Abstract
About 3% of pregnant women are treated with antidepressant drugs during gestation. After delivery the number of treated women increases to 5 to 7%. Most prescribed antidepressants in pregnancy are selective serotonin re-uptake inhibitors and/or serotonin and noradrenaline re-uptake inhibitors, such as fluoxetine, paroxetine, sertraline, citalopram and venlafaxine (VENF). Despite the fact that VENF has been assigned to pregnancy category C by the FDA, experimental studies with this drug are rare. The aim of this pilot study was to investigate the effect of prenatal administration of VENF on early postnatal development of rat offspring and selected biochemical variables at weaning of pups. Pregnant female Wistar rats were treated with VENF from day 15 to 20 of gestation at the doses of 7.5, 37.5 and 70 mg/kg. Females were allowed to spontaneously deliver their pups. After delivery the pups were inspected for viability, gross malformation and they were weighed on day 0, 4 and 21 post partum. On day 21 post partum, the pups were killed, brains were removed from the skulls and blood samples were collected for biochemical assay (proteins, glucose-GOD, glucose-HEX, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase and total antioxidant status). The study showed that prenatal VENF administration resulted in a mild maternal intoxication manifested by decreased body weight gain of pregnant females. There was no effect of the drug tested on the body and brain weights of offspring. No obvious morphological alterations were observed in the delivered pups. Similarly, there were no changes in the selected biochemical variables determined.
Keywords: selective serotonin re-uptake inhibitors and/or serotonin, noradrenaline re-uptake inhibitors, venlafaxine, pregnancy, fetus, pups, postnatal development, rat
Introduction
Affective disorder represents a serious health issue of modern society. Many women, reproductive age not excluded, suffer from major depression or bipolar disorder and in certain cases pregnancy and/or delivery may release serious affective disorders. The estimated prevalence of depression and other mood disorders in pregnancy ranges from 9 to 16% (Evans et al., 2001; Melville et al, 2010).
The major dilemma for gynecologists and obstetricians is to treat or not to treat depression in pregnancy. Consequences of untreated depression during pregnancy can be so serious that the benefit of antidepressant therapy may overweigh the possible risk for injury of fetal and/or neonatal development. Untreated depression represents a risk mostly for pregnant women. There is a higher risk of maternal morbidity, including arterial hypertension leading to preeclampsia or eclampsia, suicide attempts and post partum depression. Depression may be associated also with an increased risk of preterm or operative delivery, low birth weight, irritability, prematurity, sleep disorders, admission of the newborn to the neonatal intensive care unit and/or increased level of internalizing behaviors in childhood (Chung et al., 2004; Oberlander et al., 2010).
About 2 to 3% of pregnant women are treated with antidepressant drugs during gestation. After delivery the number of treated women increases to 5 to 7%. Most prescribed antidepressants in pregnancy and lactation are selective serotonin re-uptake inhibitors and/or serotonin and noradrenaline re-uptake inhibitors (SSRIs/SNRIs), such as fluoxetine, paroxetine, sertraline, citalopram and venlafaxine (Nonacs & Cohen, 2002; Tuccori et al., 2009; Freeman, 2011). SSRIs act mostly by inhibiting the re-uptake of serotonin after being released into synapses. In addition, several other mechanisms are suggested for the desired effect, e.g. neuroprotection and anti-inflammatory and immunomodulatory effects. SSRIs act on signal pathways such as cyclic AMP on the postsynaptic neuronal cell, which leads to the release of the brain derived neurotrophic factor (BDNF). BDNF, in turn, enhances the growth and survival of cortical neurons and synapses (Pilar-Cuéllar et al., 2012). SNRIs work by inhibiting the reuptake of both serotonin and noradrenaline. This results in an increase in the extracellular concentrations of serotonin and noradrenaline and therefore an increase in their neurotransmission (Papakostas et al., 2007). As the SSRI/SNRI drugs substantially cross the placenta (Rampono et al., 2009) and get into breast milk (Bellantuono et al., 2007), they can enter the developing brain and interfere with monoaminergic neurotransmission, which plays a key role in brain development. Therefore, this kind of drugs can represent a risk for the developing brain and postnatal neurobehavioral development. These drugs do not represent a risk factor for the developing embryo/fetus from the point of view of serious structural malformations. However, they can cause a transient decrease in uterine blood flow followed by hypoxia/ischemia and changes in fetal breathing, brain electrical activity and/or hypothalamus-pituitary-adrenal axis function. The SSRI drugs used in the 3rd trimester were found to increase the risk for neonatal behavioral syndrome, pulmonary hypertension, abortions and delayed neuromotor and behavioral development of children (Moses-Kolko et al., 2005Moses-Kolko et al., 2010; Gentile, 2005). Rare experimental studies showed the effect of fluoxetine on functional development of the frontal lobe of the cortex and the middle brain as well as on neurobehavioral development of offspring (Morrison et al., 2005). The SSRI/SNRI drugs can affect functional development of serotonergic and noradrenergic neurotransmitter systems. In turn, they can affect the development of the neuronal circuit with consequent manifestation of maladaptive behavior, which can persist until maturation, adulthood and/or even senescence as depression, anxiety, autism, and/or aggressive behavior.
Venlafaxine (VENF) belongs to the group of SNRI antidepressant drugs. It affects both serotonin and noradrenaline or even dopamine neurotransmission (Weikop et al., 2007). The mechanism of the antidepressant action of VENF in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Preclinical studies have shown that VENF and its active metabolite O-desmethylvenlafaxine are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake (Wellington and Perry, 2001). VENF has been assigned to pregnancy category C by the FDA. The FDA has classified VENF as category C with regard to pregnancy risk. This means that there have not been well-controlled studies in humans examining safety or that animal studies have demonstrated adverse effects to the developing fetus (Patil et al., 2011). Increased monoamine levels in the developing brain due to prenatal and perinatal VENF may interfere with functional maturation of the brain and increase the risk for neurobehavioral and mental disorders.
The aim of this pilot study was to investigate the effect of prenatal administration of VENF on early postnatal development of rat offspring and selected biochemical variables at weaning of pups.
Material and Methods
Animals
Female Wistar rats (initial weight 150–190 g, age 3–4 months, n=60) obtained from the breeding station of the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Dobra Voda, Slovak Republic (reg. No. SK CH 24 011), were used in the study. After 14 days of acclimatization, the females were mated with males in the ratio 1 male : 3 females. The presence of spermatozoa in vaginal smears was considered day 0 of gestation. Pregnant rats, 3 per cage, were housed in plastic cages with wooden shavings for bedding. On day 15 of gestation the females were separated and housed individually. The animals had free access to food pellets and water and were kept in a temperature and humidity controlled room (20–24 °C, relative humidity 50–70%) with 12/12 hrs light/dark cycle. The experiments were conducted in compliance with the Principles of Laboratory Animal Care issued by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Bratislava, and the experimental design was approved by the State Veterinary and Food Administration of the Slovak Republic.
Venlafaxine treatment
Venlafaxine hydrochloride (VENF) is a antidepressant for oral administration. It is designed as (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[a-[(dimethyl-amino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the empirical formula of C17H27NO2 HCl. Its molecular weight is 313.87 (Chemoz, Czech Republic, purity, 98.5%). VENF dissolved in distilled water was orally administered to pregnant rats from day 15 to 20 of gestation at doses of 7.5, 37.5 and 75 mg/kg in the application volume 0.5 ml/100 g of body weight (n = 4-9 pregnant females/group). The animals from the control group received distilled water as vehiculum.
Postnatal inspection of offspring
Pregnant females were allowed to spontaneously deliver their pups. The delivered pups were inspected for viability and possible gross malformations, number and individual weights of the pups were recorded to protocol. On day 4 post partum (PP), each litter was subjected to “culling” procedure, i.e. the number of pups in each litter was reduced to 8, 4 males and 4 females per litter, and the individual weights of the culled pups were recorded. On day 21 PP, the animals were weighed and sacrificed by decapitation, blood samples were collected for biochemical assay, the brains were removed from the skulls, weighed and frozen for further investigation.
Biochemical study
A part of the experimental animals of both sexes from the highest dose group and the control group were used in this study. The overal physiological status of the experimental animals was assessed by the following biochemical variables: blood levels of glucose (GLU), lactate dehydrogenase (LDH), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by commercially available diagnostic kits (DiaSys Diagnostic Systems, Germany). Total antioxidant status (TAS) was also assessed (Randox Life Sciences, Great Britain). All the blood samples were processed on the BioLis 24i chemistry analyzer (Tokyo Boeki Machinery Ltd., Tokyo, Japan) according to the manufacturer′s instructions.
Statistics
Raw data were analyzed by means of relevant statistical analysis (one-way ANOVA, factorial ANOVA, repeated measures ANOVA and non-parametric t-test). Differences were considered statistically significant when p≤0.05. The values are expressed as means ± S.E.M or S.D.
Results
Maternal toxicity
During the experiment, no pregnant female aborted or died. There was no effect of the treatment on the duration of pregnancy. General health status of the pregnant rats was good. The repeated measures ANOVA demonstrated asignificant effect of time on the body weight of pregnant rats with a significant increase of weight from days 15 to 20 of gestation, [F(5,160)=150.88; p<0.0001]. There was no significant effect of VENF treatment on the body weight of females [F(3,32)=0.04191; p=0.988]. Mean body weight of pregnant females treated with the highest dose of VENF on days 19 and 20 were somewhat lower compared to controls, however the differences failed to be statistically significant (Figure 1). Figure 2 shows the body weight gains from day 15 to 20 of gestation. Mean values in this variable were lower in the pregnant females treated with the middle and the highest doses of VENF compared to controls (approximately 20%), however, the differences failed to be statistically significant.
Figure 1.
Effect of prenatal VENF on body weight of pregnant females. G – gestation, V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg;
p<0.01 – statistically significant difference, repeated measures ANOVA (effect of time).
Figure 2.
Effect of prenatal VENF on body weight gains of pregnant females. G – gestation, V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Offspring
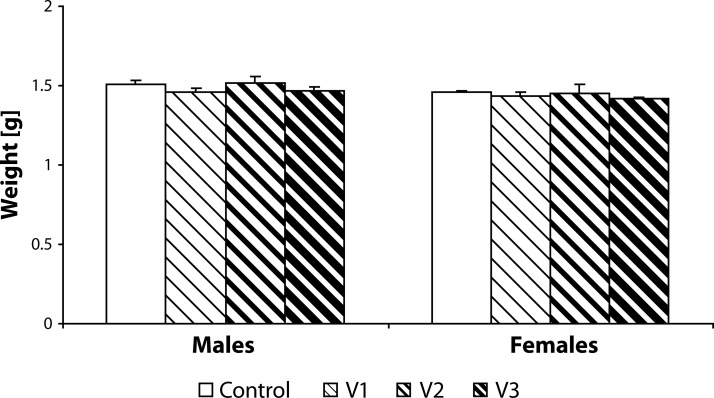
Statistical analysis did not demonstrate any statistically significant effect of VENF treatment on the body weight of pups measured on days 0, 4 and 21 PP (Figures 3–5). Not even, on days 4 and 21 PP when male and female pups were weighed separately, there were no significant differences between males and females. In the groups with the middle and the highest doses, there was one cachectic pup/litter which later died. Concerning the number of pups/litter, in the litters from females treated with VENF, there was a decrease in the mean number of pups compared to controls. However, the differences were not statistically significant (Figure 6). In two mothers treated with the highest dose of VENF, cannibalism was observed. Two-way ANOVA revealed no significant effect of treatment on brain weight, yet there was a significant effect of gender with significantly higher mean values of brain weight in males compared to females; males: 1.48 ± 0.01, females: 1.45 ± 0.01 [F(1, 50)=4.175; p=0.046] (Figure 7).
Figure 3.
Effect of prenatal VENF on body weight of pups on day 0 post partum. V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Figure 5.
Effect of prenatal VENF on body weight of pups on day 21 post partum. V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Figure 6.
Effect of prenatal VENF on the number of pups on day 0 post partum. V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Figure 7.
Effect of prenatal VENF on brain weight of pups on day 21 post partum. V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Figure 4.
Effect of prenatal VENF on body weight of pups on day 4 post partum. V1 – VENF at dose 7.5 mg/kg, V2 – VENF at dose 37.5 mg/kg, V3 – VENF at dose 70 mg/kg.
Biochemical variables
None of the biochemical variables assessed showed any significant alteration in the group with the highest VENF dose compared to controls (Table 1).
Table 1.
Effect of prenatal VENF administration on selected biochemical variables.
| Control | VENF | |
|---|---|---|
| PROT | 45.47 ± 5.01 | 48.13 ± 3.73 |
| LDH | 28.22 ± 6.49 | 28.70 ± 10.44 |
| ALAT | 1.14 ± 0.21 | 1.11 ± 0.19 |
| ASAT | 5.29 ± 1.04 | 5.47 ± 1.97 |
| GLU-GOD | 9.11 ± 0.76 | 8.95 ± 0.60 |
| GLU-HEX | 8.78 ± 0.67 | 8.94 ± 0.52 |
| TAS | 3.02 ± 0.26 | 2.99 ± 0.87 |
PROT – proteins, GLU – glucose, LDH – lactate dehydrogenase, AST – aspartate aminotransferase, ALT – alanine aminotransferase, TAS – total antioxidant status.
Discussion
Our study showed that prenatal VENF administration resulted in a mild maternal intoxication manifested by decreased body weight gain of pregnant females. There was no effect of the drug tested on body and brain weights of offspring. No obvious morphological alterations were observed in the delivered pups. Similarly, there were no changes in the selected biochemical variables determined.
A variety of behavioral and neurological symptoms including irritabilty, persistent crying, shivering, tremor, restlessness, feeding difficulties, sleep disorders, and seizures, have been reported in infants born to women who used SSRIs during pregnancy (Moses-Kolko et al., 2005). This clinical picture has been interpreted as a neonatal withdrawal syndrome (or direct toxic effect of antidepressants on the newborn) and desiganted neonatal behavioral syndrome. This syndrome includes poor tone, resipratory distress and hypoglycemia (Favrelière et al., 2010). Of the unwanted effects of SSRIs in pregnancy, Gentile (2005) reported subtle effects on motor development and motor control.
As neonatal behavioral syndrome after SSRIs withdrawal was found to be associated with hypoglycemia, we decided to investigate the basic biochemical profile of the rat offspring exposed prenatally to VENF. We did not find any marked effect of VENF on these variables. Abnormal GLU level might indicate metabolic disturbances, however in our experiments it oscillated within the physiological range. Neither was LDH, often used as a marker of tissue breakdown, changed. AST/ALT ratio is generally used in differentiating between causes of liver damage insinuating possible hepatotoxicity (Sumner et al., 2010). No such impairment was observed. Finally, the results showed that the mean serum TAS did not differ significantly compared to controls.
Prenatal administration of VENF caused non-significant decrease in the body weight gain of pregnant females treated with the middle and highest doses of VENF. Nevertheless, from the toxicological point of view we consider these decreases to be relevant. The OECD Guideline No. 426 on Developmental Neurotoxicity Study recommend to choose the highest dose level with the aim to induce some signs of maternal toxicity, such as decreased body weight gain, yet not more than 10% (OECD, 1995). However, the relatively lower number of delivered pups in VENF exposed animals might be a reason of decreased body weight gains. It can not be excluded either that the decreased body weight gain in the mothers treated with the highest dose of VENF was also associated with a higher level of distress, manifested by individual cases of cannibalism (DeSantis and Schmaltz, 1984).
Our results related to the weight of pups are in contrats to the developmenatal study with both fluoxetine and venlafaxine (da-Silva et al., 1999). They reported reduced body weight of litters, however by the time of weaning, the weight of litters from treated dams was equal to the weight of control litters. The different senzitivity of the rat strain used in this study may offer a tentative explanation of the weight divergencies.
Although, VENF has been assigned to pregnancy category C by the FDA, experimental studies with VENF are rare. Early exposure (pre-, peri- and/or neonatal periods) to VENF or other SSRIs/SNRIs may disrupt the normal maturation of the monoaminergic system and neuronal processes dependent on it. Functional alterations of brain development, in turn, can lead to various neurobehavioral dysfunctions manifested in later postnatal life (Dubovický et al., 2010). The SSRIs/SNRIs are the most common antidepressant drugs used in treatment of mood disorders in pregnancy and the postpartum period. The serotonergic system plays an important role in brain development. Thus, abnormal stimulation of serotonin receptors, as a result of increased synaptic availability of serotonin, and disruption of serotonin transporters activity during brain development due to SSRIs/SNRIs can lead to functional alterations followed by postnatal neurobehavioral dysfunctions. Delayed and/or late neurobehavioral consequences of action of prenatal and perinatal insults, including antidepressants, occur mostly in late postnatal development, in adolescence, adulthood or even senescence. Some of these dysfunctions can be hidden or masked. They can be stress-related, manifesting in response to stressful stimuli (Dubovický et al., 2008).
Effects of preventing re-uptake of presynaptic serotonin on fetal and postnatal development have not been sufficiently elucidated. Moreover, prospective epidemiological studies focused on older children, adolescents and adults exposed in utero to antidepressants are not available. Understanding of the role of monoamines in brain development is also important to identify the possible adverse effects of SSRIs/SNRIs exposure during pregnancy and lactation. Insight into the potential functional and behavioral consequences of the alterations induced by these drugs remains to be provided. Thus, scientists should focus on late consequences of prenatal and perinatal exposures with emphasis on various patterns of behavior, including emotions, aggression, sociability, coping and sexual functions.
In conclusion, the present study showed that VENF at the doses 37.5 and 70 mg/kg provoked acertain kind of maternal toxicity manifested by decreased body weight. Prenatal administration of VENF did not affect early postntal development of the rat offspring. However, possible effects of the drug tested on functional brain development in relation to postnatal neurobehavioral development should be followed up. This will be the aim of our further research.
Acknowledgement
The study was supported by The Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic for the Structural Funds of EU, OP R&D of ERDF by realization of the project „Transfer of Knowledge and Technologies from Research and Development in Toxicology on Evaluation of Environmental and Health risk“ (ITMS 26240220005) and VEGA 2/0084/11.
REFERENCES
- 1.Bellantuono C, Migliarese G, Maggioni F, Imperadore G. Anridepressant drugs and breastfeeding. Recent Prog Med. 2007;98:29–42. [PubMed] [Google Scholar]
- 2.Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med. 2001;63:830–834. doi: 10.1097/00006842-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Da-Silva VA, Alterburg SP, Malheiros LR, Thomaz TG, Lindsey CJ. Postnatal development of rats exposed to fluoxetine or venlafaxine during the third week of pregnancy. Braz J Med Biol Res. 1999;32:93–98. doi: 10.1590/s0100-879x1999000100014. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis DT, Schmaltz LW. The mother-litter relationship in developmental rat studies: cannibalism vs caring. Dev Psychobiol. 1984;17:255–62. doi: 10.1002/dev.420170306. [DOI] [PubMed] [Google Scholar]
- 5.Dubovický M, Kovačovský P, Ujházy E, Navarová J, Brucknerová I, Mach M. Evaluation of developmental neurotoxicity: some important issues focused on neurobehavioral development. Interdiscip Toxicol. 2008;1:206–210. doi: 10.2478/v10102-010-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubovický M. Neurobehavioral manifestations of developmental impairment of the brain. Interdiscip Toxicol. 2010;3:59–67. doi: 10.2478/v10102-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323:257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favreliere S, Nourrisson A, Jaafari N, Pérault Pochat MC. Treatment of depressed pregnant women by selective serotonin reuptake inhibitors: risk for the fœtus and the newborn. Encephale Suppl. 2010;2:D133–D138. doi: 10.1016/j.encep.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Freeman MP. Antidepressant medication treatment during pregnancy: prevalence of use, clinical implications, and alternatives. J Clin Psychiatry. 2011;72:977–978. doi: 10.4088/JCP.11f07206. [DOI] [PubMed] [Google Scholar]
- 10.Gentile S. SSRIs in pregnancy and lactation: emphasis on neurobehavioral outcome. CNS Drugs. 2005;19:623–633. doi: 10.2165/00023210-200519070-00004. [DOI] [PubMed] [Google Scholar]
- 11.Melville JL, Gavin A, Gup Y, Fan MY, Katon WJ. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116:1064–1070. doi: 10.1097/AOG.0b013e3181f60b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison JL, Riggs KW, Rurak DW. Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev. 2005;17:641–650. doi: 10.1071/rd05030. [DOI] [PubMed] [Google Scholar]
- 13.Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 14.Nonacs R, Cohen LS. Depression during pregnancy: diagnosis and treatment options. J Clin Psychiatry. 2002;63:24–30. [PubMed] [Google Scholar]
- 15.Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effect of selective serotonin reuptake inhibitor antidepressants, serotonin, transporter promoter genotype (SLC6A4) and maternal mood on child behavior at 3 years of age. Arch Padiatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- 16.OECD. 1995. Guideline for the testing of chemicals, Draft proposal for a new guideline 426. Developmental Neurotoxicity Study. [Google Scholar]
- 17.Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depression disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Patil AS, Kuller JA, Rhee EH. Antidepressants in pregnancy: a review of commonly prescribed medications. Obstet Gynecol Surv. 2011;66:777–8. doi: 10.1097/OGX.0b013e31823e0cbf. [DOI] [PubMed] [Google Scholar]
- 19.Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and the 5-HT(2A) antagonist ketanserin upregulates hippocampal BDNF and β-catenin in parallel with antidepressant-like effect. Br J Pharmacol. 2012;165(4b):1046–57. doi: 10.1111/j.1476-5381.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Froman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- 21.Sumner SC, Fennell TR, Snyder RW, Taylor GF, Lewin AH. Distribution of carbon-14 labeled C60 ([14C]C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J Appl Toxicol. 2010;30:354–60. doi: 10.1002/jat.1503. [DOI] [PubMed] [Google Scholar]
- 22.Tuccori M, Testi A, Antonioli L, Fomai M, Montagnani S, Ghisu N, Colucci R, Corona T, Blandizzi C, Del Tacca M. Safety concerns associated with the use of serotonin reuptake inhibitors and other serotonergic/noradrenergic antidepressants during pregnancy: a review. Clin Ther. 2009;31:1426–1453. doi: 10.1016/j.clinthera.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Weikop P, Kehr J, Scheel-Krüger J. Reciprocal effects of combined administration of serotonin, noradrenaline and dopamine reuptake inhibitors on serotonin and dopamine levels in the rat prefrontal cortex: the role of 5-HT1A receptors. J. Psychopharmacol. 2007;21:795–804. doi: 10.1177/0269881107077347. [DOI] [PubMed] [Google Scholar]
- 24.Wellington K, Perry C. Venlafaxine extended-release: a review of its use in the management of major depression. CNS Drugs. 2001;15:643–69. doi: 10.2165/00023210-200115080-00007. [DOI] [PubMed] [Google Scholar]