Abstract
A solid-phase three-component Huisgen reaction has been used to generate polar farnesol and farnesyl diphosphate analogues. The Cu(I)-catalyzed 1,3-cycloadditions of various azides with solid supported (E)-3-methylhept-2-en-6-yn-1-ol provided only the 1,4-disubstituted 1,2,3-triazole regioisomers. The organic azides were generated in situ to minimize handling of potentially explosive azides. We have employed this powerful ‘click chemistry’ to make farnesol analogues where both β- and γ-isoprenes were replaced by triazole and substituted aromatic rings, respectively.
Keywords: farnesol, farnesyldiphosphate, cancer, cycloaddition, triazoles, solid-phase synthesis, click chemistry
Farnesyl diphosphate (1, FPP) analogues have been used to study the mechanism of protein farnesyltransferase (FTase) and to examine the effect of analogue structure on prenylated protein function.1–6 FTase catalyzes transfer of the isoprenoid to the cysteine residue of proteins with C-terminal CAAX motifs, including the oncoprotein Ras.7,8 Activating mutations in Ras are found in 30% of all human cancers, and Ras prenylation is obligatory for Ras function.9–11 Ras function can be inhibited by FTase-catalyzed transfer of FPP analogues with reduced hydrophobicity, such as 2a, b and 3.12 The FTase-catalyzed transfer of FPP analogues depends on both analogue structure and CAAX sequence.13 However, there is no correlation between FPP analogue hydrophobicity and FTase-catalyzed isoprenoid transfer to protein. We have previously shown that an aniline or phenoxy group is isosteric with the terminal isoprene of FPP and that analogues with a range of substituents on the aryl group are FTase-transferable substrates.14 These observations open the door to FTase-transferable FPP analogues that act as prenyl-function inhibitors (PFI) by interfering with a prenylated protein's function.12
We have previously used solid-phase organic synthesis (SPOS) to generate a library of FTase-transferrable FPP analogues where the γ-isoprene was replaced by substituted aryl groups14,15 (Figure 1). SPOS is particularly useful for many synthetic transformations because excess reagents can be used to drive reactions to completion and facilitates the removal of leftover reagents and soluble byproducts.16 Cu(I)-catalyzed 1,3-cycloaddition of azides and alkynes to form 1,4-triazoles is a robust way of preparing small-molecule combinatorial libraries by SPOS.17–20 SPOS has been employed to prepare libraries of 1,4-triazoles where either the azide or alkyne is attached to the solid support.21–30 Utilizing alkyne-functionalized solid supports prevents formation of Cu(I)-catalyzed alkyne homocoupling side products. Recently, Gibbs et al. reported a library of S-farnesyl-thiopropionic acid 1,4-triazoles prepared by solution-phase methods to study their inhibitory activity against isoprenylcysteine methyltransferase (ICMT).31 Herein, we report a concise SPOS of FPP analogues where the β-isoprene and γ-isoprene were replaced by 1,4-triazole and substituted aryl groups, respectively. The substitutions on the aryl rings were chosen to either increase or decrease the hydrophilicity of the analogues (Scheme 1, Table 2).
Figure 1.
Structure of FPP analogues
Scheme 1.
Synthesis of resin-linked alkyne 11
Table 2.
1,2,3-Triazole-Containing Farnesol Analogues
| Entry | Compound | Structure | Yield (%)a | Purity LC–MS (%)b |
|---|---|---|---|---|
| 1 | 4a |
|
53 | 96 |
| 2 | 4b |
|
55 | 89 |
| 3 | 4c |
|
43 | 96 |
| 4 | 4d |
|
36 | 88c |
| 5 | 4e |
|
26 | 94c |
| 6 | 4f |
|
57 | 90 |
| 7 | 4g |
|
41 | 94 |
| 8 | 4h |
|
32 | 98 |
| 9 | 4i |
|
28 | 98 |
| 10 | 4j |
|
38 | 94 |
| 11 | 4k |
|
47 | 98 |
Yield (%) for 4a–k in two steps from 11. Dehydrated side products account for the bulk of the balance except for 4d and 4e.
The percentage was calculated by integrating HPLC chromatogram peaks at 230 nm.
Unidentified oxidized compounds [M + 16] account for the balance.
Treatment of previously reported1 alcohol 6 with Ph3PBr2 and Hünig's base (DIPEA) gave the pure E-isomer of allylic bromide 7. Propargyl Grignard prepared with a catalytic amount of HgCl was coupled to bromide 7 to give alkyne 8 in 72% yield. Treatment of alkyne 8 with PPTS in MeOH furnished alcohol 9 in quantitative yield.32 Coupling alcohol 9 to DHP-resin 10 generated the alkynefunctionalized resin 11 with a traceless, acid-labile THP linker.15
Resin loading of 82% was determined by recovering alcohol 9 from resin 11 treated with PPTS in MeOH and DCE. The key reaction was the 1,4-disubstituted 1,2,3-triazole formation between the resin-linked alkyne and various benzyl azides to allow introduction of diversity in the terminal aryl group.18,33,34 The required benzyl azides were prepared in situ from commercially available bromides and NaN3 in DMF. CAUTION! Due to risk of explosion,35–38 it is crucially important not to use halogenated solvents at this stage and to ensure that all azide ion is removed by thorough washing of the resin before introducing halogenated solvents in any subsequent steps.
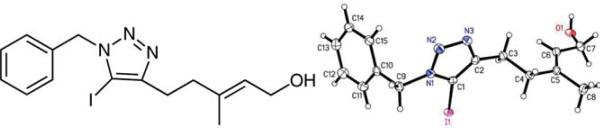
Agitating resin 11 with catalytic Cu(I)I and 10 equivalents each of BnBr, NaN3, and Hünig's base in DMF provided triazole resin 7a–k. A large excess of CuI (>5 equiv) leads to the formation of a side product where iodine is incorporated into the triazole ring 4l. The structure of (E)-5-(1-benzyl-5-iodo-1H-1,2,3-triazol-4-yl)-3-methylpent-2-en-1-ol (4l) was confirmed by X-ray crystallography (Figure 2).
Figure 2.
Structure and ellipsoid plot of compound 4l41
The in situ generation of Cu(I) from CuSO4 and ascorbic acid in the presence of water inhibited resin swelling and reduced the yield of triazole to almost nil. Triazole yield was also significantly reduced when other protic and nonprotic solvent mixtures drawn from the literature were used. Optimum triazole yields were obtained using Hünig's base and DMF as solvent (Table 1).
Table 1.
Conditions Attempted for Conversion of 7a into 8a Using CuI as Catalyst
| Entry | Solvent system | Yield (%)a |
|---|---|---|
| 1 | DMF–t-BuOH (3:1), DIPEA | 28 |
| 2 | DMF–t-BuOH–H2O (3:1:1), DIPEA | 0 |
| 3 | DMF–t-BuOH (1:1), DIPEA | 32 |
| 4 | DMF, DIPEA | 53 |
| 5 | DMF, TMED | 42 |
Yields were calculated after cleaving the resin (solvent MeOH–DCE = 1:1).
Heating the resin in MeOH–DCE (1:1) with catalytic PPTS over night provided analogues 4a–k in moderate yield (Scheme 2, Table 2). Prolonged heating of the cleavage reactions resulted in side products formed by 1,3-migration of the hydroxyl group. Exclusive formation of the 1,4-disubstituted 1,2,3-triazole regioisomer was confirmed by 1H-NOE experiments, as expected for the Cu(I)-catalyzed Huisgen reaction.39
Scheme 2.
Synthesis of Triazole-Containing Farnesols 4a–k and diphosphate 5
Triazole diphosphate 5 (19% yield in three steps) was prepared by Ph3PBr2 cleavage of THP resin 12a, followed by trapping the resulting allylic bromide with 10 equivalents of tris(tetra-n-butylammonium)hydrogen diphosphate. The crude diphosphate was purified by ion-exchange chromatography followed by RP-HPLC. All compounds in Table 1 were fully characterized by 1H NMR spectroscopy and low- and high-resolution mass spectrometry.40
In summary, we have developed a short synthetic route to triazole-containing farnesol analogues and their diphosphates through solid-phase ‘click chemistry’. The biochemical characterization of these compounds is ongoing and will be published elsewhere.
Supplementary Material
Acknowledgment
This work was supported in part by the National Institutes of Health GM66152 (to H.P.S.) and the Kentucky Lung Cancer Research Program (to H.P.S). The NMR instruments used in this work were obtained with support from NSF CRIF Grant CHE- 9974810. We acknowledge University of Kentucky Mass Spectroscopy Facility for mass data.
Footnotes
Supporting Information for this article is available online at http://www.thieme-connect.com/ejournals/toc/synlett. Included are the detailed experimental procedure and spectroscopic data of 4a–k and 5; 1H spectra and HRMS data sheet of 4a–k; and 1H, 31P, and LRMS spectra of 5.
References and Notes
- 1.Turek TC, Gaon I, Distefano MD, Strickland CL. J. Org. Chem. 2001;66:3253. doi: 10.1021/jo991130x. [DOI] [PubMed] [Google Scholar]
- 2.Kale TA, Hsieh SJ, Rose MW, Distefano MD. Curr. Top. Med. Chem. 2003;3:1043. doi: 10.2174/1568026033452087. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs BS, Zahn TJ, Mu Y, Sebolt-Leopold JS, Gibbs RA. J. Med. Chem. 1999;42:3800. doi: 10.1021/jm9902786. [DOI] [PubMed] [Google Scholar]
- 4.Chehade KA, Andres DA, Morimoto H, Spielmann HP. J. Org. Chem. 2000;65:3027. doi: 10.1021/jo991735t. [DOI] [PubMed] [Google Scholar]
- 5.Micali E, Chehade KA, Isaacs RJ, Andres DA, Spielmann HP. Biochemistry. 2001;40:12254. doi: 10.1021/bi011133f. [DOI] [PubMed] [Google Scholar]
- 6.Reigard SA, Zahn TJ, Haworth KB, Hicks KA, Fierke CA, Gibbs RA. Biochemistry. 2005;44:11214. doi: 10.1021/bi050725l. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FL, Casey PJ. Annu. Rev. Biochem. 1996;65:241. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 8.Clarke S. Annu. Rev. Biochem. 1992;61:355. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 9.Bos JL. Cancer Res. 1989;49:4682. [PubMed] [Google Scholar]
- 10.Kloog Y, Cox AD. Semin. Cancer Biol. 2004;14:253. doi: 10.1016/j.semcancer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Gschwind A, Fischer OM, Ullrich A. Nat. Rev. Cancer. 2004;4:361. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 12.Roberts MJ, Troutman JM, Chehade KA, Cha HC, Kao JP, Huang X, Zhan CG, Peterson YK, Subramanian T, Kamalakkannan S, Andres DA, Spielmann HP. Biochemistry. 2006;45:15862. doi: 10.1021/bi061704+. [DOI] [PubMed] [Google Scholar]
- 13.Troutman JM, Subramanian T, Andres DA, Spielmann HP. Biochemistry. 2007;46:11310. doi: 10.1021/bi700516m. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian T, Liu S, Troutman JM, Andres DA, Spielmann HP. ChemBioChem. 2008;9:2872. doi: 10.1002/cbic.200800248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian T, Wang Z, Troutman JM, Andres DA, Spielmann HP. Org. Lett. 2005;7:2109. doi: 10.1021/ol050386o. [DOI] [PubMed] [Google Scholar]
- 16.Blaney P, Grigg R, Sridharan V. Chem. Rev. 2002;102:2607. doi: 10.1021/cr0103827. [DOI] [PubMed] [Google Scholar]
- 17.Hein JE, Fokin VV. Chem. Soc. Rev. 2010;39:1302. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meldal M, Tornoe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 19.Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med. Res. Rev. 2008;28:278. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]
- 20.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. Engl. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Lober S, Rodriguez-Loaiza P, Gmeiner P. Org. Lett. 2003;5:1753. doi: 10.1021/ol034520l. [DOI] [PubMed] [Google Scholar]
- 22.Loaiza PR, Lober S, Hubner H, Gmeiner P. J. Comb. Chem. 2006;8:252. doi: 10.1021/cc050127q. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wang XF, Chen Y, Zhang LH, Yang ZJ. Med. Chem. Commun. 2012;3:506. [Google Scholar]
- 24.Oyelere AK, Chen PC, Yao LP, Boguslavsky N. J. Org. Chem. 2006;71:9791. doi: 10.1021/jo0618122. [DOI] [PubMed] [Google Scholar]
- 25.Papst S, Noisier A, Brimble MA, Yang Y, Krissansen GW. Bioorg. Med. Chem. 2012;20:2638. doi: 10.1016/j.bmc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Baccile JA, Morrell MA, Falotico RM, Milliken BT, Drew DL, Rossi FM. Tetrahedron Lett. 2012;53:1933. [Google Scholar]
- 27.Bouillon C, Meyer A, Vidal S, Jochum A, Chevolot Y, Cloarec JP, Praly JP, Vasseur JJ, Morvan F. J. Org. Chem. 2006;71:4700. doi: 10.1021/jo060572n. [DOI] [PubMed] [Google Scholar]
- 28.Bock VD, Hiemstra H, van Maarseveen JH. Eur. J. Org. Chem. 2006:51. [Google Scholar]
- 29.Holub JM, Kirshenbaum K. Chem. Soc. Rev. 2010;39:1325. doi: 10.1039/b901977b. [DOI] [PubMed] [Google Scholar]
- 30.Isobe H, Fujino T, Yamazaki N, Guillot-Nieckowski M, Nakamura E. Org. Lett. 2008;10:3729. doi: 10.1021/ol801230k. [DOI] [PubMed] [Google Scholar]
- 31.Bergman JA, Hahne K, Song J, Hrycyna CA, Gibbs RA. ACS Med. Chem. Lett. 2012;3:15. doi: 10.1021/ml200106d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kogl M, Brecker L, Warrass R, Mulzer J. Eur. J. Org. Chem. 2008:2714. [Google Scholar]
- 33.Presolski SI, Hong V, Cho SH, Finn MG. J. Am. Chem. Soc. 2010;132:14570. doi: 10.1021/ja105743g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohrt AE, Jensen JF, Nielsen TE. Org. Lett. 2010;12:5414. doi: 10.1021/ol102209p. [DOI] [PubMed] [Google Scholar]
- 35.Peet NP, Weintraub PM. Chem. Eng. News. 1993;71:4. (??) [Google Scholar]
- 36.Peet NP, Weintraub PM. Chem. Eng. News. 1994;72:4. (??) [Google Scholar]
- 37.Weisenburger GA, Vogt PF. Org. Process Res. Dev. 2006;10:1246. [Google Scholar]
- 38.Conrow RE, Dean WD. Org. Process Res. Dev. 2008;12:1285. [Google Scholar]
- 39.Lee BY, Park SR, Jeon HB, Kim KS. Tetrahedron Lett. 2006;47:5105. [Google Scholar]
- 40.Typical Procedure for Cycloaddition Adducts 12a–k Reaction vessels were charged with resin 11 (100 mg) and NaN3 (57.6 mg, 0.99 mmol, 10 equiv) followed by DMF (5 mL) and then CuI (3 mg) and agitate was initiated. Hünig's base (114 mg, 0.99 mmol, 10 equiv) was added, and, finally, the bromide (0.99 mmol, 10 equiv) was added. The resultant mixture was agitated for 1 d at r.t., heated to 50 °C for 3 h, then cooled. Then the resin was filtered and washed with DMF (2×), DMF–H2O (1:1, 2×), DMF (2×), CH2Cl2 (3×), Et2O (2×) and dried in vacuo.
- General Procedure for the Synthesis of Alcohols 4a–k Resin (100 mg) was heated at 80 °C with DCE–anhyd MeOH (1:1, 8 mL) in the presence of PPTS (5 mg) for 8 h. The liquid phase was collected, the resin was rinsed with CH2Cl2, and the washings were combined and sequentially washed with sat. aq NaHCO3, H2O, brine, dried over MgSO4, concentrated, and purified by silica gel column chromatography to give 4a–k.
- Compound 4a: 1H NMR (400 MHz, CDCl3): δ = 1.68 (s, 3 H), 2.35 (t, J = 8.4 Hz, 2 H), 2.81–2.85 (m, 2 H), 4.10 (d, J = 7.6 Hz, 2 H), 5.34–5.38 (m, 1 H), 5.48 (s, 2 H), 7.20 (s, 1 H), 7.21–7.27 (m, 2 H), 7.32–7.40 (m, 3 H) ppm. LRMS [M + H+]: m/z = 258.2. HRMS [M+]: m/z calcd for C15H19N3O: 257.1528; found: 257.1529.
- Compound 4b: 1H NMR (400 MHz, CDCl3): δ = 1.63 (s, 3 H), 2.31 (t, J = 8.0 Hz, 2 H), 2.81 (t, J = 8.0 Hz, 2 H), 4.10 (d, J = 6.8 Hz, 2 H), 5.33 (t, J = 6.8 Hz, 1 H), 5.58 (s, 2 H), 7.32–7.35 (m, 3 H), 8.14 (d, J = 10.8 Hz, 2 H) ppm. LRMS [M + H+]: m/z = 302.2. HRMS [M+]: m/z calcd for C15H18N4O3: 302.1379; found: 302.1375.
- Compound 4c: 1H NMR (400 MHz, CDCl3): δ = 1.29 (s, 3 H), 1.66 (s, 3 H), 2.33 (t, J = 8.0 Hz, 2 H), 2.80 (t, J = 7.6 Hz, 2 H), 4.10 (d, J = 6.8 Hz, 2 H), 5.37 (t, J = 6.8 Hz, 1 H), 5.43 (s, 2 H), 7.16–7.20 (m, 3 H), 7.36 (d, J = 8.4 Hz, 2 H) ppm. LRMS [M + H+]: m/z = 314.2. HRMS [M+]: m/z calcd for C19H27N3O: 313.2154; found: 313.2154.
- Compound 4d: 1H NMR (400 MHz, CDCl3): δ = 1.23 (d, J = 6.8 Hz, 6 H), 1.69 (s, 3 H), 2.35 (t, J = 8.4 Hz, 2 H), 2.83 (t, J = 8.4 Hz, 2 H), 2.87–2.94 (m, 1 H), 4.10 (d, J = 6.8 Hz, 2 H), 5.30 (t, J = 8.0 Hz, 1 H), 5.44 (s, 2 H), 7.16–7.26 (m, 4 H) ppm. LRMS [M + H+]: m/z = 300.2. HRMS [M+]: m/z calcd for C18H25N3O: 299.1998; found: 299.2002.
- Compound 4e: 1H NMR (400 MHz, CDCl3): δ = 1.69 (s, 3 H), 2.35 (t, J = 8.0 Hz, 2 H), 2.83 (t, J = 8.0 Hz, 2 H), 4.11 (d, J = 6.8 Hz, 2 H), 5.38 (t, J = 8.0 Hz, 1 H), 5.45 (s, 2 H), 7.02–7.09 (m, 2 H), 7.21–7.26 (m, 3 H) ppm. LRMS [M + H +]: m/z = 276.2. HRMS [M+]: m/z calcd for C15H18FN3O: 275.1434; found: 275.1431.
- Compound 4f: 1H NMR (400 MHz, CDCl3): δ = 1.69 (s, 3 H), 2.36 (t, J = 8.0 Hz, 2 H), 2.84 (t, J = 8.0 Hz, 2 H), 4.11 (d, J = 6.8 Hz, 2 H), 5.38 (t, J = 8.0 Hz, 1 H), 5.48 (s, 3 H), 6.91 (d, J = 9.2 Hz, 1 H), 7.02–7.05 (m, 2 H), 7.30–7.35 (m, 1 H) ppm. LRMS [M + H+]: m/z = 276.2. HRMS [M+]: m/z calcd for C15H18FN3O: 275.1434; found: 275.1437.
- Compound 4g: 1H NMR (400 MHz, CDCl3): δ = 1.69 (s, 3 H), 2.36 (t, J = 8.0 Hz, 2 H), 2.83 (t, J = 8.0 Hz, 2 H), 4.11 (d, J = 6.8 Hz, 2 H), 5.38 (t, J = 8.0 Hz, 1 H), 5.54 (s, 2 H), 7.08–7.37 (m, 4 H) ppm. LRMS [M + H+]: m/z = 276.2. HRMS [M+]: m/z calcd for C15H18FN3O: 275.1434; found: 275.1429.
- Compound 4h: 1H NMR (400 MHz, CDCl3): δ = 1.71 (s, 3 H), 2.38 (t, J = 8.0 Hz, 2 H), 2.86 (t, J = 8.0 Hz, 2 H), 4.12 (t, J = 6.8 Hz, 2 H), 5.39 (t, J = 8.0 Hz, 1 H), 5.52 (s, 2 H), 7.10–7.25 (m, 4 H), 7.40 (t, J = 8.0 Hz, 1 H) ppm. LRMS [M + H+]: m/z = 341.2. HRMS [M+]: m/z calcd for C16H18F3N3O2: 341.1350; found: 341.1359.
- Compound 4i: 1H NMR (400 MHz, CDCl3): δ = 1.67 (s, 3 H), 2.35 (t, J = 8.0 Hz, 2 H), 2.83 (t, J = 8.0 Hz, 2 H), 4.11 (d, J = 6.8 Hz, 2 H), 5.39 (t, J = 8.0 Hz, 1 H), 5.52 (s, 2 H), 7.18–7.27 (m, 4 H) ppm. LRMS [M + H+]: m/z = 341.2. HRMS [M+]: m/z calcd for C16H18F3N3O2: 341.1350; found: 341.1357.
- Compound 4j: 1H NMR (400 MHz, CDCl3) δ = 1.69 (s, 3H), 2.36 (t, J = 8.0Hz, 2H), 2.85 (t, J = 8.0Hz, 2H), 4.11 (d, J = 6.8 Hz, 2 H), 5.38 (t, J = 8.0 Hz, 1 H), 5.53 (s, 2 H), 7.29 (s, 1 H), 7.47–7.50 (m, 2 H), 7.61–7.63 (m, 1 H) ppm. LRMS [M + H+]: m/z = 283.2. HRMS [M+]: m/z calcd for C16H18N4O: 282.1480; found: 282.1482.
- Compound 4k: 1H NMR (400 MHz, CDCl3): δ = 1.70 (s, 3 H), 2.38 (t, J = 8.0 Hz, 2 H), 2.87 (t, J = 8.0 Hz, 2 H), 4.12 (d, J = 6.8 Hz, 2 H), 5.38 (t, J = 8.0 Hz, 1 H), 5.56 (s, 2 H), 5.56 (s, 2 H), 7.26 (s, 1 H), 7.31 (d, J = 12.0 Hz, 2 H), 7.65 (d, J = 12.0 Hz, 2 H) ppm. LRMS [M + H+]: m/z = 283.2. HRMS [M+]: m/z calcd for C16H18N4O: 282.1480; found: 282.1487.
- Procedure for the Synthesis of (E)-5-(1-Benzyl-1H-1,2,3-triazol-4-yl)-3-methylpent-2-en-1-yl Diphosphate (5) The vacuum-dried resin 12a (100 mg, 0.09 mmol) was suspended in CH2Cl2 (2 mL), Ph3PBr2 (84 mg, 0.19 mmol) was added, and the slurry was stirred at r.t. under N2 for 4 h. Tris[tetra(n-butyl)ammonium]hydrogen diphosphate (388 mg, 0.39 mmol) in anhyd MeCN (3 mL) was added and the reaction mixture was stirred at r.t. for 4 h under N2. The heterogeneous filtrate was concentrated, and the residue was suspended in 25 mM NH4HCO3 (4 mL) and extracted with Et2O (5 mL, 3×). The aqueous phase was applied to an NH4+ form DOWEX-AW-50 ion-exchange column (50 mL of resin) and eluted with four-column volume 25 mM NH4HCO3 buffer. The aqueous phase was lyophilized to obtain the crude diphosphate 5 as a white solid, which was dissolved in a minimum volume of 25 mM NH5CO3 buffer and purified by RP-HPLC (Varian Dynamax, 10 μm, 300 Å, C-4 (10 mm × 250 mm) column with a gradient mobile phase: 25 mM NH5AcO and MeCN.15 The product collected between 5.2–5.6 min was lyophilized to obtain compound 5 (9.0 mg, 19% in 2 steps). 1H NMR (400 MHz, D2O): δ = 1.44 (s, 3 H), 2.10 (t, J = 7.6 Hz, 2 H), 2.57 (t, J = 7.6 Hz, 2 H), 4.15 (t, J = 6.8 Hz, 2 H), 5.11 (t, 6.4 Hz, 1 H), 5.28 (s, 2 H), 7.03–7.05 (m, 1 H), 7.14–7.20 (m, 2 H), 7.50 (s, 1 H) ppm. 31P NMR (D2O, 161.8 MHz): δ = –6.80 (d, J = 21.36 Hz, 1 P), –10.40 (d, J = 21.36 Hz, 1 P). LRMS [M + H+]: m/z = 418.0.
- 41.X-ray crystal data of the structure 4l have been deposited at the Cambridge Crystallographic Data Center with the deposition number CCDC 871186.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.