Abstract
Decreased physical activity and marketing-driven increased consumption of “junk” food, dubbed “The Big Two”, are generally regarded as the most important contributors to the obesity epidemic. However, the full picture contains many more pieces of the puzzle. We address several additional issues and review current clinical developments in obesity research. In spite of dramatic advancements in our understanding of the adipose organ and its endocrine and immune products, the ultimate causes of the obesity epidemic remain elusive. Treatment is plagued by poor adherence to life style modifications, and available pharmacological options are marginally effective, often also associated with major side effects. Surgical treatments, albeit effective in decreasing body weight, are invasive and expensive. Thus, our approaches to finding the causes, improving the existing treatments, and inventing novel therapies must be manifold.
Keywords: Cortisol, endocrinology, environment, individualized medicine, leptin, prevention, stress
INTRODUCTION
The prevalence of obesity has increased over the last 100 years, with an accelerated rate since the 1970s, as documented by the comparison of the data from the National Health and Nutrition Examination Survey (NHANES) II in 1976, and the NHANES III in 1994 (1). In addition to the US, this is observed in Europe, and in virtually every country for which data are available (2); hence, the term obesity pandemic. Marketing-driven increases in food consumption and decreases in physical activity are typically cited as prominent causes and have thus become “The Big Two” (3, 4). However, solid evidence of causality between these factors and the pandemic still lacks. In this review (Table 1) we will focus on the following questions: Why do we eat more? Why do we expend less energy? Is it something in or around: us? Are we missing something? What are the current treatments for obesity?
Table 1.
Novel and putative factors contributing to the obesity epidemic.
| Factor | Potential mechanisms | Effects on energy balance |
|---|---|---|
| “Comfort Food” | Counterbalance chronic stress | ⇑ energy intake |
| Non-exercise-induced thermogenesis (NEAT) | Genetic | ⇓ energy expenditure |
| Microbiome | Reduced bacterial diversity in obesity | ⇓energy intake due to |
| ⇓ absorption of nutrients form the gut | ||
| Human Adenovirus 36 | Unclear: possible adipogenic effects | unclear |
| Indoor heating and air conditioning | Changes in appetite | ⇑ energy intake and |
| ⇓ energy expenditure | ||
| Epigenetic factors: | Imprinting the fetus towards a thrifty phenotype | ⇑ energy intake and |
| Alterations in the intrauterine milieu | ⇓ energy expenditure | |
| Medications: antipsychotics, (β-blockers, antidepressants, others | Variable | ⇑ energy intake and |
| ⇓ energy expenditure | ||
| Endocrine disruptors | Adipogenic effects | ⇑ energy intake and |
| ⇓ energy expenditure | ||
| Decline in smoking | Behavioral effect | ⇑ energy intake and |
| ⇓ energy expenditure | ||
| Chronic sleep deprivation | Multiple mechanisms: mostly leptin- and ghrelin-induced changes in appetite | ⇑ energy intake and |
| ? energy expenditure | ||
| Lack of seasonality | Multiple behavioral mechanisms | ⇑ energy intake and |
| ? energy expenditure |
In order to assess obesity, we need to define it. Body mass index (BMI) (weight in kilogram divided by squared height in centimeters), is typically used in epidemiological studies. Its intrinsic limitation is the lack of specific adjustments for body composition. A normal BMI ranges from 18.5 to 24.9 kg/m2, overweight from 25 to 29.9 kg/m2; and obesity is defined as a BMI≥30 kg/m2 (5). Another important anthropomorphic measure is waist circumference. It constitutes one of 5 components of the metabolic syndrome and is a good indicator of abdominal fat. In Caucasian, African American, and Hispanic individuals, generally accepted cut-offs are 40 inches (102 cm) in men and 35 inches (88 cm) in women. Another measure, neck circumference, is mostly used in Sleep Medicine and tracks well with sleep apnea (6). Data from the Framingham Heart study indicate that neck circumference is independently associated with cardiovascular risk factors, more so in women than in men (7).
In characterizing clinically obese individuals it is important to obtain a detailed weight history. While losing weight is difficult, defending the weight achieved after dieting is even more difficult. Many subjects regain the lost weight and even more within 12 to 18 months. The endocrinology of the post-obese state is characterized by decreased energy expenditure, mostly due to decreased thyroid and sympathetic tone, as well as decreased leptin (8). The decrease in energy expenditure is substantial, about 300–500 kcal/day. In a small clinical study, twice-daily leptin administration for 5 weeks in post-obese subjects who had lost 10% of their body weight restored circulating leptin levels to pre-weight-loss levels (8). Consequently, energy expenditure, sympathetic tone, and thyroid hormone levels returned to normal. Based on the available evidence, these authors suggested that adaptations to weight swings are orchestrated by the hypothalamus in a non-symmetrical fashion: a decline in leptin, as observed after weight loss, sets into motion a complex set of counter-regulatory responses that eventually defeat further weight loss. In contrast, an increase in leptin elicits few, if any, responses and fat continues to accumulate.
Next to the weight history, ethnic and age-related differences should be considered. Associations of BMI, waist circumference and fasting triglycerides with clinical outcomes such as diabetes and cardiovascular disease differ between ethnic groups (9); e.g. the BMI risk threshold may be higher in Blacks than in Whites, and higher in women than in men. Overweight black men may have a greater risk of developing diabetes than overweight black women. Other ethnic groups like Asians also have different risk thresholds (10). South Asians develop metabolic alterations at a lower BMI than other ethnic groups (11), but risk of death appears similar to European BMI norms (12). Nevertheless, consensus does not exist in how cutoff levels for overweight and obesity should be established in general, and therefore also does not exist for how, if at all, differential cut-offs should be set for different age, race, and sex groups. Ethnic specific formulations of the lipid criteria have been advocated in the definition of metabolic syndrome, based on well-conducted studies of lipid metabolism in African-Americans (13). Furthermore, aging is typically associated with sarcopenia, especially in the quadriceps, which in turn is associated with insulin resistance (14). Thus, knowledge of body composition are crucial to prevent loss of muscle mass or accumulation of abdominal fat.
RELEVANT QUESTIONS
Why do we eat more?
Food and stress are powerful modulators of the body-mind connection which is out of balance in obese individuals (15). Why do we choose chocolate over an apple when over-worked and stressed, and why does comfort food make us feel better? What is “comfort food” (Fig. 1) and why do we seek it? Could feeding to attain pleasure and/or to combat stress underlie this epidemic?
Fig. 1.
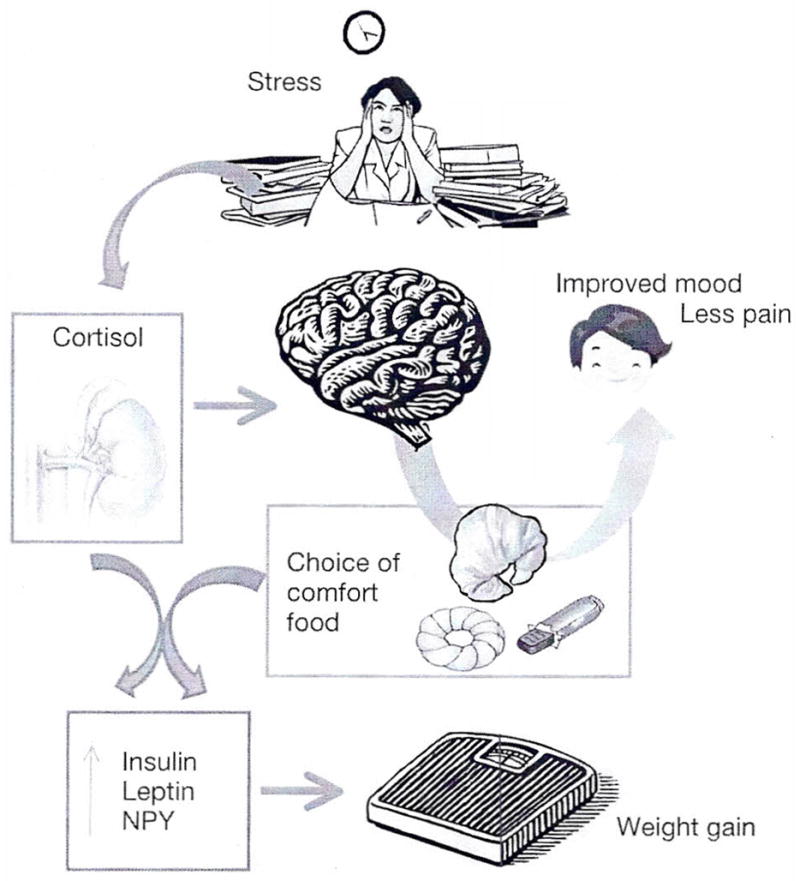
Proposed “Comfort Food Hypothesis” linking stress, e.g. induced by psychosocial subordination, to weight gain. With the exception of preferential high caloric food choices associated with stress, few experimental data exist in humans to explain the underlying neuroendocrine mechanisms.
The central control of food intake is influenced and altered by many factors, including chronic stress (16). The stress system has two main components, the hypothalamic-pituitary-adrenal axis, and the sympatho-adrenal system (17). These components have integrated metabolic effects and determine food intake and energy compart-mentalization (18). For example, high glucocorticoid levels increase the intake of palatable food, rich in fat and sugar (19). Fat and sugar act on the brain activating pleasurable responses. In return, these nutrients are able to modulate the intensity of the stress response. When rats are offered highly palatable food prior to immobilization stress, they exhibit a blunted hormonal stress response characterized by lower CRH in the paraventricular nucleus of the hypothalamus, and lower plasma levels of ACTH and Cortisol (18).
Another element in the stress-appetite link is neuropeptide Y (NPY), a 36 amino-acid orexogenic peptide present in several brain areas. During stress, NPY is secreted by the sympathetic nerve terminals together with catecholamines. Released from nerve terminals located in the adipose tissue, NPY promotes angiogenesis and adipogenesis resulting in abdominal obesity (20). Energy balance is regulated by homeostatic mechanisms mediated mostly at the hypothalamic level. However, there is increasing appreciation that “non-homeostatic” mechanisms may be also important.
The mesolimbic system is part of the reward circuits. Leptin receptors are present in the lateral hypothalamus where leptin inhibits the activity of the appetite stimulant orexin. Leptin and insulin act on the ventral tegmental area to stimulate excessive consumption of palatable food. In obese subjects insulin decreases the “liking” of palatable food, mostly via opioid-driven mechanisms (21). Leptin affects the “wanting” of food, via dopaminergic tone. Increasing leptin and insulin may progressively negate the pleasure otherwise associated with food (22). This vicious cycle leads to more food intake with less and less reward. Excessive food intake is regarded by some as an addiction, similar to smoking, alcoholism, or drug abuse (23). Thus, chronic stress may contribute to overeating to a degree comparable with substance abuse in some individuals.
Why do we move less?
Do we become obese because we move less, or do we move less because we are obese? In humans, energy expenditure is determined by several components. These are basal metabolic rate, diet-induced thermogenesis, physical activity and non-exercise activity thermogenesis (NEAT). The latter is defined by all activities of daily living minus voluntary exercise (24). NEAT is an important determinant of total energy expenditure, accounting for anywhere between 100 and 700 kcal/day. It is controlled by several neuropeptides including orexin A, CRH, NPY, cholecystokinin, agouti-related peptide, and leptin. Elevated signaling from the nervous circuits that control NEAT may protect from obesity. The following experiment shed light on how a large individual variability may relate to body weight: NEAT activity was measured by the use of tri-axial accelerometers for 10 days in 10 lean and 10 moderately obese sedentary men and women. At baseline, obese subjects spent more time (2½ h per day) seated and less time moving than did lean subjects. Once the obese study participants had lost an average of 8 kg under controlled conditions, they still moved less than the lean controls. Thus, physical activity is not a purely volitional phenomenon. It has a strong biological component, which is in part genetically determined (25). What has certainly changed over the last 3 decades is the environmental component to NEAT that may include factors such neighborhood safety, as well as suburban lifestyle. A new and very active field of research investigates the effects of design and features of the built environment such as recreational space, street connectivity, and the presence of sidewalks on obesity (26, 27).
Is it something in or around us?
The term pandemic implies that obesity may be transmissible (28). Several new lines of research support this hypothesis. The human gut harbors an ensemble of microbes, microbiome, that have the ability to extract energy from otherwise indigestible dietary components (29). A recent study documented the phylogenetic diversity for microbiota of 31 monozygotic twin female pairs, and 23 dizygotic female pairs (29). Obese twins had reduced bacterial diversity, and altered metabolic pathways. In these subjects, the microbiota were enriched for phosphotransferase enzymes involved in microbial processing of carbohydrates. An ongoing randomized clinical trial, FATLOSE, is testing whether a fecal transplant from healthy thin donors will improve obesity (30).
Further support for a different aspect of obesity comes from research on social networks. Obesity appears to spread through social ties (31). Clusters of obese persons were identified in a network that extended up to 3 degrees of separation. If one spouse became obese, the odds that the other spouse would become obese increased by 37%. Of note, a causal link should not necessarily be inferred by these observations. It may seem challenging to envision clinical studies on social ties in which never-obese subjects would be randomized to “associate” with obese individuals to see whether they develop obesity over time. Yet such studies are feasible (32, 33). We suggest “quasi-randomized” prospective studies in which never-obese subjects who move into a new social environment are followed while they associate “spontaneously” with lean or obese subjects. Similarly, complementary experiments could be designed to examine whether obese subjects lose weight when socially associating with lean people.
An infectious component to obesity has been suggested since obesity appears not only to be an independent risk factor for increased severity of certain viral illnesses (34), but the reverse also appears to be true. Human adenovirus 36 infection has been implicated in the development of human obesity and experimental obesity in laboratory animals (35).
Additional environmental aspects are the advent of indoor heating and air conditioning (36). Cold environments stimulate energy expenditure via activation of brown adipose tissue (37). Upon mild cold exposure obese subjects have been shown to increase insulation by changing temperature distribution, whereas lean subjects increased energy expenditure via changes in physical activity although intra-group differences were large (38). During the summer months, obese children have been shown to gain more weight than lean children, possibly because of a more sedentary indoor life style, adding less activity to reduced energy expenditure (39). It is a common observation that excessive heat and humidity inhibit appetite, explaining the fact that air conditioning may favor obesity. We hypothesize that low temperatures in restaurants and grocery stores not only prevent food from spoiling but also induce their customers’ appetite.
Are we missing something?
The list of novel putative contributors to the obesity epidemic is growing including the intrauterine milieu, medications and environmental effects such as endocrine disrupters, sleep deprivation, and disruptions of circadian rhythms and less obvious factors such as increased cognitive demand and mental work and eradication of helicobacter pylori (40–43). Support for the role of epigenetics comes from studies showing that children born to post-obese mothers who had undergone bariatric surgery, have a lower risk of obesity than their siblings born to the same mothers before they had lost weight (44). As women have entered the work force, mean maternal age has increased. Each 5-yr increment in maternal age increases the likelihood of obesity by 14%. The mechanisms are unclear: older mothers tend to have both under-weight and over-weight children. Under-weight children, exposed in utero to starvation because of decreased placental vascularization may imprint their metabolism towards the so-called “thrifty phenotype” (45). On the other hand, heavier infants are more likely to become obese adults (46). Furthermore, obese people tend to have children with obese people (47, 48) which may favor extreme obesity and approximately 65% of the variability in BMI is accounted for by genetic factors (49). Thus, there is growing evidence that the genetic pool is changing in ways that may favor obesity (50).
Intriguing data have been reported on endocrine disrupters: chemicals derived from plastic such as bisphenol A, pesticides, and other agents that are widespread and, unfortunately, very stable in the environment. These agents interact with estrogen receptors and may affect the amount of white adipose tissue (51, 52).
Common medications, such as anti-hypertensive (53) and hypoglycemic agents such as sulfonylureas, thiazolidinediones, and insulin, and even more so, antipsychotics such as olanzepine, are also associated with weight gain (54, 55). Decline in smoking habits and lower smoking initiation rates have played a small, albeit not trivial, role (56). Based on data from NHANES II vs NHANES III, smoking cessation has contributed a 2.3% increase in overweight in males and a little less, 1.3% in women. It is more difficult to estimate the impact of lower smoking initiation rates. Needless to say, smoking should not be regarded as a weight loss strategy.
In parallel with the rise of obesity, chronic sleep deprivation has been observed. Self-reported sleep duration has decreased by 2 h, from 9 h per night at the beginning of the 20th century to presently less than 7 h (40). For children, who clearly need more sleep, the decline in sleep duration is even more impressive (57). In 1 to 4-yr-old children, insufficient sleep is associated with a 2-fold odds increase of being obese 5 yr later (58).
Experimental support for a link between chronic sleep deprivation and obesity has been provided by Turek’s group. In 1997, they discovered a circadian gene, dubbed “Clock” (circadian, locomotor, output cycles kaput). Mice homozygous for the Clock mutation slept less and had less REM sleep. In addition, they had severe hyperphagia and developed obesity (59). In humans, scute sleep deprivation increased Cortisol, decreased GH and leptin (60), and impeded fat loss during energy restriction efforts (61). In contrast, ghrelin, a potent appetite stimulant and the proinflammatory cytokines interleukin-1 (IL-1), IL-6, and tumor necrosis factor α (TNFα) increased. These endocrine and immune changes led to heightened appetite and rapidly induced insulin resistance (62). Finally, starting with the first reports in 2004 (63, 64), at least 50 mostly cross-sectional studies confirmed an association between short sleep and obesity (65). At the National Institutes of Health, we are conducting a prospective randomized clinical trial (NCT00261898) to investigate the reverse, namely whether sleep extension is associated with weight loss (66). In addition to chronic sleep loss, attenuation of seasonality in our modern environment may contribute to weight gain during the winter, especially in susceptible individuals such as women suffering from seasonal affective disorders (67). Some propose that we are not “missing something” in the quest to understand the driving forces of the obesity epidemic, but that we are “missing the point”. The science writer Gary Taubes and others suggest focusing on why we accumulate excess fat. The argument has been made that it is our overemphasis on carbohydrates, specifically fructose that is responsible for the obesity epidemic (68). Furthermore, the connection of low-fat foods and our rising weight certainly needs further exploration. As indirect epidemiological evidence for other mechanisms than overeating and ‘under-exercising’, Taubes points to high obesity rates in underprivileged societies with no comfort food as we define it today, such as the Sioux in 1928 (69), the Trinidadians in 1961–1963 (70), and the Bantu pensioners in 1964 (71). Some of these communities had high levels of physical activity, such as the Chileans in the 1960s (72) and the Mexican-Americans oil-field workers in the early 1980s (73). These epidemiological examples underline the deleterious role of the introduction of refined carbohydrates and sudden changes in diet in populations previously not exposed to high sugar diets. Furthermore, in 2001, he investigated the dogma of saturated fat causing heart disease (74) and then went on to focus on carbohydrates’ effects on insulin arguing that obesity is primarily a disorder of insulin-induced fat accumulation rather than overeating or sedentary behavior (75).
Medical consequences of obesity
Depression and Alzheimer’s dementia are relatively underappreciated consequences of obesity. A recent meta-analysis of 15 large, prospective studies indicated a bidirectional relationship between obesity and depression: obese persons have a 55% increased risk of developing depression; vice versa subjects with depression have a 58% increased risk of becoming obese (76). The mechanisms are complex and only in part elucidated. Obesity may lead to depression via elevated cytokine production in adipose tissue (77). Additionally, higher rates of cortisone to Cortisol conversion via the enzyme 11β-hydroxysteroid dehydrogenase type-1 (HSD) in adipocytes may promote depression. Approximately ⅔ of patients with hypercortisolemia secondary to endogenous Cushing’s syndrome have psychopathological symptoms, mostly atypical depression which tends to gradually disappear 3–12 months after surgical cure, as plasma Cortisol levels normalize (78). Similarly, surgical correction of obesity favors an improvement in quality of life, with the important caveat of an unexplained increased incidence of suicides after gastric bypass surgery (79).
Additional factors include the lack of exercise, depriving obese subjects of its mood-elevating properties. As the most common forms of obesity and depression are likely polygenic, it is possible that they share a common pool of genes.
Recently, the association between obesity and Alzheimer dementia or cognitive decline has become a novel field of intense investigation. Profenno et al. reported an increased risk (effect size of 1.63; 95% confidence interval 1.39–1.92; p=0.001) of Alzheimer in obese vs control subjects in their meta-analysis (80). Potential mechanisms are obesity-induced hypercortisolemia, which induces apoptosis in the hippocampus, a brain region crucial for memory, as well as increased β amyloid deposition in the brain possibly due to chronically high levels of insulin competing with β amyloid for insulin-degrading enzymes (81, 82).
The effects of insulin on memory and cognition are difficult to establish due to the confounding factor of glucose. Subjects with Alzheimer disease have low cerebrospinal fluid-to-plasma insulin ratios (83) and intranasal administration of insulin as a way to penetrate the blood-brain barrier has been advocated as memory-enhancer, as in a randomized, pilot study it improved verbal retention (84). There may also be a genetic, obesity-related predisposition to the increased risk: the AA variant of the fat mass and obesity-associated (FTO) gene has been associated with an increased risk of Alzheimer in a prospective study of 1000 individuals who had no dementia at enrollment and were followed for 7 yr. The variant further augmented the risk in persons who had a variant of ApoE, a gene known to predispose to Alzheimer (85).
It should be noted that the existing evidence remains inconclusive and controversial. Population-based studies conducted in groups of different genetic background have indicated that mild obesity may be protective against Alzheimer. It is likely that the relationship between Alzheimer and obesity is markedly modulated by age. For example, a high BMI between the ages of 20 and 50 yr is associated with an increased risk of Alzheimer, but a BMI<20 at age 70 yr is also a risk factor for Alzheimer. Pharmacological interventions with statins or other agents effective in treatment of the metabolic syndrome aimed at preventing Alzheimer have been inconclusive, possibly due to the large number of patients needed for these trials, the heterogeneity inherent to the definition of metabolic syndrome, and the difficulty of diagnosing Alzheimer syndrome pre-mortem by pure clinical criteria. Similarly, lifestyle modifications such as increased exercise coupled with caloric restriction, although widely advocated in medical reviews and in the lay press, are not based on solid evidence. Finally, approximately 100,000 excess deaths are attributable to obesity in the US (86).
Will we ever find an effective medical treatment for obesity?
This is a frequently asked but ill-formulated question. The issue for any drug, especially for an anti-obesity drug, is not efficacy per se, but rather a favorable balance between efficacy and safety. In 1947, two amphetamines were approved by the Food and Drug Administration (FDA) as “adjunct to the dietary management of obesity” (87). In 1962, an expert panel concluded that amphetamines failed to meet the standard of effectiveness. In these days, the FDA considered an appetite suppressant effective if “it was statistically superior to placebo”. Clinical relevance was considered only much later. The safety profile of the combination drug fen-phen (fenfluramine and phentermine) became clear only after its approval, with approximately 85,000 prescriptions per week. In 1997, fen-phen was withdrawn from the market because of left-sided valvular degeneration, a risk no one saw coming. Other agents were either recently withdrawn-(sibutramine) or not approved (rimonabant) by the FDA due to cardiovascular and dose-related psychiatric side effects, respectively. The only presently available agent, orlistat, is an inhibitor of fat absorption leading to modest weight loss (2–3 kg). Side effects include steatorrhea and fecal incontinence, which are unpleasant but, to some extent, self-limiting. While there may be several promising therapeutics in development, so far no medical treatment has shown a favorable efficacy/safety profile. Given the evolution-based redundancy of appetite-stimulating circuits in the brain and its plasticity, it is more likely that a combination of centrally acting agents would be effective appetite suppressants than a single agent.
Bariatric surgery
More than 300,000 bariatric surgery procedures will be performed in the US this year. Indications are a BMI>40 kg/m2 or >35 kg/m2 with coexistent, serious medical conditions such as diabetes, hypertension and other conditions. The resultant weight loss is dramatic, 30 to 50 kg after 3 yr, and most surgical procedures are considered safe if performed by experienced surgeons. Patients with Type 2 diabetes undergoing gastric bypass procedures may experience rapid resolution of hyperglycemia independent of major weight loss, in part due to changes in incretin secretion (88, 89).
Intense efforts are underway to elucidate the underlying mechanisms. So far, data on long-term safety are promising (90, 91), but post-operative consequences can be grave (92). Examples include bowel obstruction, vitamin deficiencies and bacterial overgrowth causing severe diarrhea among others (93). Surgical procedures have become less invasive and post-operative hospital stays very short, but these interventions remain expensive. Therefore more studies of cost/effectiveness are needed (91). It is somber to note that as of today the only true effective intervention we can offer to some obese patients is an array of surgical procedures with the ultimate goal of impairing the remarkable effectiveness of the gastrointestinal tract, as it developed over evolutionary time.
CONCLUSIONS
The obesity epidemic has developed over more than a generation. It is unlikely that this problem would resolve within a generation. We need the courage and the intellectual honesty to acknowledge that, although something can currently be done at a patient level, we may be losing the battle on obesity at the societal level. To reverse the current obesity epidemic it will take more than telling people to “eat a little less” and “exercise a little more”. As noted in a recent commentary “the apparent energy imbalance for much of the US population is 5–10-fold greater than eating an extra cookie a day or walking one extra mile a day, far more beyond the ability of most individuals to address at a personal level” (94). We cannot, and should not, solely rely on the will power of the isolated individual, although each one of us will always bear the responsibility to be as healthy as possible. In brief, it will take dramatic, structural changes. It will take a concerted effort with a focus on prevention, which should start very early, not even in utero but before conception. The good news is that those societal changes would have added benefits well above and beyond obesity.
Acknowledgments
We would like to thank Dr. David Allison and Mr. Gary Taubes for critical comments.
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.Keith S, Redden DT, Katzmarzyk P, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obesity (Lond) 2006;30:1585–94. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 4.McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123:1544–50. doi: 10.1378/chest.123.5.1544. [DOI] [PubMed] [Google Scholar]
- 7.Preis SR, Massaro JM, Hoffmann U, et al. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab. 2010;95:3701–10. doi: 10.1210/jc.2009-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;3:600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 10.Pan WH, Yeh WT, Weng LC. Epidemiology of metabolic syndrome in Asia. Asia Pac J Clin Nutr. 2008;17 (Suppl 1):37–42. [PubMed] [Google Scholar]
- 11.Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr. 2007;2:267–75. doi: 10.1111/j.1559-4564.2007.07392.x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155:S7.e7–11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;9:1011–29. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 15.Cizza G, Rother KI. Was Feuerbach right: are we what we eat? J Clin Inv. 2011;121:2969–71. doi: 10.1172/JCI58595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siervo M, Wells JC, Cizza G. The contribution of psychosocial stress to the obesity epidemic: an evolutionary approach. Horm Metab Res. 2009;41:261–70. doi: 10.1055/s-0028-1119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 18.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–65. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster MT, Warne JP, Ginsberg AB, et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adreno-corticotropic and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–33. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnanský R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–7. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JF, Choi DL, Benoit SC. Insulin, leptin and reward. Trends Endocrinol Metab. 2010;2:68–74. doi: 10.1016/j.tem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warne JP, Dallman MF. Stress, diet and abdominal obesity: Y? Nat Med. 2007;7:781–3. doi: 10.1038/nm0707-781. [DOI] [PubMed] [Google Scholar]
- 23.Pelchat ML. Food addiction in humans. J Nutr. 2009;139:620–2. doi: 10.3945/jn.108.097816. [DOI] [PubMed] [Google Scholar]
- 24.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 25.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lopez RP, Hynes HP. Obesity, physical activity, and the urban environment: public health research needs. Environ Health. 2006;5:25. doi: 10.1186/1476-069X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- 28.Klimentidis YC, Beasley TM, Lin H, et al. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci. 2011;278:1626–32. doi: 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–84. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–58. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacerdote B. Peer effects with random assignment: results for dart-mouth roommates. Q J Econ. 2001;116:681–704. [Google Scholar]
- 33.Hahn J, Hirano K. Design of randomized experiments to measure social interaction effects. Economics Letters. 106:51–3. [Google Scholar]
- 34.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–33. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 35.Arnold J, Jánoska M, Kajon AE, et al. Genomic characterization of human adenovirus 36, a putative obesity agent. Virus Res. 2010;149:152–61. doi: 10.1016/j.virusres.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson F, Mavrogianni A, Ucci M, Vidal-Puig A, Wardle J. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes Rev. 2011;12:543–51. doi: 10.1111/j.1467-789X.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- 37.Celi FS. Brown adipose tissue--when it pays to be inefficient. N Engl J Med. 2009;360:1553–6. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obesity (Silver Spring) 2010;18:1092–9. doi: 10.1038/oby.2010.74. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Kobayashi M. The relationship between obesity and seasonal variation in body weight among elementary school children in Tokyo. Econ Hum Biol. 2006;4:253–61. doi: 10.1016/j.ehb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Van Cauter E, Spiegel K. Sleep as a mediator of the relationship between socioeconomic status and health: a hypothesis. Ann N Y Acad Sci. 1999;896:254–61. doi: 10.1111/j.1749-6632.1999.tb08120.x. [DOI] [PubMed] [Google Scholar]
- 41.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–62. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaput JP, Tremblay A. Acute effects of knowledge-based work on feeding behavior and energy intake. Physiol Behav. 2007;90:66–72. doi: 10.1016/j.physbeh.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Loffeld RJ. Helicobacter pylori, obesity and gastro-oesophageal reflux disease. Is there a relation? A personal view. Neth J Med. 2005;63:344–7. [PubMed] [Google Scholar]
- 44.Krai JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e 1644–9. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson PW, Parkin JM, Pearlson J, Philips PR, Sykes P. Obesity in childhood: A community study in Nawcastle upon Tyne. Lancet. 1977;1:350–2. doi: 10.1016/s0140-6736(77)91147-3. [DOI] [PubMed] [Google Scholar]
- 46.Stark O, Atkins E, Wolff OH, Douglas JW. Longitudinal study of obesity in the National Survey of Health and Development. Br Med J (Clin Res Ed) 1981;283:13–7. doi: 10.1136/bmj.283.6283.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speakman JR, Djafarian K, Stewart J, Jackson DM. Assortative mating for obesity. Am J Clin Nutr. 2007;86:316–23. doi: 10.1093/ajcn/86.2.316. [DOI] [PubMed] [Google Scholar]
- 48.Hebebrand J, Wulftange H, Goerg T, et al. Epidemic obesity: are genetic factors involved via increased rates of assortative mating? Int J Obes Relat Metab Disord. 2000;24:345–53. doi: 10.1038/sj.ijo.0801135. [DOI] [PubMed] [Google Scholar]
- 49.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 50.Byars SG, Ewbank D, Govindaraju DR, Steams SC. Colloquium papers: Natural selection in a contemporary human population. Proc Natl Acad Sci U S A. 2010;107 (Suppl 1 ):1787–92. doi: 10.1073/pnas.0906199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243–50. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messerli FH, Bell DS, Fonseca V, et al. GEMINI Investigators. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610–5. doi: 10.1016/j.amjmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 55.Wu RR, Zhao JP, Guo XF, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165:352–8. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- 56.Flegal KM. The effects of changes in smoking prevalence on obesity prevalence in the United States. Am J Public Health. 2007;97:1510–4. doi: 10.2105/AJPH.2005.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 58.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010;164:840–5. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 59.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheen AJ, Byrne MM, Plat L, Leproult R, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol. 1996;271:E261–70. doi: 10.1152/ajpendo.1996.271.2.E261. [DOI] [PubMed] [Google Scholar]
- 61.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knutson KL, Van Cauter E. Associations, between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 64.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cizza G, Marincola P, Mattingly M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010;3:274–85. doi: 10.1177/1740774510368298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cizza G, Requena M, Galli G, de Jonge L. Chronic sleep deprivation and seasonality: implications for the obesity epidemic. J Endocrinol Invest. 2011;34:793–800. doi: 10.3275/7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307–21. doi: 10.1016/j.jada.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Stene JA, Roberts IL. A Nutrition Study on an Indian Reservation. J Am Diet Assoc. 1928;4:215–22. [Google Scholar]
- 70.McCarthy C. Dietary and Activity Patterns of Obese Women in Trinidad. J Am Diet Assoc. 1966;48:33–7. [PubMed] [Google Scholar]
- 71.Walker AR. Overweight and hypertension in emerging populations. Am Heart J. 1964;68:581–5. doi: 10.1016/0002-8703(64)90265-0. [DOI] [PubMed] [Google Scholar]
- 72.Arteaga A. The Nutritional Status of Latin American Adults. In: Scrimshaw NS, Moises B, editors. Nutrition and Agricultural Development. New York: Plenum Press; 1974. pp. 67–76. [Google Scholar]
- 73.Reichley KB, Mueller WH, Hanis CL, et al. Centralized obesity and cardiovascular disease risk in Mexican Americans. Am J Epidemiol. 1987;125:373–86. doi: 10.1093/oxfordjournals.aje.a114544. [DOI] [PubMed] [Google Scholar]
- 74.Taubes G. Nutrition. The soft science of dietary fat. Science. 2001;291:2536–45. doi: 10.1126/science.291.5513.2536. [DOI] [PubMed] [Google Scholar]
- 75.Taubes G. Why we get fat and what to do about it. New York: Knopf; 2010. [Google Scholar]
- 76.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 77.Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–99. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–9. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 79.Tindle HA, Omalu B, Courcoulas A, Marcus M, Hammers J, Kuller LH. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med. 2010;123:1036–42. doi: 10.1016/j.amjmed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does Cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 82.Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24:445–9. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–8. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 84.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 85.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis. 2011;23:461–9. doi: 10.3233/JAD-2010-101068. [DOI] [PubMed] [Google Scholar]
- 86.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143:380–5. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- 88.Laferrère B, Swerdlow N, Bawa B, et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4072–6. doi: 10.1210/jc.2009-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nandagopal R, Brown RJ, Rother Kl. Resolution of type 2 diabetes following bariatric surgery: implications for adults and adolescents. Diabetes Technol Ther. 2010;12:671–7. doi: 10.1089/dia.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 92.Abell TL, Minocha A. Gastrointestinal complications of bariatric surgery: diagnosis and therapy. Am J Med Sci. 2006;331:214–8. doi: 10.1097/00000441-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–190. 215–357. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 94.Katan MB, Ludwig DS. Extra calories cause weight gain--but how much? JAMA. 2010;303:65–6. doi: 10.1001/jama.2009.1912. [DOI] [PubMed] [Google Scholar]


