Abstract
Lipoblastoma is a rare benign tumour arising from embryonic white fat. The tumours occur primarily in infancy and early childhood and usually arise from the limbs and the trunk, but neck involvement is rare. We report three cases of head and neck lipoblastoma. In all cases, imaging showed a well-delineated, fat-containing tumour. After surgical resection, the outcome of these patients was uneventful.
Keywords: Head and neck tumour, lipoma, lipoblastoma, paediatric
Introduction
There is a wide variety of different paediatric benign soft tissue tumours. The most frequent benign paediatric soft tissue tumours in the head and neck are hemangiomas[1]. Lipoblastoma is a rare cause of a paediatric head and neck mass.
Case report
Case 1
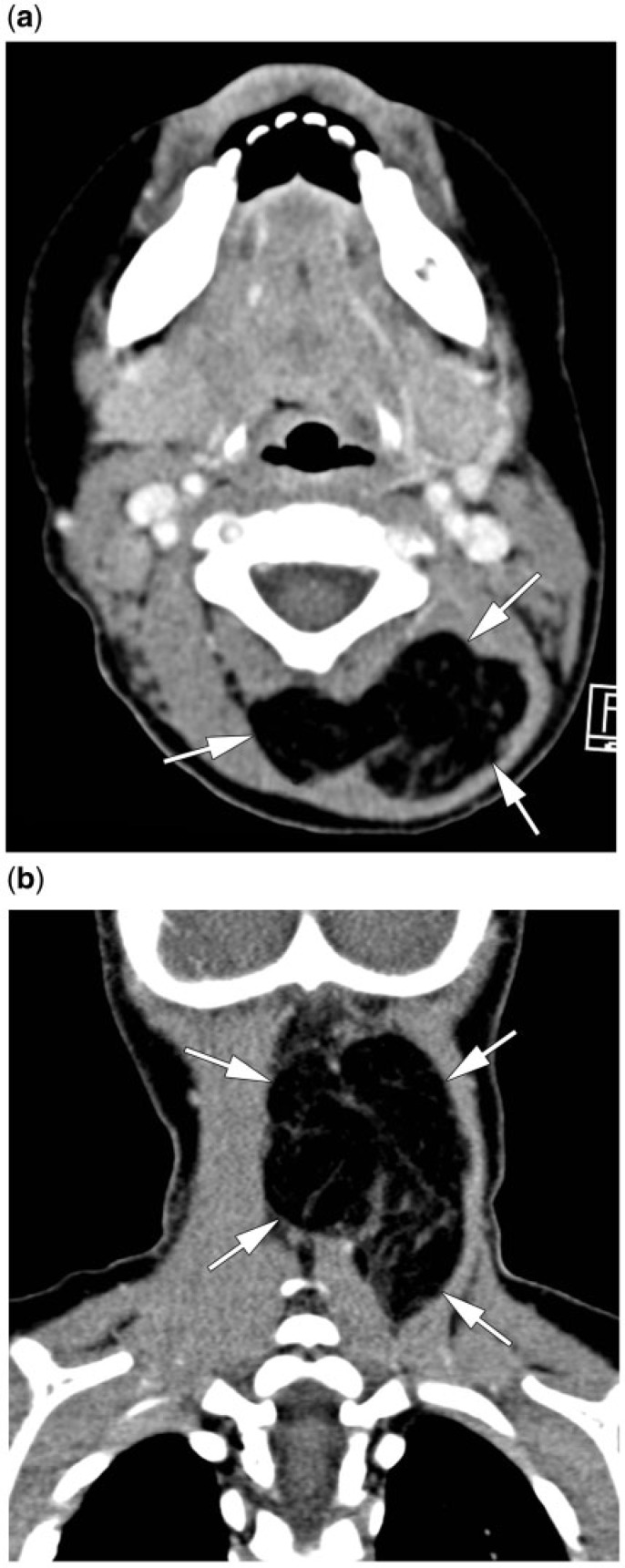
A 6-year-old girl presented with a painless, progressive growing left neck mass, first noticed about 1 month earlier. She had no other symptoms, a normal clinical examination and normal laboratory findings. Ultrasonography and magnetic resonance imaging (MRI) were performed. Ultrasonography revealed a homogeneous, hypovascular, hyperechogenic mass next to the submandibular gland. On MRI, the soft tissue lesion was seen to be localized in the left parapharyngeal space, extending into the submandibular space, having a mass effect on the oro- and nasopharynx. The diameter was about 8 cm. The lesion showed an overall hyperintense signal on T1- and T2-weighted images, similar to the signal intensities of the subcutaneous fat, with streaky hypointensities (Fig. 1a,b). No enhancement was seen after administration of gadolinium. Complete surgical resection was performed. Histological examination showed an adipose lesion, fitting with the overall T2 and T1 hyperintense aspect, divided into lobules by fibrous septa, compatible with the streaky hypointensities. The adipose tissue consisted of mature adipocytes of various size and a few lipoblasts. These findings are compatible with lipoblastoma.
Figure 1.
Axial plain T1-weighted image (a) and coronal gadolinium-enhanced T1-weighted image (b). A sharply demarcated, spontaneously hyperintense soft tissue mass (arrowheads) is seen in the prestyloid compartment of the left parapharyngeal space, displacing the surrounding structures, and extending into the submandibular space. Non-enhancing streaky hypointensities are present throughout the mass.
Case 2
A 6-month-old girl presented with a painless, progressive growing left neck mass with a diameter of approximately 4 cm, detected 2 months earlier. She had no other symptoms, a normal clinical examination and normal laboratory findings. On computed tomography (CT) the mass had a peripheral fatty rim and higher density in the centre in which some enhancement was visible (Fig. 2a). On MRI, the T1-weighted images showed a hyperintense peripheral rim, compatible with fat, and a hypointense centre, which showed intense enhancement after administration of gadolinium (Fig. 2b,c), thought to correspond to vascularized fibrous tissue. This lesion was resected. Histological examination confirmed the diagnosis of lipoblastoma, showing a lobulated fatty tumour, partially consisting of mature adipocytes and lipoblasts, while the central part of the lesion contained fibrous tissue and vascular elements.
Figure 2.
(a) Axial contrast-enhanced CT image. A large mass lesion is seen in the left side of the neck (arrowheads), largely consisting out of fatty tissue. The mass contains a central tissue portion of higher density (asterisk); a vessel-like structure is seen running through the mass (arrow). (b,c) Coronal plain (b) and gadolinium-enhanced fat-saturated (c) T1-weighted images confirm the fatty nature of the peripheral tumour rim (arrows); the largest part of the central portion shows enhancement.
Case 3
An 18-month-old boy presented with bronchitis and a painless mass posterior in the neck. This mass was initially considered to correspond to an adenopathy; however, the lesion showed slow progressive enlargement over a period of 6 weeks. There were no other symptoms, the clinical examination was within normal limits, and no abnormal laboratory findings. Ultrasonography revealed a heterogeneous mainly hyperechogenic, hypovascular mass, with a diameter of about 4 cm, just below the trapezius muscle. On CT, the lesion appeared as a well-circumscribed, lobulated mass in the paraspinal muscles, showing an overall low attenuation, similar to fat, with some intralesional septations (Fig. 3a,b). After surgical resection, histological examination showed an adipose tumour with an overall lobular structure, adipocytes of varying size and multiple lipoblasts. Between the adipocytes, a few scarce myxoid fields and numerous small blood vessels were seen.
Figure 3.
Axial and coronal contrast-enhanced CT image. A well-delineated mass lesion (arrows), located in the paraspinal muscles, showing fat density is seen. Within the lesion, some streaky areas of higher tissue density are present.
Discussion
Lipoblastoma is a rare, benign, rapidly growing tumour of infancy and early childhood that arises from embryonal white fat, accounting for up to 30% of adipocytic tumours in children. In contrast, true lipomas (accounting for two-thirds of adipocytic tumours in children) consist of mature adipocytes identical to those in normal fat, and do not display the rapid growth rate[1].
Lipoblastoma is categorized into two types: the circumscribed lipoblastoma (approximately 70% of cases), a superficial and encapsulated lesion, and diffuse lipoblastomatosis (about 30% of cases), a deeply located, poorly circumscribed lesion with infiltrative growth pattern that may affect surrounding muscle structures[2].
Lipoblastoma occurs almost exclusively in infants and children, and is usually diagnosed within the first 3 years of life, the median age of onset being 1 year. Lipoblastoma is more common in boys than girls (3:1 ratio). The site of origin is most often in the limbs, followed by the trunk, the retroperitoneum, and the head and neck. Within this last region, the neck is the most common location. Other locations include the parotid gland, cheek, skin and orbit. Other uncommon sites include the mediastinum, heart, lung, mesentery, omentum, scrotum, labia, axillary, inguinal and perineal regions[3,4].
The most common presenting symptom is a painless, progressively growing mass if localized superficially. Other symptoms are related to the location and size or mass effect of the lesion. Airway obstruction and respiratory symptoms have been described in patients with pleural, mediastinal, pulmonary, and lower neck lipoblastomas. Gastrointestinal symptoms, such as emesis, diarrhoea, anorexia and abdominal pain occur in patients with mesenteric or retroperitoneal lipoblastomas. Depending on the location, nerve compression and related symptoms can be present[3,5].
On imaging, lipoblastoma appears as a well-defined soft tissue mass, often with lobular appearance and showing internal septations. The imaging appearance of lipoblastoma depends on the proportion of fat relative to the amount of myxocollagenous stroma. Fat in lipoblastoma appears as hyperechogenic areas on ultrasonographs, areas of low attenuation on CT images and signal intensity identical to that of subcutaneous adipose tissue (high signal intensity on T1- and T2- weighted images). The myxoid components are hypoechoic on ultrasonographs, have low attenuation on CT images (but less hypodense than fat) and on MRI have low signal intensities on T1-weighted images and high signal intensities on T2-weighted images; contrast enhancement of these areas reflects the rich capillary network[1,5,6].
Histologically, the lesions are composed of immature fat cells (lipoblasts) in varying stages of maturity, mesenchymal cells, a plexiform capillary network, myxoid stroma and mature adipocytes organized in lobules by fibrous septa. There is no nuclear atypia. Cytogenic and molecular genetic analysis is useful to differentiate lipoblastoma from myxoid liposarcoma and atypical lipomatous tumour: lipoblastoma shows a characteristic rearrangement of the long arm of chromosome 8 (8q11-13) affecting PLAG1, myxoid liposarcoma, a t(12;16)(q13;p11) translocation, and atypical lipomatous tumour amplification of the MDM2/CDK4 genes on 12q[7–10].
The differential diagnosis of this fat-containing tumour includes liposarcoma, lipoma, teratoma, dermoid cyst, hibernoma and involuting hemangioma. Most teratomas and dermoid cysts contain calcifications or cystic areas in addition to soft tissue and fatty elements[11]. True lipomas have the same characteristics as subcutaneous fat. Masses consisting entirely of fat are diagnostic for lipoma; a few thin septa may be present, possibly enhancing[1]. A fat-rich lipoblastoma may have a similar appearance, however, in such a case, the clinical history of a progressive growing mass would serve to differentiate a fat-rich lipoblastoma from a lipoma. Involuting hemangiomas may include a small amount of fat, but have a characteristic clinical history; hemangiomas undergo a proliferation phase and an involuting phase. The proliferation phase is characterized by rapid growth in the first months of life, stabilizing in size at about 9–10 months of age. The involuting phase then continues slowly for the next several years, during which the hemangioma is replaced by fibrofatty tissue; usually this process is completed by 7–10 years of age[1]. Hibernomas are mostly seen in the 3rd to 4th decade of life; often these lesions have typical prominent branching and serpentine high- and low-flow vascular structures[5].
The most important differential diagnosis is myxoid liposarcoma. Lipoblastoma cannot be distinguished from myxoid liposarcoma on imaging. However, the age of the patient is vital; liposarcomas are extraordinarily rare in patients less than 10 years of age. Histology can differentiate on the basis of architectural changes and cytogenetic analysis as described above.
Thus, on imaging, a lesion containing fat in a young child (less than 2 years old), even with prominent or predominant non-lipomatous components, is nearly always a lipoblastoma[1,11].
Complete excision of lipoblastoma has traditionally been recommended. However, recent reports suggest that subtotal resection is acceptable, to avoid mutilation. Although recurrence rates after surgery vary between 9% and 25%, and this is largely associated with the infiltrative lipoblastomatosis, maturation into lipoma and spontaneous resolution may occur. Metastasis has not been reported[3,4].
In conclusion, head and neck lipoblastoma is a rare childhood tumour, usually presenting as a progressive painless swelling, rarely causing airway obstruction or nerve compression. Imaging is helpful showing the precise location and extent of the lesion. Although the ratio of fat to myxocollagenous tissue in the tumour is variable, the diagnosis can be suggested in most cases based on the imaging characteristics. Recommended treatment is complete surgical excision, if possible; subtotal, non-mutilating surgery seems to be acceptable.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Navarro OM, Laffan EE, Ngan BY. Pediatric soft-tissue tumors and pseudotumors: MRI imaging features with pathologic correlation. Radiographics. 2009;29:887–906. doi: 10.1148/rg.293085168. . PMid:19448123. [DOI] [PubMed] [Google Scholar]
- 2.Papaioannou G, Sebire N, McHugh K. Imaging of the unusual pediatric ‘blastomas’. Cancer Imaging. 2009;9:1–11. doi: 10.1102/1470-7330.2009.0001. . PMid:19237343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham N, Poirier B, Fuller S, et al. Pediatric lipoblastoma in the head and neck: a systematic review of 48 reported cases. Int J Pediatr Otorhinolaryngol. 2010;74:723–728. doi: 10.1016/j.ijporl.2010.04.010. . PMid:20472310. [DOI] [PubMed] [Google Scholar]
- 4.Kok K, Telisinghe P. Lipoblastoma: clinical features, treatment and outcome. World J Surg. 2010;34:1517–1522. doi: 10.1007/s00268-010-0466-8. . PMid:20151124. [DOI] [PubMed] [Google Scholar]
- 5.Murphey M, Carroll J, Flemming D, et al. From the archive from AFIP. Benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433–1466. doi: 10.1148/rg.245045120. . PMid:15371618. [DOI] [PubMed] [Google Scholar]
- 6.Castellote A, Vazquez E, Vera J, et al. Cervicothoracic lesions in infants and children. Radiographics. 1999;19:583–600. doi: 10.1148/radiographics.19.3.g99ma08583. PMid:10336190. [DOI] [PubMed] [Google Scholar]
- 7.de Saint Aubain Somerhausen N, Coindre JM, Debiec-Rychter M, et al. Lipoblastoma in adolescents and young adults: report of six cases with FISH analysis. Histopathology. 2008;52:294–298. doi: 10.1111/j.1365-2559.2007.02954.x. . PMid:18269579. [DOI] [PubMed] [Google Scholar]
- 8.Hibbard MK, Kozakewich HP, Dal Cin P, et al. PLAG1 fusion oncogenes in lipoblastoma. Cancer Res. 2000;60:4869–4872. PMid:10987300. [PubMed] [Google Scholar]
- 9.Tallini G, Akerman M, Dal Cin P, et al. Combined morphologic and karyotypic study of 28 myxoid liposarcomas. Implications for a revised morphologic typing (a report from the CHAMP Group) Am J Surg Pathol. 1996;20:1047–1055. doi: 10.1097/00000478-199609000-00002. . PMid:8764741. [DOI] [PubMed] [Google Scholar]
- 10.Sciot R, De Wever I, Debiec-Rychter M. Lipoblastoma in a 23-year-old male: distinction from atypical lipomatous tumor using cytogenetic and fluorescence in-situ hybridization analysis. Virchows Arch. 2003;442:468–471. doi: 10.1007/s00428-003-0799-x. PMid:12684772. [DOI] [PubMed] [Google Scholar]
- 11.Moholkar S, Sebire N, Roebuck D. Radiological-pathological correlation in lipoblastoma and lipoblastomatosis. Pediatr Radiol. 2006;36:851–856. doi: 10.1007/s00247-006-0175-5. . PMid:16775739. [DOI] [PubMed] [Google Scholar]