Abstract
The study provides new information on the effect of natural polyphenols (derivatives of stilbene – resveratrol, pterostilbene, pinosylvin and piceatannol and derivatives of ferulic acid – curcumin, N-feruloylserotonin) on the activity of human neutrophils in influencing oxidative burst. All the polyphenols tested were found to reduce markedly the production of reactive oxygen species released by human neutrophils on extra-and intracellular levels as well as in cell free system. Moreover, pinosylvin, curcumin, N-feruloylserotonin and resveratrol decreased protein kinase C activity involved in neutrophil signalling and reactive oxygen species production. Our results suggest that due to their anti-neutrophil activity, the polyphenols tested might be attractive candidates in therapeutic development.
Keywords: activity of neutrophils, reactive oxygen species, natural polyphenols
Introduction
Numerous studies reported polyphenols as potential therapeutic agents against inflammatory diseases including obesity, diabetes, cardiovascular and neurodegenerative diseases, rheumatoid arthritis, cancer and aging (Stevenson & Hurst, 2007; Pandey & Rizvi, 2009; Obrenovich et al., 2010; Štefek, 2011). Several mechanisms of the anti-inflammatory effect of polyphenols have been proposed, yet information about their favourable effects on neutrophils is rare. Activated neutrophils release large amounts of enzymes and reactive oxygen species (ROS) to the extracellular milieu, overpowering the local antioxidant defense systems and contributing to tissue damage and the amplification of the inflammatory process. In the physiopathology of many inflammatory diseases, the involvement of ROS produced by neutrophils has been attracting interest in the discovery of new compounds with antioxidant and immunomodulatory properties, which might modulate neutrophil activity.
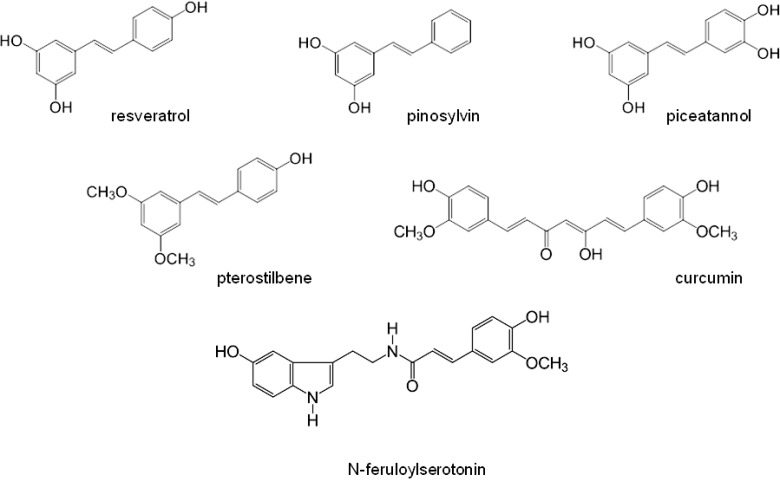
In this context, we investigated the effect of some compounds of natural origin (derivatives of stilbene, resveratrol, pinosylvin, pterostilbene, piceatannol and derivatives of ferulic acid – curcumin, N-feruloylserotonin, Figure 1) on the activity of human neutrophils in vitro with respect to their influence on oxidative burst. Here we summarize the results obtained.
Figure 1.
Structure of resveratrol, pinosylvin, piceatannol, pterostilbene, curcumin and N-feruloylserotonin.
Effect on extra- and intracellular ROS generation
For activation of human isolated neutrophils we used the soluble stimulus phorbol 12-myristate 13-acetate (PMA) which stimulates ROS generation by direct activation of protein kinase C (PKC). PMA is useful in investigating signal transduction pathways leading to NADPH-oxidase activation in plasma (extracellular – potentially dangerous for host tissues) and granule membranes (intracellular – involved in elimination of phagocytosed pathogens and fulfilling a regulatory role) (Karsson, 2002). Neutrophil activation was evaluated by using luminol/isoluminol-amplified chemiluminescence (CL), which allows to differentiate the effect of polyphenols on extracellular and intracellular oxidant production (Jančinová et al., 2006). Table 1 shows the effects of the substances tested on extracellular CL of isolated human neutrophils stimulated with PMA (0.05 µmol/l) in the 0.01–100 µmol/l concentration scale. The most effective concentration tested was 100 µmol/l of all substances, reaching 99% inhibition for curcumin, piceatannol, resveratrol and N-feruloylserotonin, and 87% for pinosylvin and pterostilbene. The effective rank order of the substances tested producing 50% inhibition of control extracellular CL of neutrophils is: resveratrol > N-feruloylserotonin ≥ curcumin > piceatannol > pterostilbene > pinosylvin (Table 1) (Perečko et al., 2008; Jančinová et al., 2009, 2011; Nosáľ et al., 2010Nosáľ et al., 2011).
Table 1.
Effect of curcumin, N-feruloylserotonin, resveratrol, piceatannol, pterostilbene and pinosylvin on extracellular neutrophil chemiluminescence stimulated with PMA.
| Inhibition of extracellular chemiluminescence (%) | ||||||
|---|---|---|---|---|---|---|
| µmol/l | Curcumin | N-feruloyl serotonin | Resveratrol | Piceatannol | Pterostilbene | Pinosylvin |
| 0.01 | 9.9±3.8 | 4.0±1.0 | 17.4±2.6 | 6.0±2.8 | 20.6±3.4 | –1.6±1.2 |
| 0.1 | 15.7±3.8 | 1.8±2.3 | 22.2±3.7 | 9.7±3.8 | 19.8±4.5 | 1.2±3.1 |
| 1 | 31.0±1.9 | 30.7±2.6 | 44.3±3.0 | 23.0±3.5 | 31.0±2.8 | 9.4±2.8 |
| 10 | 86.1±2.3 | 91.1±0.8 | 88.6±1.4 | 92.5±1.2 | 70.9±5.8 | 39.6±2.1 |
| 100 | 99.7±0.0 | 99.6±0.1 | 99.5±0.1 | 100±0 | 88.2±3.4 | 86.2±1.0 |
| IC 50 | 1.84 | 1.82 | 0.85 | 1.87 | 2.25 | 15.07 |
Percentage inhibition was calculated on the basis of integrated values of chemiluminescence (CL) over 1800s. Mean ± SEM, n=6–8.
IC50 – doses producing 50% inhibition of control extracellular CL.
In the intracellular milieu of human neutrophils, no effects of the substances tested were observed at concentrations 0.01–1 µmol/l. However, the effects differed at concentrations 10 µmol/l, the inhibitory effect of resveratrol, pinosylvin and curcumin increased rapidly, whereas pterostilbene, piceatannol and N-feruloylserotonin were less operative. In the concentration of 100 µmol/l all compounds tested (except pterostilbene) caused at least 90% inhibition of intracellular CL (Perečko et al., 2008; Jančinová et al., 2009, 2011; Nosáľ et al., 2010, 2011). The effective rank order of the substances tested producing 50% inhibition of control intracellular CL of neutrophils is: curcumin > pinosylvin > resveratrol > N-feruloylserotonin > piceatannol > pterostilbene (Table 2).
Table 2.
Effect of curcumin, N-feruloylserotonin, resveratrol, piceatannol, pterostilbene and pinosylvin on intracellular neutrophil chemiluminescence stimulated with PMA.
| Inhibition of intracellular chemiluminescence (%) | ||||||
|---|---|---|---|---|---|---|
| µmol/l | Curcumin | N-feruloyl serotonin | Resveratrol | Piceatannol | Pterostilbene | Pinosylvin |
| 0.01 | –8.2±4.6 | –1.1±3.2 | –0.2±1.7 | –1.7±2.6 | –3.5±2.8 | 2.1±3.2 |
| 0.1 | –6.9±5.5 | –1.1±2.2 | –3.6±2.8 | –2.9±1.4 | –2.5±1.1 | 0.1±3.0 |
| 1 | 8.1±6.1 | 7.9±4.6 | 4.9±1.6 | –0.2±1.3 | 3.9±1.9 | 8.4±5.2 |
| 10 | 81.4±2.4 | 38.9±4.7 | 71.4±3.2 | 39.6±2.1 | 39.5±3.4 | 66.0±4.3 |
| 100 | 93.9±1.4 | 90.0±1.0 | 96.4±0.7 | 99.3±0.1 | 69.3±5.5 | 90.5±1.4 |
| IC 50 | 3.57 | 8.40 | 6.19 | 13.17 | 21.58 | 4.45 |
Percentage inhibition was calculated on the basis of integrated values of chemiluminescence (CL) over 1800s. Mean ± SEM, n=6–8.
IC50 – doses producing 50% inhibition of control intracellular CL.
Effect on protein kinase C activity
Polyphenols have been suggested to affect cell function by modifying plasma membrane structure and physical characteristics such as fluidity and electrical properties. These effects can be observed both when polyphenols are adsorbed on the membrane (polyphenols could provide a physical barrier for hydrosoluble radicals) and when they are inserted into the bilayer (polyphenols would be in close proximity so as to scavenge lipid soluble radicals) (Fraga et al., 2010). In biological systems, ROS are generated by a number of enzymatic systems and the modifications of plasma membrane structure can result in functional changes including the activity of membrane-associated enzymes, ligand-receptor interactions, ion and/or metabolite fluxes, and the modulation of signal transduction (Khlebnikov et al., 2007). Stimulation of neutrophils with PMA is accompanied by increased phosphorylation of protein kinase C (PKC) isoenzymes α and β II, which directly participate in the activation of neutrophil NADPH oxidase (Fontayne et al., 2002; Klink et al., 2009). On the other hand, inhibition of PKC or down-regulation of its intracellular expression and activity has also been proposed as an important mechanism of polyphenol antioxidant effect (Khlebnikov et al., 2006). Moreover, evidence has been increasing on the selective inhibition of PKC beneficially applied in a new therapeutic strategy for treating diseases related to oxidative stress (Lee et al., 2009).
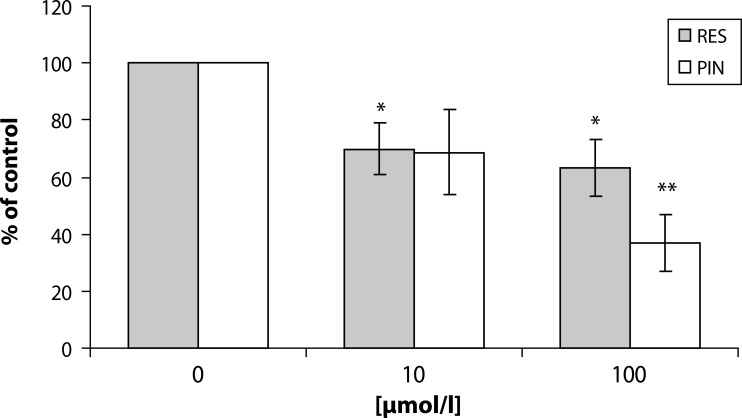
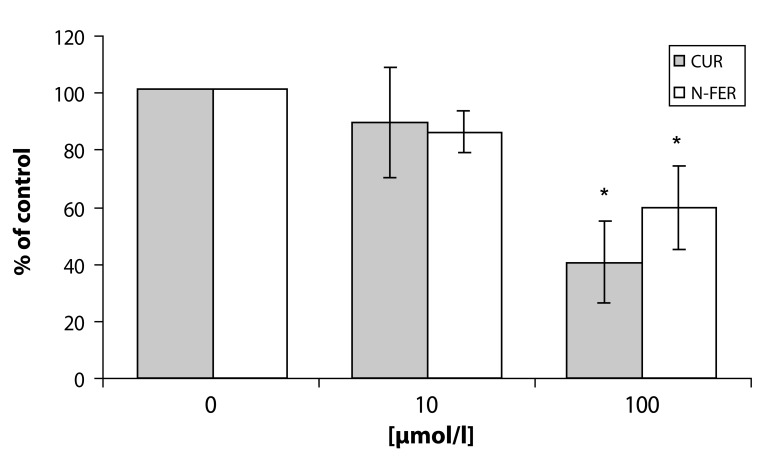
In the attempt to elucidate the molecular mechanisms involved in the reduction of ROS production by human neutrophils, we examined the effect of resveratrol, pinosylvin, pterostilbene, piceatannol, curcumin and N-feruloylserotonin on the phosphorylation of PKC α/βII (Thr638/641). Pterostilbene and piceatannol did not influence PKCα/βII phosphorylation after PMA stimulation, and this effect could be explained by low accessibility of these compounds to the cell compartments containing the enzyme. However resveratrol, pinosylvin, curcumin and N-feruloylserotonin in the concentrations of 10 and 100 µmol/l effectively reduced PKCα/βII phosphorylation (Figures 2, 3) (Jančinová et al., 2009; Perečko et al., 2010; Nosáľ et al., 2011). As described for resveratrol (Slater et al., 2003), the inhibitory effect on PKC activity might result from competition between polyphenols and phorbol ester for binding to the C1 domains of the enzyme, or from the conformational change in the membrane-associated enzyme. Moreover, studies of docking simulation into PKC showed efficient inhibition of PKC by polyphenols (Račková et al., 2009). The structure-dependence of the inhibitory effect of polyphenolic antioxidants on signal transduction enzymes, such as PKC, has been suggested also by Ursini et al. (1994) and Varga et al. (2006).
Figure 2.
PKC phosphorylation in PMA stimulated human neutrophils treated with 10 and 100 µmol/l resveratrol (RES) and pinosylvin (PIN). The values of phosphorylation are presented as percentage of stimulated (PMA) control. Phosphorylated PKC isoenzymes (α and βII) were isolated by Western blotting and detected by Phospho-PKC α/βII (Thr638/641) Antibody. Mean ± SEM, n=4. **p<0.01, p<0.05 as compared with the control in the absence of the substances tested.
Figure 3.
PKC phosphorylation in PMA stimulated human neutrophils treated with 10 and 100 µmol/l curcumin (CUR) and N-feruloylserotonin (NFER). The values of phosphorylation are presented as percentage of stimulated (PMA) control. Phosphorylated PKC isoenzymes (α and βII) were isolated by Western blotting and detected by Phospho-PKC α/βII (Thr638/641) Antibody. Mean ± SEM, n=4. *p<0.05 as compared with the control in the absence of the substances tested.
Effect on cell free system
We investigated the participation of direct antioxidant activity of polyphenols in decreasing peroxyl radical formation in a cell free CL system consisting of luminol, horseradish peroxidase and hydrogen peroxide. The luminol reaction is highly dependent on the participation of myeloperoxidase, thus the reduction of the CL signal might be the result of decreased availability of peroxidase, due either to its decrease of activity or liberation from azurophilic granules of neutrophils. The possible interaction of polyphenolic antioxidants with peroxidase is supported by findings of Franck et al. (2008) who demonstrated the interaction of curcuminoids with the active site of myeloperoxidase. The inhibitory effect of pinosylvin and pterostilbene on MPO release was described by Pečivová et al. (2010). Our results showed that the observed reduction of oxidants, produced by neutrophils extra- and intracellularly, may involve antioxidant activity of the polyphenols tested, as manifested by the effective inhibition of CL generated by cell free system (Table 3). The effective rank order of the substances tested producing 50% inhibition of cell free CL is: piceatannol > resveratrol ≥ pterostilbene = N-feruloylserotonin > pinosylvin (Table 3).
Table 3.
Effect of N-feruloylserotonin, resveratrol, piceatannol, pterostilbene and pinosylvin on cell free chemiluminescence.
| Inhibition of cell-free chemiluminescence (%) | |||||
|---|---|---|---|---|---|
| µmol/l | N-feruloyl serotonin | Resveratrol | Piceatannol | Pterostilbene | Pinosylvin |
| 0.01 | 0.8±1.0 | 0.3±0.7 | 0.6±1.2 | –0.3±0.4 | –0.4±1.3 |
| 0.1 | 3.6±1.2 | 3.8±1.6 | 6.2±0.6 | –1.4±0.6 | 0.1±0.9 |
| 1 | 25.5±0.8 | 31.6±0.7 | 66.4±0.1 | 12.8±0.0 | 5.4±0.8 |
| 10 | 97.8±0.1 | 98.1±0.0 | 99.3±0.0 | 76.6±0.5 | 62.7±0.5 |
| 100 | 98.8±0.1 | 99.4±0.1 | 99.3±0.0 | 99.2±0.0 | 99.1±01.1 |
| IC 50 | 1.70 | 1.50 | 0.60 | 1.70 | 6.60 |
Percentage inhibition was calculated on the basis of integrated values of chemiluminescence (CL) over 600s. Mean ± SEM, n=3.
IC50 – doses producing 50% inhibition of control cell free CL system.
The mechanisms involved in antioxidant activity of polyphenols are complex, related to the structure of the compound. Stilbenes are naturally occurring more in Z form, which is also more effective compared to the E form (Aggarwal et al., 2004; Šmidrkal et al., 2010). The molecule has a common C6–C2–C6 structure, consisting of two aromatic rings linked through a two-carbon bridge with a double bond. Depending on the character of the substituent, the phenols in stilbene could be either saturated or drawn off with electrons. This may influence electron donor/acceptor properties of stilbene derivatives and thus their antioxidant activity.
Resveratrol is a phytoalexin structurally related to stilbenes. Resveratrol has been an effective scavenger of hydroxyl, superoxide, and metal induced radicals. In cells producing ROS, its antioxidant abilities have also been documented (Rizvi & Pandey, 2010). The authors further showed its protective effect against lipid peroxidation in cell membranes. The three hydroxyl groups of resveratrol were found to participate in an extensive three-dimensional hydrogen-bonding network. The hydrogen bonding due to the molecular packing in the crystal structure demonstrates the ready mobility of up to three hydrogen atoms per resveratrol molecule (Rizvi & Pandey, 2010).
Removal of the hydroxyl group from resveratrol in position 4′ results in pinosylvin. By this change, pinosylvin (partition coefficient-logP: 3.8) is more lipophilic than resveratrol (logP: 3.1). Both pinosylvin hydroxyl groups are located in meta position (with respect to the ethylene bridge of the stilbene molecule), i.e. in an arrangement less favourable both for electron abstraction and for the distribution of the unpaired electron (Fan et al., 2009; Queiroz et al., 2009).
In our experiments with human neutrophils, both pinosylvin and resveratrol at the concentrations 10 and 100 µmol/l, were effective in reducing intracellular ROS production and PKCα/βII phosphorylation (Table 2, Figure 2). Thus, we suggest that the removal of 4′-OH does not affect either intracellular antioxidant activity or PKCα/βII phosphorylation. In agreement with other studies (Stojanovič et al., 2001; Roupe et al., 2006), we found that the 4′-OH group in the structure of resveratrol was crucial for a strong extracellular antioxidant effect (Perečko et al., 2008). The less potent antioxidant effect of pinosylvin in comparison with resveratrol was established also in the cell free CL system (Table 3).
The change in resveratrol, i.e. methoxylation in 3,5 – position, leads to pterostilbene (logP 4.1). The peroxyl radical scavenging activity of pterostilbene appears to be similar to that of resveratrol. The antioxidant activity of pterostilbene was first demonstrated in vitro by its inhibition of methyl linoleate oxidation. Pterostilbene was reported to scavenge 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radicals, 2,2′azo-bis(2amidinopropane) (ABAP) derived peroxyl radicals and to inhibit the oxidation of citronellal, as well as lipid peroxidation in rat liver microsomes and in cultured human fibroblasts (Roupe et al., 2006; Pan et al., 2008).
Substitution of two hydroxyl groups with methoxy groups increased the lipophilicity parameter RM (retention constant) of resveratrol from 0.21 to 1.45 which applies for pterostilbene. This may enhance the bioavailability of pterostilbene in contrast to the low bioavailability of resveratrol. On the other side, lipophilicity may influence the transition of substances through the cell membrane into cytosol. Also the number and position of hydroxyl groups play a role in the antioxidant effects of polyphenols in different cell systems. Considering the effects of different resveratrol derivatives on the production of thiobarbituric acid reactive substances (TBARS) in normal human fibroblasts, pterostilbene was as good as resveratrol. Its 4′-methoxy derivative as well as 3, 4′, 5-trimethoxystilbene did not exert a significant inhibition of TBARS production (Stivala et al., 2001). These results support our findings with chemiluminescence assay in whole blood. The 3, 5-methoxy groups increased the antioxidant properties of pterostilbene compared to resveratrol in whole human blood (Perečko et al., 2008). In the extracellular space of isolated neutrophils, we found that pterostilbene at the concentration 10 µmol/l was less effective than resveratrol (Table 1). Despite the highest lipophilicity among the substances tested, pterostilbene was the least effective against intracellular CL of isolated neutrophils. This may be due to the requirement of free 3,5-OH groups in intracellular activity. The results are indicating that 3,5-meta-methoxyl groups decrease the extracellular and especially the intracellular activity of pterostilbene compared to resveratrol. After PMA stimulation, pterostilbene in either concentration used (10 and 100 µmol/l), failed to induce significant changes in PKC α/β II (Thr638/641) phosphorylation. Thus logP is not the only condition operative in intracellular activity (Perečko et al., 2008Perečko et al., 2010).
Due to the structural similarities between piceatannol and resveratrol, it has been hypothesised that piceatannol may also possess potent antioxidant activity. Piceatannol was shown to be a more effective scavenger of nitric oxide and hydrogen peroxide compared to resveratrol. The additional hydroxyl group of piceatannol makes it more reactive compared to resveratrol. In our experiments, piceatannol exerted a more intensive inhibition of chemiluminescence in free cell system in comparison to resveratrol, yet in the inhibition of extra-and intracellular ROS production resveratrol was more effective (Tables 1,2,3) (Jančinová et al., 2011). The mechanism by which the naturally occurring polyphenolic compound resveratrol and its metabolite piceatannol scavenge free radicals was studied using experimental and density functional theory methods (Rossi et al., 2008). Piceatannol was found to be more efficient than resveratrol because (i) by sharing its 3′-OH hydrogen atom with its adjacent neighbour, O-4′, the abstraction and transfer of the 4′-H atom to the free radical becomes easier, and (ii) the resulting piceatannol semiquinone radical is more stable. The interaction of both resveratrol and piceatannol with model membranes composed of phosphatidylcholine (DMPC and DPPC) was investigated by means of fluorescence spectroscopy, differential scanning calorimetry and electron spin resonance spectroscopy pointing to the preferential interaction of resveratrol and piceatannol with the headgroup region of lipid bilayer (Wesołowska et al., 2009).
Curcumin was found to be an effective antioxidant in different in vitro assays including: 2,2-diphenyl-1-picryhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and N,N-dimethyl-p-phenylenediamine (DMPD). The antioxidant activity of curcumin results from the presence of phenolic and central methylene hydrogens in its molecule (Ak & Gülçin, 2008; Lin et al., 2008). In our experiments, curcumin reduced dose-dependently oxidant formation in human neutrophils at extra- and intracellular level and in cell free system, and it effectively reduced protein kinase C activation (Jančinová et al., 2009). This is consistent with the results found by Franck et al. (2008), Deby-Dupont et al. (2005), in which curcumin inhibited ROS generation of neutrophils and lymphocytes stimulated with PMA, as well as the fibroblast PKC. The antioxidant properties of curcumin are based on its lipid peroxidation lowering effects through the ability to maintain the cellular status of antioxidant enzymes, like superoxide dismutase, catalase and glutathione peroxidase. Indeed, curcumin has been shown to increase reduced glutathione (GSH) levels, which leads to lowered ROS production (Rahman et al., 2006; Rahman, 2008). When curcumin was compared to other antioxidants in a lipid peroxidation assay of linoleic acid, it inhibited the lipid peroxidation by 97.3% as compared to standard antioxidants: 84.6% for α-tocopherol and 95.6% for trolox (Ak & Gülçin, 2008). In the micro to millimolar range, curcumin was shown to scavenge ROS, i.e. superoxide anion, hydrogen peroxide and nitric oxide (NO), both in vitro and in vivo (Obrenovich et al., 2010). Curcumin between 1 and 50 mmol/l, scavenged ROS as determined by electron pulse resonance spectroscopy and it was much faster in terms of quenching ROS than other polyphenols (resveratrol and quercetin).
N-feruloylserotonin conjugate was identified as the major and unique phenolic constituent of defatted safflower seeds. We found that N-feruloylserotonin markedly diminished oxidant formation in cell free CL system as well as in human neutrophils, both at extra- and intracellular level. Our further results suggest that one of the molecular effects of N-feruloylserotonin might involve the inhibition of PKCα/βII activity (Nosáľ et al., 2011). N-feruloylserotonin was demonstrated to exert an inhibitory effect on overproduction of mitochondrial superoxide by acting as scavenger of the superoxide, and its ROS-scavenging activity was comparable with that exerted by 40 µmol/l α-tocopherol (Piga et al., 2009). The antioxidant effect of N-feruloylserotonin was shown to be dependent on its structure (Piga et al., 2009; Takahashi & Miyazawa, 2011). Compared to serotonin, the authors reported a higher ability of N-feruloylserotonin to reduce ROS, suggesting a strong effect of the serotonin and ferulic acid moieties and their amide linkage on the antioxidant activity of N-feruloylserotonin.
Conclusion
The presented findings indicate that the derivatives of stilbene – resveratrol, pterostilbene, pinosylvin and piceatannol and the derivatives of ferulic acid – curcumin, N-feruloylserotonin may be suitable inhibitors of neutrophil activation, implying their anti-inflammatory potential.
Acknowledgement
The work was supported by The Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic for the Structural Funds of EU, OP R&D of ERDF by realization of the “Project Transfer of Knowledge and Technologies from Research and Development in Toxicology on Evaluation of Environmental and Health risk” (ITMS Code 26240220005) and by grant APVV-0052-10.
REFERENCES
- 1.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chemico-Biological Interactions. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Deby-Dupont G, Mouithys-Mickalad A, Serteyn D, Lamy M, Deby C. Resveratrol and curcumin reduce the respiratory burst of Chlamydia-primed THP-1cells. Biochem Biophys Res Commun. 2005;333:21–27. doi: 10.1016/j.bbrc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 4.Drábiková K, Perečko T, Nosáľ R, Bauerová K, Poništ S, Mihalová D, Kogan G, Jančinová V. Glucomannan reduces neutrophil free radical production in vitro and in rats with adjuvant arthritis. Pharmacol Res. 2009;59:399–403. doi: 10.1016/j.phrs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Fan GJ, Liu XD, Qian YP, Shang YJ, Li XZ, Dai F, Fang JG, Jin XL, Zhou B. 4,4’-Dihydroxy-trans-stilbene, a resveratrol analogue, exhibited enhanced antioxidant activity and cytotoxicity. Bioorg Med Chem. 2009;17:2360–2365. doi: 10.1016/j.bmc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Fontayne A, Dang PMC, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of p47phox sites by PKC α, β II, δ and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 7.Fraga CG, Galleano M, Verstraten S, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Asp Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Franck T, Kohnene S, Grulke S, Neven P, Goutman Y, Peters F, Pirotte B, Deby-Dupont G, Serteyn D. Inhibitory effect of curcuminoids and tetrahydrocurcuminoids on equine activated neutrophils and myeloperoxidase activity. Physiol Res. 2008;57:577–587. doi: 10.33549/physiolres.931086. [DOI] [PubMed] [Google Scholar]
- 9.Jančinová V, Drábiková K, Nosáľ R, Račková L, Májeková M, Holománová D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox Rep. 2006;1:110–116. doi: 10.1179/135100006X116592. [DOI] [PubMed] [Google Scholar]
- 10.Jančinová V, Perečko T, Nosáľ R, Koštálová D, Bauerová K, Drábiková K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol. 2009;612:161–166. doi: 10.1016/j.ejphar.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 11.Jančinová V, Perečko T, Drábiková K, Nosáľ R, Sviteková K. Piceatannol, a natural analogue of resveratrol, inhibits oxidative burst of human neutrophils. Interdisciplinary Toxicol. 2011;4:A35–A36. [Google Scholar]
- 12.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 13.Khlebnikov AI, Schepetkin I, Domina NG, Kirpotina LN, Quinn MT. Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic and cellular systems. Bioorg Med Chem. 2007;15:1749–1770. doi: 10.1016/j.bmc.2006.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klink M, Jastrzembska K, Bednarska K, Banasik M, Sulowska Z. Effect of nitric oxide donors on NADPH oxidase signaling pathway in human neutrophils in vitro . Immunobiology. 2009;214:692–702. doi: 10.1016/j.imbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee MR, Duan W, Tan SL. Protein kinase isoenzymes as potential targets in immune disorders. Expert Opin Ther Targets. 2008;12:535–552. doi: 10.1517/14728222.12.5.535. [DOI] [PubMed] [Google Scholar]
- 16.Lin HC, Tsai SH, Chen CS, Chang YC, Lee CM, Lai ZY, Lin CM. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem Pharmacol. 2008;75:1416–1425. doi: 10.1016/j.bcp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Nosáľ R, Perečko T, Jančinová V, Drábiková K, Harmatha J, Sviteková K. Suppression of oxidative burst in human neutrophils with the naturally occurring serotonin derivative isomer from Leuzea carthamoides . Neuroendocrinol Lett. 2010;31:69–72. [PubMed] [Google Scholar]
- 18.Nosáľ R, Perečko T, Jančinová V, Drábiková K, Harmatha J, Sviteková K. Naturally appearing N-feruloylserotonin isomers supress oxidative burst of human neutrophils at the protein kinase C level. Pharmacol Rep. 2011;63:790–798. doi: 10.1016/s1734-1140(11)70591-6. [DOI] [PubMed] [Google Scholar]
- 19.Obrenovich ME, Nair NG, Beyaz A, Aliev G, Reddy VP. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res. 2010;13:631–664. doi: 10.1089/rej.2010.1043. [DOI] [PubMed] [Google Scholar]
- 20.Pan Z, Agarwal AK, Xu T, Feng Q, Baerson SR, Duke SO, Rimando AM. Identification of molecular pathways affected by pterostilbene, a natural dimethylether analog of resveratrol. BMC Med Genomics. 2008;1:1–13. doi: 10.1186/1755-8794-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perečko T, Jančinová V, Drábiková K, Nosáľ R, Harmatha J. Structure – efficiency relationship in derivatives of stilbene. Comparison of resveratrol, pinosylvin and pterostilbene. Neuroendocrinol Lett. 2008;29:802–805. [PubMed] [Google Scholar]
- 23.Perečko T, Drábiková K, Račková L, Číž M, Podborská M, Lojek A, Harmatha J, Šmidrkal J, Nosáľ R, Jančinová V. Molecular targets of the natural antioxidant pterostilbene: effect on protein kinase C, caspase-3 and apoptosis in human neutrophils in vitro . Neuroendocrinol Lett. 2010;31:84–90. [PubMed] [Google Scholar]
- 24.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Inhibitory effect of serotonin derivatives on high glucose-induced adhesion and migration of monocytes on human aortic endothelial cells. Br J Nut. 2009;102:264–72. doi: 10.1017/S0007114508201947. [DOI] [PubMed] [Google Scholar]
- 25.Queiroz AN, Gomes BAQ, Moraes WM, Borges RS. A theoretical antioxidant pharmacophore for resveratrol. Eur J Med Chem. 2009;44:1644–1649. doi: 10.1016/j.ejmech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Račková L, Drábiková K, Jančinová V, Perečko T, Šmidrkal J, Harmatha J, Tóth J, Koštálová D, Bezek Š, Štefek M, Nosáľ R. Structural apects of antioxidant action of selected natural polyphenols. Free Rad Res. 2009;4:S39. [Google Scholar]
- 27.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Rahman I. Dietary polyphenols mediated regulation of oxidative stress and chromatin remodelling in inflammation. Nutrition Reviews. 2008;66:S42–S45. doi: 10.1111/j.1753-4887.2008.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi SI, Pandey KB. Activation of the erythrocyte plasma membrane redox system by resveratrol: a possible mechanism for antioxidant properties. Pharmacol Rep. 2010;62:726–732. doi: 10.1016/s1734-1140(10)70330-3. [DOI] [PubMed] [Google Scholar]
- 30.Rossi M, Caruso F, Opazo C, Salciccioli J. Crystal and molecular structure of piceatannol; scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals and docking with transthyretin. J Agric Food Chem. 2008;26:10557–10566. doi: 10.1021/jf801923j. [DOI] [PubMed] [Google Scholar]
- 31.Roupe KA, Remsberg CM, Yañez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 32.Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochim Biophys Acta. 2003;1637:59–69. doi: 10.1016/s0925-4439(02)00214-4. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson DE, Hurst RD. Plyphenolic phytochemicals-just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, Forti L, Pagnoni UM, Albini A, Prosperi E, Vannini V. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 35.Stojanovič S, Sprinz H, Brede O. Efficiency and mechanism of the antioxidant action of trans-Resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys. 2001;391:79–89. doi: 10.1006/abbi.2001.2388. [DOI] [PubMed] [Google Scholar]
- 36.Šmidrkal J, Harmatha J, Buděšínský M, Vokác K, Zídek Z, Kmonícková E, Merkl R, Filip V. Modified approach for preparing (E)-stilbenes related to resveratrol, and evaluation of their potential immunobiological effects. Collect Czech Chem Commun. 2010;7:175–186. [Google Scholar]
- 37.Štefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdisciplinary Toxicol. 2011;4:69–77. doi: 10.2478/v10102-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Miyazawa M. Synthesis and structure-activity relationships of phenylpropanoid amides of serotonin on tyrosinase inhibition. Bioorg Med Chem Lett. 2011;21:1983–1986. doi: 10.1016/j.bmcl.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Ursini F, Maiorino M, Morazzoni P, Roveri A, Pifferi G. A novel antioxidant flavonoid (ldB 1031) affecting molecular mechanisms of molecular activation. Free Radic Biol Med. 1994;16:547–553. doi: 10.1016/0891-5849(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 40.Varga ZS, Seres I, Nagy E, Ujhelyi L, Balla G, Balla J, Antus S. Structure prerequisite for antioxidant activity of silybin in different biochemical systems in vitro. Phytomedicine. 2006;13:85–93. doi: 10.1016/j.phymed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Wesołowska O, Kużdżal, Štrancar J, Michalak K. Interaction of the chemopreventive agent resveratrol and its metabolite, piceatannol, with model membranes. Biochim Biophys Acta. 2009;1788:1851–1860. doi: 10.1016/j.bbamem.2009.06.005. [DOI] [PubMed] [Google Scholar]