maternal 25(OH)D results. Birth weight was recorded at delivery. A whole-body DEXA scan of the newborn was performed within the first 2 wk of age to assess the total BMC and total BMD, using a pencil beam densitometer Lunar DPX-L (GE Lunar, Madison, WI). A typical scan takes approximately 10 min, and the estimated radiation dose received per examination (0.002 mSv) is only a fraction of the average daily background radiation (17) (0.01 mSv); in comparison, a typical chest x-ray generates 0.06 mSv and a CT scan up to 20 mSv (18). All scans were performed after parental informed consent.
Continuous maternal characteristics were maternal age, height, weight, body mass index (BMI), triceps skinfold thickness (TSF), and serum 25(OH)D concentration; categorical binary variables were parity, smoking, alcohol, exercise, and season. Fetal outcome measures were the ultrasound measurements (FL, PMD, MSD, and FV), birth weight, total BMC, and total BMD. Normality of distributions was checked, and logarithmic transformations (log10) were applied on the FV and TSF measurements. All ultrasound measurements were adjusted for gestational age; birth weight was adjusted for gestational age at delivery; BMC and BMD were adjusted for gestational age at birth and postnatal age at the time of the DEXA examination.
Differences of mean ultrasound measurements between subjects with 25(OH)D insufficiency (<75 nmol/liter) and those with sufficient 25(OH)D (≥75 nmol/liter) were assessed using the independent samples t test. Univariate linear regression analyses were performed between fetal outcomes (dependent variable) and continuous maternal characteristics (independent variable), whereas the independent samples t test was used to assess the effect of other binary maternal variables.
After univariate analysis, all maternal predictors of the femur ultrasound measurements with a P value of less than 0.1 were fitted into a multiple regression model. Statistical analyses and figures were performed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY).
Results
FV validation
Six cases undergoing medical pregnancy termination were enrolled in the CT validation study. In all cases, the FL was between the 5th and 95th percentiles for gestational age. The fetal skeleton was clearly visualized on CT without imaging artifact in all cases. The ultrasound FV was compared against the volume derived from CT and showed excellent agreement with a mean difference of −0.01 ml (paired t test P = 0.739). Table 1 summarizes the case characteristics and measurements taken.
Table 1.
Characteristics of cases in the CT validation study
| Case | GA (wk) | Diagnosis | CT volume (ml) | 3D US volume (ml) |
|---|---|---|---|---|
| 1 | 18+3 | Anencephaly | 0.29 | 0.39 |
| 2 | 19+3 | Trisomy 21 | 0.41 | 0.31 |
| 3 | 21+2 | Trisomy 18 | 0.37 | 0.40 |
| 4 | 22+6 | Trisomy 21 | 0.88 | 0.79 |
| 5 | 24+0 | Trisomy 13 | 1.18 | 1.29 |
| 6 | 28+6 | Severe brain abnormality | 2.33 | 2.36 |
GA, Gestational age at termination; 3D US, 3D ultrasound.
Rendered CT views of both femoral metaphyses revealed that the proximal and distal metaphyses are not identical; the distal metaphysis is flattened and ovoid rather than circular, highlighting the importance to properly identify the proximal metaphysis for PMD measurement.
Observational study of maternal vitamin D, fetal FV, and neonatal DEXA
The cohort consisted of 357 consecutive subjects, with a complete set of ultrasound measurements and serum 25(OH)D concentrations, which were performed at a mean gestation of 34.4 wk (sd, 0.5 wk). Neonatal DEXA examinations were available for 203 of 357 subjects (57%) and were performed at a mean postnatal age of 7.8 d (sd, 4.8 d).
Median 25(OH)D concentration was 63 nmol/liter (minimum, 14; maximum, 144; interquartile range, 42). Vitamin D deficiency defined as below 25 nmol/liter was encountered in 19 cases (5.3%); insufficiency defined as below 50 nmol/liter was found in 123 cases (34.5%); whereas insufficiency defined as below 75 nmol/liter was found in 220 cases (61.6%). Maternal and fetal characteristics are listed in Table 2.
Table 2.
Maternal and fetal characteristics
| n | Median (IQR) or n (%) | |
|---|---|---|
| Maternal characteristics | ||
| Age at delivery (yr) | 356 | 31.6 (5.3) |
| Height (cm) | 355 | 164 (8.2) |
| Weight (kg) | 355 | 63.5 (13.1) |
| BMI (kg/m2) | 355 | 23.7 (4.6) |
| Parity | 357 | |
| Nullipara | 211 (59.1%) | |
| Multipara | 146 (40.9%) | |
| Smoking | 353 | |
| Yes | 48 (13.6%) | |
| No | 305 (86.4%) | |
| Alcohol | 357 | |
| ≤4 U/wk | 267 (74.8%) | |
| >4 U/wk | 90 (25.2%) | |
| Strenuous exercise | 357 | |
| Yes | 84 (23.5%) | |
| No | 273 (76.5%) | |
| Season at ultrasound | 352 | |
| Winter | 160 (45.5%) | |
| Summer | 192 (54.5%) | |
| TSF (mm) | 347 | 19.9 (7.9) |
| Maternal 25(OH)D (nmol/liter) | 357 | 63 (42) |
| Fetal characteristics | ||
| FL (mm) | 357 | 64.3 (3.6) |
| PMD (mm) | 357 | 13.6 (1.8) |
| MSD (mm) | 357 | 7.00 (1.1) |
| FV (ml) | 357 | 4.86 (1.21) |
| Gestation at delivery (wk) | 356 | 40.3 (2.0) |
| Birth weight (g) | 356 | 3444 (628) |
| BMC (g) | 203 | 61.53 (18.44) |
| BMD (g/cm2) | 203 | 0.5249 (0.033) |
Height, weight, and BMI were measured before conception. Smoking and alcohol consumption were documented at 11 wk. Strenuous exercise, TSF, and 25(OH)D levels were assessed at 34 wk. FL, PMD, MSD, and FV were measured by 3D ultrasound at 34 wk. BMC and BMD were assessed by DEXA within 2 wk after birth. IQR, Interquartile range.
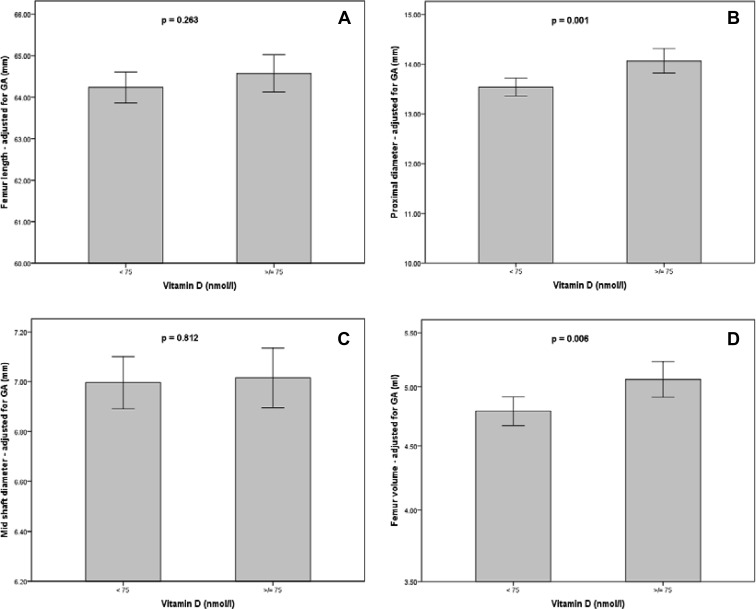
Smaller fetal ultrasound measurements were demonstrated in women with 25(OH)D below 75 nmol/liter, compared with those with levels of at least 75 nmol/liter: mean PMD, 13.5 vs. 14.1 mm (P = 0.001), and mean logFV, 0.67 vs. 0.70 (P = 0.006); the latter corresponds to a difference of median FV 4.7 vs. 5.0 ml. No significant differences were seen for FL (P = 0.263) or MSD (P = 0.812). These results are presented graphically in Fig. 2. These differences persisted at weaker statistical significance, when using different 25(OH)D cutoffs: deficiency of less than 50 nmol/liter and less than 25 nmol/liter was linked with smaller PMD (P = 0.021 and 0.011, respectively) and smaller logFV (P = 0.051 and 0.043, respectively).
Fig. 2.
The effect of maternal vitamin D concentration, expressed as a binary variable, on FL (A), PMD (B), MSD (C), and FV (D); all ultrasound measurements are adjusted for GA; FV axis (D) is shown in logarithmic scale; box plots demonstrate mean values; error bars show the 95% confidence intervals; p = statistical significance from independent samples t-test.
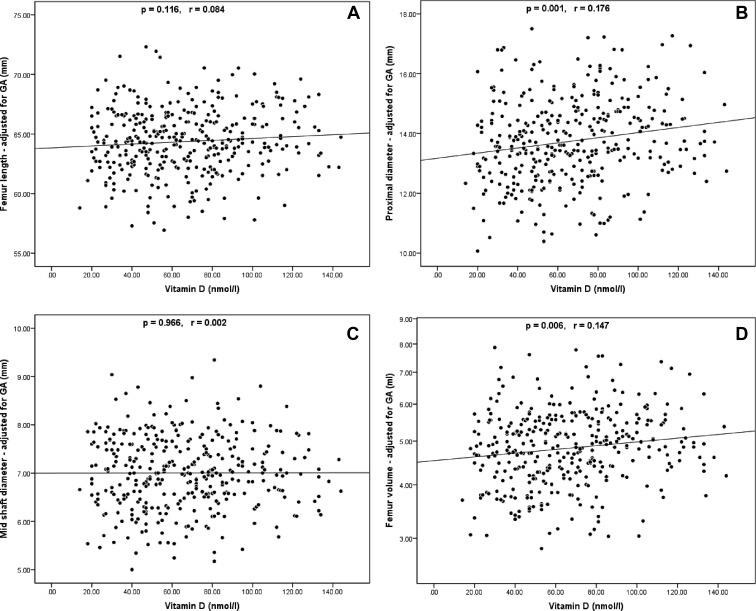
A summary of the univariate linear regression analysis between ultrasound measurements and each independent maternal variable is shown in Table 3. Maternal height and weight were significant positive predictors of almost every ultrasound parameter. logTSF was a positive predictor of FL, PMD, and logFV. Maternal serum 25(OH)D concentration demonstrated significant positive correlations with PMD and logFV. The linear regression scatterplots between ultrasound measurements and 25(OH)D are presented in Fig. 3.
Table 3.
Univariate effect of maternal characteristics on fetal and neonatal outcome measures
| FL |
PMD |
MSD |
logFV |
BW |
BMC |
BMD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | r | P | r | P | r | P | r | P | r | P | r | P | r | |
| Maternal age | 0.175 | −0.072 | 0.745 | −0.017 | 0.044 | −0.108 | 0.193 | −0.070 | 0.761 | −0.016 | 0.065 | −0.130 | 0.119 | −0.110 |
| Height | <0.001 | 0.260 | 0.001 | 0.172 | 0.004 | 0.153 | <0.001 | 0.246 | <0.001 | 0.248 | 0.096 | 0.117 | 0.453 | 0.053 |
| Weight | 0.025 | 0.119 | 0.004 | 0.155 | 0.335 | 0.052 | 0.003 | 0.160 | <0.001 | 0.260 | 0.260 | 0.079 | 0.950 | 0.004 |
| BMI | 0.956 | 0.003 | 0.153 | 0.076 | 0.683 | −0.022 | 0.387 | 0.046 | 0.004 | 0.152 | 0.653 | 0.032 | 0.806 | −0.017 |
| Paritya | 0.756 | 0.595 | 0.156 | 0.451 | 0.001 | 0.231 | 0.140 | |||||||
| Smokinga | <0.001 | 0.096 | 0.558 | 0.061 | 0.014 | 0.054 | 0.340 | |||||||
| Alcohola | 0.060 | 0.345 | 0.780 | 0.760 | 0.048 | 0.523 | 0.048 | |||||||
| Strenuous exercisea | 0.266 | 0.106 | 0.388 | 0.095 | 0.708 | 0.854 | 0.619 | |||||||
| Season at ultrasounda | 0.952 | 0.232 | 0.934 | 0.405 | 0.611 | 0.420 | 0.987 | |||||||
| logTSF | 0.018 | 0.128 | 0.010 | 0.139 | 0.884 | 0.008 | 0.013 | 0.134 | 0.008 | 0.143 | 0.107 | 0.115 | 0.563 | 0.041 |
| Maternal 25(OH)D | 0.116 | 0.084 | 0.001 | 0.176 | 0.966 | 0.002 | 0.006 | 0.147 | 0.276 | 0.058 | 0.824 | −0.016 | 0.095 | −0.117 |
Statistical significance (P) and Pearson correlation coefficient (r) were derived from linear regression analysis. For categorical variables (a), the P value is derived from the independent samples t test. FL, PMD, MSD, and logFV are adjusted for gestational age at ultrasound. Birth weight is adjusted for gestational age at delivery. BMC and BMD are adjusted for gestational age at delivery and postnatal age at DEXA. BW, Birth weight.
Fig. 3.
Linear regression analyses of FL (A), PMD (B), MSD (C), and FV (D) against maternal vitamin D concentration; all ultrasound measurements are adjusted for GA; FV axis (D) is shown in logarithmic scale; p = statistical significance from univariate linear regression analysis; r = Pearson correlation coefficient.
The univariate associations between maternal characteristics and neonatal outcomes are also included in Table 3. In summary, birth weight, BMC, and BMD demonstrate no correlation with serum 25(OH)D concentration. BMC is correlated with maternal age, height, and smoking; whereas birth weight has significant correlations with most maternal predictors of soft tissue growth (height, weight, BMI, parity, smoking, alcohol, and TSF).
The association between sonographic logFV and neonatal DEXA indices was assessed separately: logFV is positively correlated with BMC (P < 0.001; r = 0.257), and this persists on multiple regression analysis after controlling for the effect of maternal age, height, and smoking. However, logFV is not correlated with BMD (P = 0.461; r = −0.052).
On multiple regression analysis, the only independent predictors of logFV were maternal height and logTSF. To assess which of the component linear measurements contributed to this relation, multiple regression analysis of all femur measurements was undertaken: FL demonstrated independent significant correlations with maternal height, logTSF, and smoking; PMD was correlated with maternal height and 25(OH)D; and MSD was correlated with maternal height and maternal age.
Discussion
This study has demonstrated that maternal height, TSF, and serum vitamin D concentration are significant predictors of the fetal femur size on prenatal 3D ultrasound. To achieve this, we developed and validated a simple and quick method for measurement of FV.
Recent evidence suggests that the target range of serum vitamin D for optimal health outcomes is 75–100 nmol/liter (19, 20); a review of observational studies (21) and a randomized controlled trial of vitamin D supplementation in pregnancy (22) have indicated a possible association with a reduced incidence of preeclampsia, gestational diabetes, and primary cesarean section in replete mothers. However, low maternal vitamin D levels are commonly encountered in developed countries: studies of pregnant women from the United States (1), United Kingdom (23), and Australia (24) demonstrate a 7–18% prevalence of deficiency (<25 nmol/liter), whereas the prevalence of insufficiency defined as below 50 nmol/liter is 33–49%. This is an important public health issue because it has been hypothesized that low maternal vitamin D may be associated with reduced fetal growth (24). A number of epidemiological studies support the theory of fetal programming, according to which suboptimal bone development in pregnancy may increase the lifetime risk of osteoporosis (2). Intrauterine life—like adolescence and puberty—is a period of intensive skeletal expansion; failure of the developing fetus to achieve its potential for bone growth could reprogram its developmental trajectory.
The evidence linking reduced maternal vitamin D and fetal skeletal growth has been indirect. For instance, vitamin D deficiency has been associated with reduced birth weight (24). It has also been demonstrated that children born to vitamin D-deficient mothers have reduced BMC measured by DEXA at the age of 9 yr (23). One explanation is that the intrauterine lack of vitamin D altered their growth trajectories, with results becoming evident several years after birth. Conversely, it is also possible that postnatal nutritional deficiencies during childhood are responsible: children share lifestyle and nutritional habits with their parents, and this may result in vitamin D deficiency during childhood, similar to maternal deficiency during pregnancy.
The present study demonstrates a direct link between maternal vitamin D and fetal bone size in utero. Univariate analysis suggests that 25(OH)D is a significant predictor for FV and PMD; a vitamin D level in excess of 75 nmol/liter, as recommended by The Endocrine Society in their recent Clinical Practice Guideline (20), is associated with higher mean values for both these measurements, although no statistical difference is noted for FL and MSD. Maternal height and TSF also have significant correlations with FL, PMD, and FV. Some interesting negative correlations were also demonstrated: the negative effect of smoking on FL and FV may be explained by the overall adverse effect of smoking on fetal growth and placental function; there is also a borderline negative effect on FL from alcohol consumption; and there is a negative effect of advancing maternal age on MSD.
To assess the independent contributions of these factors, multiple regression analysis was performed for the ultrasound parameters. The effect of maternal weight—although strongly significant on univariate analysis—is excluded from the final model, when height and maternal adiposity (logTSF) are taken into account. The effect of 25(OH)D remains significant for PMD, but in the case of FV it becomes attenuated below significance. The reason may be that maternal height and 25(OH)D are positive predictors of FV, but they are also correlated with each other (P = 0.022; r = 0.121); in other words, taller mothers have higher vitamin D levels and also fetuses with larger femurs. One explanation is that the maternal height influences FV directly as a result of genetic determination and that vitamin D is merely a confounder. The alternative explanation is that in taller mothers, there is increased fetal bone size as a result of the biological effect of higher vitamin D levels.
Maternal height, adiposity, and serum vitamin D concentration affect fetal skeletal size, each through a different mechanism. The first such mechanism is the effect of nonmodifiable anthropometric factors like maternal height; although the association is strong its clinical value is minimal because no intervention for optimization is possible. Secondly, bone size is indirectly regulated by the growth of the fetus as a whole; for instance, increased maternal adiposity is associated with higher fetal weight, and this could also lead to increased bone size. Thirdly, skeletal growth is affected by specific determinants of bone physiology, such as maternal vitamin D, where antenatal intervention is possible.
The distinction between the latter two mechanisms has an important implication: direct determinants of bone metabolism, such as maternal vitamin D, could be specifically targeted by public health interventions to optimize bone health. Conversely, higher maternal adiposity—although it could result in increased fetal bone size—may not always be physiological or desirable; such intervention may only be acceptable in very thin and underweight women.
This study has several strengths. A simple FV method was developed, and it was validated using an independent “gold standard,” namely CT. This ultrasound method allowed for improved assessment of femoral size, including not only its length but also its cross-sectional thickness. Previous studies have indicated a possible effect of maternal vitamin D on the FL, but this has only been confirmed in pregnant adolescents (10) and not in adult women (9). The present study indicates that the positive effect of vitamin D on the fetal femur is exerted primarily in the proximal metaphysis rather than its length. Several other correlations between femoral size and maternal characteristics were also demonstrated, which have strong biological plausibility. For instance, the effects of smoking and maternal TSF have been demonstrated in a previous study using neonatal BMC measured by DEXA (4).
A previous analysis of the same cohort demonstrated a negative correlation between maternal vitamin D and the cross-sectional area of the distal metaphysis (9); the present study found a positive effect of vitamin D on the diameter of the proximal metaphysis. It is possible that vitamin D may have discordant biological effects between the proximal and distal metaphyseal poles. In our analysis, we did not assess the distal femoral metaphysis because CT showed that this metaphysis is not circular, and therefore, measurement of a single diameter could be misleading. In addition, we measured metaphyseal diameters instead of the cross-sectional area, using a different measurement technique, because cross-sectional area measurement is often made difficult by the effect of acoustic shadowing.
The present study has some limitations. Our method of validation consisted of paired ultrasound and CT examinations up to 28 wk gestation, but we were unable to recruit cases at later gestation because these terminations are rare; however, the results indicated satisfactory agreement, and it is reasonable to assume similar validity for older fetuses. DEXA examinations were only available in a subset of participants, and this did not allow us to examine the effect of maternal vitamin D on neonatal indices with the same power as for the ultrasound measurements. When interpreting the FV, an underlying assumption was made that higher volume is beneficial. However, size and mineralization are not necessarily synonyms; it is possible that in certain circumstances FV may increase in the absence of adequate mineralization and that bone “quality” may be inferior as a result. It is reassuring that, in the present data, FV had a strong positive correlation with neonatal BMC without any significant or negative effect on BMD.
Finally, the univariate effect of vitamin D on fetal femur size, although statistically significant, is clinically small. Nevertheless, 3D ultrasound is a simple and safe investigation that does not carry radiation risk and is therefore likely to be acceptable to pregnant women. It can be used as a research measure in population studies or interventional trials of vitamin D supplementation.
Conclusions
In conclusion, we demonstrated that maternal height, adiposity, and serum vitamin D concentration are significant predictors of femoral size. Maternal height is a nonmodifiable pregnancy factor, whereas lifestyle interventions to increase maternal adiposity cannot be recommended because the risks associated with maternal obesity would outweigh any potential benefit on fetal bone mineralization. Conversely, vitamin D supplementation is an intervention that carries minimal risk and may be beneficial for the fetus. Future trials of pregnancy supplementation should use ultrasonographic fetal femur size as an outcome measure of efficacy, which could help in establishing the optimal supplement dose and would provide direct evidence of the potential to influence intrauterine fetal programming.
Acknowledgments
The authors thank Dr. K. Platt, consultant radiologist; H. Nicholl, radiographer; and J. Scott, research manager at the Stillbirth and Neonatal Death Society, for their assistance during the CT validation study. We are grateful to the staff of the Southampton Women Survey Study Group for their work in recruiting and interviewing the participants and processing the data and the samples. We also thank Philips Healthcare for providing the HD9 ultrasound machine and technical assistance. The SWS is grateful for financial support from the UK Medical Research Council, the University of Southampton, the Dunhill Medical Trust, the British Heart Foundation, and the Food Standards Agency.
A.T.P. and C.I. are supported by the Oxford Partnership Comprehensive Biomedical Research Centre, with funding from the Department of Health National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme. K.M.G. is supported by the NIHR through the NIHR Southampton Biomedical Research Centre.
Disclosure Summary: K.M.G. has acted as a consultant to Abbott Nutrition and Nestle Nutrition and has received reimbursement for speaking at an Abbott Nutrition Conference on Pregnancy Nutrition and Later Health Outcomes and at a Nestle Nutrition Institute Workshop. He is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. The other authors have nothing to disclose.
Footnotes
- BMC
- Bone mineral content
- BMD
- bone mineral density
- BMI
- body mass index
- CT
- computed tomography
- 3D
- three-dimensional
- DEXA
- dual-energy x-ray absorptiometry
- FL
- femur length
- FV
- femur volume
- MSD
- midshaft diameter
- 25(OH)D
- 25-hydroxyvitamin D
- PMD
- proximal metaphyseal diameter
- TSF
- triceps skinfold thickness.
References
- 1. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA., Jr 2010. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol 202:436.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Javaid MK, Cooper C. 2002. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab 16:349–367 [DOI] [PubMed] [Google Scholar]
- 3. Dror DK, King JC, Fung EB, Van Loan MD, Gertz ER, Allen LH. 2012. Evidence of associations between feto-maternal vitamin D status, cord parathyroid hormone and bone-specific alkaline phosphatase, and newborn whole body bone mineral content. Nutrients 4:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, Cooper C. 2001. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res 16:1694–1703 [DOI] [PubMed] [Google Scholar]
- 5. Chitty LS, Altman DG, Henderson A, Campbell S. 1994. Charts of fetal size: 4. Femur length. Br J Obstet Gynaecol 101:132–135 [DOI] [PubMed] [Google Scholar]
- 6. Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. 1984. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 150:535–540 [DOI] [PubMed] [Google Scholar]
- 7. Smith-Bindman R, Hosmer W, Feldstein VA, Deeks JJ, Goldberg JD. 2001. Second-trimester ultrasound to detect fetuses with Down syndrome: a meta-analysis. JAMA 285:1044–1055 [DOI] [PubMed] [Google Scholar]
- 8. Papageorghiou AT, Fratelli N, Leslie K, Bhide A, Thilaganathan B. 2008. Outcome of fetuses with antenatally diagnosed short femur. Ultrasound Obstet Gynecol 31:507–511 [DOI] [PubMed] [Google Scholar]
- 9. Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K. 2010. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res 25:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O'Brien KO. 2012. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr 95:1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang CH, Tsai PY, Yu CH, Ko HC, Chang FM. 2007. Prenatal detection of fetal growth restriction by fetal femur volume: efficacy assessment using three-dimensional ultrasound. Ultrasound Med Biol 33:335–341 [DOI] [PubMed] [Google Scholar]
- 12. Ioannou C, Sarris I, Salomon LJ, Papageorghiou AT. 2011. A review of fetal volumetry: the need for standardization and definitions in measurement methodology. Ultrasound Obstet Gynecol 38:613–619 [DOI] [PubMed] [Google Scholar]
- 13. Hadlock FP, Kent WR, Loyd JL, Harrist RB, Deter RL, Park SK. 1982. An evaluation of two methods for measuring fetal head and body circumferences. J Ultrasound Med 1:359–360 [DOI] [PubMed] [Google Scholar]
- 14. Harris J, Stocker H. 1998. Handbook of mathematics and computational science. New York, London: Springer [Google Scholar]
- 15. Braillon PM, Buenerd A, Lapillonne A, Bouvier R. 2002. Skeletal and total body volumes of human fetuses: assessment of reference data by spiral CT. Pediatr Radiol 32:354–359 [DOI] [PubMed] [Google Scholar]
- 16. Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. 2006. Cohort profile: the Southampton Women's Survey. Int J Epidemiol 35:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hull H, He Q, Thornton J, Javed F, Allen L, Wang J, Pierson RN, Jr, Gallagher D. 2009. iDXA, Prodigy, and DPXL dual-energy X-ray absorptiometry whole-body scans: a cross-calibration study. J Clin Densitom 12:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brenner DJ, Hall EJ. 2007. Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284 [DOI] [PubMed] [Google Scholar]
- 19. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. 2006. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28 [DOI] [PubMed] [Google Scholar]
- 20. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 21. Hollis BW, Wagner CL. 24 May 2012. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int doi: 10.1007/s00223-012-9607-4 [DOI] [PubMed] [Google Scholar]
- 22. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. 2011. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. 2006. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367:36–43 [DOI] [PubMed] [Google Scholar]
- 24. Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. 2009. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 70:372–377 [DOI] [PubMed] [Google Scholar]