Abstract
We previously showed that survival signaling in TNFα-treated, human THP1-derived macrophages (TDMs) has an obligatory requirement for constitutive Ca2+ influx through a mechanism involving calmodulin/calmodulin kinase II (CAM/CAMKII). We also demonstrated that such requirement also applies to the protective actions of TNFα in murine bone marrow-derived macrophages (BMDMs) and that TRPC3 channels mediate constitutive Ca2+ influx. Using a pharmacological approach we here examined if in BMDMs, similarly to TDMs, TNFα-induced survival signaling also involves CAM/CAMKII. In BMDMs, TNFα induced rapid activation of the survival pathways NFkB, AKT and p38MAPK. All these routes were activated in a PI3K-dependent fashion. Activation of AKT and NFkB, but not that of p38MAPK, was abrogated by the CAM inhibitor W7, while KN-62, a CAMKII inhibitor, prevented activation of AKT and p38MAPK but not that of NFkB. Inhibition of CAM or CAMKII completely prevented the protective actions of TNFα. Our observations indicate that in BMDMs CAM and CAMKII have differential contributions to the components of TNFα-dependent survival signaling and underscore a complex interplay among canonical survival routes. These findings set a signaling framework to understand how constitutive Ca2+ influx couples to macrophage survival in BMDMs.
Keywords: calcium influx, calcium channels, macrophage survival, apoptosis, calmodulin, calmodulin kinase
INTRODUCTION
Macrophages are critical players during inflammation resolution and as such, they are determinant to the progression of inflammatory vascular disease such as atherosclerosis [1]. In fact, within the context of the cellular and molecular events that underlie atherosclerotic lesion formation and progression, the balance between survival and apoptosis of lesional macrophages and their timely clearance from the lesion site by resident phagocytes –efferocytosis- shape the lesion cellularity and thus its progression and fate. A rapidly growing area of research in the field is focused on characterizing mechanisms that regulate macrophage survival, apoptosis and efferocytosis during atherogenesis with the hope of identifying novel targets to develop alternative strategies to manage the disease [1; 2]. In cells other than macrophages some of the typical cell survival pathways –i.e., the phosphatidylinositol-3-kinase (PI3K)/AKT axis and Nuclear Factor kappa B (NFκB)- are modulated, directly or indirectly, by Ca2+ influx [3; 4; 5]. While studying the actions of the atherorelevant cytokine tumor necrosis factor alpha (TNFα) on macrophage survival and apoptosis, we recently showed for the first time that in human, THP-1-derived macrophages (TDMs), as in other cell types, Ca2+ influx is also critical to support survival signaling [6]. In that instance we found that constitutive, non-regulated Ca2+ influx couples to cell survival through a calmodulin/calmodulin-dependent kinase II (CAM/CAMKII) axis [6]. In TDMs however, TNFα-induced survival signaling is the result of a compensatory response of the macrophages against the pro-apoptotic actions of the cytokine. In more recent work using murine bone marrow-derived macrophages (BMDMs), in which TNFα exerts an unambiguous pro-survival effect [7; 8], we found, once again, that a Ca2+ influx dependent mechanism exists and that TRPC3, a member of the TRPC family of Ca2+-permeable cation channels [9; 10], is the channel responsible for mediating constitutive Ca2+ influx in these cells [11]. However, if a CAM/CAMKII-dependent mechanism also operates in BMDMs remains to be determined. To examine this, in the present work we explored TNFα-dependent activation of survival pathways in BMDMs and the impact of selective inhibitors of CAM and CAMKII on cell survival and apoptosis.
MATERIALS AND METHODS
Preparation of bone marrow-derived macrophages
In our recent studies on the role of TRPC3 channels in macrophage constitutive Ca2+ influx we used BMDMs derived from 129SvTrpc3−/− mice, while BMDMs from 129SvTrpc3lox/lox mice were used as control cells. Although in pilot studies we did not find any significant differences in the phenotype of macrophages from 129SvTrpc3lox/lox mice compared to those from wild-type animals, for consistency, in the present work all BMDMs used for experiments were obtained from 129SvTrpc3lox/lox (provided by Dr. Lutz Birnbaumer, NIEHS, NC). Generation and full characterization of these mice have been previously described [12]. All animal procedures were approved by University of Toledo IACUC. Bone marrow-derived macrophages were obtained essentially as described by us in [11]. Briefly, femurs and tibias were flushed with sterile RPMI (containing 2% FBS + 5 U/ml heparin + 1% penicillin/streptomycin) and cells were plated with L929-conditioned medium for 7 days (37°C, 5% CO2 atmosphere); after that, cells were recovered with ice-cold phosphate-buffered saline solution (PBS) and replated in 6- (immunoblots) or 96-well (TUNEL) plates for experiments. Macrophage phenotype was confirmed as we described in [6] (not shown). Immunoblotting conditions were as we described in [6; 11]. Briefly, following cell lysis, solubilized proteins were separated in 10% acrylamide gels, electrotransferred to PVDF membranes and immunoblotted with the indicated primary antibody. After incubation with appropriate HRP-conjugated secondary antibodies, immunoreactive bands were visualized by ECL (Amersham, PA). Phosphorylation of IκBα is immediately followed by its degradation; therefore, immunodetection of phospho-IκBα was normalized against GAPDH (see also [6]). Primary antibodies used were: cleaved PARP (Asp214, clone 7C9), phospho-IκBα (Ser32/36, clone 5A5), phospho-AKT (Ser473, clone 587F11), total AKT, phospho-GSK3β (Ser9, clone D85E12), total GSK3β (clone 27C10), phospho-p38 MAPK (Thr180/Tyr182, clone D3F9), total p38MAPK, all from Cell Signaling (MA); anti-GAPDH (clone 0411) was from Santa Cruz (CA).
TUNEL assay
apoptosis was assayed by using the in situ cell death detection kit, TMR red (Roche, IN) as described in [13]. Macrophages were grown on 96-well plates under the culture conditions described above; visualization and analysis was performed by fluorescence microscopy. Hoechst co-staining was used to count total cells. TMR-positive cells in 5 fields were counted and expressed as % of total cells.
Statistical analysis
Comparison of mean values was by using a two-tailed t test for two means, using Graph Pad InStat version 3.00 for Windows 95 (Graph Pad Software, San Diego CA, www.graphpad.com). All biochemical experiments were repeated at least 3 times. P<0.05 was considered significant.
RESULTS
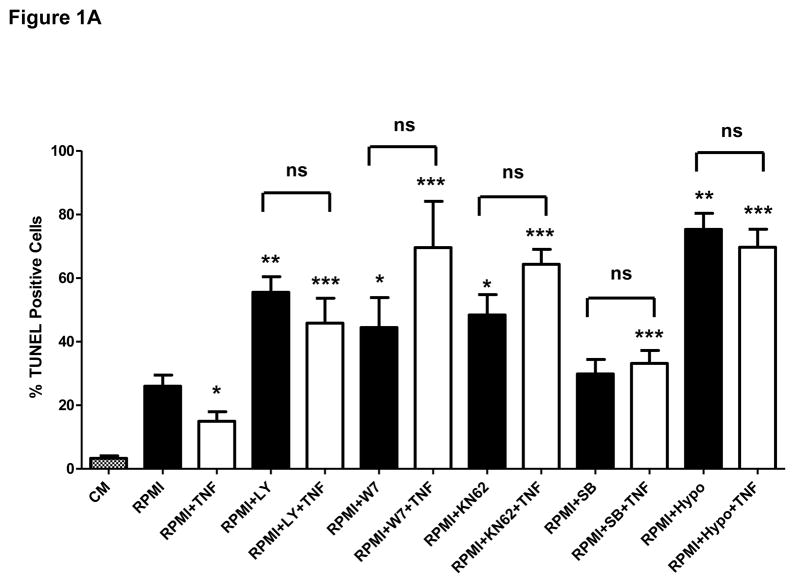
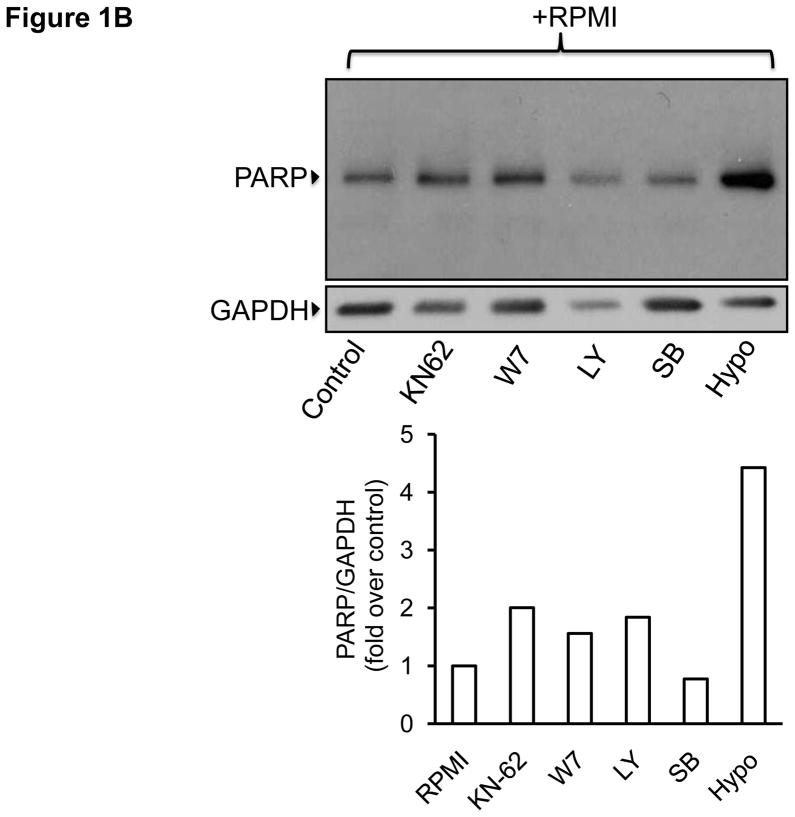
We recently showed that in THP-1-derived macrophages (TDMs) CAM and CAMKII are critical players within the compensatory survival signaling that takes place in response to the pro-apoptotic actions of TNFα [6]. Contrarily to the actions of this cytokine in TDMs, in bone marrow-derived macrophages (BMDMs) TNFα exerts an unambiguous pro-survival effect [7; 8] (see also Discussion). Yet, similar to TDMs, survival signaling in BMDMs also exhibits an obligatory requirement for constitutive Ca2+ influx [11] (and see Supplementary figure I). To determine if in BMDMs the survival mechanism underlying the protective actions of TNFα is similar to that mediating compensatory survival in TDMs, we first examined the contribution of different survival pathways to the protective actions of TNFα against apoptosis induced by macrophage-colony stimulating factor (M-CSF) withdrawal –i.e., serum free RPMI-. BMDMs were incubated for 24 hours in complete growth medium, serum free medium (RPMI) or RPMI containing TNFα (10ng/ml) in the presence or absence of inihibitors that selectively target PI3K/AKT, NFκB or p38MAPK –typical macrophage survival molecules-, or in the presence or absence of selective inhibitors of CAM and CAMKII. Following treatments apoptosis was examined by terminal deoxynucleotidyl-transferase-dUTP-nick end labeling (TUNEL) assay, as we described in [6]. As previously shown by others [7; 8], TNFα treatment exerted a clear protective action against M-CSF withdrawal-induced apoptosis, manifested by a significant reduction in the number of TUNEL-positive cells when compared to the control (Figure 1A). Notably, when macrophages were pre-treated with selective inhibitors for PI3-kinase (LY294002, 10 μM), calmodulin (W7, 10 μM), CAMKII (KN62, 25 μM), p38MAPK (SB203580, 10 μM) or the IκBα kinase IKK (hypoestoxide, 50 μM), the protective effect of TNFα was completely abrogated and the number of apoptotic cells increased by 2–4 fold, clearly indicating the involvement of these pathways in both basal and cytokine-dependent survival of the macrophages. Unlike to what we observed upon inhibition of PI3K, CAM, CAMKII or IKK, inhibition of p38MAPK did not affect apoptosis induced by M-CSF withdrawal. All these observations were positively correlated with increased levels of cleaved poly (ADP-ribose) polymerase (PARP; Fig. 1B).
Figure 1.
A) Bone marrow-derived macrophages were incubated for 24 hours in complete growth medium (CM), serum-free RPMI medium (RPMI) or RPMI containing TNFα (TNF, 10 ng/ml) in the presence or absence of selective inhibitors of PI3K (LY294002 or LY, 10 μM), CAM (W7, 10 μM), CAMKII (KN62, 25 μM), p38MAPK (SB203580 or SB, 10 μM) or IkBα (hypoestoxide, “Hypo”, 50 μM). Alternatively, cells were incubated (24 h) in RPMI containing those inhibitors but in the absence of TNFα. Following treatments macrophages were processed for evaluation of apoptosis by TUNEL assay (see details in Materials and Methods). *P<0.05, **P<0.0001, respect to RPMI; ***P<0.0003 respect to RPMI+TNF. “ns”: not statistically significant difference. Averages are from four independent experiments.
B) Bone marrow-derived macrophages were incubated for 24 hours in serum-free RPMI medium (RPMI) or RPMI containing inhibitors of survival pathways at the concentrations indicated in panel “A”. Following treatments cells were processed for immunodetection of cleaved PARP (89 kDa) in whole cell lysates. Membranes were reprobed for GAPDH to control for protein loading. Shown is a blot representative of three independent experiments and its corresponding densitometric analysis.
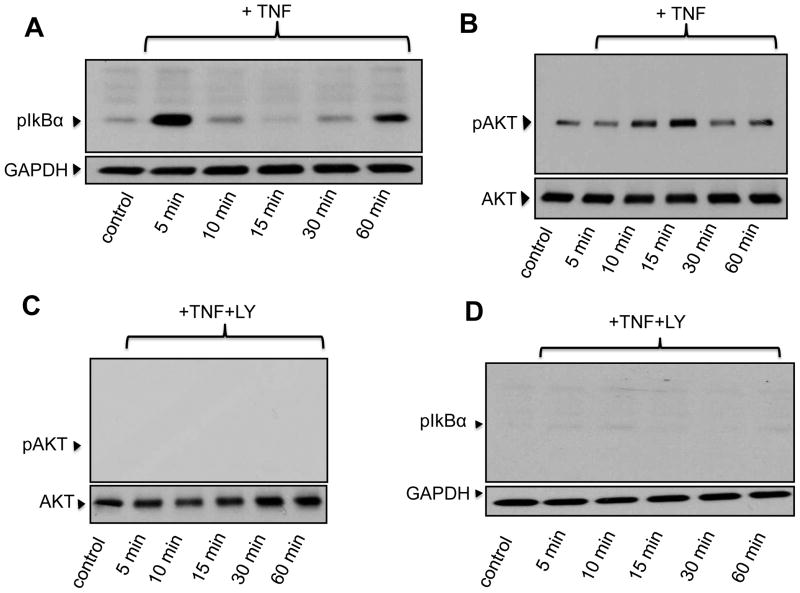
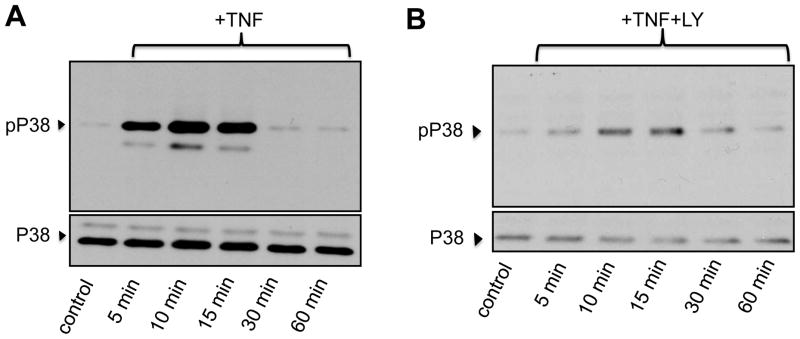
We next examined if treatment of BMDMs with TNFα promoted activation of survival signaling pathways. Canonical survival mechanisms in macrophages, as in other cell types, involve rapid, sometimes transient activation but with a long lasting impact in anti-apoptotic mechanisms [6; 11]. BMDMs were exposed to TNFα (10 ng/ml) for 5, 10, 15, 30 or 60 min; following treatments, cells were lysed and cellular proteins processed for immunoblotting to examine the phosphorylation status of typical survival molecules. NFκB is a key regulator of survival gene expression in macrophages [14], and we have previously shown that the phosphorylation status of IκBα, rather than its degradation, is a more reliable indicator of rapid activation of the NFκB route [6; 11]. Treatment of BMDMs with TNFα caused a rapid (within 5- min) and transient phosphorylation of IκBα (Fig. 2A). Activation of AKT, another critical regulator of macrophage survival [13; 15] occurs in a strictly PI3K-dependent manner and is evidenced by phosphorylation of AKT on Ser473 [15]. Figure 2B shows that TNFα treatment resulted in a time-dependent activation of AKT (10–15 min) which declined to pre-stimulation levels after 60 min of treatment. No significant changes were observed in the amount of total AKT. The PI3K inhibitor LY294002 (10 μM) completely abrogated TNFα-dependent phosphorylation of AKT (Fig. 2C) confirming activation downstream of PI3K. As we recently described in TDMs [6], pre-treatment of BMDMs with LY294002 abrogated TNFα-dependent IκBα phosphorylation (Fig. 2D) supporting the notion that AKT and NFκB can crosstalk to promote macrophage survival [3]. The mitogen-activated protein kinase p38MAPK also represents an important player in macrophage survival [16]. As shown in Fig. 2E, TNFα treatment promoted a robust and transient (5–15 min) increase in phosphorylation of p38MAPK (Thr180/Tyr182). Total levels of p38MAPK remained unchanged. Similarly to what we observed for AKT and IκBα, inhibition of PI3K with LY294002 resulted in marked reduction of TNFα-dependent phosphorylation of p38MAPK (Fig. 2F).
Figure 2.
Bone marrow-derived macrophages were treated for the indicated times with TNFα (TNF, 10 ng/ml) in the absence (A, B, E) or presence (C, D, F) of LY294002 (LY, 10 μM) and then processed for immunodetection of phospho-IκBα (Ser32/36, 40 kDa, A, D), phospho-AKT (Ser473; 60 kDa, B, C), or phospho-p38MAPK (pP38, Thr180/Tyr182; 43 kDa, E, F) in whole cell lysates. Membranes were reprobed for GAPDH, total AK or total p38MAPK (P38) to control for protein loading. Blots are representative from three independent experiments.
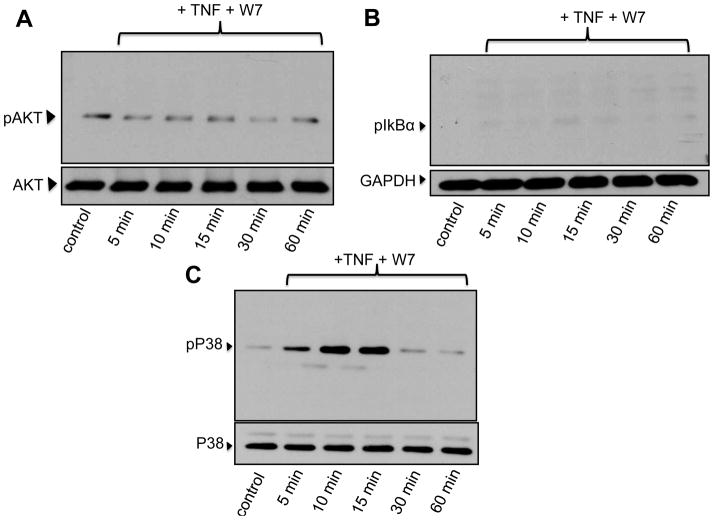
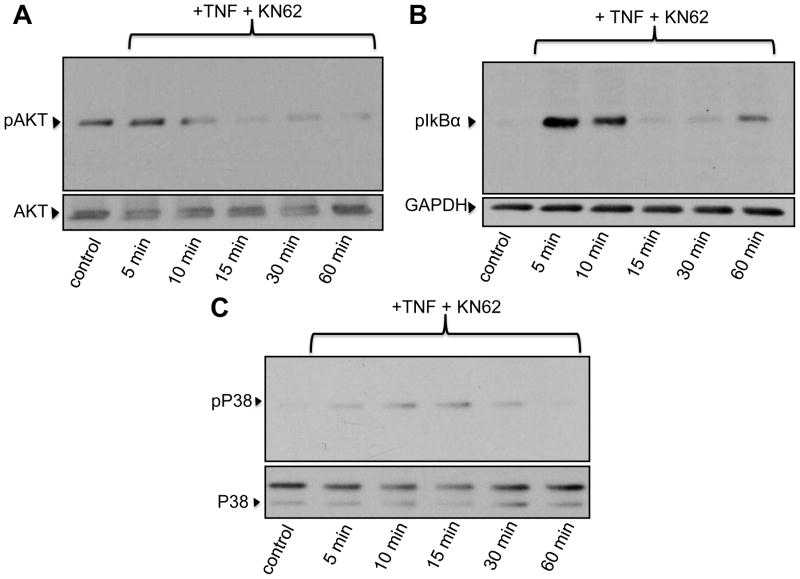
In TDMs we have shown that constitutive Ca2+ influx is an obligatory component of survival signaling through a mechanism that involves the calmodulin (CAM)/CAM-dependent kinaseII (CAMKII) axis [6]. In more recent work we demonstrated that TRPC3-mediated constitutive Ca2+ influx is also mandatory for survival of BMDMs [11]. However, if CAM and/or CAMKII are required in the survival mechanism that operates in BMDMs remained to be determined. To specifically address this issue, we examined TNFα-dependent activation of survival pathways in the presence of selective inhibitors of CAM and CAMKII. As shown in Figure 3, TNFα-induced phosphorylation of IκBα and AKT, but not that of p38MAPK, were suppressed when macrophages were pre-treated with the CAM inhibitor W7 (10 μM). Notably, the CAMKII antagonist KN62 (25 μM) significantly reduced TNFα-induced phosphorylation of AKT and p38MAPK but did not affect IκBα phosphorylation (Fig. 4). Neither W7 nor KN62 affected the basal phosphorylation status of AKT (“Control” in Figures 3A and 4A). GSK3β is a downstream target of AKT and AKT-mediated phosphorylation of Ser9 inhibits GSK3β pro-apoptotic actions [17]. Notably, GSK3β exhibited constitutive phosphorylation of Ser9 over the time course examined, and this was not significantly increased by TNFα treatment (Supplementary figure IIA). As expected, pre-treatment with LY294002 completely abrogated GSK3β phosphorylation on Ser9 (Supplementary figure IIB).
Figure 3.
Bone marrow-derived macrophages were pre-incubated for 15 min with the calmodulin inhibitor W7 (25 μM), treated with TNFα (TNF, 10 ng/ml) for the indicated times and then processed for immunodetection of phospho-AKT (Ser473; 60 kDa, A), phospho-IκBα (Ser32/36, 40 kDa, B) or phospho-p38MAPK (pP38, Thr180/Tyr182Ser473; 43 kDa, C) in whole cell lysates. Membranes were reprobed for total AKT, GAPDH or total p38MAPK to control for protein loading. Blots are representative from three independent experiments.
Figure 4.
Bone marrow-derived macrophages were pre-incubated for 15 min with the calmodulin-dependent kinase II inhibitor KN62 (10 μM), treated with TNFα (TNF, 10 ng/ml) for the indicated times and then processed for immunodetection of phospho-AKT (Ser473; 60 kDa, A), phospho-IκBα (Ser32/36, 40 kDa, B) or phospho-p38MAPK (pP38, Thr180/Tyr182Ser473; 43 kDa, C) in whole cell lysates. Membranes were reprobed for total AKT, GAPDH or total p38MAPK to control for protein loading. Blots are representative from three independent experiments.
DISCUSSION
In recent work from our laboratory we showed that in TDMs constitutive Ca2+ influx, acting through CAM and CAMKII, is mandatory for proper operation of survival signaling [6]. In TDMs however, activation of survival mechanisms in response to TNFα mostly results from a compensatory response of the cells against the pro-apoptotic actions of the cytokine. In BMDMs, unlike TDMs, TNFα exerts a pro-survival, protective action [7; 18]. Yet, we recently found that constitutive Ca2+ influx is also obligatory for TNFα-dependent activation of survival molecules in BMDMs and that TRPC3, a member of the TRPC family of non-selective Ca2+-permeable channels, is responsible of mediating such constitutive Ca2+ entry [11]; however, it remained to be determined if a CAM/CAMKII-dependent mechanism was also operational in BMDMs. In the present work we addressed this important question by examining the impact of selective inhibition of CAM and CAMKII on M-CSF withdrawal-induced apoptosis of BMDMs and on TNFα-dependent activation of survival molecules. Our findings show that treatment of BMDMs with TNFα results in reduced number of apoptotic cells in response to M-CSF withdrawal, confirming the observations by others that in BMDMs TNFα exerts a protective action. This pro-survival effect of the cytokine was positively correlated with activation of typical survival routes such as NFκB, AKT and p38MAPK. Notably, although based on a pharmacological approach, our findings suggest that in BMDMs CAM and CAMKII also contribute, although differentially, to TNFα-dependent activation of these survival molecules. Therefore, similarly to our findings in TDMs [6], CAM and CAMKII presumably couple TRPC3-mediated constitutive Ca2+ entry to intracellular survival mechanisms. When CAM or CAMKII were selectively inhibited, not only was TNFα-dependent activation of survival signaling markedly reduced but it also augmented the apoptosis rate of BMDMS. This was accompanied by a two-fold increase in the levels of cleaved PARP, which is a direct substrate of caspase-3, a key player in the execution stages of macrophage apoptosis [19]. Interestingly, inhibition of CAM but not CAMKII some prevented TNFα-dependent phosphorylation of IκBα, suggesting that in BMDMs CAM can promote NFκB activation independently of CAMKII, as it has been shown in certain non-macrophage cell types [20; 21]. On the other hand, inhibition of CAMKII, but not CAM, significantly reduced TNFα-induced phosphorylation of p38MAPK. Whereas inhibition of CAM and CAMKII both completely prevented TNFα-dependent activation of AKT, neither W7 nor KN62 affected the basal phosphorylation level of AKT; such constitutive AKT activity, which is not a rare finding in macrophages from different sources and species [6; 13; 15], might explain the constitutive phosphorylation of GSK3β which was not modified by TNFα treatment but completely suppressed by inhibition of PI3K, an obligatory upstream regulator of AKT. The transcription factor β-catenin can promote cell survival by directly regulating expression of survival genes [22]. Phosphorylation of β-catenin by GSK3β targets β-catenin for ubiquitination and proteasomal degradation [23] and thus, signals that abrogate GSK3β-mediated β-catenin phosphorylation –such as AKT mediated phosphorylation of GSK3β on Ser9, which inactivates GSK3β- result in β-catenin dependent cell survival. Our findings therefore suggest that constitutive, AKT-mediated phosphorylation of GSK3β on Ser-9 in BMDMs can promote β-catenin accumulation and probably help in keeping a tonic survival status of the macrophage (see model in Supplementary figure III). Importantly, results derived from TUNEL assays confirm a critical role of PI3K/AKT and NFκB pathways in survival of BMDMs. Interestingly our data indicate that a pro-survival action of p38 MAPK may require concomitant activation of other survival routes. This is suggested by the observation that treating BMDMs with SB203580, a selective inhibitor of the p38α and p38β isoforms of MAPK [24], was not able to rescue macrophages from apoptosis induced by M-CSF withdrawal but it did prevent the protective actions of TNFα. Also, there was a significant reduction in p38MAPK phosphorylation when BMDMs were treated with the PI3K inhibitor LY294002, indicating partial dependence upon the PI3K/AKT axis. Previous studies aimed at understanding the mechanism underlying oxidized-LDL induced survival of BMDMs unraveled a pro-apoptotic role of p38δMAPK through phosphorylation and inhibition of eukaryotic elongation factor-2 (eEF2) kinase, a pro-survival molecule [25]. However, the p38α and p38β isoforms of p38MAPK had no effect on eEF2 kinase activity [25]. Our findings showing that selective inhibition of p38α and p38β isoforms of MAPK with SB203580 impairs the ability of TNFα to protect BMDMs from M-CSF withdrawal-induced apoptosis, suggest that different isoforms of p38MAPK –i.e., p38α/p38β vs. p38δ- may play quite opposite roles in the context of macrophage survival. Because TNFα-induced phosphorylation of p38MAPK was drastically reduced by inhibition of PI3K or CAMKII, it is possible that in BMDMs either both kinases contribute to p38MAPK activation, or CAMKII acts upstream of AKT which then promotes p38MAPK activity. Of note, our results are in agreement with those from Tabas’s group showing a critical role of p38αMAPK in suppressing macrophage apoptosis [26]. Because the antibody used in our experiments only detects phosphorylation of GSK3β on Ser9 –target site for AKT- it remains to be determined if in BMDMs p38MAPK survival actions are in part mediated by p38MAPK-mediated phosphorylation and inhibition of GSK3β, a recently described novel mechanism of p38MAPK-mediated, β-catenin-dependent cell survival [27].
Identifying and characterizing components of signaling mechanisms that mediate macrophage survival is of most importance to identify novel targets for pharmacological manipulation of inflammation resolution. The present findings represent a contribution to that goal, and provide evidence for the first time of CAM and CAMKII involvement in TNFα-induced survival of BMDMs, drawing a potential mechanism by which constitutive Ca2+ influx can couple to survival mechanisms in these cells. We have recently identified TRPC3 as the channel responsible for most of the constitutive Ca2+ influx that supports survival signaling in BMDMs [11]. Additional pharmacological and molecular studies are now required to determine the molecular determinants that link channel constitutive activity to CAM/CAMKII function.
Supplementary Material
Acknowledgments
This work has been supported by NIH (grant NHLBI1R01HL111877-01 to G.V.) and University of Toledo College of Medicine (G.V.).
ABBREVIATIONS
- CAM
calmodulin
- CAMKII
calmodulin dependent kinase II
- GSK3β
glycogen synthase kinase 3 beta
- PI3K
phosphatidylinositol-3-kinase
- NFkB
nuclear factor kappa B
- M-CSF
macrophage-colony stimulating factor
- TNFα
tumor necrosis factor alpha
- TRPC
Transient Receptor Potential Canonical
Footnotes
DISCLOSURES
None.
References
- 1.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tano JY, Lee RH, Vazquez G. Macrophage function in atherosclerosis: potential roles of TRP channels. Channels (Austin) 2012;6:141–148. doi: 10.4161/chan.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lilienbaum A, Israel A. From Calcium to NF-{kappa}B Signaling Pathways in Neurons. Mol Cell Biol. 2003;23:2680–2698. doi: 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentili C, Picotto G, Morelli S, Boland R, de Boland AR. Effect of ageing in the early biochemical signals elicited by PTH in intestinal cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2003;1593:169–178. doi: 10.1016/s0167-4889(02)00387-7. [DOI] [PubMed] [Google Scholar]
- 5.Sameermahmood Z, Balasubramanyam M, Saravanan T, Rema M. Curcumin Modulates SDF-1α/CXCR4–Induced Migration of Human Retinal Endothelial Cells (HRECs) Investigative Ophthalmology & Visual Science. 2008;49:3305–3311. doi: 10.1167/iovs.07-0456. [DOI] [PubMed] [Google Scholar]
- 6.Tano JY, Vazquez G. Requirement for non-regulated, constitutive calcium influx in macrophage survival signaling. Biochemical and Biophysical Research Communications. 2011;407:432–437. doi: 10.1016/j.bbrc.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Lo S, Steer J, Joyce D. Tumor necrosis factor-alpha promotes survival in methotrexate-exposed macrophages by an NF-kappaB-dependent pathway. Arthritis Research & Therapy. 2011;13:R24. doi: 10.1186/ar3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardo E, Alvarez-Barrientos A, Maroto B, Boscá L, Knaus UG. TLR4-Mediated Survival of Macrophages Is MyD88 Dependent and Requires TNF-α Autocrine Signalling. The Journal of Immunology. 2007;178:3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney J, James W. The mammalian TRPC cation channels. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tano JY, Smedlund K, Lee R, Abramowitz J, Birnbaumer L, Vazquez G. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochemical and Biophysical Research Communications. 2011;410:643–647. doi: 10.1016/j.bbrc.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–8. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, Fazio S. Macrophage LRP-1 Controls Plaque Cellularity by Regulating Efferocytosis and Akt Activation. Arterioscler Thromb Vasc Biol. 2010;30:787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 Deficiency Increases Apoptosis and Suppresses Early Atherosclerosis. Cell metabolism. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively Activated Akt-1 Is Vital for the Survival of Human Monocyte-differentiated Macrophages: Role of Mcl-1, Independent of Nuclear Factor (NF)-{kappa}B, Bad, or Caspase Activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zeigler MM, Lam GK, Hunter MG, Eubank TD, Khramtsov VV, Tridandapani S, Sen CK, Marsh CB. The Role of the NADPH Oxidase Complex, p38 MAPK, and Akt in Regulating Human Monocyte/Macrophage Survival. American Journal of Respiratory Cell and Molecular Biology. 2007;36:68–77. doi: 10.1165/rcmb.2006-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 18.Rushworth SA, MacEwan DJ. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood. 2008;111:3793–3801. doi: 10.1182/blood-2007-07-104042. [DOI] [PubMed] [Google Scholar]
- 19.Asmis R, Begley JG. Oxidized LDL Promotes Peroxide-Mediated Mitochondrial Dysfunction and Cell Death in Human Macrophages: A Caspase-3-Independent Pathway. Circ Res. 2003;92:e20–29. doi: 10.1161/01.res.0000051886.43510.90. [DOI] [PubMed] [Google Scholar]
- 20.Edin S, Oruganti SR, Grundström C, Grundström T. Interaction of calmodulin with Bcl10 modulates NF-κB activation. Molecular Immunology. 47:2057–2064. doi: 10.1016/j.molimm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Antonsson Å, Hughes K, Edin S, Grundström T. Regulation of c-Rel Nuclear Localization by Binding of Ca2+/Calmodulin. Molecular and Cellular Biology. 2003;23:1418–1427. doi: 10.1128/MCB.23.4.1418-1427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 23.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Letters. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 25.Chen JH, Riazy M, Smith EM, Proud CG, Steinbrecher UP, Duronio V. Oxidized LDL-Mediated Macrophage Survival Involves Elongation Factor-2 Kinase. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:92–98. doi: 10.1161/ATVBAHA.108.174599. [DOI] [PubMed] [Google Scholar]
- 26.Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38α MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. The Journal of Clinical Investigation. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton T, Pedraza-Alva G, Deng B, Wood D, Aronshtam A, Clements J, Sabio G, Davis R, Matthews D, Doble B, Rincon M. Phosphorylation by p38 MAPK as an Alternative Pathway for GSK3β Inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.