Abstract
Oxygen metabolism has an important role in the pathogenesis of rheumatoid arthritis. A certain correlation was observed between oxidative stress, arthritis and the immune system. Reactive oxygen species produced in the course of cellular oxidative phosphorylation and by activated phagocytic cells during oxidative burst, exceed the physiological buffering capacity and result in oxidative stress. The excessive production of ROS can damage protein, lipids, nucleic acids, and matrix components. Patients with rheumatoid arthritis have an altered antioxidant defense capacity barrier. In the present study the effect of substances with antioxidative properties, i.e. pinosylvin and carnosine, was determined in monotherapy for the treatment of adjuvant arthritis (AA). Moreover carnosine was evaluated in combination therapy with methotrexate. Rats with AA were administered first pinosylvin (30 mg/kg body mass daily per os), second carnosine (150 mg/kg body mass daily per os) in monotherapy for a period of 28 days. Further, rats with AA were administered methotrexate (0.3 mg/kg body mass 2-times weekly per os), and a combination of methotrexate+carnosine, with the carnosine dose being the same as in the previous experiment. Parameters, i.e. changes in hind paw volume and arthritic score were determined in rats as indicators of destructive arthritis-associated clinical changes. Plasmatic levels of TBARS and lag time of Fe2+-induced lipid peroxidation (tau-FeLP) in plasma and brain were specified as markers of oxidation. Plasmatic level of CRP and activity of γ-glutamyltransferase (GGT) in spleen and joint were used as inflammation markers. In comparison to pinosylvin, administration of carnosine monotherapy led to a significant decrease in the majority of the parameters studied. In the combination treatment with methotrexate+carnosine most parameters monitored were improved more remarkably than by methotrexate alone. Carnosine can increase the disease-modifying effect of methotrexate treatment in rat AA.
Keywords: arthritis, oxidative stress, pinosylvin, carnosine, methotrexate, combination therapy
Introduction
Rheumatoid arthritis (RA) is characterized by persistent synovitis, systemic inflammation, and presence of autoantibodies. Genetic factors are implicated for 50% of the risk of developing RA. Smoking is the main environmental risk. In industrialized countries, RA affects 0.5–1.0% of adults, with 5–50 per 100,000 new cases annually. The disorder is most typical in women and elderly people. Uncontrolled active RA causes joint damage, disability, decreased quality of life, and cardiovascular and other comorbidities. Disease-modifying antirheumatic drugs (DMARDs), the key therapeutic agents, reduce synovitis and systemic inflammation and improve function of the musculoskeletal system. The leading DMARD is methotrexate, which can be combined with other DMARDs. Biological agents are used when arthritis is uncontrolled or toxic effects arise with DMARDs. Tumor necrosis factor alpha inhibitors were the first biological agents, followed by abatacept, rituximab, and tocilizumab. Infections and high costs restrict prescription of biological agents. RA has 19th century roots. Its name was introduced in the 1850s. First classification criteria were developed only 50 years ago (Storey et al., 1994; Ropes et al., 1959; Arnett et al., 1988). Several clinical studies as well as preclinical animal models of RA have documented an imbalance in the body redox homeostasis to a more pro-oxidative environment, suggesting that therapies that restore the balance have a beneficial effect (Kunsch et al., 2005). Bauerova and Bezek (1999) and Jaswal et al. (2003) described oxidative stress as a primary factor involved in the RA pathogenetic changes. Our previous results with different antioxidants and immunomodulators showed a beneficial effect of these substances on the development of adjuvant arthritis (AA) (Bauerova et al., 2005a, 2005b 2008a, 2008b 2009, 2011, Drabikova et al., 2009; Drafi et al., 2010; Jancinova et al., 2009; Kogan et al., 2005; Macickova et al., 2010; Ponist et al., 2010; Sotnikova et al., 2009; Strosova et al., 2008, 2009). The advantage of AA as an experimental arthritis model is its great similarity to RA, such as symmetrical joint involvement, persistent joint inflammation, synovial hyperplasia, and a good response to most therapies effective in RA (Bina & Wilder, 1999).
According to Babior (2000) reactive oxidants are essential tools for the pathogenesis of RA. The world of plants is an unlimited source of compounds with healing effects, including anti-inflammatory, antioxidative and immunomodulating properties. For this experimental overview, we chose to study the effect of pinosylvin (PIN). PIN [3′,5′-dihydroxystilbene] is a natural substance from the stilbenoid group, wide-spread in a variety of plants. PIN is chemically related to resveratrol, which is well known by its anti-oxidative activity. Structural analogues of resveratrol possess some of the beneficial effects of the parent drug and may provide even further benefits. PIN has been studied for its anticancer, antifungal and antioxidative properties (Roupe et al., 2008). Further we chose the endogenous antioxidant carnosine (CARN) for monotherapy and as a suitable candidate for combination therapy with methotrexate (MTX). CARN is a dipeptide consisting of β–alanine and L-histidine. It was shown to be a specific constituent of excitable tissues of all vertebrates accumulating in amounts exceeding that of ATP (Boldyrev and Severin, 1990). The antioxidant capacity of this compound is well documented, as well as its pH buffering, osmoregulation, and metalchelating abilities (Boldyrev, 1990).
Methods
Animals, experimental design and treatments
Male Lewis rats, weighing 160–180 g, were obtained from the “Breeding Station Dobra Voda” (Slovakia). The rats had free access to standard pelleted diet and tap water ad libitum. The animal facilities comply with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes. The experimental protocol was approved by the Ethics Committee of the Institute of Experimental Pharmacology and Toxicology and by the State Veterinary and Food Administration of the Slovak Republic. Induction of arthritis in rats was performed by injecting a suspension of heat-killed Mycobacterium butyricum (MB) in incomplete Freund's adjuvant (Difco Laboratories, Detroit, MI, USA) intradermally into the tail base.
After 7-days quarantine, the animals were randomized as follows in 3 experiments:
1st experiment included: 3 groups of eight – ten animals:
group 1 – healthy untreated controls (CO);
group 2 – untreated rats with adjuvant arthritis (AA);
group 3 – AA rats treated with carnosine (AA-CARN).
2nd experiment included: 3 groups of eight – ten animals:
group 1 – healthy untreated controls (CO);
group 2 – untreated rats with adjuvant arthritis (AA);
group 3 – AA rats treated with pinosylvin (AA-PIN).
3rd experiment included: 4 groups of eight – ten animals:
group 1 – healthy untreated controls (CO);
group 2 – untreated rats with adjuvant arthritis (AA);
group 3 – AA rats treated with methotrexate (AA-MTX);
group 4 – AA rats in combination therapy of carnosine and methotrexate (AA-CARN+MTX).
Pinosylvin (daily dose of 30 mg/kg b.w. per os) and carnosine (daily dose of 150 mg/kg b.w. per os) in monotherapy were administered to the arthritic animals from day 1 (day of arthritic insult) to the 28 (end of experiment). MTX in dose 0.3 mg/kg body mass was applied per os twice a week. The combination of carnosine and methotrexate (CARN – daily dose of 150 mg/kg b.w. per os + MTX in the dose of 0.3 mg/kg body mass twice a week per os) was administered to the animals during the whole experiment. PIN was synthesized and purified by Šmidrkal et al. (2010) and Harmatha et al. (2011). METHOTREXATE-TEVA® 25 mg/ml (TEVA Pharmaceuticals Slovakia s.r.o.– SVK) was used. CARN was purchased from Hamary Chemicals Ltd., Japan. After the animals had been sacrificed under deep anesthesia, blood for plasma preparation and tissues for brain, spleen and hind paw joint homogenate preparation were taken on day 28. Heparinized plasma was stored at –70°C until biochemical and immunological analysis.
Clinical parameters evaluated – hind paw volume change and arthrogram
Hind paw volume (HPV) was calculated as the percentage increase of the hind paw of each animal, compared to the HPV measured of the beginning of the experiment. HPV was recorded by means of an electronic water plethysmometer (UGO BASILE, Comerio-Varese, Italy). The arthritic score was measured as the total score of HPV (ml, max. points 8) + paw diameter of forelimb (mm, max. points 5) + diameter of scab, in the site of MB application, measured parallel to the spinal column (mm, max. points 5) for each animal.
Tissue activity of cellular γ-glutamyltransferase in spleen and hind paw joint homogenates
The activity of cellular γ-glutamyltransferase (GGT) in hind paw joint and spleen tissue homogenates was measured by the method of Orlowski and Meister (1970) as modified by Ondrejickova et al. (1993). Samples were homogenized in a buffer (2.6 mM NaH2PO4, 50 mM Na2HPO4, 15 mM EDTA, 68 mM NaCl; pH 8.1) at 1:9 (w/v) by UltraTurax TP 18/10 (Janke & Kunkel, Germany) for 1 min at 0°C. Substrates (8.7 mM γ-glutamyl-p-nitroaniline, 44 mM methionine) were added to 65% isopropylalcohol to final concentrations of 2.5 mM and 12.6 mM, respectively. After incubation for 60 min at 37°C, the reaction was stopped with 2.3 ml cold methanol and the tubes were centrifuged for 20 min at 5,000 rpm. Absorbance of supernatant was measured in a Specord 40 (Jena, Germany) spectrophotometer in a 0.5 cm cuvette at 406 nm. Reaction mixtures in the absence of either substrate or acceptor were used as reference samples.
Thiobarbituric acid reactive substances (TBARS) in plasma
The reaction with TBA occurs by attack of the monoenolic form of malondialdehyde (MDA) on the active methylene groups of TBA. Visible and ultraviolet spectrophotometry of the pigment confirms the primary maximum at 535 nm. TBARS were measured in heparinized blood plasma. The amount of 750 μL of 0.67% TBA (Merck), 750 μL of 20% trichloroacetic acid (Fluka), 350 μL of phosphate buffer (pH 7.4) were added to 150 μL of plasma, then mixed and incubated in a water bath at 90°C for 30 min. The reaction was stopped by dipping the test tubes into ice for 10 min. Samples were centrifuged at 3,000 rpm (centrifuge Eppendorf 5702 R, Germany). The supernatant was removed and absorbance measured at 535 nm (Specord 40, Jena, Germany) in a 0.5 cm cuvette.
Lag time of Fe2+-induced lipid peroxidation (tau-FeLP) of plasma and brain
This measurement was analyzed using the chemiluminescence (CHL) signal derived from addition of ferrous ions to plasma and brain homogenates. After addition of 100 μl of 25 mM FeSO4 to the samples, the lag period between initial fast flash and the following slow rising CHL signal reflecting the rate of lipid oxidation was measured. The lag time (τ-tau) is referring to the stability of the sample to the Fe2+-induced oxidation (the longer the lag period the more stable the resistance of the biological material to oxidation) and is dependent on the intrinsic antioxidant capacity of plasma and brain. CHL signal was monitored using LKB 1251 Chemiluminometer (Sweden).
C-reactive protein
For the determination of rat C-reactive protein (CRP) concentration in plasma the ELISA kit from Immunology Consultant Laboratories, Inc. (ICL) was used. The reaction of secondary biotin-conjugated anti-rat CRP antibody is evaluated by streptavidin-HRP. The tetramethyl-benzidine reaction with HRP bound to immune complex was measured at 450 nm (Microplate reader Labsystems Multiskan RC). The results were calculated using the standard calibration curve on internal standards.
Statistical analysis
The data for all parameters are expressed as arithmetic mean ± S.E.M with eight – ten animals in each experimental group. For statistical analysis the unpaired Student‘s t-test was applied. The following symbols were used: *p<0.05 (significant), **p<0.01 (very significant), ***p<0.001 (extremely significant). The arthritis group (AA) was compared with healthy control animals (CO) (*). The treated arthritis groups in monotherapy (AA-PIN / AA-CARN) and the combination therapy (AA-CARN+MTX) were compared with untreated arthritis (AA) (+). The combination treatment (AA-CARN+MTX) was compared to the group treated with methotrexate (AA-MTX) (#).
Results
Our results concern three experiments: 1st monotherapy with pinosylvin (PIN), 2nd monotherapy with carnosine (CARN), and 3rd the combination therapy of carnosine and methotrexate (CARN+MTX).
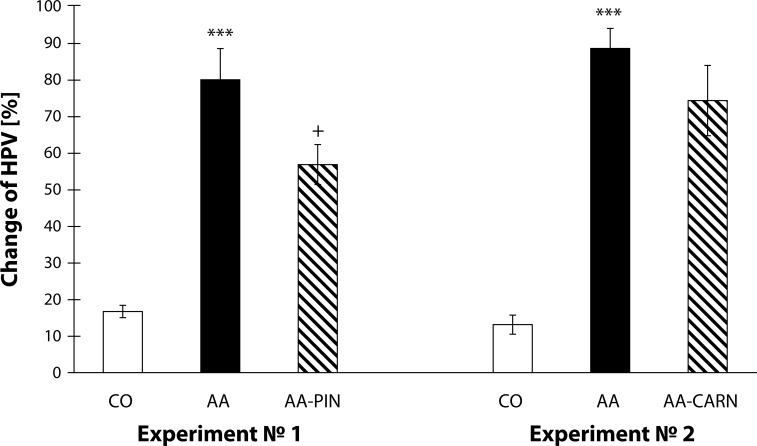
Figure 1 shows the results from the 1st and 2nd experiment on the clinical parameter change of HPV [%]. The change of HPV shows an increase in the arthritic groups compared to the control groups (AA vs. CO, ***p<0.001). Further the administration of pinosylvin decreased the change of HPV by 28.96% in comparison with the untreated arthritis group in a beneficially way (AA-PIN vs. AA, +p<0.05). In the second experiment, administration of carnosine decreased slightly the changed HPV by 16.09% in comparison with the untreated arthritis group (Figure 1).
Figure 1.
Change of HPV measured on day 28 in the model of adjuvant arthritis treated with pinosylvin and carnosine in monotherapy.
CO – control group, AA – Adjuvant arthritis group, AA-PIN – Adjuvant arthritis group administered pinosylvin, AA-CARN – Adjuvant arthritis group administered carnosine. Hind paw volume (HPV) is expressed in percentages [%]. Results are mean ± S.E.M., n=8-10. The symbol (*) shows significant difference ***p<0.001 versus CO, +p<0.05 versus AA.
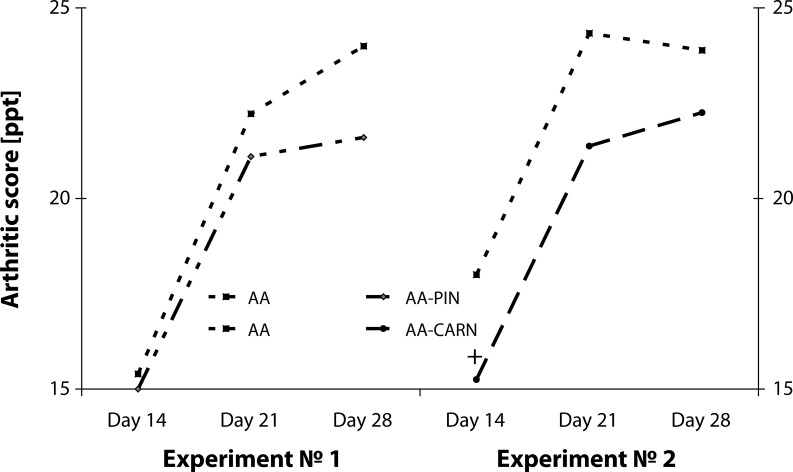
The points of arthritic score in Figure 2 show a trend to decrease the score for the treated groups in both experiments administered PIN or CARN in comparison to untreated arthritic animals. With CARN administration, the effect was clearly significant only on day 14 (AA-CARN vs. AA, +p<0.05) (Figure 2).
Figure 2.
Arthritic score in the model of adjuvant arthritis treated with pinosylvin and carnosine in monotherapy.
CO – control group, AA – Adjuvant arthritis group, AA-PIN – Adjuvant arthritis group administered with pinosylvin, AA-CARN – Adjuvant arthritis group administered carnosine. Arthrogram is expressed in points [pts]. Results are mean ± S.E.M., n=8-10. The symbol (+) shows significant difference +p<0.05 vs AA.
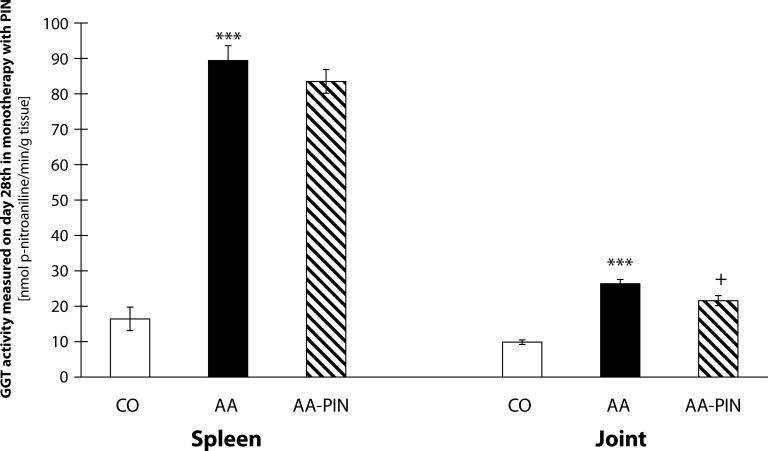
The results in Figure 3 show the activity of cellular γ-glutamyltransferase (GGT) in hind paw joint and spleen tissue homogenates on PIN administration. The value of the activity of GGT in the spleen in the 1st experiment shows an increase in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of PIN decreased the activity of GGT in spleen by 6.58% compared with the untreated arthritis group. Further, the value of the activity of GGT increased significantly in the joint in the 1st experiment in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of pinosylvin decreased the activity of GGT in the joint by 18.14% in comparison with the untreated arthritis group (AA-PIN vs. AA, +p<0.05) (Figure 3).
Figure 3.
GGT activity in spleen and joint on day 28 in the model of adjuvant arthritis treated with pinosylvin in monotherapy.
CO – control group, AA – Adjuvant arthritis group, AA-PIN – Adjuvant arthritis group administered with pinosylvin. The activity of cellular γ-glutamyltransferase (GGT) in hind paw joint and spleen tissue homogenates is expressed in [nmoln p-nitroaniline/min/g tissue]. Results are mean ± S.E.M., n=8-10. The symbol (*) shows significant difference ***p<0.001 vs CO, +p<0.05 vs AA.
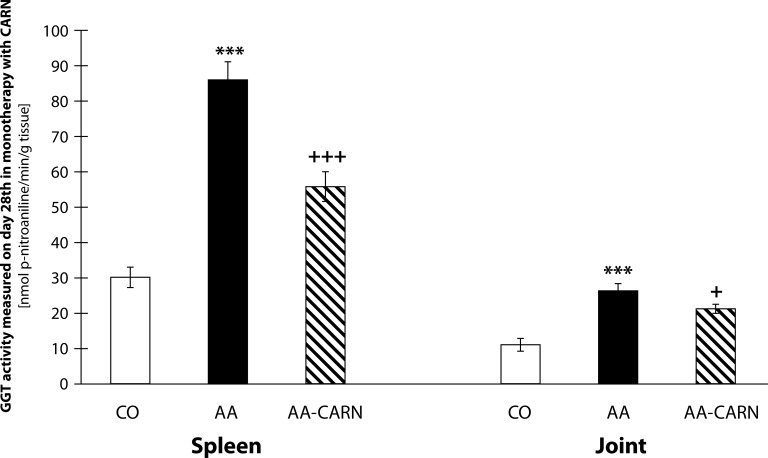
In the 2nd experiment (Figure 4), the activity of GGT in the spleen shows significant increase in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of CARN decreased significantly the activity of GGT in the spleen by 35.17% compared with the untreated arthritis group (AA-CARN vs. AA, +++p<0.001). The activity of GGT in the joint showed also significant increase in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of carnosine decreased significantly the activity of GGT in the joint by 19.30% compared with the untreated arthritis group (AA-CARN vs. AA +p<0.05). The GGT activity assessed in the joint homogenate achieved results which showed the same trend in both experiments (Figure 4).
Figure 4.
GGT activity in spleen and joint on day 28 in the model of adjuvant arthritis treated with carnosine in monotherapy.
CO – control group, AA – Adjuvant arthritis group, AA-CARN – Adjuvant arthritis group administered carnosine. The activity of cellular γ-glutamyltransferase (GGT) in hind paw joint and spleen tissue homogenates is expressed in [nmoln p-nitroaniline / min / g tissue]. Results are mean ± S.E.M., n=8-10. The symbol (*) shows significant difference ***p<0.001 vs CO, +p<0.05 vs AA, +++p<0.001 vs AA.
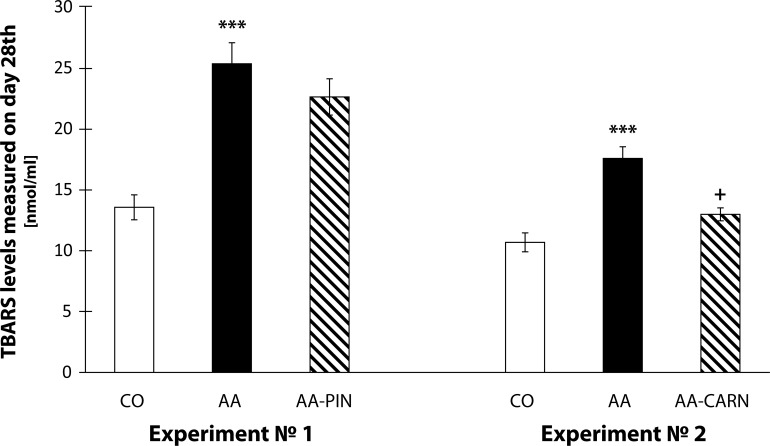
The results in Figure 5 show the levels of plasmatic TBARS measured on day 28 [nmol/ml]. The levels of plasmatic TBARS increased significantly in the 1st experiment in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of pinosylvin decreased the level of TBARS in plasma by 10.76% compared with the untreated arthritis group (AA-PIN vs. AA). In the 2nd experiment, the increased levels of TBARS measured on day 28 showed also significance in the arthritis group compared to the control group (AA vs. CO, ***p<0.001). Administration of carnosine decreased significantly the level of TBARS in plasma by 26.11% in comparison with the untreated arthritis group (AA-CARN vs. AA, +p<0.05). The results show that carnosine improved more efficiently the TBARS levels in plasma than PIN (Figure 5).
Figure 5.
TBARS measured on day 28 in the model of adjuvant arthritis treated with pinosylvin and carnosine in monotherapy.
CO – control group, AA – Adjuvant arthritis group, AA-PIN – Adjuvant arthritis group administered with pinosylvin, AA-CARN – Adjuvant arthritis group administered with carnosine. Levels of TBARS in plasma are expressed in [nmoln/ml]. Results are mean ± S.E.M., n=8–10. The symbol (*) shows significant difference ***p<0.001 vs CO, +p<0.05 vs AA.
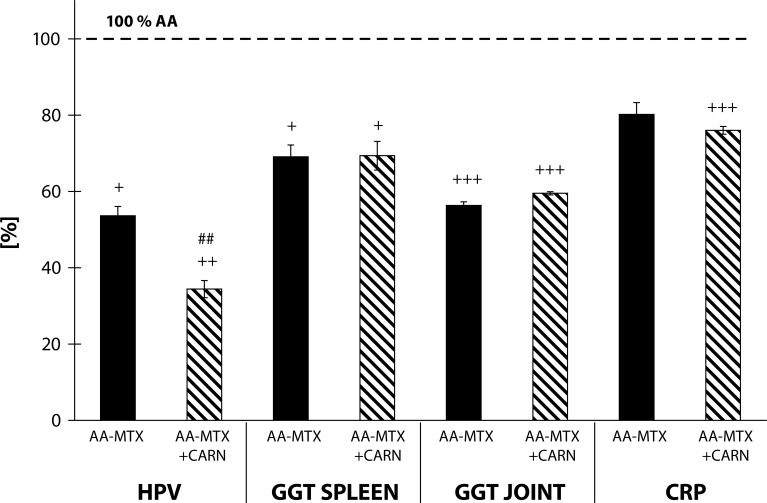
The results in Figure 6 show the values from the 3rd experiment on selected clinical and inflammation parameters, i.e. change of HPV, GGT activity (spleen and joint homogenates) and levels of C-reactive protein (CRP) in plasma. The HPV decreased in the arthritis group treated with MTX by 46.38% in comparison to AA (AA-MTX vs. AA, +p<0.05). Administration of carnosine+methotrexate decreased HPV by 65.59% in comparison with the untreated arthritis group (AA-CARN+MTX vs. AA, ++p<0.01). The activity of GGT decreased by 30.93% in the spleen in the arthritis group treated with MTX in comparison to AA (AA-MTX vs. AA, +p<0.05). Administration of carnosine+methotrexate decreased the activity of GGT by 30.62% compared to the untreated arthritis group (AA-CARN+MTX vs. AA, +p<0.01). In the arthritis group treated with MTX the activity of GGT in the joint decreased by 43.38% significantly in comparison to AA (AA-MTX vs. AA, +++p<0.001). Administration of carnosine+methotrexate significantly decreased the activity of GGT by 40.47% in comparison to the untreated arthritis group (AA-CARN+MTX vs. AA, +++p<0.001). The level of CRP decreased by 19.78% in plasma measured on day 28 in the arthritis group treated with MTX in comparison to AA. Administration of carnosine+methotrexate decreased the level of CRP in plasma by 23.98% in comparison to the untreated arthritis group (AA-CARN+MTX vs. AA, +++p<0.001). Figure 6 shows a statistically significant difference between MTX monotherapy used as reference treatment and its combination with CARN only for the clinical parameter – change of HPV.
Figure 6.
Effect of methotrexate and the combination of carnosine + methotrexate on adjuvant arthritis on the parameters of hind paw volume (HPV), activity of cellular γ-glutamyltransferase (GGT) in hind paw joint and spleen tissue homogenates and C-reactive protein (CRP) in plasma measured on day 28, expressed in percentage compared to the arthritis group considered as 100%.
AA – Adjuvant arthritis group (100%), AA- MTX – Adjuvant arthritis group administered methotrexate and AA-CARN+MTX – Adjuvant arthritis group administered with carnosine+methotrexate. Results are mean ± S.E.M., n=8–10. The symbol (+) shows significant difference +p<0.05 vs AA, ++p<0.01 vs AA, +++p<0.001 vs AA, ##p<0.01 vs AA-MTX.
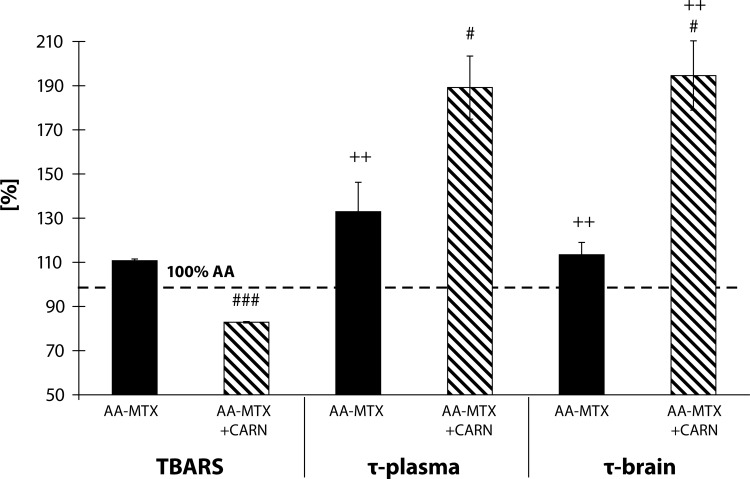
The results in Figure 7 show the values from the 3rd experiment on the parameters: plasmatic levels of TBARS, lag time of Fe2+-induced lipid peroxidation (tau-FeLP) in plasma and brain expressed in percentages in comparison to the arthritis group considered as 100%. The levels of TBARS in the arthritis group treated with MTX increased by 10.76% in comparison to AA. Administration of carnosine+methotrexate decreased the level of TBARS by 17.16% in comparison with the arthritis group. In plasma in the arthritis group treated with MTX, the tau-FeLP was increased by 32.96% in comparison to AA (AA-MTX vs. AA, ++p<0.01). Administration of carnosine+methotrexate increased significantly the tau-FeLP plasma in comparison to the untreated arthritis group by 89.14% (AA-CARN+MTX vs. AA, ++p<0.01). In the arthritis group treated with MTX, the lag time of Fe2+-induced lipid peroxidation in the brain was increased significantly in comparison to AA by 13.43% (AA-MTX vs. AA,++p<0.01). Administration of carnosine+methotrexate increased significantly the lag time of Fe2+-induced lipid peroxidation in the brain in comparison to the untreated arthritis group by 94.53% (AA-CARN+MTX vs. AA, ++p<0.01). For all oxidative stress parameters we found a statistically significant difference between MTX monotherapy used as reference treatment and its combination with CARN.
Figure 7.
Effect of methotrexate and the combination of carnosine + methotrexate on adjuvant arthritis on the parameters of TBARS, tau-FeLP in plasma and brain on day 28, expressed in percentages in comparison to the arthritis group considered as 100%.
AA – Adjuvant arthritis group (100%), AA- MTX – Adjuvant arthritis group administered methotrexate and AA-CARN+MTX – Adjuvant arthritis group administered carnosine+methotrexate. Results are mean ± S.E.M., n=8–10. The symbol (+) shows significant difference ++p<0.01 vs AA, #p<0.05 vs AA-MTX, ###p<0.001 vs AA-MTX.
Discussion
In the presented experiments we showed the effect of two antioxidative substances tested (pinosylvin and carnosine) and the combination of the latter one with methotrexate in the model of adjuvant arthritis. Pinosylvin in monotherapy decreased signigficantly the basic clinical parameter HPV in comparison to untreated animals. Our previous experiments (Bauerova et al., 2007, 2008a, 2008b 2009, Rovensky et al., 2008, Rovensky et al., 2009a) reported that clinical parameters, such as HPV and body weight, became significantly worse due to arthritis and were efficiently decreased by administration of substances with antioxidative properties. The administration with carnosine did not exert significant effect on HPV but showed a potency to decrease the changes in HPV (Figure 1). On the other hand, the combination of carnosine with methotrexate decreased the HPV more effectively than did treatment with methotrexate alone (Figure 6). This result is in compliance with other studies on combination of naturally occurring substances and methotrexate, where the combination proved more effective than monotherapy (Bauerova et al., 2009, 2010; Rovensky et al., 2002, 2009a, 2009b). The substances pinosylvin and carnosine, failed to decrease significantly the arthritic score in comparison to untreated arthritic animals (Figure 2). This is due to the fact that the arthritic score consists of five different inputs (two forelimbs, two hind limbs and scab) and these are influenced variably.
The activity of GGT is considered a reliable biochemical marker of oxidative stress as it is sensitive to immunomodulating effects of yeast polysaccharides (Bauerova et al., 2006). Ishizuka et al. (2007) found that neutralizing antibodies against GGT had a therapeutic effect on joint destruction in collagen-induced arthritis in mice. Increased GGT activity was observed in serum and also in the synovial fluid of affected joints in patients with RA (Rambabu et al., 1990) and also in joints of AA-animals (Ponist et al., 2010). Previously we found that the activity of GGT was approximately 3–6 times higher in AA animals than in healthy controls in the spleen and 1.4–2.3 higher in the joint (Bauerova et al., 2006; 2008a; 2009; Sotnikova et al., 2009). Elevated expression and activity of GGT in joint tissue is a good marker for synovial inflammation and bone resorption, thus molecules able to reduce the activity and/or expression of GGT could be important for RA therapy. In our study, we observed increased activity of GGT in joint tissues and also in the spleen in animals with induced AA. Administration of pinosylvin slightly decreased the activity of GGT in the spleen (Figure 3). On the other hand, administration with carnosine remarkably decreased the activity of GGT in this important organ of the immune system (Figure 4). After pinosylvin or carnosine administration to arthritic rats, the activity of GGT in joint tissue decreased significantly in the same manner for both compounds tested (Figures 3, 4). Administration of MTX led to significantly decreased values of GGT activity in the spleen and joint (Figure 6). This beneficial effect of MTX was reported also in our previous experiments (Feketeova et al., 2011; Bauerova et al., 2010; Ponist et al., 2010; Jurcovicova et al., 2009) with a similar experimental design and setting. The combination of carnosine with methotrexate failed to yield a better effect on the improvement of GGT activity in the spleen and joint than did methotrexate treatment alone (Figure 6).
A frequently used marker of lipid peroxidation is MDA assessed as an adduct with TBA. Clinical studies have shown increased plasmatic levels of MDA in patients with RA (Baskol et al., 2005; 2006; Sarban et al., 2005). Also in animal models of AA, an increase in oxidative stress, represented by evaluated plasmatic levels of TBARS, was detected (Tastekin et al., 2007; He et al., 2006; Bauerova et al., 2008b; 2009; Strosova et al., 2008, 2009; Sotnikova et al., 2009). In our previous work using the model of AA we found evidence of systemic oxidative stress, specifically: increased protein carbonyls (Bauerova et al., 2005a), increased lipid peroxidation (Bauerova et al., 2005b), and a significant reduction of total antioxidant status (Mihalova et al., 2007) in plasma. Measurements of plasma TBARS in the present study confirmed that AA induced a systemic oxidative damage. Administration of carnosine was more effective than pinosylvin in reducing this oxidative damage monitored as TBARS levels in plasma (Figure 5). Moreover the combination of carnosine+methotrexate was more effective than methotrexate alone (Figure 7).
The levels of C-reactive protein (CRP), a protein found in plasma, rise in response to inflammation (Thompson et al., 1999). Methotrexate decreased significantly the level of CRP in plasma of arthritic animals, as described by Bauerova et al. (2011). The level of CRP in AA rats was even more decreased by the combination therapy of carnosine+methotrexate (Figure 6). Furthermore, the group with combination therapy of carnosine and methotrexate increased the lag time of Fe2+-induced lipid peroxidation (tau-FeLP) in plasma and in brain samples, which refers to its ability to restore the systemic antioxidant capacity in plasma and locally in brain tissue (Figure 7). Carnosine exhibited a good protective activity against Fe2+-induced lipid peroxidation of human plasma lipoproteins in vitro and in the brain of experimental animals (Dobrota et al., 2005; Fedorova et al., 1999). In the present experiment, we report for the first time the protective effect of carnosine in combination therapy on tau-FeLP in Lewis rats with AA (Figure 7).
The results of our investigation confirmed the previously reported effect of methotrexate treatment in rats with AA (Welles et al., 1985; Connolly et al., 1988). Methotrexate at a dose of 0.6 mg/kg body mass per week (Bauerova et al., 2010) suppressed but did not prevent arthritis development. The therapeutic effect of MTX was confirmed also by a significant reduction in the edema of the affected joints. Such effect could be ascribed also to the oxidative stress reducing properties of MTX (Koner et al., 1997), as already reported in patients with RA (Herman et al., 2008; Kageyama et al., 2007). The anti-inflammatory effect of MTX is accompanied with suppression of oxidative burst of stimulated blood phagocytes in animals treated with MTX and was proved in our previous experiments (Bauerova et al., 2010; Nosal et al., 2007). Finally, the reduction of hind paw volume and CRP were more pronounced in rats treated with the combination of carnosine+methotrexate than in those treated with methotrexate alone. Moreover, comparable results were observed for TBARS levels in plasma. Thus we can conclude that oxidative stress plays an important role in AA and could be controlled by suitable combination therapy of MTX and an antioxidant substance, as demonstrated for carnosine.
Acknowledgements
The work was supported by The Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic for the Structural Funds of EU, OP R&D of ERDF by realization of the Project: “Transfer of Knowledge and Technologies from Research and Development in Toxicology on Evaluation of Environmental and Health risk” (ITMS Code 26240220005).
Further this work was supported by: Bilateral project SAS-RAMS 2010-2012: “Regulation of cytokine synthesis during inflammation development in brain and other tissues.” and by projects VEGA 2/0045/11, APVV-0315-07 and APVV-0052-10.
REFERENCES
- 1.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 2.Babior BM. Phagocytes and oxidative stres. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 3.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951–955. doi: 10.1016/j.clinbiochem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Karakukcu C, Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Func. 2006;24:307–311. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 5.Bauerova K, Bezek S. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys. 1999;18:15–20. [PubMed] [Google Scholar]
- 6.Bauerova K, Kucharska J, Mihalova D, Navarova J, Gvozdjakova A, Sumbalova Z. Effect of coenzyme Q(10) supplementation in the rat model of adjuvant arthritis. Biomed pap. 2005a;149:501–503. doi: 10.5507/bp.2005.090. [DOI] [PubMed] [Google Scholar]
- 7.Bauerova K, Valentova J, Ponist S, Navarova J, Komendova D, Mihalova D. Effect of copper complexes on the development of adjuvant arthritis: Therapeutic and toxicological aspects. Biologia. 2005b;60:65–68. [Google Scholar]
- 8.Bauerova K, Ponist S, Ondrejickova O, Komendova D, Mihalova D. Association between tissue gamma-glutamyl-transferase and clinical markers of adjuvant arthritis in Lewis rats. Neuroendocrinol Lett. 2006;27:172–175. [PubMed] [Google Scholar]
- 9.Bauerova K, Kucharska J, Ponist S, Gvozdjakova A. Coenzyme Q10 supplementation in adjuvant arthritis (pre-clinical study) In: Gvozdjakova A, editor. Mitochondrial medicine: mitochodrial metabolism, diseases, diagnosis and therapy. Netherlands: Springer; 2008a. pp. 340–342. [Google Scholar]
- 10.Bauerova K, Ponist S, Navarova J, Dubnickova M, Paulovicova E, Pajtinka M, Kogan G, Mihalova D. Glucomannan in prevention of oxidative stress and infammation occuring in adjuvant arthritis. Neuroendocrinol Lett. 2008b;29:691–696. [PubMed] [Google Scholar]
- 11.Bauerova K, Paulovicova E, Mihalova D, Svik K, Ponist S. Study of new ways of supplementary and combinatory therapy of rheumatoid arthritis with immunomodulators. Glucomannan and Imunoglukan in adjuvant arthritis. Toxicology and Industrial Health. 2009;25:329–335. doi: 10.1177/0748233709102945. [DOI] [PubMed] [Google Scholar]
- 12.Bauerova K, Paulovičova E, Mihalova D, Drafi F, Ktrosova M, Mascia C, Biasi F, Rovensky J, Kucharska J, Gvozdjakova A, Ponint S. Combined methotrexate and coenzyme Q10 therapy in adjuvant-induced arthritis evaluated using parameters of inflammation and oxidative stress. Acta Biochim Pol. 2010;57:347–354. [PubMed] [Google Scholar]
- 13.Bauerova K, Ponist S, Nosal R, Stancikova M, Rovensky J. Modern pharmacological approaches to therapies: substances tested in animal models of rheumatoid arthritis, in Rheumatoid arthritis – Treatment 1 th. In: Lemmey AD, editor. 2011. pp. 233–268. Intech, Rijeka. ISBN 978-953-307-850-2. [Google Scholar]
- 14.Bauerova K, Ponist S, Kuncirova V, Mihalova D, Paulovicova E, Volpi N. Chondroitin sulfate effect on induced arthritis in rats. Osteoarthritis Cartilage. 2011;19:1373–1379. doi: 10.1016/j.joca.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Bina J, Wilder RL. Animal models of rheumatoid arthritis. Mol Med Today. 1999;5:367–369. doi: 10.1016/s1357-4310(99)01528-2. [DOI] [PubMed] [Google Scholar]
- 16.Boldyrev AA, Severin SE. The histidine-containing dipeptides, carnosine, and anserine: distribution, properties, and biological significance. Advan Enzym Regul. 1990;30:175–193. doi: 10.1016/0065-2571(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 17.Boldyrev AA. Retrospectives and perspectives on the biological activity of histidine- containing dipeptides. Int J Biochem. 1990;22:129–132. doi: 10.1016/0020-711x(90)90173-z. [DOI] [PubMed] [Google Scholar]
- 18.Connolly KM, Stecher VJ, Danis E, Pruden DJ, LaBrie T. Alteration of interleukin-1 production and the acute phase response following medication of adjuvant arthritic rats with cyclosporin-A or methotrexate. Int J Immunopharmacol. 1988;10:717–728. doi: 10.1016/0192-0561(88)90025-2. [DOI] [PubMed] [Google Scholar]
- 19.Dobrota D, Fedorova T, Stvolinsky S, Babushikova E, Licavcanova K, Drgova A, Strapkova A, Boldrev A. Carnosine protects the brain of rats and mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res. 2005;30:1283–1288. doi: 10.1007/s11064-005-8799-7. [DOI] [PubMed] [Google Scholar]
- 20.Drabikova K, Perecko T, Nosal R, Bauerova K, Ponist S, Mihalova D, Kogan G, Jancinova V. Glucomannan reduces neutrophil free radical production in vitro and in rats with adjuvant arthritis. Pharmacol Res. 2009;59:399–403. doi: 10.1016/j.phrs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Drafi F, Bauerova K, Valachova K, Poništ S, Mihalova D, Juranek I, Boldyrev A, Hrabarova E, Soltes L. Carnosine inhibits degradation of hyaluronan induced by free radical processes in vitro and improves the redox imbalance in adjuvant arthritis in vivo. Neuroendocrinol Lett. 2010;31:96–100. [PubMed] [Google Scholar]
- 22.Fedorova TN, Boldyrev AA, Gannushkina IV. Lipid peroxidation in experimental ischemia of the brain. Biochemistry (Mosc) 1999;64:75–79. [PubMed] [Google Scholar]
- 23.Feketeova L, Jančova P, Moravcova P, Janegova A, Bauerova K, Poništ S, Mihalova D, Janega P, Babal P. Effect of methotrexate on inflammatory cells redistribution in experimental adjuvant arthritis. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2177-3. [DOI] [PubMed] [Google Scholar]
- 24.Harmatha J, Zídek Z, Kmoníčkova E, Šmidrkal J. Immunobiological properties of selected natural and chemically modified phenylpropanoids. Interdisc Toxicol. 2011;4:5–10. doi: 10.2478/v10102-011-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He YH, Zhou J, Wang YS, Xiao C, Tong Y, Tang JC, Chan AS, Lu AP. Anti-infammatory and anti-oxidative effects of cherries on Freund's adjuvant-induced arthritis in rats. Scand J Rheumatol. 2006;35:356–358. doi: 10.1080/03009740600704155. [DOI] [PubMed] [Google Scholar]
- 26.Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin Exp Rheumatol. 2008;26:317–323. [PubMed] [Google Scholar]
- 27.Ishizuka Y, Moriwaki S, Kawahara-Hanaoka M, Uemura Y, Serizawa I, Miyauchi M, Shibata S, Kanaya T, Takata T, Taniguchi N, Niida S. Treatment with anti-gamma-glutamyl transpeptidase antibody attenuates osteolysis in collagen-induced arthritis mice. J Bone Miner Res. 2007;22:1933–42. doi: 10.1359/jbmr.070726. [DOI] [PubMed] [Google Scholar]
- 28.Jancinova V, Perecko T, Nosal R, Kostalova D, Bauerova K, Drabikova K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol. 2009;612:161–6. doi: 10.1016/j.ejphar.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 29.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2003;338:123–129. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Jurcovicova J, Svik K, Scsukova S, Bauerova K, Rovensky J, Stancikova M. Methotrexate treatment ameliorated testicular suppression and anorexia related leptin reduction in rats with adjuvant arthritis. Rheumatol Int. 2009;29:1187–1191. doi: 10.1007/s00296-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama Y, Takahashi M, Nagafusa T, Torikai E, Nagano A. Methotrexate reduces the levels of pentosidine and 8- hydroxy-deoxy guanosine in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:398–402. doi: 10.1007/s10165-007-0607-6. [DOI] [PubMed] [Google Scholar]
- 32.Kogan G, Staško A, Bauerova K, Polovka M, Šoltés L, Brezova V, Navarova J, Mihalova D. Antioxidant properties of yeast (1/3)-β-D-glucan studied by electron paramagnetic resonance spectroscopy and its activity in adjuvant arthritis. Carbohydr Polym. 2005;61:18–28. [Google Scholar]
- 33.Koner BC, Banrejee BD, Ray A. Efects of stress on gamma- glutamyl transpeptidase activity in lymphoid system of rats: modulation by drugs. Indian J Exp Biol. 1997;35:222–224. [PubMed] [Google Scholar]
- 34.Kunsch CH, Sikorski JA, Sundell CL. Oxidative stress and the use of antioxidants for the treatment of rheumatoid arthritis. Cur Med Chem Immunol Endocr Metab Agents. 2005;5:249–258. [Google Scholar]
- 35.Macickova T, Drabikova K, Nosal R, Bauerova K, Mihalova D, Harmatha J, Pecivova J. In vivo effect of pinosylvin and pterostilbene in the animal model of adjuvant arthritis. Neuroendocrinol Lett. 2010;31:91–95. [PubMed] [Google Scholar]
- 36.Mihalova D, Ponist S, Kucharska J, Komendova D, Bauerova K. Total antioxidant status-systemic marker of oxidative stress in adjuvant arthritis. Chemicke listy. 2007;101:225–226. [Google Scholar]
- 37.Nosal R, Jančinova V, Petríkova M, Ponint S, Bauerova K. Suppression of oxidative burst of neutrophils with methotrexate in rat adjuvant arthritis. Chemicke Listy. 2007;101:243–244. [Google Scholar]
- 38.Ponist S, Mihalova D, Jancinova V, Snirc V, Ondrejickova O, Mascia C, Poli G, Stancikova M, Nosal R, Bauerova K. Reduction of oxidative stress in adjuvant arthritis. Comparison of efficacy of two pyridoindoles: stobadine dipalmitate and SMe1.2HCl. Acta Biochim Pol. 2010;57:223–228. [PubMed] [Google Scholar]
- 39.Rambabu K, Ansari AA, Shaafe IA, Chelvam AP, Ziu MM. Gamma-glutamyl transpeptidas in synovial fluid, serum, and urine of patients with rheumatoid arthritis. Biochem Med Metab Biol. 1990;43:183–192. doi: 10.1016/0885-4505(90)90024-u. [DOI] [PubMed] [Google Scholar]
- 40.Ropes MW, Bennett GA, Cobb S, Jacox R, Jessar RA. Revision of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1958;2:16–203. [PubMed] [Google Scholar]
- 41.Roupe KA, Remsberg CM, Yańez JA, Davies NM. Pharmacometric of Stilbenes: Seguing Towards the Clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 42.Sarban A, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem. 2005;38:981–986. doi: 10.1016/j.clinbiochem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Šmidrkal J, Harmatha J, Buděšínsky M, Vokač K, Zídek Z, Kmoníčkova E, Merkl R, Filip V. Modified approach for preparing (E)-stilbens related to resveratrol, and evaluation of their potential immunobiological effects. Collection of Czechoslovak Chemical Communications. 2010;75:175–186. [Google Scholar]
- 44.Sotnikova R, Ponist S, Navarova J, Mihalova D, Tomekova V, Strosova M, Bauerova K. Effects of sesame oil in the model of adjuvant arthritis. Neuroendocrinol Lett. 2009;30:22–24. [PubMed] [Google Scholar]
- 45.Storey GO, Comer M, Scott DL. Chronic arthritis before 1876: early British cases suggesting rheumatoid arthritis. Ann Rheum Dis. 1994;53:557–60. doi: 10.1136/ard.53.9.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strosova M, Tomaskova I, Ponist S, Bauerova K, Karlovska J, Spickett CM, Horakova L. Oxidative impairment of plasma and skeletal muscle sarcoplasmic reticulum in rats with adjuvant arthritis – effects of pyridoindole antioxidants. Neuroendocrinol Lett. 2008;29:706–711. [PubMed] [Google Scholar]
- 47.Strosova M, Karlovska J, Spickett CM, Orszaghova Z, Ponist S, Bauerova K, Mihalova D, Horakova L. Modulation of SERCA in the chronic phase of adjuvant arthritis as a possible adaptation mechanism of redox imbalance. Free Radic Res. 2009;43:852–864. doi: 10.1080/10715760903089708. [DOI] [PubMed] [Google Scholar]
- 48.Tastekin N, Aydogdu N, Dokmeci D, Usta U, Birtane M, Erbas H, Ture M. Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res. 2007;56:303–310. doi: 10.1016/j.phrs.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;2:169–77. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 50.Welles WL, Silkworth J, Oronsky AL, Kerwar SS, Galivan J. Studies on the effect of low dose methotrexate in adjuvant arthritis. J. Rheumatol. 1985;12:904–906. [PubMed] [Google Scholar]