Abstract
This study reports for the first time the complete liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS) and tandem mass spectrometry (MS/MS) analyses performed in negative ion mode of saturated unsulfated chondroitin oligosaccharides up to 16-mers and comparison with hyaluronic acid (HA) oligomers differing only in the nature of the hexosamine residue. MS/MS of the chondroitin disaccharide on the singly charged precursor at m/z 396.1 afforded a glycosidic cleavage C1 product ion at m/z 192.9. In the tetrasaccharide, C2 (m/z 396.0) and C3 (m/z 572.0) product anions were generated by glycosidic cleavage. A C5 [M–2H]2− production at m/z 475.1 was generated by the glycosidic cleavage of the hexasaccharide, and a C7 ion (m/z 664.6, charge state of −2) was produced from the octasaccharide. The same fragmentation pattern of deprotonated oligomers was observed for the largest oligosaccharides, from 10- to 16-mers. There has been no previous report of MS/MS spectra for unsulfated chondroitin oligomers of these sizes. Unsulfated saturated chondroitin oligosaccharides with x-mer units and larger than a tetrasaccharide dissociate to almost exclusively form CX–1-type ions. Saturated HA oligomers also afforded the same fragmentation pattern as deprotonated oligomers by ESI-MS and MS/MS analyses. Thus, under the experimental conditions used in the current study, we were unable to distinguish between unsulfated chondroitin and HA.
Escherichia coli K4 bacteria (05:K4:H4) produces a polysaccharide composed of repeating disaccharides [GlcA (β1 → 3) GalNAc (β1 ar=r 4)]n to which β-fructofuranose units are linked to C-3 of D-glucuronic acid (GlcA) residues.1,2 D-Fructose is easily and rapidly removed by acid hydrolysis3–5 to produce an unsulfated chondroitin backbone. This uncommon unsulfated polymer has a very similar structure to the more common nonsulfated hyaluronic acid or hyaluronan (HA) formed of repeating disaccharides [GlcA (β1 → 3) GlcNAc (β1 → 4)]n differing only in the nature of the hexosamine residue.
Oligosaccharides prepared from glycosaminoglycans (GAGs) by controlled enzymatic depolymerization are often studied to define the structural requirements for biological activity6,7 and electrospray ionization/tandem mass spectrometry (ESI-MS/MS) coupled online with reversed-phase high-performance liquid chromatography (RP-HPLC) is particularly useful for characterizing the structure of these GAG-derived oligomers, such as N-acetylheparosan,8,9 heparin,10Escherichia coli K4 polysaccharide,2 and HA.9,11 Isobaric oligosaccharides enzymatically prepared from HA and N-acetylheparosan, differing from each other only in the position of the linkage between GlcA and GlcNAc, have been distinguished using MS/MS.9 Although the ESI-MS spectra of unsaturated chondroitin disaccharide12 and tetrasaccharide13 are known, this work examines the ESI-MS and ESI-MS/MS analyses of saturated unsulfated chondroitin oligosaccharides as large as 16-mers (hesadecasaccharides). Furthermore, MS/MS analyses were performed to evaluate differences and similarities between the isobaric saturated oligosaccharides derived from chondroitin and HA.
EXPERIMENTAL
E. coli strain K4 U1-41, serotype O5:K4(L):H4, was from the American Type Culture Collection (LGC Promochem s.r.l., Milan, Italy). HA sodium salt from rooster comb and hyaluronidase from bovine testes [E.C. 3.2.1.35, 300 units/mg solid] were purchased from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile, MS-grade, and all other reagents, of the purest grade available, were from Sigma Aldrich.
K4 polysaccharide was produced and purified as previously reported2 and totally defructosylated by acid treatment3–5 to produce the chondroitin polymer. This unsulfated polysaccharide (and HA) was partially degraded by using bovine testicular hyaluronidase to produce saturated oligosaccharides of various lengths, up to 16-mers. To a solution containing 20mg of polysaccharide in 2mL of 100mM Na acetate buffer adjusted to pH 5.2 with acetic acid containing 150mM NaCl were added 5000 units of hyaluronidase, and enzymatic digestion was performed at 37°C for 1–8 h. The incubation time of the hyaluronidase varied according to the size of oligosaccharides to be obtained, assessed at various time points by running 5μL of the reaction mixture by strong anion exchange (SAX)-HPLC.3 The reactions were stopped by boiling for 20 min. The sample was centrifuged at 10 000 rpm for 30 min at 5°C, and the supernatant was studied by HPLC/ESI-MS as previously reported2,11 on a 3-μm Gemini C18 110Å column (4.6×150mm; Phenomenex, Torrance, CA, USA). Eluent A was water/acetonitrile (80:20) and eluent B was water/acetonitrile (35:65). Tributylamine (15mM) and ammonium acetate (50mM) were added to both eluents.10 The pH of the mobile phase was adjusted to 7.0 with acetic acid. The sample (20μL, approx. 200 μg) was injected, and a linear gradient (from 0 to 100% eluent B in 90 min) at a flow rate of 0.3 mL/min was used for elution. Samples were directly on-line injected at 0.3 mL/min into the ESI source of an Agilent Technologies LC/MSD Trap VL ion trap mass spectrometer fitted with an ESI interface with an orthogonal nebulizer (Agilent Technologies, Palo Alto, CA, USA). The oligosaccharides were detected in the negative ion mode using the following parameters: capillary voltage 3500V (to obtain the maximum value of signal-to-noise ratio), nebulizer gas pressure 40 psi (275.8×103 N/m2), trap drive 47.2 V, skim-mer one −43.2 V, capillary exit offset −77.2 V. Nitrogen was used as the drying gas at a flow rate of 9 L/min and temperature of 350°C. The scan range used was m/z 200–2200, with a maximum accumulation time of 300 ms, and a ion charge control (ICC) target of 20 000. Auto MS/MS, a procedure for performing MSn analyses to analyze multiple precursor ions, was turned on in these experiments giving an estimated cycle time of 0.1 min. The collision energy was optimized in order to obtain all structurally informative product ions. The isolation Software versions were LC/MSD trap control version 4.2 and Data Analysis version 2.2 (Agilent Technologies).
RESULTS AND DISCUSSION
After removal of fructose, the chondroitin polysaccharide was depolymerized by partial controlled digestion with testicular hyaluronidase, and separated into fully saturated oligosaccharides from 2- to 16-mers, having the general structure [GlcA (1 → 3) GalNAc (1− > 4)]n with n from 1 to 8 (identified by ESI-MS), by HPLC in approx. 30 min. (Figure 1 shows the total ion chromatogram (TIC) of the chondroitin oligosaccharides in negative ion mode.) According to Thanawiroon et al.,10 the choice of a volatile ion-pairing reagent represents a compromise between low alkyl chain length affording high volatility, required for compatibility with on-line MS, and longer alkyl chain length affording higher on-column retention of oligosaccharides associated with enhanced resolution. Tributylamine provided optimal retention with MS compatibility and good detection sensitivity. The choice of a small analytical RP-C18 column with a particle size of 3 mm and a pore size of 110Å enabled the separation and analysis of discrete amounts of chondroitin (or HA) digest mixture, about 200 μg sample size suitable for ESI-MS detection, by maintaining the capacity to resolve each single oligosaccharide species in a relatively short time. Calculated and experimentally derived m/z values for oligomers are shown in Table 1 (the ESI-MS spectra of several oligomers are shown in Fig. 2 as examples). More highly charged molecules were produced with the increasing length of the oligosaccharides.
Figure 1.
Total ion chromatograms (TICs) of unsulfated saturated chondroitin oligosaccharides up to 16-mers in negative ion mode separated by reversed-phase ion-pairing (RPIP)-HPLC. According to Thanawiroon and Linhardt,17 the presence of tributylamine may result in peak doubling, presumably through the resolution of the α and β anomeric forms of the individual oligosaccharides.
Table 1.
Calculated monoisotopic m/z values for anions derived from fully saturated unsulfated chondroitin oligomer species separated by means of reversed-phase ion pairing (RPIP)-HPLC, and calculated MS/MS C1 product ions derived from precursor ions by glycosidic cleavage (according to the nomenclature of Domon and Costello14). The main ions detected for each oligosaccharide are highlighted in bold. m.m.=molecular mass. RT=retention time
| Possible ions (charge) m/z |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chondroitin oligomers | m.m. | (−1) | (−2) | (−3) | (−4) | (−5) | (−6) | (−7) | (−8) |
| 2-mers (RT=6.3 min) | |||||||||
| GlcA-GalNAc | 397.1 | 396.1 | |||||||
| C1 product | 194.0 | 193.0 | |||||||
| 4-mers (RT=7.8 min) | |||||||||
| [GlcA-GalNAc]2 | 776.2 | 775.2 | 387.1 | ||||||
| C3 product | 573.1 | 572.1 | 285.1 | ||||||
| 6-mers (RT=10.2 min) | |||||||||
| [GlcA-GalNAc]3 | 1155.3 | 1154.3 | 576.7 | 384.1 | |||||
| C5 product | 952.2 | 951.2 | 474.6 | 316.1 | |||||
| 8-mers (RT=13.6 min) | |||||||||
| [GlcA-GalNAc]4 | 1534.4 | 1533.4 | 766.2 | 510.5 | 382.6 | ||||
| C7 product | 1331.3 | 1330.3 | 664.2 | 442.4 | 442.4 | ||||
| 10-mers (RT=18.6 min) | |||||||||
| [GlcA-GalNAc]5 | 1913.5 | 1912.5 | 955.8 | 636.8 | 477.4 | 381.7 | |||
| C9 product | 1710.4 | 1709.4 | 853.7 | 568.8 | 426.4 | 340.9 | |||
| 12-mers (RT=23.2 min) | |||||||||
| [GlcA-GalNAc]6 | 2292.6 | 2291.6 | 1145.3 | 763.2 | 572.2 | 457.5 | 381.1 | ||
| C11 product | 2089.5 | 2088.5 | 1043.3 | 695.2 | 521.1 | 416.7 | 347.1 | ||
| 14-mers (RT=26.5 min) | |||||||||
| [GlcA-GalNAc]7 | 2671.7 | 2670.7 | 1334.9 | 889.6 | 666.9 | 533.3 | 444.3 | 380.7 | |
| C13 product | 2468.6 | 2467.6 | 1232.8 | 821.5 | 615.9 | 492.5 | 410.3 | 351.5 | |
| 16-mers (RT=29.2 min) | |||||||||
| [GlcA-GalNAc]8 | 3050.8 | 3049.8 | 1524.4 | 1015.9 | 761.7 | 609.2 | 507.5 | 434.8 | 380.4 |
| C15 product | 2847.7 | 2846.7 | 1422.4 | 947.9 | 710.7 | 568.3 | 473.5 | 405.7 | 354.8 |
Figure 2.
ESI-MS spectra recorded in negative ion mode of various single unsulfated saturated chondroitin oligosaccharide species, in particular tetrasaccharide (A), octasaccharide (B), dodecasaccharide (C), and the 16-mer oligomer (D), separated by RPIP-HPLC.
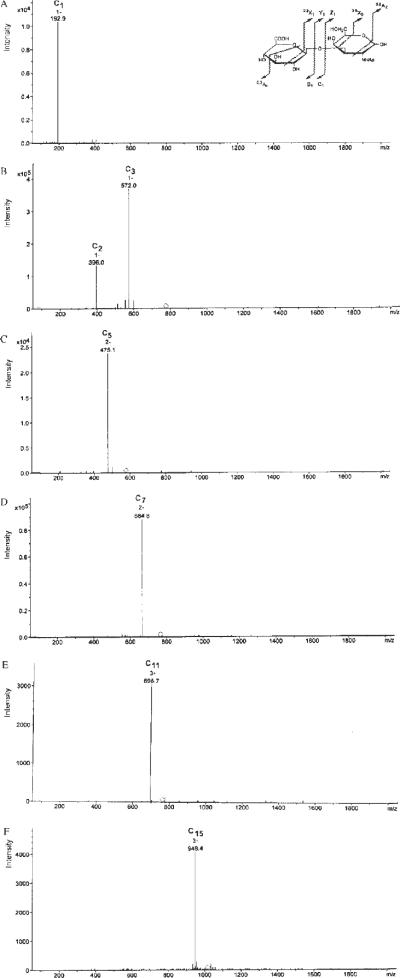
The ESI-MS/MS spectrum of the [M–H]1− precursor ion at m/z 396.1 of the disaccharide (2-mer) from chondroitin (Fig. 3(A) and Table 1) afforded a glycosidic cleavage C1 product ion (according to the nomenclature of Domon and Costello14) at m/z 192.9 and the same ion was found for the smallest saturated HA oligomer, 2-mers (not shown). C2 (m/z 396.0) and C3 (m/z 572.0) productions14 were generated by the glycosidic cleavage of the 4-mer oligosaccharide ([M–H]1− ion at m/z 775.2) (Fig. 3(B)). A C5 [M–2H]2− ion at m/z 475.1 was produced by the glycosidic cleavage of the 6-mer oligosaccharide (ion [M–2H]2− ion at m/z 576.7) (Fig. 3(C)), a C7 ion (m/z 664.6, charge state of −2) was generated from the octasaccharide ([M–2H]2− ion at m/z 766.2) (Fig. 3(D)), and a C9 [M–3H]3− ion (m/z 568.8) was produced from the decasaccharide [M–3H]3− ion at m/z 636.8) (not shown). The same fragmentation of the deprotonated oligomers was observed for larger oligomers. In fact, MS/MS on the [M–3H]3− ion (m/z 763.2) of the 12-mer oligosaccharide afforded the C11 [M–3H]3− ion (m/z 695.7) (Fig. 3(E)). MS/MS on the [M–3H]3− ion (m/z 889.6) of the 14-mer species produced a C13 [M–3H]3− ion (m/z 821.8) (not shown), and a C15 [M–3H]3− product ion (m/z 948.4) was generated from the 16-mer [M–3H]3− precursor ion (m/z 1015.9) (Fig. 3(F)). The MS/MS spectra of unsulfated chondroitin oligomers of sizes as large as 16-mers have not previously been reported. It is noteworthy that the same fragmentation pattern was found for the saturated HA oligomers (not shown).
Figure 3.
ESI-MS/MS spectra recorded in negative ion mode of various single unsulfated saturated chondroitin oligosaccharide species, in particular disaccharide (A), tetrasaccharide (B), hexasaccharide (C), octasaccharide (D), dodecasaccharide (E), and 16-mer oligomer (F), separated by means of RPIP-HPLC. See also Table 1 for the calculated MS/MS C1 product ions derived from specific precursor ions. The inset in (A) shows the nomenclature for disaccharide fragmentation proposed by Domon and Costello.14 Ions that contain a non-reducing terminus are labeled with upper-case letters from the beginning of the alphabet (A, B, C), and those that contain the reducing end of the oligosaccharide are labeled with letters from the end of the alphabet (X, Y, Z). Subscripts indicate the cleaved ions. The A and X ions are produced by cleavage across the glycosidic ring, and are labeled by assigning each ring bond a number and counting clockwise. Ions produced from cleavage of successive residues are labeled Am, Bm, and Cm, with m=1 for the non-reducing end, and Xn, Yn, and Zn, with n=1 for the reducing-end residue.
This study provides MS/MS analyses of unsulfated chondroitins, composed of repeating [GlcA (β1→3) GalNAc (β1→4)n disaccharides as large as 16-mers. Zaia et al.13 found that the [M–H]1− ion of an unsulfated unsaturated chondroitin tetrasaccharide dissociated to form an abundant series of C-type product ions. The same product ions have been observed in this study for the saturated chondroitin 4-mer oligomer, in particular the formation of the C3 and C2 anions. Furthermore, we found that unsulfated saturated chondroitin oligosaccharides, formed of x-mer units and larger than a tetrasaccharide, dissociate to form CX–1-type ions almost exclusively. According to Zaia et al.,13 C-type ions are favored in the negative ion mode when either sulfate or carboxylate groups are present in the oligomer structure. In contrast, the formation of B- and Y-type ion pairs is favored in the presence of both sulfate and carboxylate groups.
These data should be useful for the structural characterization of oligosaccharides having the previously reported disaccharide sequences, as for example for the enzymatic synthesis of chondroitin by using chondroitin sulfate N-acetylgalactosaminyltransferase.15 Furthermore, a comparison was made of the isobaric HA saturated oligosaccharides and no differences were observed in the spectra of these two polymers, despite differences in the nature of the hexosamine residue. Thus, other MS approaches are needed to differentiate between these two isobars. Studies are in progress to evaluate the possibility to distinguish unsulfated chondroitin and HA oligosaccharides by using MSn experiments.
CONCLUSIONS
The current study reports for the first time the complete MS and MS/MS spectra of saturated unsulfated chondroitin oligosaccharides up to 16-mers. Several studies15,16 have been undertaken to enzymatically produce and elongate chondroitin with specific transferases. As a consequence, chondroitin oligosaccharides of various sizes are being generated. The rapid analytical methodology presented here for the characterization of saturated unsulfated chondroitin oligosaccharides up to 16-mers may be useful for the understanding of their fine structure and related properties of such enzymatically synthesized chondroitins.
REFERENCES
- 1.Rodriguez ML, Jann B, Jann K. Eur. J. Biochem. 1988;177:117. doi: 10.1111/j.1432-1033.1988.tb14351.x. [DOI] [PubMed] [Google Scholar]
- 2.Volpi N. Rapid Commun. Mass Spectrom. 2007;21:3459. doi: 10.1002/rcm.3245. [DOI] [PubMed] [Google Scholar]
- 3.Volpi N. Glycobiology. 2003;13:635. doi: 10.1093/glycob/cwg074. [DOI] [PubMed] [Google Scholar]
- 4.Volpi N. Electrophoresis. 2003;24:1063. doi: 10.1002/elps.200390123. [DOI] [PubMed] [Google Scholar]
- 5.Volpi N. Electrophoresis. 2004;25:692. doi: 10.1002/elps.200305563. [DOI] [PubMed] [Google Scholar]
- 6.Seyfried NT, McVey GF, Almond A, Mahoney DJ, Dudhia J, Day AJ. J. Biol. Chem. 2005;280:5435. doi: 10.1074/jbc.M411297200. [DOI] [PubMed] [Google Scholar]
- 7.Blundell CD, Mahoney DJ, Almond A, DeAngelis PL, Kahmann JD, Teriete P, Pickford AR, Campbell ID, Day AJ. J. Biol. Chem. 2003;278:49261. doi: 10.1074/jbc.M309623200. [DOI] [PubMed] [Google Scholar]
- 8.Kuberan B, Lech M, Zhang L, Wu ZL, Beeler DL, Rosenberg RD. J. Am. Chem. Soc. 2002;124:8707. doi: 10.1021/ja0178867. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Xie J, Liu J, Linhardt RJ. J. Am. Soc. Mass Spectrom. 2008;19:82. doi: 10.1016/j.jasms.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanawiroon C, Rice KG, Toida T, Linhardt RJ. J. Biol. Chem. 2004;279:2608. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- 11.Volpi N. Anal. Chem. 2007;79:6390. doi: 10.1021/ac070837d. [DOI] [PubMed] [Google Scholar]
- 12.Ii T, Okuda S, Hirano T, Ohashi M. Glycoconjugate J. 1994;11:123. doi: 10.1007/BF00731152. [DOI] [PubMed] [Google Scholar]
- 13.Zaia J, Miller MJ, Seymour JL, Costello CE. J. Am. Soc. Mass Spectrom. 2007;18:952. doi: 10.1016/j.jasms.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domon B, Costello CE. Glycoconjugate J. 1988;5:397. [Google Scholar]
- 15.Gotoh M, Sato T, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, Yada T, Inaba N, Zhang Y, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Nishihara S, Watanabe H, Kimata K, Narimatsu H. J. Biol. Chem. 2002;277:38189. doi: 10.1074/jbc.M203619200. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Gotoh M, Kiyohara K, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, Yada T, Inaba N, Togayachi A, Kudo T, Asada M, Watanabe H, Imamura T, Kimata K, Narimatsu H. J. Biol. Chem. 2003;278:3063. doi: 10.1074/jbc.M208886200. [DOI] [PubMed] [Google Scholar]
- 17.Thanawiroon C, Linhardt RJ. J. Chromatogr. A. 2003;1014:215. doi: 10.1016/s0021-9673(03)00779-9. [DOI] [PubMed] [Google Scholar]