Abstract
Purpose
Current therapies for male lower urinary tract symptoms secondary to prostate enlargement prevent hormonal effects on prostate growth and inhibit smooth muscle contraction to ease bladder neck and urethral pressure. However, lower urinary tract symptoms can be refractory to these therapies, suggesting that additional biological processes not addressed by them may also contribute to lower urinary tract symptoms. Aging associated fibrotic changes in tissue architecture contribute to dysfunction in multiple organ systems. Thus, we tested whether such changes potentially have a role in impaired urethral function and perhaps in male lower urinary tract symptoms.
Materials and Methods
Periurethral tissues were obtained from a whole prostate ex vivo and from 28 consecutive men treated with radical prostatectomy. Lower urinary tract symptoms were assessed using the American Urological Association symptom index. Prostate tissues were subjected to mechanical testing to assess rigidity and stiffness. Fixed sections of these tissues were evaluated for collagen and elastin content, and glandularity to assess fibrosis. Statistical analysis included the Student t test and calculation of Pearson correlation coefficients to compare groups.
Results
Periurethral prostate tissues demonstrated nonlinear viscoelastic mechanical behavior. Tissue from men with lower urinary tract symptoms was significantly stiffer (p = 0.0016) with significantly higher collagen content (p = 0.0038) and lower glandularity than that from men without lower urinary tract symptoms (American Urological Association symptom index 8 or greater vs 7 or less).
Conclusions
Findings show that extracellular matrix deposition and fibrosis characterize the periurethral prostate tissue of some men with lower urinary tract symptoms. They point to fibrosis as a factor contributing to lower urinary tract symptom etiology.
Keywords: prostate, prostatic hyperplasia, fibrosis, collagen, lower urinary tract symptoms
Lower urinary tract symptoms secondary to prostate enlargement manifest as urinary frequency and urgency, decreased force of stream, incomplete bladder emptying, hesitancy, intermittency and nocturia.1 If left untreated, LUTS can progress to bladder dysfunction, urinary retention and renal impairment.1–5
In men medical treatment for LUTS includes 5α-reductase inhibitors and/or α1-adrenergic receptor antagonists. Although these therapies ameliorate symptoms, they can produce adverse effects that require termination of the therapeutic regimen and LUTS can become refractory to these approaches.1–5 Thus, there is a need to explore other therapeutic targets to provide LUTS relief with improved tolerability and duration.
A potential target is prostatic fibrosis, which to our knowledge has been unexplored. Fibrosis is an aberrant version of the normal wound healing process that is characterized by myofibroblast accumulation, collagen deposition, extracellular matrix remodeling and tissue rigidization.6–8 Numerous studies show that aging and inflammation associated fibrotic changes in tissue architecture contribute to dysfunction and disease in multiple organ systems, including pancreatic dysfunction in type 2 diabetes,9,10 chronic obstructive pulmonary disease,11,12 cirrhotic nonalcoholic fatty acid liver disease13,14 and Crohn disease, which is part of the spectrum disorder termed inflammatory bowel disease.15,16 If the prostate, like other soft tissues, is susceptible to fibrotic changes associated with inflammation and aging, such changes could produce stiffer tissue architecture and a consequent negative impact on urethral function, promoting urinary obstructive symptoms.
To investigate prostatic fibrosis as a potential contributing factor to LUTS we established parameters to measure prostatic tissue stiffness and determined whether these measures correlated with histopathology and collagen content indicating fibrosis in a series of patients with or without evidence of LUTS. Results revealed that extracellular matrix deposition characterizes the periurethral prostate tissue of some men with LUTS and point to fibrosis as a previously unrecognized factor contributing to LUTS etiology.
MATERIALS AND METHODS
Tissue Procurement and Preparation
An entire prostate was resected at autopsy within 24 hours after death from a 66-year-old male who died of chronic lymphocytic leukemia. Information on LUTS or prostate cancer was not available in the medical record for this patient. Periurethral tissue was also procured at surgery from 28 consecutive men undergoing radical prostatectomy, of whom 21 had completed the AUASI within 30 days before surgery. All prostate tissues, and annotated clinical and pathology information were obtained with institutional review board approval. All tissues were received in 10% RPMI medium and processed immediately for mechanical and histological studies.
Mechanical Testing
The postmortem prostate was processed within 48 hours after death. Nine tissue slices were taken parallel and 9 were taken perpendicular to the urethra. Slices were trimmed to a mean ± SD of 12.3 ± 1.7 × 3.5 ± 0.4 × 3.3 ± 0.4 mm (fig. 1, A). Prostatectomy tissues were processed within 24 hours of procurement and trimmed to a mean of 9.5 ± 2.2 × 1.7 ± 0.5 × 2.0 ± 0.6 mm. All trimmed tissues were subjected to uniaxial load-unload mechanical testing using a constant strain rate of 0.01 per second to attain a maximum strain of 0.3 with a custom-built tensile tester (fig. 1, B). Synchronized force and image recordings were compiled using LabVIEW™.17 The terminal slope of the nominal stress vs nominal strain response is the tangent modulus or passive stiffness of prostate tissue in kPa, expressed as force per unit area, with higher tangent modulus values corresponding to greater tissue stiffness.
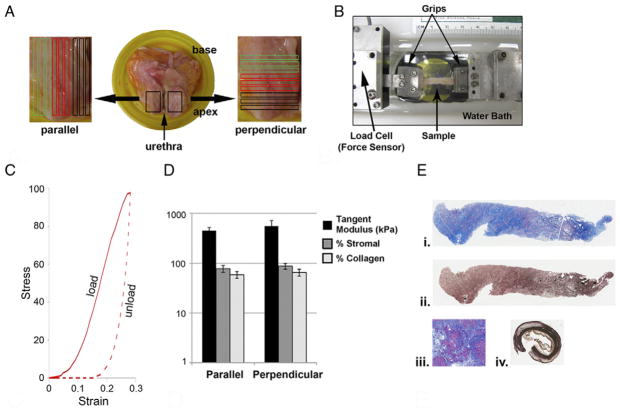
Figure 1.
Postmortem prostate tissue mechanical testing and histological assessment. A, prostate was trimmed of excess tissue and opened along urethra. Periurethral tissue showed some hyperplasia. Nine sections were taken parallel and 9 perpendicular to urethra. B, tensile tester had dual actuators driven by stepper motors and mounted on crossed roller slides with load cell on 1 actuator and grips at actuator ends. Specimen was gripped by micro artery clamp in larger grips. Grips hung in trough to submerge specimen in saline. Samples were loaded to maximum strain and unloaded at constant true strain rate. Synchronized force and image recordings were compiled. C, typical stress-strain uniaxial load-unload curve generated from mechanical testing of tissue sections parallel or perpendicular to urethra. Response curve was consistent with nonlinear and time dependent or viscoelastic mechanical behavior. D, tangent modulus, and collagen and stromal content of 9 tissue sections were each averaged and SD (error bars) was calculated. E, photomicrographs show parallel inner tissue sections. Deep blue areas indicate high collagen content (i). Masson trichrome stain, reduced from ×50. Same section (ii). Verhoeff elastin stain, reduced from ×50. Human cirrhotic liver serving as positive control for collagen was assessed at about 80% collagen (iii). Masson trichrome stain, reduced from ×50. Human aorta (iv). Brown areas indicate nonspecific staining. Black areas indicate elastin fibers. Verhoeff elastin stain, reduced from ×50.
Histological Evaluation
The 18 tissue slices from the postmortem prostate and tissues adjacent to those subjected to mechanical testing from the prostatectomy specimens were fixed in 10% phosphate buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin. We determined the percent of glandular epithelium and stroma, and the extent of inflammatory infiltrate, which was assessed as absent, minimal, mild or severe. Elastin content was assessed by Verhoeff staining. Collagen content was assessed by Masson trichrome staining using a previously described methodology.18 Briefly, sections were digitally imaged with a PathScan Enabler IV (Advanced imaging Concepts, Princeton, New Jersey) and color segmented using a subprogram in MATLAB (R2010a, MathWorks®) that separates and quantifies color elements from trichrome images, permitting quantitation of blue stained areas corresponding to extracellular collagen. The extracellular collagen I portion of the tissue section (numerator) was divided by the area of the tissue section (denominator) and multiplied by 100 to provide the percent of collagen in that tissue section.
Statistical Analysis
The Student t test with p <0.05 considered statistically significant and Pearson correlation coefficients were calculated to compare groups.
RESULTS
Periurethral Prostate Tissues Demonstrate Nonlinear Viscoelastic Mechanical Behavior
Nodular hyperplasia was evident upon opening the postmortem prostate along the urethra (fig. 1, A). Stress-strain curves generated from uniaxial load-unload mechanical testing of the 9 tissue sections parallel and 9 perpendicular to the urethra were similar between sections and nodules, and also nonlinear and time dependent (fig. 1, C). This suggested that, like other soft tissues, periurethral prostate tissues showed viscoelastic mechanical behavior.19–22 In the 9 tissue sections taken parallel vs the 9 taken perpendicular to the urethra average tangent modulus was 450 ± 77 vs 560 ± 180 kPa, average stromal content was 78% ± 12% vs 88% ± 12% and average collagen content was 59% ± 9% vs 66% ± 10% (fig. 1, D). Thus, despite some localized differences in tensile mechanics, and glandular and collagen content these periurethral tissues were largely homogeneous for these properties.
Histologically Homogenous Periurethral Prostate Tissues are Mechanically Isotropic
Postmortem prostate tissues were relatively aglandular with a 5% to 35% glandular content and 65% to 95% stromal content (fig. 1, D). All tissue sections showed minimal or mild inflammatory infiltrate and the extent of inflammation evaluated for all sections was similar. Masson trichrome and Verhoeff staining revealed an average collagen content of 49% to 67% and minimal elastin content, respectively (fig. 1, E). The average tangent modulus, stromal content and collagen content of the 9 tissue sections parallel or perpendicular to the urethra were similar and not significantly different within or between groups (fig. 1, D). This suggested that the periurethral tissues examined were largely homogeneous for these properties and mechanically isotropic.
Periurethral Tissue Collagen Content and Mechanical Stiffness/Rigidity are Positively Correlated with LUTS
After validating the approaches used to mechanically test postmortem prostate tissue we used these methods to similarly test periurethral tissue from 28 prostatectomy specimens. One piece of tissue was available for testing from each of 10 patients and 2 or more were available for each of the remaining 18. The tangent modulus for these tissues was 9 to 2,390 kPa (median 502). The median collagen content of these tissues was 32%. The average tangent modulus of tissues with a collagen content of greater than 32% was significantly higher than that of tissues with a collagen content of less than 32%. This indicated that greater tissue stiffness directly correlated with higher collagen content due to fibrosis associated extracellular matrix deposition (r = 0.60, p = 0.0038, table 1 and fig. 2, A).
Table 1.
Tangent modulus
| No. Pts | Median/Cutoff (range) | Mean ± SD Tangent Modulus (kPa)
|
p Value | ||
|---|---|---|---|---|---|
| Median or Less | Greater Than Median | ||||
| Content: | 28 | ||||
| Collagen | 32% (1.6–58.3) | 477 ± 340 | 1,211 ± 794 | 0.0038 | |
| Glandular | 40% (0–70) | 1,113 ± 862 | 654 ± 431 | 0.08 | |
| AUASI | 21 | 7 (0–31) | 390 ± 215 | 1,184 ± 758 | 0.0016 |
| Transrectal ultrasound prostate vol | 19 | 60, 40 cc (23.4–196) | 829 ± 770, 910 ± 890 | 930 ± 713, 857 ± 652 | 0.77, 0.88 |
| Age at surgery | 28 | 58 yrs (43–70) | 842 ± 683 | 846 ± 760 | 0.99 |
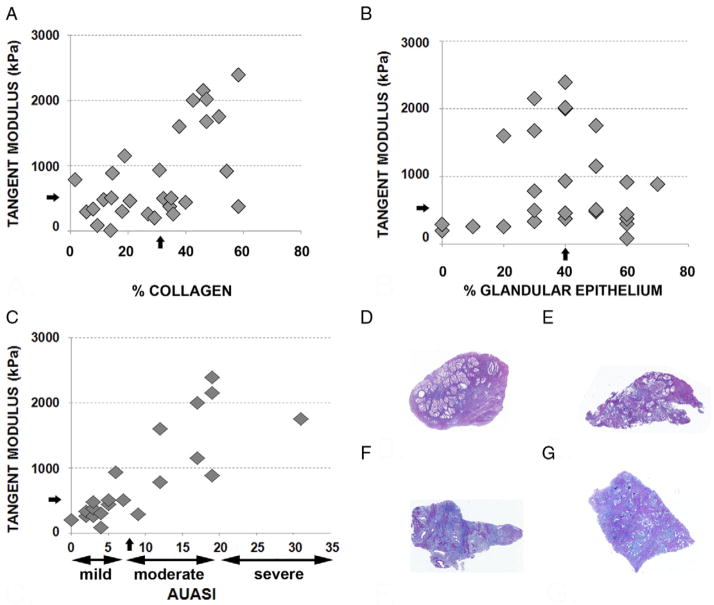
Figure 2.
Mechanical testing and histological assessment of periurethral prostatectomy tissue obtained at prostatectomy. A, percent collagen content and tangent modulus of tissues from 28 patients. Arrows indicate median. B, percent glandular epithelium and tangent modulus of tissues from 28 patients. Arrows indicate median. C, AUASI and tangent modulus of tissues from 21 of 28 patients. D and E, representative photomicrographs show high glandular and low collagen content associated with low mechanical stiffness. D, patient 090611a. E, patient 061411. F and G, photomicrographs show representative low glandular and high collagen content associated with high mechanical stiffness. F, patient 090611b. G, patient 092010. D to F, Masson trichrome stain, reduced from ×50.
The median glandular content of these tissues was 40% (range 0% to 70%). The average tangent modulus of tissues with less than 40% glandular contents was higher than that of tissues with greater than 40% glandular contents. Although these differences were not statistically significant, they indicated a trend toward a correlation of greater tissue stiffness with lower tissue glandular content (p = 0.080, fig. 2, B).
The average tangent modulus of tissues of the 12 patients with an AUASI score of 7 or less was less than that of the 9 with a score of 8 or greater. This was a highly statistically significant difference, indicating that greater tissue stiffness directly correlated with moderate/severe LUTS in these patients (r = 0.82, p = 0.0016).
Figure 2, D and E shows examples of trichrome stained tissues representative of periurethral prostate tissues with high glandular and low collagen content associated with low mechanical stiffness. Figure 2, F and G show those with low glandular and high collagen content associated with high mechanical stiffness.
Mild/moderate histological inflammation was observed in 5 of 13 (38%) vs 9 of 15 patient tissues (60%) with a tangent modulus below vs above the median of 502 kPa. These differences were not statistically significant but they suggested a trend toward higher inflammation levels associated with greater tissue stiffness. Elastin staining was evaluable for periurethral tissues from 24 of the 28 patients but it was largely negative with staining evident in tissue from only 8 of 24 patients (33%).
AUASI scores, including 0 to 7—absent/mild, 8 to 19 —moderate and 20 to 35—severe, were available for 21 of the 28 study patients. Of the men 12, 8 and 1 described symptoms that were absent/mild, moderate and severe, respectively. The average tangent modulus of tissues from the 9 patients describing a moderate/severe AUASI score was significantly higher than that of tissues from the 12 with a score in the absent/mild range (p = 0.0016). This indicated that greater tissue stiffness directly correlated with moderate/severe LUTS (r = 0.82, table 1). Men with moderate/severe symptoms scored significantly worse in several categories, particularly intermittent stream (table 2).
Table 2.
AUASI score components
| Pt No. | Incomplete Emptying | Frequent Urination | Intermittent Stream | Urgency | Weak Stream | Straining | Nocturia |
|---|---|---|---|---|---|---|---|
| AUASI 7 or less: | |||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 3 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 4 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| 5 | 0 | 2 | 1 | 0 | 1 | 0 | 1 |
| 6 | 0 | 1 | 1 | 0 | 2 | 0 | 1 |
| 7 | 1 | 1 | 0 | 0 | 4 | 0 | 0 |
| 8 | 2 | 2 | 0 | 0 | 1 | 0 | 2 |
| – | – | – | – | – | – | – | |
| Totals | 3 | 8 | 3 | 2 | 9 | 1 | 7 |
| AUASI 8 or greater: | |||||||
| 1 | 0 | 4 | 0 | 2 | 0 | 0 | 2 |
| 2 | 0 | 0 | 2 | 0 | 5 | 3 | 2 |
| 3 | 3 | 1 | 2 | 0 | 2 | 0 | 2 |
| 4 | 2 | 2 | 2 | 2 | 2 | 1 | 1 |
| 5 | 1 | 2 | 4 | 1 | 5 | 3 | 1 |
| 6 | 5 | 4 | 5 | 1 | 3 | 0 | 1 |
| 7 | 3 | 3 | 2 | 2 | 5 | 1 | 3 |
| 8 | 0 | 5 | 2 | 5 | 4 | 0 | 3 |
| – | – | – | – | – | – | – | |
| Totals | 14 | 21 | 19 | 13 | 26 | 8 | 15 |
| p Value | 0.069 | 0.026 | 0.003 | 0.035 | 0.020 | 0.089 | 0.018 |
Periurethral Tissue Stiffness was Independent of Prostate Volume and Patient Age
Transrectal ultrasound measurements, available for 19 of the 28 prostates subjected to mechanical testing, were used to calculate prostate volume. The average tangent modulus for periurethral tissues from prostates smaller or larger than the median volume of 60 cc was not statistically significant. Since median prostate volume was skewed toward higher volume prostates, data were reevaluated using 40 cc as a cutoff. The average tangent modulus for tissues from prostates smaller or larger than 40 cc was again not statistically significant (table 1). Together these results suggest that periurethral tissue stiffness was independent of prostate volume.
Median age at surgery of these 28 patients was 58 years (range 43 to 73). The average tangent modulus for periurethral tissues from men younger or older than 58 years was not statistically significant (table 1). Notably average age at surgery was 59 ± 6 years and in 20 of the 28 men (70%) ages clustered between 53 and 64 years.
DISCUSSION
We propose what is to our knowledge a previously unexplored mechanism that might promote LUTS in men, namely prostate tissue stiffening consequent to fibrosis. Results demonstrate the feasibility of measuring prostate tissue stiffness, the association of high levels of tissue stiffness with collagen content and fibrosis, and the further association of these measures with LUTS. Such periurethral tissue fibrosis could decrease urethral flexibility and compromise the ability of the prostatic urethra to expand to accommodate urinary flow during micturition, which could manifest as obstructive symptoms.
A limitation of the current study was the inability to collect and examine transurethral resection of the prostate tissues procured after treatment for benign prostatic hyperplasia and prostatic enlargement. This inability was due to the current standard of care at our institution, which is based on laser ablation of prostate tissue. Although it is effective, this method does not permit tissue collection for research studies. Another limitation is the lack of information on LUTS in study patients other than preoperative AUASI scores. Since most patients are referred to our institution for prostatectomy after the prostate cancer diagnosis, information on a history of LUTS is not available. Also, it is unclear whether periurethral fibrosis reflects fibrosis only of the transitional zone of the prostate or whether it may indicate more widespread fibrosis including the peripheral zone and lower urinary tract. It is also unclear whether such more extensive fibrosis would impact urethral function. Furthermore, in 20 of the 28 men (70%) treated with prostatectomy ages clustered between 53 and 64 years. Thus, we could not adequately test the role of aging in the development of fibrotic changes in prostate tissue architecture. Despite these limitations we believe that our study clearly associates periurethral fibrosis with LUTS and provides the rationale for further study and perhaps therapeutic targeting of the mechanisms contributing to prostatic fibrosis.
Although to our knowledge it is novel in the setting of LUTS, aging associated fibrotic changes in tissue architecture contribute to dysfunction and disease in multiple organ systems.6 Fibrosis can generally be considered an errant wound healing process in response to chronic inflammation. Chronic inflammation has been noted in the prostate in the context of prostatitis and histological inflammatory infiltrate.6,8 Prostatitis is a common condition that accounts for almost 2 million ambulatory care visits annually in the United States.23 Epidemiological data reveal an association between chronic prostatic inflammation (ie prostatitis) and subsequent development of LUTS.24,25 Also, chronic inflammation has been noted in several histological studies of the prostate.25–29 Together these studies suggest that, as in other organ systems, inflammatory changes in the prostate consequent to aging or infection may promote progressive fibrosis and changes in tissue architecture, contributing to urinary obstructive symptoms.
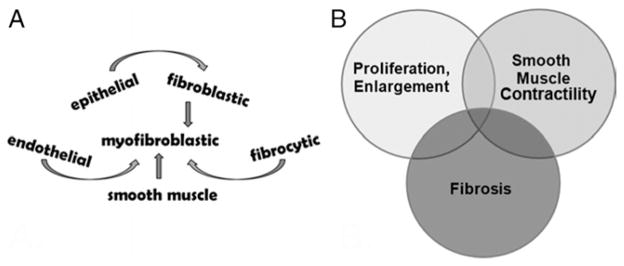
Multiple cell types can differentiate or dedifferentiate into myofibroblasts (fig. 3, A).30 Many if not all of these cell types comprise the prostatic tissue microenvironment, suggesting that dynamic changes in tissue composition may develop in the prostate due to aging and inflammatory processes. Our data suggest that fibrosis is one of these processes and it is associated with LUTS. Tissue fibrosis along with cellular proliferation/prostatic enlargement and smooth muscle hypercontractility may act independently or in combination to promote male LUTS (fig. 3, B). Moreover, these 3 pathological processes likely overlap, in that the cell types involved in these processes can accumulate and transdifferentiate. If so, therapy targeting these 3 pathological processes may be needed to adequately alleviate the symptoms and pathobiology contributing to male LUTS.
Figure 3.
Prostate fibrotic development and contribution to male LUTS. A, multiple cell types that can differentiate into myofibroblastic cells. B, 3 major pathobiological processes that can act alone or in combination to promote male LUTS.
CONCLUSIONS
To our knowledge we report the first study showing that fibrotic changes affecting periurethral prostatic tissue are associated with increased mechanical stiffness and LUTS. Thus, some patients with LUTS may benefit from antifibrotic treatment alone or combined with the currently prescribed regimen of 5α-reductase inhibitors and α1-adrenergic receptor antagonists.22
Supplementary Material
Acknowledgments
Supported by Grant P20DK090770 (JAM).
Abbreviations and Acronyms
- AUASI
American Urological Association symptom index
- LUTS
lower urinary tract symptoms
Footnotes
Study received institutional review board approval.
Supplementary material for this article can be obtained at http://jurology.com.
References
- 1.Laborde EE, McVary KT. Medical management of lower urinary tract symptoms. Rev Urol, suppl. 2009;11:S19. [PMC free article] [PubMed] [Google Scholar]
- 2.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 3.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 4.Parsons JK, Bergstrom J, Silberstein J, et al. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology. 2008;72:318. doi: 10.1016/j.urology.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin DE, Kopp ZS, Agatep B, et al. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 6.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 8.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detlefsen S, Sipos B, Feyerabend B, et al. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 10.Donath MY, Schumann DM, Faulenbach M, et al. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care, suppl. 2008;31:S161. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 11.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 12.Gharaee-Kermani M, Hu B, Phan SH, et al. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 13.Frith J, Day CP, Henderson E, et al. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55:607. doi: 10.1159/000235677. [DOI] [PubMed] [Google Scholar]
- 14.Novo E, di Bonzo LV, Cannito S, et al. Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int J Biochem Cell Biol. 2009;41:2089. doi: 10.1016/j.biocel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 16.Goldacre MJ. Demography of aging and the epidemiology of gastrointestinal disorders in the elderly. Best Pract Res Clin Gastroenterol. 2009;23:793. doi: 10.1016/j.bpg.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Larkin LM, Calve S, Kostrominova TY, et al. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler J, Swanson SD, Schmiedlin-Ren P, et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology. 2011;259:127. doi: 10.1148/radiol.10091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RE. Stress-strain characteristics of fresh and frozen human aortic and mitral leaflets and chordae tendineae. Implications for clinical use. J Thorac Cardiovasc Surg. 1973;66:202. [PubMed] [Google Scholar]
- 20.Ghista DN, Rao AP. Mitral-valve mechanics—stress-strain characteristics of excised leaflets, analysis of its functional mechanics and its medical application. Med Biol Eng. 1973;11:691. doi: 10.1007/BF02478657. [DOI] [PubMed] [Google Scholar]
- 21.Lanir Y, Fung YC. Two-dimensional mechanical properties of rabbit skin. IExperimental results. J Biomech. 1974;7:29. doi: 10.1016/0021-9290(74)90058-x. [DOI] [PubMed] [Google Scholar]
- 22.Lanir Y, Fung YC. Two-dimensional mechanical properties of rabbit skin. IIExperimental results. J Biomech. 1974;7:171. doi: 10.1016/0021-9290(74)90058-x. [DOI] [PubMed] [Google Scholar]
- 23.Habermacher GM, Chason JT, Schaeffer AJ. Prostatitis/chronic pelvic pain syndrome. Annu Rev Med. 2006;57:195. doi: 10.1146/annurev.med.57.011205.135654. [DOI] [PubMed] [Google Scholar]
- 24.St Sauver JL, Jacobson DJ, McGree ME, et al. Longitudinal association between prostatitis and development of benign prostatic hyperplasia. Urology. 2008;71:475. doi: 10.1016/j.urology.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger JN, Lee SW, Jeon J, et al. Epidemiology of prostatitis. Int J Antimicrob Agents, suppl. 2008;31:S85. doi: 10.1016/j.ijantimicag.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roehrborn CG, Kaplan SA, Noble WD, et al. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPH: results from the MTOPS study. J Urol, suppl. 2005;173:346, abstract 1277. [Google Scholar]
- 27.Robert G, Descazeaud A, Nicolaiew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69:1774. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delongchamps NB, de la Roza G, Chandan V, et al. Evaluation of prostatitis in autopsied prostates—is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179:1736. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierhoff E, Vogel J, Benz M, et al. Stromal nodules in benign prostatic hyperplasia. Eur Urol. 1996;29:345. doi: 10.1159/000473774. [DOI] [PubMed] [Google Scholar]
- 30.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.