Abstract
Multidetector-row computed tomography (MDCT) has become the primary imaging test for the staging and follow-up of most malignancies that originate outside of the central nervous system. Technical advances in this imaging technique have led to significant improvement in the detection of metastatic disease to the liver. An unintended by-product of this improving diagnostic acumen is the discovery of incidental hepatic lesions in oncology patients that in the past remained undetected. These ubiquitous, incidentally identified hepatic lesions have created a management dilemma for both clinicians and radiologists: are these lesions benign or do they represent metastases? Naturally, the answer to this question has profound prognostic and therapeutic implications. In this review, guidelines concerning the diagnosis and management of some of the more common hepatic incidental lesions detected in patients with extrahepatic malignancies are presented.
Keywords: Hepatic metastases, incidentaloma, hepatic cyst, bile duct hamartoma, hepatic hemangioma, focal nodular hyperplasia, hepatic adenoma, nodular regenerative hyperplasia
The problem
Metastatic disease to the liver is one of the most common problems encountered in patients with cancer. Although the true prevalence of metastatic disease is unknown, some 24–26% of individuals who die of malignancy will have liver metastases at autopsy[1–4]. Autopsy series also reveal the presence of benign hepatic lesions in up to 52% of the general population[1–4]. Recent advances in multidetector-row computed tomography (MDCT) have led to the more accurate and earlier detection of hepatic metastases.
Pari passu, incidental hepatic lesions that in the past remained undetected are being depicted with maddening regularity in the oncology and non-oncology patient population alike[5–9]. These incidental hepatic lesions have created a management conundrum for both clinicians and radiologists: does every hepatic lesion found in a patient with cancer require immediate further investigation with magnetic resonance imaging (MRI), positron emission tomography (PET)/computed tomography (CT), PET/MR, or biopsy to exclude metastases or a primary hepatic neoplasm? Faced with this tantalizing array of diagnostic imaging tests, exhaustive evaluation is advocated by some physicians who are unwilling to accept any degree of diagnostic uncertainty. This unwillingness is in part driven by a paucity of data on the topic, the lack of clear-cut algorithms with regard to diagnostic and treatment strategies, fear of potential malpractice liability, and/or the anxiety of the patient.
Strategies for optimizing patient management of these incidental hepatic lesions are only beginning to emerge in terms of deciding which ones are incidental and can be ignored, which can simply be monitored over time, and which lesions require more aggressive workup[10–12]. In this article, guidelines concerning the diagnosis and management of some of the more common hepatic incidental lesions detected in patients with extrahepatic cancer are suggested. While this discussion focuses on incidentalomas found on MDCT, many of its lessons also apply to ultrasonography and MRI.
Why the liver?
In oncologic terms, the liver has the dubious distinction of having a dual blood supply, being perfused by both the portal and the systemic circulations. Hepatic metastases from gastrointestinal tract malignancies are common, since the liver is the first capillary bed encountered by metastasizing cells and the hepatic sinusoids are fenestrated, permitting tumor cells to establish themselves and grow. Extra-abdominal tumors such as bronchogenic carcinoma, breast cancer, and malignant melanoma spread hematogenously to the liver and are discovered as the initial site of metastases in 15%, 4%, and 24% of these patients, respectively[1].
This dual blood supply to the liver is also responsible for a number of benign entities that can simulate metastases on MDCT that are described more fully below: focal fatty deposition, focal sparing in a diffusely fatty liver, and transient hepatic attenuation differences (THADs).
What is at stake
Although isolated hepatic metastases are a common clinical problem frequently associated with a very poor prognosis, there are evolving systemic, regional, and local treatment strategies that have significantly expanded therapeutic options. Metastases to the liver represent the sole or life-limiting component of disease for many patients with colorectal cancer, ocular melanoma, genitourinary and neuroendocrine tumors[2].
In patients with limited hepatic metastatic disease from neuroendocrine tumors, transplantation is an increasingly popular therapeutic choice[13]. For selected patients with resectable hepatic metastases primarily of colorectal, neuroendocrine, or genitourinary origin, the benefit of resection in terms of overall survival or palliation is well established[14]. In patients with limited liver metastases from colorectal cancer, resection is associated with an overall 5-year survival of between 30% and 50%[15]. The development of highly effective systemic chemotherapy regimens has markedly expanded the criteria used to define resectability.
For an even larger group of patients with unresectable metastatic hepatic disease, multimodal approaches that integrate locoregional and systemic treatment are being increasingly used, providing an expectation that durable complete disease control within the liver with an associated improvement in quality of life and overall survival may be routinely achieved. The approaches that provide an opportunity to dose-intensify therapy to the liver include: percutaneous or surgical chemical, thermal, or cryoablation; chemoembolization; hepatic artery infusion pump therapy; isolated hepatic perfusion; whole-liver radiation therapy; highly conformed partial liver radiation therapy; and yttrium-90 microsphere therapy[16]. The selection and deployment of these treatment options is first based on the detection of the metastases and the ability to confidently differentiate them from incidentalomas.
The odds
A number of studies have evaluated the clinical significance of small low-density hepatic lesions found on MDCT in patients with cancer. Jones et al.[17] found at least one hepatic lesion ≤1.5 cm in 17% of all patients in a retrospective study performed on non-helical CT scanners. In patients with a known malignancy, 51% were benign, 26% were malignant, and 23% were indeterminate. The likelihood of malignancy was 5% with one lesion, 19% with 2–4 lesions, and 74% with 5 or more lesions. In a second study of 2978 patients with cancer, Schwartz et al.[18] found small (≤1.0 cm) lesions in 12.7% of patients, of which 80.2% were benign, 11.6% were malignant, and 8.2% were indeterminate.
Studies performed with helical scanner technology have also found that the majority of small, low-attenuating lesions within the liver in the oncology patient are benign. In a review of 1133 patients with colorectal and gastric cancer, Jang et al.[19] found small (≤1.5 cm) hypoattenuating lesions in 25.5% of cases. Some 94% of lesions that were smooth and of low density (≤20 HU) proved to be benign. In a study of 941 women with breast cancer, Khalil et al.[20] found one or more small, hypoattenuating lesions in 29.4%. In 92.7% of patients these lesions showed no change, in 4.2% they disappeared, and in 3.1% they became larger. Khalil et al. concluded that finding a small, hypodense lesion in the liver in a patient with otherwise no definite metastases was a benign finding. Krakora et al.[21], in a study of 153 patients with breast cancer, discovered small hypoattenuating hepatic lesions in 35%. They found that the presence of these small lesions without definite hepatic metastases did not contribute an increased risk of developing subsequent hepatic metastases.
The players
Metastases
Metastases are the most common malignant liver tumors, and occur 20 times more frequently than primary hepatic neoplasms[2]. Except for infiltrative tumors such as lymphoma, most metastases manifest as multiple discrete lesions. The imaging appearance of metastases can vary greatly depending on differences in blood supply, hemorrhage, cellular differentiation, fibrosis, and necrosis[22].
Most liver metastases are hypovascular and as a result are hypodense on CT in comparison with normal liver parenchyma during the portal venous phase (PVP). Colon, lung, breast, and gastric cancers are the most common causes of hypovascular liver metastases. They are best visualized during the PVP and typically show perilesional enhancement or a target appearance[22]. If the lesions do not show this appearance, small hypodense metastases may be difficult to differentiate from a host of benign hepatic lesions. Benign low-attenuation lesions tend to be smaller, more frequently have a discrete margin, and markedly low attenuation compared with metastases. Target enhancement is far more frequent in metastases than in benign low-attenuation lesions[19].
Hypervascular metastases enhance earlier and are most conspicuous on the hepatic arterial phase (HAP). In addition, they demonstrate variable degrees of washout on delayed images. The most common causes of hypervascular hepatic metastases include neuroendocrine tumors (e.g., carcinoid, pheochromocytoma, and islet cell tumors), renal cell carcinoma, melanoma, choriocarcinoma, and thyroid carcinoma. Breast carcinoma and pancreatic adenocarcinoma uncommonly cause hypervascular metastases[23].
Hypervascular metastases <1.5 cm can be difficult to distinguish from flash-filling hepatic hemangiomas (HHs), since both can display rapid enhancement during the HAP. On PVP or delayed-phase imaging, however, they do have a distinctly different appearance[24]. HHs retain their contrast material and appear isodense with the blood pool during the PVP, while hypervascular metastases tend to wash out. Another potential distinguishing feature is the “peripheral washout” sign, a specific but insensitive sign for malignancy that favors the diagnosis of metastasis or hepatocellular carcinoma over HH. Malignant lesions often show peripheral washout of contrast on delayed contrast-enhanced images and a target appearance, with the rim appearing hypodense relative to the center. This target appearance has been reported to be highly specific for hypervascular metastasis (100% specificity), and is frequently observed in neuroendocrine and carcinoid tumors[24].
Hypodense incidental liver lesions
Low-density liver lesions are commonly found incidentally in both the oncology and non-oncology populations. These lesions are listed in Table 1 and discussed more fully below.
Table 1.
Differential diagnosis of cystic–hypodense hepatic lesions on multidetector-row computed tomography
| Metastases |
| Cysts |
| Bile duct hamartomas |
| Polycystic liver disease |
| Peribiliary cysts |
| Sclerosing cholangitis |
| Abscesses |
| Bilomas |
| Cystic primary hepatic neoplasms |
| Post-traumatic cysts |
| Hydatid disease |
| Caroli disease |
| Choledochal cysts |
| Focal fat |
Hepatic cysts
The incidence of simple hepatic cysts ranges up to 14% in autopsy series, 17% in CT series, and 20% in surgery series[2]. The vast majority of cysts are found incidentally and do not require any treatment or further evaluation. On CT (Fig. 1b), uncomplicated hepatic cysts manifest as well-defined, water-attenuation (<20 HU) intrahepatic masses, with smooth, thin walls, no internal structure, and no enhancement following contrast administration. The attenuation values of small hepatic cysts can be influenced by partial volume averaging, pixel size, matrix size, kilovoltage and milliamperage of the x-ray beam, slice thickness, reconstruction algorithm, patient diameter, and pseudo-enhancement. All these factors can influence the attenuation of the cyst contents. If the patient is scanned using dual-energy CT, the lack of contrast enhancement is easy to confirm, as described below.
Figure 1.
Multiple bile duct hamartomas. Axial (a) and coronal (b) contrast-enhanced CT scans show innumerable tiny, non-enhancing cystic hepatic lesions.
Hemorrhage and infection can also lead to increased density of hepatic cysts. The differential diagnosis of a hepatic cyst includes cystic metastases from cystic primary tumors (i.e., ovarian and cystic pancreatic primaries) and solid tumors that can produce cystic metastases (i.e., gastrointestinal stromal tumor and endometrial carcinoma). MRI with diffusion-weighted imaging (DWI), which is described more fully below, can help differentiate these lesions.
Bile duct hamartomas
Bile duct hamartomas (BDH), also known as biliary microhamartomas or von Meyenburg complexes, are a focal disorderly collection of bile ducts that result from failure of involution of embryonic bile ducts. BDHs are found in 0.69–5.6% of individuals at autopsy. They manifest on CT (Fig. 1) as multiple, widely scattered, small (<1.5 cm), low-attenuation lesions, which do not demonstrate discernible contrast enhancement[25,26]. Typically they are not uniform in size. If needed, dual-energy CT can confirm the absence of contrast enhancement and MRI can confirm the cystic nature of these lesions, but in the vast majority of cases these techniques are not needed.
The differential diagnoses of BDHs include: metastatic disease, multiple microabscesses, Caroli disease, peribiliary cysts, primary sclerosing cholangitis, and simple hepatic cysts. BDHs are usually not uniform in size, whereas metastatic lesions are usually more heterogeneous in size and attenuation.
Focal hepatic steatosis
The risk of developing high-grade steatosis and steatohepatitis doubles in patients undergoing chemotherapy[27,28]. When fatty infiltration is diffuse or lobar, segmental, or wedge shaped, differentiation from other focal hepatic disease is straightforward. In these cases the region of fat has a straight-line margin with normal parenchyma, typically extending to the liver capsule without associated bulging of the hepatic contour, or vascular displacement or invasion to suggest an underlying mass. When steatosis is nodular or focal, differentiation from metastatic disease and other masses can be problematic on CT[29]. Absolute CT-attenuation values are unreliable indicators because fatty infiltration does not produce a fat-density lesion, rather the steatosis merely diminishes the density of the region to lower than that of normal liver parenchyma[30]. There are several features that are helpful in this differentiation: focal fat does not cause local contour abnormalities; portal and hepatic venous branches course normally through the fatty areas; and these lesions may improve in a matter of days[31].
The two most common areas of focal fatty infiltration and focal sparing in an otherwise normal or diffusely fatty liver are surrounding the gallbladder fossa and adjacent to the falciform ligament in segments II, III, and IV (Fig. 2). In the gallbladder fossa, direct vascular communications to the portal system through aberrant gastric venous flow or accessory cystic veins permit perfusion of this portion of the liver by systemic blood flow rather than by splanchnic venous blood from the portal veins. The liver adjacent to the falciform ligament has also been shown to have aberrant direct venous flow. Consequently, a third blood supply to these areas may help spare them the adverse effects of toxic agents entering through the portal circulation. This variant vascular blood supply is also key in the development of THADs (see below)[32].
Figure 2.
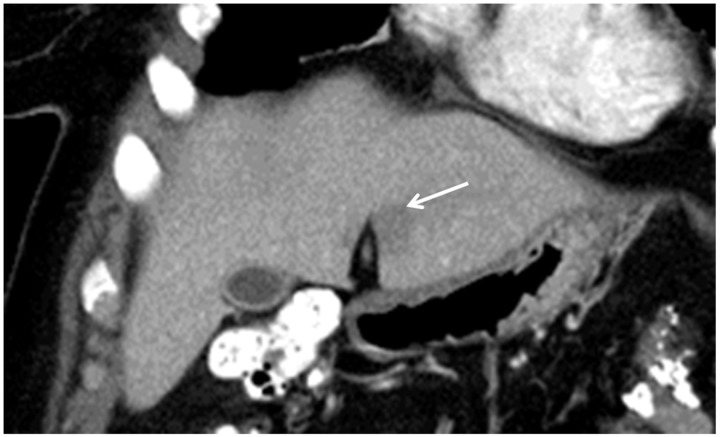
Focal hepatic steatosis adjacent to the falciform ligament in a patient with breast cancer. Coronal reformatted contrast-enhanced CT scan shows a hypodense “lesion” (arrow) in segment III. Its location strongly suggests that it represents focal fat.
During the HAP, a hypovascular region that can simulate a metastasis is often seen in segment IV (Fig. 3) adjacent to the falciform ligament which on PVP or delayed images becomes isodense with the liver. This may result from the aberrant venous blood flow or be attributable to this region being is a watershed area of hepatic arterial and portal venous blood flow[32].
Figure 3.
Transient hepatic attenuation difference (THAD) adjacent to the falciform ligament in a patient with colon cancer. Contrast-enhanced CT shows a focal hypodense region in segment IV that became isodense in later phases. This appearance and location are classic for a THAD.
Hypervascular flash-filling incidentalomas
Small hypervascular, flash-filling hepatic defects are commonly found incidentally on contrast-enhanced MDCT in both the oncology and non-oncology populations. The differential diagnosis of these lesions (Table 2) is extensive, and most are usually benign.
Table 2.
Differential diagnosis of flash-filling hepatic lesions on multidetector-row computed tomography
| Hypervascular metastases |
| Hemangiomas |
| Focal nodular hyperplasia |
| Transient hepatic attenuation differences |
| Adenomas |
| Nodular regenerative hyperplasia |
| Hepatocellular carcinoma |
| Fibrolamellar carcinoma |
| Arteriovenous malformations |
| Peliosis |
| Arterioportal shunts |
| Arteriovenous shunts |
| Portovenous shunts |
Hepatic hemangiomas
HHs are the most common benign tumor of the liver, with a reported incidence ranging from 1% to 20%[2]. On unenhanced CT scans, HHs have low attenuation compared with adjacent normal hepatic parenchyma, and when small may be impossible to differentiate from metastases or cysts. The classic enhancement pattern of HHs on the HAP is highly characteristic: peripheral, nodular, discontinuous enhancement isodense with the aorta, with progressive centripetal fill-in on subsequent phases. On PVP images, the lesions may become uniformly hyperenhancing compared with the normal parenchyma, and isodense with hepatic and portal veins, which generally persists into delayed phases.
Small HHs often show robust, uniform, flash filling (Fig. 4) and may be difficult to differentiate from other HAP-enhancing neoplasms, such as hepatocellular carcinoma or hypervascular metastases. Distinguishing features, however, can be found on hepatic venous phase and delayed images. Hypervascular neoplasms often show washout, whereas HHs show contrast retention that is isodense with the aorta, portal vein, or hepatic veins[33]. In questionable cases MRI with DWI can usually make this differentiation.
Figure 4.
Flash-filling hemangioma with THAD in a patient with ovarian cancer. Axial (a and b) contrast-enhanced CT images show a robustly enhancing hepatic mass (white arrow) associated with a prominent THAD (black arrows).
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is the second most common benign hepatic neoplasm, constituting 8% of primary hepatic tumors with an estimated prevalence of 0.9% and an 8:1 female-to-male predominance. These lesions have a very characteristic appearance on contrast-enhanced CT scans and are unlikely to be confused with metastases. On uninfused CT scans, however, this differentiation is not possible because FNH appears as a homogeneous hypo- or isodense mass. In one-third of cases, a low-density central scar is visualized.
During the HAP, FNH shows intense enhancement and the central scar remains hypodense (Fig. 5). During the PVP, the difference in attenuation between FNH and normal liver promptly diminishes so that the tumor may be slightly hyperdense or isodense, with a hypo- or isodense central scar. On delayed imaging the lesion is isodense, but the central scar typically becomes hyperdense[34,35]. If there is still doubt, MRI can provide confirmation[36–38].
Figure 5.
Focal nodular hyperplasia. Axial image made during a pulmonary embolism study performed in a patient with osteosarcoma shows a large incidental hypervascular hepatic mass with a central scar. Note the hypertrophied feeding artery and early draining vein (black arrow). White arrow indicates central scar.
Hepatic adenomas
Hepatocellular adenomas (HCAs) are rare, histologically benign neoplasms that have a small risk for malignant transformation into hepatocellular carcinoma, as well as a propensity for hemorrhage and rupture. There is an increased risk of developing these tumors in patients with cancer who are taking tamoxifen, estrogen replacement therapy, anabolic and androgenic steroid, and/or erythropoietin as part of their chemotherapeutic regimen[39]. The imaging features of HCAs depend on the amount of lipid, hemorrhage, or fibrosis within the tumor, and the status of the surrounding hepatic parenchyma. Of all the benign hepatic incidentalomas in the patient with cancer, these are the most troublesome to differentiate from metastases[12].
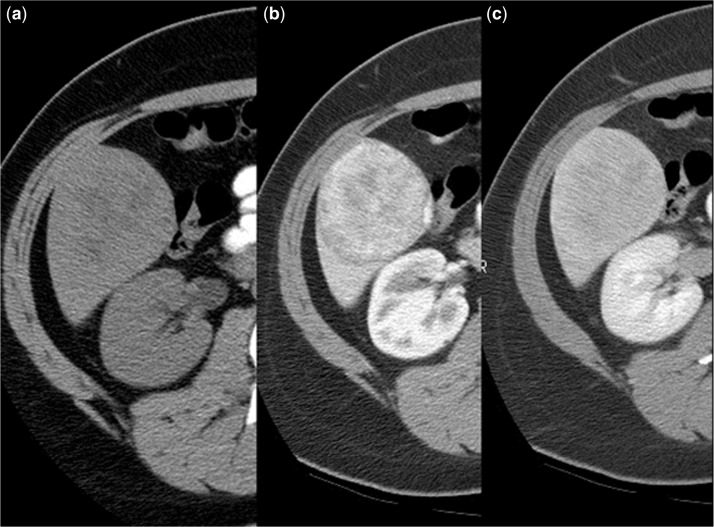
On unenhanced CT (Fig. 6a), uncomplicated HAs are isodense or hypodense to the surrounding liver. Hyperdense areas corresponding to hemorrhage can be noted as well. Low-density regions may correspond to regions of intratumoral fat. Following contrast administration, CT (Fig. 6b,c) shows homogeneous enhancement on HAP imaging in 81–90% of cases, particularly if the lesions are small (<3 cm).The enhancement is moderate and remains less than that of the arterial vasculature. It is less impressive and more heterogeneous than seen in FNH. On PVP and delayed imaging, the lesion is nearly isodense to the surrounding liver. Because of the presence of necrosis, fat, and hemorrhage, some 25% of lesions will have a more heterogeneous appearance. Fat has been identified in 7% of lesions and calcifications are present in 5–15%. By virtue of its ability to depict internal lipid content (and hemorrhage) with in-phase and opposed-phase sequences and superior contrast resolution, MRI can often better characterize HAs. PET/CT has also shown promise in diagnosing adenomas[40,41].
Figure 6.
Hepatic adenoma in a patient with breast cancer on tamoxifen. (a) Axial unenhanced CT scan shows a low-density hepatic mass (black arrows) with focal areas of fat. This lesions exhibits moderate, inhomogeneous contrast enhancement on hepatic arterial-phase image (b) that shows gradual washout on delayed phase (c).
Nodular regenerative hyperplasia
Nodular regenerative hyperplasia (NRH) is defined as a diffuse nodularity of the liver produced by many regenerative nodules that are not associated with fibrosis. NRH grossly is characterized by the presence of multiple bulging subcapsular nodules that on cut surface appear as discrete, round, flat nodules resembling diffuse involvement with metastatic carcinoma. These nodules vary in size from a few millimeters to several centimeters, and are diffusely scattered. Microscopically the nodules are composed of cells resembling normal hepatocytes, and no fibrosis is noted. This is an important difference between NRH and regenerating nodules of cirrhosis[42].
NRH is associated with various systemic diseases and drugs that are also associated with the Budd–Chiari syndrome: polycythemia vera, chronic myelogenous leukemia, myeloid metaplasia, Hodgkin disease and non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, Felty syndrome, polyarteritis nodosa, scleroderma, systemic lupus erythematosus, and steroid and antineoplastic medications[42].
The CT appearance of NRH is variable. On unenhanced scans, these lesions are usually hypodense but when hemorrhagic, they may produce a complex mass with variable density. On contrast-enhanced scans, HAP imaging shows hypervascular lesions (Fig. 7) that may become almost imperceptible during the PVP[43–45].
Figure 7.
Nodular regenerative hyperplasia with Budd–Chiari syndrome in a patient with Hodgkin disease undergoing chemotherapy. Axial contrast-enhanced CT scan shows multiple hyperenhancing hepatic masses.
Transient hepatic attenuation differences
THADs are epiphenomena of alterations of the dual vascular supply of the liver. They manifest as areas of parenchymal enhancement visible during the HAP. These “lesions” are an increasingly common cause of hepatic incidentalomas. While THADs may be seen in association with malignant and benign hepatic masses, they are more commonly seen secondary to: portal hypoperfusion due to portal branch compression or thrombosis; flow diversion by arterioportal shunts or by an anomalous blood supply; or inflammation or obstruction of the bile ducts or gallbladder[46–52].
Sectorial THADs are usually caused by portal hypoperfusion due to portal vein or hepatic vein thrombosis, long-standing biliary obstruction, or an arterioportal shunt that may be congenital, traumatic, or due to cirrhosis. These THADs can have a globular shape especially when they are adjacent to the Glisson capsule[46–52].
Polymorphous THADs have four major causes: external compression by a rib or subcapsular fluid collection; anomalous blood supply from atypical arteries, collateral venous vessels or accessory veins, especially in segment IV of the liver; inflammation of adjacent organs such as cholecystitis and pancreatitis that spreads inflammatory mediators and reduces portal inflow due to interstitial edema; and post-traumatic, post-biopsy, post-radiofrequency ablation of hepatic tumors[46–52].
In patients with obstruction of the superior vena cava, the medial segment of the left lobe (segment IV) of the liver will often show hyperenhancement (Fig. 8) owing to collateral veins. The internal mammary vein connects to the left portal vein via the paraumbilical vein. Diffuse THADs can be seen in right-sided heart failure, the Budd–Chiari syndrome, and biliary obstruction, leading to abnormal attenuation and signal intensity adjacent to the portal triads[53].
Figure 8.
THAD caused by superior vena cava obstruction by metastatic lung cancer. (a) Axial CT scan shows flash-filling lesions (arrows) along the anterior aspect of the medial segment of the left hepatic lobe. (b) Axial contrast-enhanced chest CT scan shows tumor (T) obstructing the superior vena cava (arrow).
Problem-solving tools
Using the imaging characteristics described above, MDCT should be able to successfully characterize the majority of incidentalomas in the oncology patient. If the lesion cannot be confidently characterized by CT, several possible imaging options are available before a biopsy is needed: dual-energy MDCT, MRI with DWI, PET/CT, and most recently, PET/MR.
Dual-energy MDCT
Dual-energy MDCT is very useful in evaluating incidental hepatic cysts with a density >20 HU because it can provide a virtual unenhanced image and can depict the presence or absence of real enhancement. There is good correlation between virtual unenhanced and true unenhanced CT Hounsfield units of the hepatic parenchyma. Using dual-energy post-processing software, the contrast agent can be digitally subtracted from the image. The dual-energy data can also be used to generate a color-coded image that shows the distribution of iodine within the volume of tissue examined by CT. This color-coded display is very sensitive to subtle enhancement. In our experience, high-density hepatic cysts and BDHs can be reliably identified and characterized based on measured HU values, as those correlate well between unenhanced and virtual unenhanced data sets[54–56]. This discussion, however, is only relevant if the patient was scanned on a dual-energy scanner. If an indeterminate lesion is found on conventional MDCT then MRI rather than dual-energy CT is the next step.
MRI
MRI is recognized as the most sensitive and specific examination for the detection and characterization of hepatic masses including metastases. In one study, the sensitivity, specificity, positive predictive value, and negative predictive value in differentiation of benign from malignant lesions on MDCT were 81.2%, 77.3%, 60.5%, 90.6%, and on MR were 83.3%, 97.5%, 92.1%, and 94.4%, respectively[57]. In another study performed in women with newly diagnosed breast cancer and no definite liver metastasis on initial CT, immediate further evaluation of hepatic lesions too small to characterize with MRI found that 5% of these lesions represented metastases[58].
The addition of DWI (Fig. 9) and liver-specific agents improves the confidence of this examination in differentiating benign from malignant masses[59–63]. Because they are more cellular, malignant hepatic lesions typically demonstrate impeded diffusion, which is manifested as high residual signal intensity on images obtained with high b values compared with background liver parenchyma as opposed to benign, non-solid lesions such as liver cysts and HHs. As a consequence the apparent diffusion coefficient (ADC) values of malignant lesions are visually lower than that of surrounding liver parenchyma. The ADC values of benign lesions such as HHs and cysts are significantly higher than those of malignant lesions such as metastases and hepatocellular carcinoma: an ADC value <1.5 usually indicates a malignant lesion and an ADC >1.6 usually indicates a benign lesion. FNHs and HCAs, however, may show restricted diffusion, but most have ADC values close to that of normal liver and less restriction than metastases[59–63].
Figure 9.
Utility of magnetic resonance imaging with diffusion-weighted imaging (DWI) in characterizing a small hepatic defect in a patient with colon cancer. This metastatic lesion manifested as a hypointense mass (arrow) on the post-Gd T1-weighted fat-saturation image (a) but was not depicted on the T2-weighted image (b). The lesion has high conspicuity (arrow) on the b-500 DWI (c).
PET/CT
PET/CT has become a well-accepted method for staging a number of malignancies including lung, breast, colorectal, and esophageal cancer, because of its ability to provide superb simultaneous anatomic and metabolic information[64]. However, PET/CT, especially when performed without an infused CT scan, is less sensitive than contrast-enhanced MR in the depiction and characterization of small (<1 cm) liver metastases. Several studies have shown the superiority of ethoxybenzyl diethylenetriaminepentaacetic acid (EOB-DTPA)-enhanced MRI and PET/MRI for the detection and characterization of liver metastases, especially for lesions <1 cm in size. For lesions >1 cm, diagnostic confidence was better with PET/MRI than with PET/CT. For lesions of this size, however, the functional information of PET did not significantly increase sensitivity or diagnostic confidence in comparison with gadolinium EOB-DTPA-enhanced MRI[65,66]. PET/MR, however, is available at only a few centers.
Conclusions and recommendations
The vast majority of small low-density or flash-filling hepatic defects found on MDCT in the oncology patient are benign. Using the imaging criteria described herein, most of these lesions can be confidently characterized and do not require further evaluation. Knowing the primary neoplasm and its usual metastatic patterns is also helpful in increasing confidence levels. In the minority of cases, the imaging findings are not characteristic and in these patients several fundamental questions need to be considered. First, if the lesion is potentially malignant, will this alter the stage, therapy, or prognosis of the patient? If the answer is no, no further workup is needed. If the answer is yes, the next step depends upon the clinical situation. In a patient with a new diagnosis of cancer, MRI with DWI is the next step. If more disseminated disease is suspected and the lesion is 1 cm or larger in size, PET/CT or PET/MR should be considered. If this lesion is discovered in an established patient during follow-up scanning, can further evaluation of this lesion await the next interval follow-up scan? If so, the tincture of time can permit the lesion to declare itself. If not, MRI with DWI is the next step. If the lesion is 1 cm or larger and/or more disseminated disease is suspected, PET/CT or PET/MR should be considered.
The American College of Radiology has created a series of guidelines[10] for the management of incidentally discovered hepatic masses discovered on MDCT based on the following patient risk factors (Fig. 10).
Low-risk individuals: Young patients (≤40 years old), with no malignancy, hepatic dysfunction, hepatic malignant risk factors, or symptoms related to the liver.
Average-risk individuals: Patients >40 years old, with no known malignancy, hepatic dysfunction, hepatic malignant risk factors, or symptoms related to the liver.
High-risk individuals: Patients with a known primary malignancy with a propensity to metastasize to the liver, cirrhosis, and/or other hepatic risk factor. Hepatic risk factors include cirrhosis, hepatitis, chronic active hepatitis, sclerosing cholangitis, hemosiderosis, hepatic dysfunction, and long-term use of anabolic steroids or oral contraceptives.
Figure 10.
American College of Radiology algorithm for management of hepatic incidentalomas. (1) Low-risk individuals: young patient (≤40 years old), with no known malignancy, hepatic dysfunction, hepatic malignant risk factors, or symptoms attributable to the liver. (2) Average-risk individuals: patient >40 years old, with no known malignancy, hepatic dysfunction, or hepatic malignant risk factors or symptoms attributable to the liver. (3) High-risk individuals: known primary malignancy with a propensity to metastasize to the liver, cirrhosis, and/or other hepatic risk factors. Hepatic risk factors include hepatitis, chronic active hepatitis, sclerosing cholangitis, primary biliary cirrhosis, hemochromatosis, hemosiderosis, oral contraceptive use, anabolic steroid use. (4) Follow-up computed tomography or magnetic resonance imaging (MRI) in 6 months. May need more frequent follow-up in some situations, such as a cirrhotic patient who is a liver transplant candidate. (5) Benign imaging features: typical hemangioma (see below), sharply marginated, homogeneous low attenuation up to about 20 HU, no enhancement. May have sharp but irregular shape. (6) Benign low-attenuation masses: cyst, hemangioma, hamartoma, von Meyenburg complex (bile duct hamartomas). (7) Suspicious imaging features: ill-defined margins, enhancement (more than about 20 HU), heterogeneous, enlargement. To evaluate, prefer multiphasic MRI. (8) Hemangioma features: Nodular discontinuous peripheral enhancement with progressive enlargement of enhancing foci on subsequent phases. Nodule isodense with vessels, not parenchyma. (9) Small robustly enhancing lesion in average-risk, young patient: hemangioma, focal nodular hyperplasia (FNH), transient hepatic attenuation difference (THAD), flow artifact; and in average-risk, older patient: hemangioma, THAD flow artifact. Other possible diagnoses: adenoma, arteriovenous malformation (AVM), nodular regenerative hyperplasia. Differentiation of FNH from adenoma important especially if larger than 4 cm and subcapsular. (10) Hepatocellular or common metastatic enhancing malignancy: islet cell, neuroendocrine, carcinoid, renal cell carcinoma, melanoma, choriocarcinoma, sarcoma, breast, some pancreatic lesions. (From Berland et al.[10])
Patients with extrahepatic malignancies fall into category 3. Using these guidelines can help direct further management.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Vauthey J-N, Audisio RA, Hoff PMG, Poston GJ. Liver metastases. Berlin: Springer; 2009. [Google Scholar]
- 2.Ishak KG, Goodman ZD, Stocker JT, editors. Tumors of the liver and intrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology; 2001. [Google Scholar]
- 3.Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press; 2000. [Google Scholar]
- 4.Baker ME, Pelley R. Hepatic metastases in perspective. Radiology. 1995;197:329–337. doi: 10.1148/radiology.197.2.7480672. PMid:7480672. [DOI] [PubMed] [Google Scholar]
- 5.Green DE, Woodward PJ. The management of indeterminate incidental findings detected at abdominal CT. Semin Ultrasound CT MRI. 2005;26:2–13. doi: 10.1053/j.sult.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Tsung A, Geller DA. Workup of the incidental liver lesion. Adv Surg. 2005;39:331–341. doi: 10.1016/j.yasu.2005.03.002. . PMid:16250559. [DOI] [PubMed] [Google Scholar]
- 7.Ather MH, Memon W, Rees J. Clinical impact of incidental diagnosis on non-contrast-enhanced helical CT for acute ureteral colic. Semin Ultrasound CT MRI. 2005;26:20–23. doi: 10.1053/j.sult.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Flicker MS, Tsoukas AT, Hazra A, Dachman AH. Economic impact of extracolonic findings at computed tomographic colonography. J Comput Assist Tomogr. 2008;32:497–503. doi: 10.1097/RCT.0b013e3181692091. . PMid:18664832. [DOI] [PubMed] [Google Scholar]
- 9.Munk MD, Peitzman AB, Hostler DP, Wolfson AB. Frequency and follow-up of incidental findings on trauma computed tomography scans: experience at a level one trauma center. J Emerg Med. 2010;38:346–350. doi: 10.1016/j.jemermed.2008.01.021. . PMid:18804935. [DOI] [PubMed] [Google Scholar]
- 10.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2010;7:754–773. doi: 10.1016/j.jacr.2010.06.013. . PMid:20889105. [DOI] [PubMed] [Google Scholar]
- 11.Beatty JS, Williams HT, Aldridge BA, et al. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery. 2009;146:274–281. doi: 10.1016/j.surg.2009.04.024. . PMid:19628085. [DOI] [PubMed] [Google Scholar]
- 12.Gore RM, Newmark GM, Thakrar KH, Mehta UK, Berlin JW. Hepatic incidentalomas. Radiol Clin North Am. 2011;49:291–322. doi: 10.1016/j.rcl.2010.10.004. . PMid:21333779. [DOI] [PubMed] [Google Scholar]
- 13.Mathe Z, Tagkalos E, Paul A, et al. Liver transplantation for hepatic metastases of neuroendrocrine pancreatic tumors: a survival-based analysis. Transplantation. 2011;91:575–582. doi: 10.1097/TP.0b013e3182081312. . PMid:21200365. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–1077. doi: 10.1007/s11605-006-0061-3. . PMid:17530336. [DOI] [PubMed] [Google Scholar]
- 15.Quan D, Gallinger S, Nhan C, et al. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery. 2012;151:860–870. doi: 10.1016/j.surg.2011.12.018. . PMid:22316439. [DOI] [PubMed] [Google Scholar]
- 16.Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. . PMid:22039320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones EC, Chezmar JL, Nelson RC, et al. The frequency and significance of small (less than or equal to 15 mm) hepatic lesions detected by CT. AJR. 1992;158:535–539. doi: 10.2214/ajr.158.3.1738990. PMid:1738990. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz LH, Gandras EJ, Colangelo S, et al. Prevalence and importance of small hepatic lesions found at CT in patients with cancer. Radiology. 1999;210:71–74. doi: 10.1148/radiology.210.1.r99ja0371. PMid:9885589. [DOI] [PubMed] [Google Scholar]
- 19.Jang HJ, Lim HK, Lee WJ, et al. Small hypoattenuating lesions in the liver on single-phase helical CT in preoperative patients with gastric and colorectal cancer: prevalence, significance, and differentiating features. J Comput Assist Tomogr. 2002;26:718–724. doi: 10.1097/00004728-200209000-00009. . PMid:12439304. [DOI] [PubMed] [Google Scholar]
- 20.Khalil HI, Patterson SA, Panicek DM. Hepatic lesions deemed too small to characterize at CT: prevalence and importance in women with breast cancer. Radiology. 2005;235:872–878. doi: 10.1148/radiol.2353041099. . PMid:15833992. [DOI] [PubMed] [Google Scholar]
- 21.Krakora GA, Coakley FV, Williams G, et al. Small hypoattenuating hepatic lesions at contrast-enhanced CT: Prognostic importance in patients with breast cancer. Radiology. 2004;233:667–673. doi: 10.1148/radiol.2333031473. . PMid:15516602. [DOI] [PubMed] [Google Scholar]
- 22.Ros PR, Taylor HM. Malignant tumors of the liver. In: Gore RM, Levine MS, editors. Textbook of gastrointestinal radiology. Philadelphia, PA: WB Saunders; 2008. pp. 1523–1568. [Google Scholar]
- 23.Bioulac-Sage P, Laumonier H, Laurent C, et al. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis. 2008;28:302–314. doi: 10.1055/s-0028-1085098. . PMid:18814083. [DOI] [PubMed] [Google Scholar]
- 24.Kamaya A, Maturen KE, Tye GA, Liu YI. Hypervascular liver lesions. Semin Ultrasound CT MRI. 2009;22:387–407. doi: 10.1053/j.sult.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Brancatelli G, Federle MP, Vilgrain V, et al. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25:659–670. doi: 10.1148/rg.253045114. . PMid:15888616. [DOI] [PubMed] [Google Scholar]
- 26.Lev-Toaff AS, Bach AM, Wechsler RJ, et al. The radiologic and pathologic spectrum of biliary hamartomas. AJR. 1995;165:309–313. doi: 10.2214/ajr.165.2.7618546. PMid:7618546. [DOI] [PubMed] [Google Scholar]
- 27.Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. . PMid:17315288. [DOI] [PubMed] [Google Scholar]
- 28.Pilgrim CH, Satgunaseelan L, Pham A, et al. Correlations between histopathological diagnosis of chemotherapy-induced hepatic injury, clinical features, and perioperative morbidity. HPB (Oxford) 2012;14:333–340. doi: 10.1111/j.1477-2574.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gore RM. Diffuse liver disease. In: Gore RM, Levine MS, editors. Textbook of gastrointestinal radiology. 3rd. Philadelphia, PA: WB Saunders; 2008. pp. 1685–1730. [Google Scholar]
- 30.Browning JD. New imaging techniques for non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:607–1944. doi: 10.1016/j.cld.2009.07.002. . PMid:19818308. [DOI] [PubMed] [Google Scholar]
- 31.Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637–1653. doi: 10.1148/rg.266065004. . PMid:17102041. [DOI] [PubMed] [Google Scholar]
- 32.Desser TS. Understanding transient hepatic attenuation differences. Semin Ultrasound CT MRI. 2009;30:408–417. doi: 10.1053/j.sult.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Oto A, Kulkarni K, Nishikawa R, Baron RL. Contrast enhancement of hepatic hemangiomas on multiphase MDCT: can we diagnose hepatic hemangiomas by comparing enhancement with blood pool? AJR. 2010;195:381–386. doi: 10.2214/AJR.09.3324. . PMid:20651193. [DOI] [PubMed] [Google Scholar]
- 34.Lin MC, Tsay PK, Ko SF, et al. Triphasic dynamic CT findings of 63 hepatic focal nodular hyperplasia in 46 patients: correlation with size and pathological findings. Abdom Imaging. 2008;33:301–307. doi: 10.1007/s00261-007-9258-5. . PMid:17632749. [DOI] [PubMed] [Google Scholar]
- 35.Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: Findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. RadioGraphics. 2004;24:3–17. doi: 10.1148/rg.241035050. . PMid:14730031. [DOI] [PubMed] [Google Scholar]
- 36.Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520–529. doi: 10.1148/radiol.11101742. . PMid:22282184. [DOI] [PubMed] [Google Scholar]
- 37.Purysko AS, Remer EM, Coppa CP, Obuchowski NA, Schneider E, Veniero JC. Characteristics and distinguishing features of hepatocellular adenoma and focal nodular hyperplasia on gadoxetate disodium-enhanced MRI. AJR. 2012;198:115–123. doi: 10.2214/AJR.11.6836. . PMid:22194486. [DOI] [PubMed] [Google Scholar]
- 38.van den Esschert JW, Bieze M, Beuers UH, van Gulik TM, Bennink RJ. Differentiation of hepatocellular adenoma and focal nodular hyperplasia using 18F-fluorocholine PET/CT. Eur J Nucl Med Mol Imaging. 2011;38:436–440. doi: 10.1007/s00259-010-1584-0. . PMid:20717825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011;258:673–693. doi: 10.1148/radiol.10100376. [DOI] [PubMed] [Google Scholar]
- 40.Hussain SM, van den Bos IC, Dwarkasing RS, et al. Hepatocellular adenoma: Findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16:1873–1886. doi: 10.1007/s00330-006-0292-4. . PMid:16708218. [DOI] [PubMed] [Google Scholar]
- 41.Brancatelli G, Federle MP, Vullierme MP, et al. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30:745–750. doi: 10.1097/01.rct.0000224630.48068.bf. . PMid:16954922. [DOI] [PubMed] [Google Scholar]
- 42.Ibarrola C, Castellano VM, Colina F. Focal hyperplastic hepatocellular nodules in hepatic venous outflow obstruction: a clinicopathological study of four patients and 24 nodules. Histopathology. 2004;44:172–179. doi: 10.1111/j.1365-2559.2004.01795.x. . PMid:14764061. [DOI] [PubMed] [Google Scholar]
- 43.Maetani Y, Itoh K, Egawa H, et al. Benign hepatic nodules in Budd-Chiari syndrome: radiologic-pathologic correlation with emphasis on the central scar. AJR. 2002;178:869–875. doi: 10.2214/ajr.178.4.1780869. PMid:11906865. [DOI] [PubMed] [Google Scholar]
- 44.Rha SE, Lee MG, Lee YS, et al. Nodular regenerative hyperplasia of the liver in Budd-Chiari syndrome: CT and MR features. Abdom Imaging. 2000;25:255–258. doi: 10.1007/s002610000027. . PMid:10823445. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi S, Matsui O, Gabata T, et al. Radiological and histopathological manifestations of hepatocellular nodular lesions concomitant with various congenital and acquired hepatic hemodynamic abnormalities. Jpn J Radiol. 2009;27:53–68. doi: 10.1007/s11604-008-0299-7. . PMid:19373534. [DOI] [PubMed] [Google Scholar]
- 46.Colegrande S, Centi N, Galdiero R, et al. Transient hepatic intensity differences: Part 1. Those associated with focal lesions. AJR. 2007;188:154–159. doi: 10.2214/AJR.05.1368. [DOI] [PubMed] [Google Scholar]
- 47.Colegrande S, Centi N, Galdiero R, et al. Transient hepatic intensity differences: Part 2. Those not associated with focal lesions. AJR. 2007;188:160–166. doi: 10.2214/AJR.05.1367. [DOI] [PubMed] [Google Scholar]
- 48.Eberhardt SC, Choi PH, Bach AM, et al. Utility of sonography for small hepatic lesions found on computed tomography in patients with cancer. J Ultrasound Med. 2003;22:335–343. doi: 10.7863/jum.2003.22.4.335. PMid:12693617. [DOI] [PubMed] [Google Scholar]
- 49.Kim HJ, Kim AY, Kim TK, et al. Transient hepatic attenuation differences in focal hepatic lesions: dynamic CT features. AJR. 2005;184:83–90. doi: 10.2214/ajr.184.1.01840083. PMid:15615955. [DOI] [PubMed] [Google Scholar]
- 50.Colagrande S, Centi N, Pradella S, et al. Transient hepatic attenuation differences and focal liver lesions: sump effect due to primary arterial hyperperfusion. J Comput Assist Tomogr. 2009;33:259–265. doi: 10.1097/RCT.0b013e31818050bc. . PMid:19346856. [DOI] [PubMed] [Google Scholar]
- 51.Choi SH, Lee JM, Lee KH, et al. Relationship between various patterns of transient increased hepatic attenuation on CT and portal vein thrombosis related to acute cholecystitis. AJR. 2004;183:437–442. doi: 10.2214/ajr.183.2.1830437. PMid:15269038. [DOI] [PubMed] [Google Scholar]
- 52.Pradella S, Centi N, La Villa G, et al. Transient hepatic attenuation difference (THAD) in biliary duct disease. Abdom Imaging. 2009;34:626–633. doi: 10.1007/s00261-008-9445-z. . PMid:18682878. [DOI] [PubMed] [Google Scholar]
- 53.Gore RM. Vascular disorders of the liver. In: Gore RM, Levine MS, editors. Textbook of gastrointestinal radiology. 4th . Philadelphia, PA: Saunders-Elsevier; 2008. pp. 1685–1730. [Google Scholar]
- 54.Karçaaltıncaba M, Aktaş A. Dual-energy CT revisited with multidetector CT: review of principles and clinical applications. Diagn Interv Radiol. 2011;17:181–194. doi: 10.4261/1305-3825.DIR.3860-10.0. [DOI] [PubMed] [Google Scholar]
- 55.Robinson E, Babb J, Chandarana H, Macari M. Dual source dual energy MDCT: comparison of 80 kVp and weighted average 120 kVp data for conspicuity of hypo-vascular liver metastases. Invest Radiol. 2010;45:413–418. doi: 10.1097/RLI.0b013e3181dfda78. PMid:20458250. [DOI] [PubMed] [Google Scholar]
- 56.De Cecco CN, Buffa V, Fedeli S, et al. Dual energy CT (DECT) of the liver: conventional versus virtual unenhanced images. Eur Radiol. 2010;20:2870–2875. doi: 10.1007/s00330-010-1874-8. . PMid:20623126. [DOI] [PubMed] [Google Scholar]
- 57.Holalkere NS, Sahani DV, Blake MA, et al. Characterization of small liver lesions: added value of MR after MDCT. J Comput Assist Tomogr. 2006;30:591–596. doi: 10.1097/00004728-200607000-00007. . PMid:16845289. [DOI] [PubMed] [Google Scholar]
- 58.Patterson SA, Khalil HI, Panicek DM. MRI evaluation of small hepatic lesions in women with breast cancer. AJR. 2006;187:307–312. doi: 10.2214/AJR.04.1030. . PMid:16861531. [DOI] [PubMed] [Google Scholar]
- 59.Kim YK, Lee ME, Lee WJ, et al. Diagnostic accuracy and sensitivity of diffusion-weighted and of gadoxetic acid-enhanced 3-T MR imaging alone or in combination in the detection of small liver metastasis (≤ 1.5 cm in diameter) Invest Radiol. 2012;47:159–166. doi: 10.1097/RLI.0b013e31823a1495. PMid:22330426. [DOI] [PubMed] [Google Scholar]
- 60.Xia D, Jing J, Shen H, Wu J. Value of diffusion-weighted magnetic resonance images for discrimination of focal benign and malignant hepatic lesions: a meta-analysis. J Magn Reson Imaging. 2012;23:130–137. doi: 10.1002/jmri.22211. [DOI] [PubMed] [Google Scholar]
- 61.Bonekamp S, Corona-Villalobos CP, Kamel IR. Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging. 2012;35:257–279. doi: 10.1002/jmri.22786. . PMid:22271274. [DOI] [PubMed] [Google Scholar]
- 62.Taouli B, Koh D-M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. . PMid:20032142. [DOI] [PubMed] [Google Scholar]
- 63.Agnello F, Ronot M, Valla DC, Sinkus R, Van Beers BE, Vilgrain V. High-b-value diffusion-weighted MR imaging of benign hepatocellular lesions: quantitative and qualitative analysis. Radiology. 2012;262:511–519. doi: 10.1148/radiol.11110922. . PMid:22143926. [DOI] [PubMed] [Google Scholar]
- 64.Mittra E, Quon A. Positron emission tomography/computed tomography: the current technology and applications. Radiol Clin North Am. 2009;47:147–160. doi: 10.1016/j.rcl.2008.10.005. . PMid:19195540. [DOI] [PubMed] [Google Scholar]
- 65.Donati OF, Reiner CS, Hany TF, et al. 18F-FDG-PET and MRI in patients with malignancies of the liver and pancreas. Accuracy of retrospective multimodality image registration by using the CT-component of PET/CT. Nuklearmedizin. 2010;49:106–114. doi: 10.3413/nukmed-0263. . PMid:20407733. [DOI] [PubMed] [Google Scholar]
- 66.Donati OF, Hany TF, Reiner CS, et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51:692–699. doi: 10.2967/jnumed.109.068510. . PMid:20395324. [DOI] [PubMed] [Google Scholar]