Abstract
Context:
A central/visceral fat distribution and excess free fatty acid (FFA) availability are associated with dyslipidemia and insulin resistance. However, these two characteristics often coexist, making it difficult to detect the independent contributions of each. Whether FFA suppression is more closely linked to metabolic abnormalities is not clear.
Objective:
The aim of the study was to examine the relationship between FFA suppression, body fat distribution, and fitness as contributors toward insulin resistance and hypertriglyceridemia.
Design:
We measured systemic palmitate turnover using an iv infusion of [9,10-3H]palmitate; upper body sc adipose tissue (UBSQ) and visceral adipose tissue (VAT) with dual-energy x-ray absorptiometry and a single-slice abdominal computed tomography scan; fitness with a graded exercise treadmill test; and insulin sensitivity with both the iv glucose tolerance test (IVGTT) (SIIVGTT) and mixed meal tolerance test (SIMeal).
Setting:
The study was conducted at a General Clinical Research Center.
Participants:
Baseline data were obtained from 140 elderly adults (age, 60–88 yr; 83 males) and 60 young adults (age, 18–31 yr; 31 males) who participated in a previously published trial assessing the effects of 2-yr supplementation of dehydroepiandrosterone or testosterone on body composition, glucose metabolism, and bone density.
Interventions:
There were no interventions.
Main Outcome Measures:
We measured fasting plasma triglyceride (TG) concentrations, SIIVGTT, and SIMeal.
Results:
Using multivariate regression analysis, the strongest combined predictors of TG concentrations were VAT, postmeal nadir FFA concentrations, sex, and age. The best predictors of SIIVGTT were IVGTT nadir palmitate concentration, VAT, UBSQ fat, fitness, and age, whereas the best predictors of SIMeal were meal nadir palmitate concentration, UBSQ fat, fitness, and sex.
Conclusions:
FFA suppression is associated with both fasting TG concentrations and insulin sensitivity, independent of measures of adiposity.
Obesity, especially when accompanied by greater visceral adipose tissue (VAT) (1) and free fatty acids (FFA) (2, 3), is associated with increased plasma triglyceride (TG) concentrations and insulin resistance. In healthy, normoglycemic postabsorptive adults, FFA supply fuel to many organs and tissues (4, 5). However, undesirable metabolic consequences can result from persistent elevation of FFA. Abnormally high FFA concentrations are thought to create hypertriglyceridemia by stimulating hepatic very low-density lipoprotein (VLDL)-TG production (2, 3, 6). Both elevated fasting FFA (7) and impaired FFA suppression after a glucose, meal, or insulin challenge are associated with hypertriglyceridemia (8–11). Examining VAT and upper body sc (UBSQ) adipose tissue mass and FFA as individual predictors of TG concentrations is problematic because of the interrelatedness of these factors. Some investigators found that indices of FFA suppression are associated with fasting TG concentrations independently of body mass index (BMI) and/or waist-to-hip ratio (10, 11). However, few studies have examined the relationship between postprandial and insulin-suppressed FFA and fasting plasma TG concentrations independent of robust measures of abdominal fat distribution.
Increased FFA can also mediate muscle (12, 13) and hepatic (14, 15) insulin resistance. However, in upper body obesity, this relationship is somewhat circuitous because adipose tissue FFA release is also insulin resistant (16, 17). Some report that insulin-mediated FFA suppression predicts insulin sensitivity with respect to glucose metabolism to a better degree than basal FFA flux (18) and independent of adiposity (19, 20), but others have not found this association after adjusting for VAT (21).
Our goal was to understand whether insulin-mediated FFA suppression is related to fasting plasma TG concentrations and insulin sensitivity after controlling for the effects of body composition. We measured plasma palmitate concentrations and flux before and after a meal and an insulin-modified iv glucose tolerance test (IVGTT) in a large group of young and elderly men and women. We hypothesized that a reduced ability of insulin to suppress FFA would be positively associated with TG concentrations and inversely associated with insulin sensitivity independent of age group, sex, and body fat distribution. We also tested whether measures of FFA kinetics would be more informative than measures of FFA concentrations.
Subjects and Methods
Subjects
This report provides preintervention data from 140 elderly adults (mean age ± sd, 69 ± 6 yr; range, 60–88 yr; 83 males) who participated in a published trial assessing changes in body composition, glucose metabolism, and bone density after 2-yr supplementation with dehydroepiandrosterone (DHEA) or testosterone (22). Elderly men with bioavailable testosterone below 103 ng/dl and sulfated DHEA below 1.57 μg/dl and elderly women with sulfated DHEA below 0.95 μg/dl who were not on hormonal replacement therapy were included in the study. Of the elderly participants included in the study, 13 men and six women had impaired fasting glucose (100–125 mg/dl). Data from 60 young adults (mean age ± sd, 23 ± 3 yr; range, 18–31 yr; 31 males) recruited as part of the original parent study for a one-time evaluation to serve as a comparison group for outcome measures in the elderly are included as well. The study was approved by the Mayo Institutional Review Board, and all participants provided informed written consent before enrollment in the study.
Protocol
The study protocol details have been published (23). Briefly, fasting TG concentrations and sex hormone blood levels were obtained as part of the screening labs to determine eligibility for the study. Within a 4-wk period, all volunteers underwent the following tests in this order: 1) outpatient treadmill exercise test for maximum oxygen uptake (VO2 max); 2) outpatient dual-energy x-ray absorptiometry and single-slice abdominal computed tomography for body composition; 3) consumption of meals provided by the Mayo General Clinical Research Center (GCRC) kitchen for 3 d before the inpatient study to assure weight stability and standardized macronutrient intake; 4) inpatient GCRC study using the IVGTT model with palmitate tracer and resting energy expenditure (REE); 5) repeat of 3-d outpatient meals for dietary control; and 6) inpatient GCRC study using the mixed meal model approach with palmitate tracer and REE. For inpatient studies, participants were admitted to the GCRC the afternoon before the study day, and they fasted overnight after consuming a standard 10-kcal/kg meal (55% carbohydrate, 15% protein, and 30% fat) between 1700 and 1730 h. The next morning they underwent the IVGTT or mixed meal tolerance test (MMTT). A continuous infusion of [9,10-3H]palmitate was administered on both days to measure FFA flux before and during the IVGTT and the MMTT.
MMTT and IVGTT
On the morning of both days, a catheter was inserted into the forearm vein for the palmitate tracer infusion. A separate catheter was inserted into the dorsal vein of the opposite hand in a retrograde fashion for collection of arterialized blood via the heated box technique. Over a 15-min period, starting at time zero (0900 h), participants consumed a solid mixed meal (10 kcal/kg, 45% carbohydrate, 15% protein, and 40% fat), which consisted of Canadian bacon, three scrambled eggs, and Jell-O containing 1.2 g/kg body weight of dextrose. Blood samples were collected at −120, −30, −20, −10, 0, 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 120, 150, 180, 210, 240, 260, 280, 300, 360, and 420 min relative to time zero for measurement of insulin and glucose concentrations.
The modified IVGTT was performed using infusion and sampling iv catheters as described above. The [9,10-3H]palmitate infusion was also as outlined above. Glucose (0.3 g/kg body weight) was infused over 2 min starting at time zero (0900 h). At time 20 (relative to zero), a square-wave bolus of insulin (0.02 U/kg body weight) was infused over 5 min. Blood was collected at the following time points for determination of insulin and glucose concentrations: −120, −30, −20, −10, 0, 2, 4, 6, 8, 10, 15, 20, 22, 25, 26, 28, 31, 35, 45, 60, 75, 90, 120, 180, and 240 min.
Glucose and insulin data obtained during the MMTT were used in conjunction with the oral glucose minimal model to generate an index of insulin sensitivity after a meal [SIMeal (24, 25)]. The iv glucose minimal model was used to calculate an insulin sensitivity index based on glucose and insulin data obtained during the IVGTT [SIIVGTT (26)].
Palmitate kinetic protocol
Plasma palmitate concentration and kinetics were determined using samples collected during the [9,10-3H]palmitate infusions for both the MMTT and IVGTT studies. Before tracer infusion, baseline blood samples were collected for background palmitate concentration and specific activity. The tracer infusion was started at time zero, and blood samples for measuring palmitate concentration and rate of appearance were collected every 30 min for 3 h during the MMTT and at the following times for the IVGTT: 20, 31, 45, 90, and 120 min.
Nadir palmitate concentration was the lowest concentration observed after the meal and insulin infusion. The Steele equation modified for non-steady state was used for determining palmitate rate of appearance (Ra, μmol/min) (27).
Body composition, indirect calorimetry, and VO2 max
Total body, UBSQ, and VAT fat mass were measured using a combination of dual-energy x-ray absorptiometry (DXP-IQ scanner; Lunar Radiation, Madison, WI) and a single-slice computed tomography scan at the level of L2-L3 as previously described (28, 29). REE was measured for at least 20 min using indirect calorimetry (DeltaTrac, Yorba Linda, CA) before starting the [9,10-3H]palmitate infusion on the morning of both the MMTT study day and the IVGTT study day. Average REE was then calculated from these two baseline measures. VO2 max was determined using a graded-intensity treadmill test (30).
Sample analysis
Samples were separated and stored at −70 C until analyzed. The glucose oxidase method was used for analyzing plasma glucose concentrations (YSI, Yellow Springs, OH). A chemiluminescence assay with reagents (Access Assay; Beckman Coulter Inc., Chaska, MN) was used to measure insulin concentrations. TG concentrations (31), palmitate concentrations, and specific activity were measured using HPLC (32).
Statistical methods
Descriptive and demographic data are presented as means ± sd. Age and sex group differences, as well as age by sex interactions, were determined by two-way ANOVA for descriptive and metabolic characteristics, fat distribution, and nadir FFA concentrations. For significant interactions, Tukey's post hoc analysis was used to determine significant group differences. Because REE is highly associated with suppressed FFA Ra and differs by sex (20), differences in nadir FFA Ra during the MMTT and IVGTT were determined by two-way analysis of covariance adjusted for REE. Pearson correlation analysis was used to determine simple bivariate relationships among outcome variables of interest. A multistage modeling process was performed to determine significant predictors of fasting TG concentrations and insulin sensitivity assessed by a MMTT and IVGTT with the design variables sex and age group included in all models and a prespecified set of candidate covariates including nadir FFA concentration and Ra, VAT, and UBSQ added to the models to assess improvement in fit as measured by R2. Final models were analyzed using multiple linear regression analysis, and type II squared partial correlations were calculated as a means of demonstrating the independent contribution of each predictor on the variance in the outcome variable (i.e. contribution of each predictor adjusted for all other predictors in the model). The square root of VAT and the natural log of metabolic variables, nadir FFA concentration and Ra, and REE were used to transform the variables to approximate a normal distribution. Two-sided statistical tests and a 5% significance level were used for all analyses. All analyses were performed using an SAS software package (version 9.2; SAS Institute, Cary, NC).
Results
Participant characteristics
The descriptive and demographic characteristics of our volunteers are presented in Table 1. Some of the preintervention information from this 2-yr trial, including insulin sensitivity data, have been published (22, 23, 33–35), but are provided in this report juxtaposed to the novel FFA data. Fasting plasma TG concentrations were significantly lower in younger than in elderly participants. There was a significant age by sex interaction for insulin sensitivity measured by the mixed meal approach such that young males had significantly greater SIMeal compared with elderly males (P < 0.001) as well as elderly and younger females (P < 0.001 and P < 0.01, respectively), but there was no significant difference in SIMeal between young and elderly females (P = 0.838) or young females and elderly males (P = 0.650). A significant age by sex interaction was also evident for insulin sensitivity measured by the IVGTT such that SIIVGTT was significantly greater in younger than elderly men (P < 0.001) but was not significantly different between young and elderly women (P = 0.214). As reported (22, 36), VAT and UBSQ were greater in older compared with younger participants, men had more VAT than women, and women had more UBSQ than men.
Table 1.
Volunteer characteristics and nadir palmitate concentration and Ra during a MMTT and IVGTT
| Variable | Young |
Elderly |
P age | P sex | P interaction | ||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||
| n | 31 | 29 | 83 | 57 | |||
| Age (yr) | 24 ± 3 | 22 ± 3 | 69 ± 6 | 70 ± 6 | |||
| BMI (kg/m2) | 25.0 ± 2.8 | 24.1 ± 2.8 | 27.5 ± 3.1 | 27.0 ± 3.5 | <0.001 | 0.175 | 0.745 |
| REE (kcal/d) | 1795 ± 300 | 1450 ± 164 | 1679 ± 209 | 1313 ± 155 | <0.001 | <0.001 | 0.374 |
| TG (mg/dl) | 105 ± 40 | 99 ± 37 | 120 ± 47 | 125 ± 54 | 0.008 | 0.772 | 0.399 |
| SIMeal [×10−4 dl/kg min−1/(μU/ml)] | 15.8 ± 10.0 | 8.7 ± 5.0 | 9.4 ± 6.8 | 8.1 ± 6.0 | 0.002 | 0.001 | 0.048 |
| SIIVGTT [×10−4 dl/kg min−1/(μU/ml)] | 6.7 ± 3.1 | 5.0 ± 2.3 | 3.6 ± 2.3 | 4.2 ± 2.8 | <0.001 | 0.396 | 0.024 |
| VAT (kg) | 2.2 ± 1.2 | 1.5 ± 0.8 | 4.7 ± 1.9 | 3.8 ± 1.7 | <0.001 | 0.002 | 0.888 |
| UBSQ (kg) | 8.6 ± 3.2 | 11.1 ± 3.7 | 11.4 ± 3.6 | 14.5 ± 3.7 | <0.001 | <0.001 | 0.537 |
| VO2 max (ml/kg FFM/min) | 58.82 ± 8.60 | 56.39 ± 8.45 | 40.64 ± 6.79 | 38.39 ± 6.92 | <0.001 | 0.046 | 0.934 |
| Meal nadir palmitate concentration (μmol/liter) | 12 ± 3 | 10 ± 4 | 15 ± 7 | 10 ± 4 | 0.244 | <0.001 | 0.062 |
| IVGTT nadir palmitate concentration (μmol/liter) | 13 ± 4 | 12 ± 4 | 18 ± 7 | 16 ± 8 | <0.001 | 0.043 | 0.807 |
| Meal nadir palmitate Ra (μmol/min) | 27 ± 9 | 22 ± 8 | 32 ± 13 | 20 ± 9 | 0.731 | <0.001 | 0.046 |
| IVGTT nadir palmitate Ra (μmol/min) | 32 ± 12 | 32 ± 13 | 40 ± 16 | 35 ± 15 | 0.012 | 0.112 | 0.359 |
Data are expressed as means ± sd. Differences by age group, sex, and the interaction were determined by two-way ANOVA. The palmitate Ra data are not adjusted for REE. For some variables, n = 30 for young males and n = 52 for elderly females.
Plasma palmitate concentrations and kinetics responses to a MMTT and IVGTT
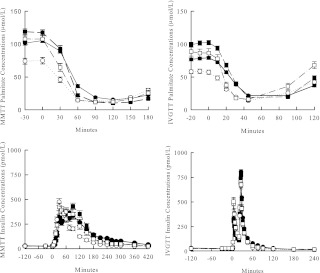
Nadir plasma palmitate concentrations during the MMTT were significantly lower in females than males (Table 1), but no age difference was detected. We observed a more rapid suppression of FFA in younger than elderly participants during the MMTT (Fig. 1A, left panel), with nadir concentrations observed at 90 min for the younger group and 120 min for the elderly group. This may relate to the delayed meal insulin response in the elderly (Fig. 1B, left panel) because peak insulin concentrations occurred 30 min after the meal in young males and females and at 60 and 90 min for elderly males and females, respectively. The palmitate Ra responses (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) on the two study days were quite similar in pattern to the concentration responses.
Fig. 1.
Top, Plasma palmitate concentrations during a MMTT (left) and IVGTT (right). Bottom, Plasma insulin concentrations during a MMTT (left) and IVGTT (right) in elderly males (●), elderly females (■), young males (○), and young females (□).
During the IVGTT, nadir palmitate concentrations were significantly greater in the elderly than the young participants and greater in males than females (Table 1). However, the time to reach nadir was similar for all groups with nadir palmitate concentrations occurring at 45 min (Fig. 1A, right panel). Note that insulin concentrations during the IVGTT were identical in all groups (Fig. 1B, right panel).
Nadir palmitate Ra was correlated with REE during both the MMTT (r = 0.61; P < 0.001) and IVGTT (r = 0.38; P < 0.001). Analysis of unadjusted nadir palmitate Ra data suggested a significant age by sex interaction during the MMTT. Both elderly and younger males had significantly greater Ra than elderly females. Elderly males had greater nadir palmitate Ra than younger females, but no significant differences in Ra were observed between young males and females or between young and elderly participants of the same sex (Table 1). After adjusting for REE, the age by sex interaction was no longer significant (P = 0.074), and there was no significant sex difference in nadir FFA Ra (P = 0.850). However, nadir palmitate Ra in older participants was greater than younger participants (P = 0.005).
During the IVGTT, older men and women had significantly greater nadir palmitate Ra than younger men and women (unadjusted for REE; Table 1), which remained after adjusting for REE (P < 0.001). There was no significant difference between males and females when unadjusted Ra data were analyzed (Table 1). However, after adjusting for REE, females had significantly greater nadir palmitate Ra than males (P = 0.002). The time course of palmitate Ra during MMTT and IVGTT mirrored the palmitate concentration shown in Fig. 1.
Associations of FFA suppression with plasma TG concentrations and insulin sensitivity
Using univariate regression analysis, nadir FFA (palmitate) concentrations and Ra during the MMTT and IVGTT were correlated significantly with the respective insulin sensitivity measurements as well as fasting TG concentrations (Table 2). As expected, VAT and UBSQ fat were positively correlated with TG concentrations and inversely correlated with both measures of insulin sensitivity. Additionally, VAT was correlated with both MMTT and IVGTT nadir palmitate concentration (r = 0.41 and r = 0.43, respectively; P < 0.001 for both) and nadir palmitate Ra (r = 0.40 and r = 0.39, respectively; P < 0.001 for both).
Table 2.
Pearson correlation coefficients to describe the linear associations of nadir palmitate concentration, Ra, and fat distribution with fasting TG concentrations and SIMeal and SIIVGTT
| TG | SIMeal | SIIVGTT | |
|---|---|---|---|
| Meal nadir palmitate concentration | 0.43b | −0.28b | |
| Meal nadir palmitate Ra | 0.33b | −0.20a | |
| IVGTT nadir palmitate concentration | 0.38b | −0.42b | |
| IVGTT nadir palmitate Ra | 0.33b | −0.27b | |
| VAT | 0.49b | −0.31b | −0.56b |
| UBSQ | 0.35b | −0.37b | −0.44b |
| VO2 max | 0.27b | 0.40b |
P < 0.01.
P < 0.001.
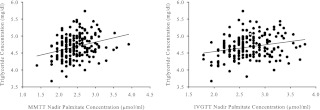
To better understand whether the univariate correlations were all driven by some single factor such as visceral fat, we used a multistage modeling process to examine the following as possible predictors of TG concentrations: sex, age group (young vs. elderly), body composition variables, as well as MMTT and IVGTT nadir palmitate concentration and Ra using separate models for concentration and Ra. The model with MMTT nadir palmitate concentration (Table 3, model 1) explained approximately 36% (P < 0.001) of the total variance in TG, and the model including IVGTT palmitate concentration (Table 3, model 2) explained approximately 33% (P < 0.001) of the variance. Both MMTT and IVGTT nadir palmitate concentrations were significantly and positively associated with TG concentrations independent of VAT, sex, and age group (Fig. 2, partial r = 0.34 and 0.26, respectively; both P < 0.001). However, after accounting for the other variables in the model, MMTT nadir palmitate concentration explained ∼11% of the variance in TG and IVGTT nadir palmitate concentration explained only 7% of the variance. More of the variance in TG was explained by VAT in the model with IVGTT nadir palmitate concentration (21%) than the model with MMTT nadir palmitate concentration (approximately 16%). Including MMTT or IVGTT palmitate Ra instead of concentration did not improve the results (for both, model R2 = 30%; P < 0.001) and contributed to less of the variation in TG (partial R2 = 4%, P = 0.009; and partial R2 = 3%, P = 0.012, respectively) than concentration. Including basal palmitate concentration as a covariate in the model did not alter the significant, positive associations observed for MMTT or IVGTT nadir palmitate concentration with TG concentrations (partial r = 0.36 and 0.30, respectively; P < 0.001 for both), and did not predict higher fasting plasma TG concentrations.
Table 3.
Predictors of fasting plasma TG concentrations and SIMeal and SIIVGTT
| Model R2 | Partial R2 | P | |
|---|---|---|---|
| Models for plasma TG | |||
| Model 1 | |||
| Total variance in plasma TG | 35.8% | <0.001 | |
| Meal nadir palmitate concentration (μmol/liter) | 11.2% | <0.001 | |
| VAT (kg) | 16.4% | <0.001 | |
| Sex | 5.4% | 0.002 | |
| Age group (young/elderly) | 1.6% | 0.088 | |
| Model 2 | |||
| Total variance plasma TG | 32.6% | <0.001 | |
| IVGTT nadir palmitate concentration (μmol/liter) | 6.7% | <0.001 | |
| VAT (kg) | 20.6% | <0.001 | |
| Sex | 2.4% | 0.032 | |
| Age group (young/elderly) | 3.7% | 0.008 | |
| Models for insulin sensitivity | |||
| Model 3 | |||
| Total variance in SIMeal | 25.6% | <0.001 | |
| Meal nadir palmitate concentration (μmol/liter) | 9.9% | <0.001 | |
| UBSQ (kg) | 4.5% | 0.004 | |
| VO2 max (ml/kg FFM/min) | 4.0% | 0.007 | |
| Sex | 4.2% | 0.006 | |
| Model 4 | |||
| Total variance in SIIVGTT | 44.4% | <0.001 | |
| IVGTT nadir palmitate concentration (μmol/liter) | 9.7% | <0.001 | |
| VAT (kg) | 8.3% | <0.001 | |
| UBSQ (kg) | 5.1% | 0.002 | |
| VO2 max (ml/kg FFM/min) | 7.2% | <0.001 | |
| Age group (young/elderly) | 3.0% | 0.020 |
Model R2 measures the percentage of the total variance in dependent variable (fasting TG and insulin sensitivity) explained by the collection of independent variables determined by multiple linear regression analysis. Partial R2 measures the percentage of residual variance explained by each independent variable when adjusted for the remaining independent variables included in the model.
Fig. 2.
Independent associations of nadir palmitate concentrations during a MMTT (left) and IVGTT (right) with fasting plasma TG concentrations adjusted for age group, sex, and VAT. Partial R, 0.34 (MMTT) and 0.45 (IVGTT); P < 0.001 for both.
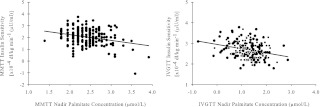
Significant predictors of SIMeal were nadir palmitate concentration during the MMTT, UBSQ fat mass, sex, and VO2 max (Table 3, model 3). This model explained approximately 26% of the variance in SIMeal. Figure 3 (left panel) shows the independent association of nadir palmitate concentration with SIMeal (partial R = −0.31; P < 0.001), which explained approximately 10% of the variance after accounting for UBSQ, VO2 max, and sex. UBSQ fat contributed approximately 5% and VO2 max and sex each contributed approximately 4% to the unexplained variance in SIMeal. When basal palmitate concentration was included as a covariate in the model, it was not significantly associated with SIMeal (partial r = −0.12; P = 0.11) and did not alter the significant, inverse association observed for MMTT nadir palmitate concentration with SIMeal (partial r = −0.26; P < 0.001). Therefore, it was not included in the final model. Including MMTT nadir palmitate Ra instead of concentration did not improve the results (model R2 = 23%; P < 0.001) and contributed to less of the unexplained variance than concentration (partial R2 = 6%; P < 0.001).
Fig. 3.
Independent associations of nadir palmitate concentration with insulin sensitivity determined from a MMTT (left) adjusted for UBSQ, VO2 max, and sex (partial R, −0.31; P < 0.001) and from an IVGTT (right) adjusted for VAT and UBSQ, VO2 max, and age group (partial R, −0.31; P < 0.001).
SIIVGTT was predicted by nadir IVGTT palmitate concentration, VAT, UBSQ, VO2 max, and age group (Table 3, model 4). This model explained approximately 44% of the variance in SIIVGTT with IVGTT nadir palmitate concentration explaining approximately 10%, VAT approximately 8%, UBSQ approximately 5%, VO2 max approximately 7%, and age group 3% of the explained variance in SIIVGTT. Figure 3 (right panel) depicts the significant, inverse association of IVGTT nadir palmitate concentration with SIIVGTT adjusted for VAT, UBSQ, VO2 max, and age group (partial R = −0.31; P < 0.001). Including basal palmitate concentration did not alter the significant, inverse association observed for IVGTT nadir palmitate concentration with SIIVGTT (partial r = −0.24; P = 0.001), nor was it significantly associated with SIIVGTT (partial r = −0.06; P = 0.45). Nadir palmitate Ra during the IVGTT was not a significant predictor of SIIVGTT when substituted for IVGTT nadir palmitate concentration (partial R2 = 0.6%; P = 0.294), and less of the total variance was explained by this model (model R2 = 39%; P < 0.001).
Discussion
Using baseline data from a large, prospective randomized trial (21), we tested whether suppression of FFA, a measure of adipose tissue functional regulation, predicts fasting plasma TG concentrations and insulin sensitivity over and above the known correlates such as adiposity, fitness, age, and sex. We found that nadir palmitate concentrations after both a MMTT and an IVGTT were significant predictors of fasting plasma TG concentrations, independent of age, sex, and VAT. Likewise, nadir palmitate concentrations significantly predicted insulin sensitivity determined from the MMTT and the IVGTT independent of sex, age group, measures of fat distribution, and VO2 max. Of interest, we found that body fat distribution predictors of SIMeal and SIIVGTT differed such that UBSQ was a significant predictor of SIMeal, but both UBSQ and VAT were significant predictors of SIIVGTT.
Our findings support previous reports that suppression of FFA after a glucose, meal, or insulin challenge predicts fasting TG concentrations (8–11, 37, 38). A possible explanation for the relationship between FFA suppression and hypertriglyceridemia is that FFA may drive hepatic VLDL-TG production, which is considered one of the primary causes of hypertriglyceridemia, especially in insulin-resistant states (2, 39, 40). Thus, inadequate suppression of FFA may result in greater FFA delivery to the liver and increased VLDL-TG production. Because FFA, TG concentrations, and fat mass, particularly VAT, are interrelated, it is often difficult to tease out the independent effect of FFA above that contributed by adiposity. In support of previous studies relying on indirect measures of adiposity such as BMI and/or waist-to-hip ratio (10, 11, 38), we found that suppression of FFA was predictive of TG concentrations independent of VAT, but not UBSQ. This suggests that VAT is serving as a surrogate for direct portal delivery of FFA to the liver, which cannot be assessed by plasma FFA concentrations. However, systemic FFA concentrations largely reflect lipolysis from UBSQ (41–43), which suggests that the direct assessment of this aspect of adipose tissue function may have displaced the possible effects of UBSQ fat mass per se from our model. Overall, these findings suggest that FFA suppression as an indicator of adipose tissue functional regulation offers additional information above that provided by VAT in the association of hypertriglyceridemia.
Similar to elevated TG concentrations, elevated FFA concentrations are also associated with measures of insulin resistance (13–15, 19, 20, 44). Our results are consistent with findings that FFA concentrations and/or Ra during a euglycemic-hyperinsulinemic clamp were significantly associated with glucose disposal (18) independent of VO2 max and body fat (20) and age, sex, and BMI (19). Our results extend the previous data by demonstrating that the inability to appropriately suppress FFA predicts insulin sensitivity even among healthy adults and after adjusting for more robust measures of fat distribution. The relationship was consistent even when insulin sensitivity was measured on two different days by separate methods (MMTT and IVGTT).
As expected, we observed significant univariate associations between VAT and UBSQ fat with the MMTT and IVGTT measures of insulin sensitivity. However, insulin sensitivity determined from the MMTT and IVGTT were predicted by different fat depots in multivariate analysis. Although UBSQ, but not VAT, was a significant predictor of SIMeal, both VAT and UBSQ were significant predictors of SIIVGTT. This result might suggest that these two measures of insulin sensitivity are impacted by somewhat different physiological inputs or that the relative contribution of VAT and UBSQ to insulin sensitivity might be test dependent, which is possible given that the two tissue beds respond differently to insulin, with VAT being more resistant to its antilipolytic effects compared with UBSQ (45). However, we cannot exclude the possibility that the multivariate models imputed statistical contributions of these two, closely related depots imperfectly despite the very large sample size. Whether insulin sensitivity from the MMTT and IVGTT are differentially associated with different adipose tissue depots requires further study.
Because our volunteers were Caucasian, these results may not be applicable to other ethnic groups. Additionally, elderly participants included in this study were selected because of low, but not deficient, DHEA and testosterone as part of an intervention trial. However, given that TG concentrations and insulin sensitivity did not change significantly over 2 yr of treatment, it seems unlikely that lower DHEA and testosterone concentrations among the elderly participants at baseline would have an effect on our findings (22, 33, 34). Although there is day-to-day variation in fasting FFA (46), there is less variability in the suppression of FFA by insulin (17), suggesting that “noisy” results obtained from the MMTT and IVGTT are unlikely to have had a significant impact on our findings. In addition, the insulin sensitivity determined from these tests was well correlated and performed under similar fasting conditions.
In conclusion, meal- and IVGTT-mediated FFA suppression is associated with both fasting TG concentrations and insulin sensitivity, independent of measures of adiposity. This suggests that suppression of FFA, which is a measure of adipose tissue functional regulation, offers additional information to that provided by fat mass alone with regard to hypertriglyceridemia and insulin sensitivity. We suggest that these results indicate that suppressed rather than overnight postabsorptive FFA are superior indices of the functional link between adipose tissue function and metabolic health. Understanding the mechanism of impaired insulin suppression of lipolysis may lead to novel interventions for obesity-related disorders.
Acknowledgments
We thank the volunteers who participated in this study. We also thank Jean Feehan, Barbara Norby, and the members of the Mayo Clinic Clinical Research Unit nursing, dietary, and support laboratory staff for technical assistance in performing the study.
The manuscript was supported by grants DK07352, PO1 AG14283, DK40484, and RR00585 from the U.S. Public Health Service and the Mayo Foundation.
Clinical Trial Registration no.: NCT00254371.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- DHEA
- dehydroepiandrosterone
- FFA
- free fatty acids
- IVGTT
- iv glucose tolerance test
- MMTT
- mixed meal tolerance test
- Ra
- rate of appearance
- REE
- resting energy expenditure
- SIIVGTT
- insulin sensitivity measured by IVGTT
- SIMeal
- insulin sensitivity measured by MMTT
- TG
- triglyceride
- UBSQ
- upper body sc adipose tissue
- VAT
- visceral adipose tissue
- VLDL
- very low-density lipoprotein
- VO2 max
- maximum oxygen uptake.
References
- 1. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- 2. Havel RJ, Kane JP, Balasse EO, Segel N, Basso LV. 1970. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest 49:2017–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kissebah AH, Adams PW, Wynn V. 1974. Plasma free fatty acid and triglyceride transport kinetics in man. Clin Sci Mol Med 47:259–278 [DOI] [PubMed] [Google Scholar]
- 4. Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. 2003. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spitzer JJ, Gold M. 1964. Free fatty acid metabolism by skeletal muscle. Am J Physiol 206:159–164 [DOI] [PubMed] [Google Scholar]
- 6. Kissebah AH, Alfarsi S, Adams PW, Wynn V. 1976. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12:563–571 [DOI] [PubMed] [Google Scholar]
- 7. Baynes C, Henderson AD, Hughes CL, Richmond W, Johnston DG, Elkeles RS. 1991. Determinants of mild fasting hypertriglyceridaemia in non-insulin-dependent diabetes. J Intern Med 229:267–273 [DOI] [PubMed] [Google Scholar]
- 8. Byrne CD, Wareham NJ, Brown DC, Clark PM, Cox LJ, Day NE, Palmer CR, Wang TW, Williams DR, Hales CN. 1994. Hypertriglyceridaemia in subjects with normal and abnormal glucose tolerance: relative contributions of insulin secretion, insulin resistance and suppression of plasma non-esterified fatty acids. Diabetologia 37:889–896 [DOI] [PubMed] [Google Scholar]
- 9. Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Pugh WL, Getz GS, Polonsky KS. 1991. Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab 72:934–944 [DOI] [PubMed] [Google Scholar]
- 10. McKeigue PM, Laws A, Chen YD, Marmot MG, Reaven GM. 1993. Relation of plasma triglyceride and apoB levels to insulin-mediated suppression of nonesterified fatty acids. Possible explanation for sex differences in lipoprotein pattern. Arterioscler Thromb 13:1187–1192 [DOI] [PubMed] [Google Scholar]
- 11. Yki-Järvinen H, Taskinen MR. 1988. Interrelationships among insulin's antilipolytic and glucoregulatory effects and plasma triglycerides in nondiabetic and diabetic patients with endogenous hypertriglyceridemia. Diabetes 37:1271–1278 [DOI] [PubMed] [Google Scholar]
- 12. Boden G, Chen X. 1995. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest 96:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. 1996. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebrin K, Steil GM, Getty L, Bergman RN. 1995. Free fatty acids as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 44:1038–1045 [DOI] [PubMed] [Google Scholar]
- 15. Saloranta C, Franssila-Kallunki A, Ekstrand A, Taskinen MR, Groop L. 1991. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 34:409–415 [DOI] [PubMed] [Google Scholar]
- 16. Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. 1989. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. J Clin Invest 84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen MD, Caruso M, Heiling V, Miles JM. 1989. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38:1595–1601 [DOI] [PubMed] [Google Scholar]
- 18. Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. 2012. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. J Clin Endocrinol Metab 97:E1219–E1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrannini E, Camastra S, Coppack SW, Fliser D, Golay A, Mitrakou A. 1997. Insulin action and non-esterified fatty acids. The European Group for the Study of Insulin Resistance (EGIR). Proc Nutr Soc 56:753–761 [DOI] [PubMed] [Google Scholar]
- 20. Shadid S, Kanaley JA, Sheehan MT, Jensen MD. 2007. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab 292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- 21. Goree LL, Darnell BE, Oster RA, Brown MA, Gower BA. 2010. Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity 18:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, 3rd, Smith GE, Khosla S, Jensen MD. 2006. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- 23. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. 2003. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action and clearance. Diabetes 52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 24. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. 2001. Oral glucose tolerance test minimal model indexes of β-cell function and insulin sensitivity. Diabetes 50:150–158 [DOI] [PubMed] [Google Scholar]
- 25. Dalla Man C, Caumo A, Cobelli C. 2002. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49:419–429 [DOI] [PubMed] [Google Scholar]
- 26. Bergman RN, Ider YZ, Bowden CR, Cobelli C. 1979. Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 27. Jensen MD, Heiling V, Miles JM. 1990. Measurement of non-steady-state free fatty acid turnover. Am J Physiol 258:E103–E108 [DOI] [PubMed] [Google Scholar]
- 28. Jensen MD, Kanaley JA, Reed JE, Sheedy PF. 1995. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61:274–278 [DOI] [PubMed] [Google Scholar]
- 29. Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW. 1993. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 68:867–873 [DOI] [PubMed] [Google Scholar]
- 30. Proctor DN, Beck KC. 1996. Delay time adjustments to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol 81:2495–2499 [DOI] [PubMed] [Google Scholar]
- 31. Humphreys SM, Fisher RM, Frayn KN. 1990. Micromethod for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem 27:597–598 [DOI] [PubMed] [Google Scholar]
- 32. Miles JM, Ellman MG, McClean KL, Jensen MD. 1987. Validation of a new method for determination of free fatty acid turnover. Am J Physiol 252:E431–E438 [DOI] [PubMed] [Google Scholar]
- 33. Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. 2007. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care 30:1972–1978 [DOI] [PubMed] [Google Scholar]
- 34. Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. 2007. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes [Erratum (2007) 56:1486] 56:753–766 [DOI] [PubMed] [Google Scholar]
- 35. Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. 2006. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55:2001–2014 [DOI] [PubMed] [Google Scholar]
- 36. Koutsari C, Ali AH, Nair KS, Rizza RA, O'Brien P, Khosla S, Jensen MD. 2009. Fatty acid metabolism in the elderly: effects of dehydroepiandrosterone and testosterone replacement in hormonally deficient men and women. J Clin Endocrinol Metab 94:3414–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensen MD, Nielsen S. 2007. Insulin dose response analysis of free fatty acid kinetics. Metabolism 56:68–76 [DOI] [PubMed] [Google Scholar]
- 38. Laws A, Hoen HM, Selby JV, Saad MF, Haffner SM, Howard BV. 1997. Differences in insulin suppression of free fatty acid levels by gender and glucose tolerance status. Relation to plasma triglyceride and apolipoprotein B concentrations. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Arterioscler Thromb Vasc Biol 17:64–71 [DOI] [PubMed] [Google Scholar]
- 39. Kissebah AH, Alfarsi S, Adams PW, Seed M, Folkard J, Wynn V. 1976. Transport kinetics of plasma free fatty acid, very low density lipoprotein triglycerides and apoprotein in patients with endogenous hypertriglyceridaemia. Effects of 2,2-dimethyl, 5(2,5-xylyloxy) valeric acid therapy. Atherosclerosis 24:199–218 [DOI] [PubMed] [Google Scholar]
- 40. Hassing HC, Surendran RP, Mooij HL, Stroes ES, Nieuwdorp M, Dallinga-Thie GM. 2012. Pathophysiology of hypertriglyceridemia. Biochim Biophys Acta 1821:826–832 [DOI] [PubMed] [Google Scholar]
- 41. Guo Z, Hensrud DD, Johnson CM, Jensen MD. 1999. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48:1586–1592 [DOI] [PubMed] [Google Scholar]
- 42. Jensen MD. 1995. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96:2297–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin ML, Jensen MD. 1991. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boden G, Chen X, Rosner J, Barton M. 1995. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44:1239–1242 [DOI] [PubMed] [Google Scholar]
- 45. Meek SE, Nair KS, Jensen MD. 1999. Insulin regulation of regional free fatty acid metabolism. Diabetes 48:10–14 [DOI] [PubMed] [Google Scholar]
- 46. Jensen MD, Bajnárek J, Lee SY, Nielsen S, Koutsari C. 2009. Relationship between postabsorptive respiratory exchange ratio and plasma free fatty acid concentrations. J Lipid Res 50:1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]