Abstract
Approximately 215,000 people younger than 20 yr of age, or 1 in 500 children and adolescents, had diabetes in the United States in 2010 – and the incidence is rising. We still have insufficient knowledge about the precise mechanisms leading to the autoimmune mediated β-cell destruction in Type 1 diabetes, and the β-cell failure associated with insulin resistance in Type 2 diabetes. Long-term complications are similar: micro-and macrovascular disease occurs prematurely and presents an enormous burden on affected individuals, often as early as in middle age. In Type 1 diabetes, technological advances have clearly improved blood glucose management, but chronic peripheral over-insulinization remains a problem even with the most advanced systems. Thus, in Type 1 diabetes our research must focus on 1) finding the stimulus that ignites the immune response and 2) developing treatments that avoid hyperinsulinemia. In Type 2 diabetes in youth, the challenges start much earlier: most young patients do not even benefit from existing therapies due to non-compliance. Therefore, prevention of Type 2 diabetes and improvement of compliance, especially with non-pharmacological interventions, are the greatest challenges.
Keywords: Autoimmunity, chronic stress, genetic, leptin, obesity, prevention, sleep deprivation, socioeconomic status, Vitamin D
INTRODUCTION
Childhood diabetes comes in many forms including rare conditions such as neonatal diabetes, chronic disease associated (e.g. with cystic fibrosis) and monogenic diabetes (e.g. maturity onset diabetes of the young). This presentation focuses on the two most common diabetes forms, Type 1 and Type 2 diabetes.
At the start of the 20th century, Type 1 diabetes was infrequently observed and Type 2 diabetes was a diagnosis made only in adults. Even in the 1980s, there were hardly any pediatric cases of Type 2 diabetes with the exception of reports in minorities such as the Pima Indians (1). With the rise of obesity, this has dramatically changed over the past 30 years. Ever more frequently, the distinction between Type 1 and Type 2 diabetes is difficult since some youths present with features characteristic for both diabetes types (2).
In this review, we address the following relevant questions: What are the important etiologic factors leading to the continuously rising incidence of pediatric Type 1 and Type 2 diabetes? Which are the greatest challenges to be addressed in order to prevent and better treat childhood diabetes?
INCIDENCE AND ETIOLOGY OF TYPE 1 DIABETES IN CHILDREN AND ADOLESCENTS
Formerly known as juvenile or insulin-dependent diabetes, Type 1 diabetes mellitus’ became the generally accepted term with the recognition that older age does not protect from autoimmune diabetes and that many patients with long-standing Type 2 diabetes also require insulin. In contrast to the prevailing dogma that patients with Type 1 diabetes gradually lose all of their insulin-producing β-cells due to a T-cell mediated autoimmune attack, we and others have shown that a few, functional β-cells can be detected in almost all individuals irrespective of the duration of disease (3, 4). These remaining β-cells may present an opportunity for regenerative efforts in the future.
Worldwide, Sardinia in Italy and Finland are hot spots for Type 1 diabetes. In 2005, Finnish adolescents had an annual incidence of 64.2 per 100,000 persons per year (5). In the US, the current estimate is slightly below 30 new diagnoses per 100,000 per year across all ages with a considerable variation in different races and ethnicities (6) (Fig. 1). Overall, the worldwide incidence increases approximately 2–3% per year. In the European Diabetes (EURODIAB) study, it was shown that children <5 years of age belonged to the fastest growing group (7).
Fig. 1.
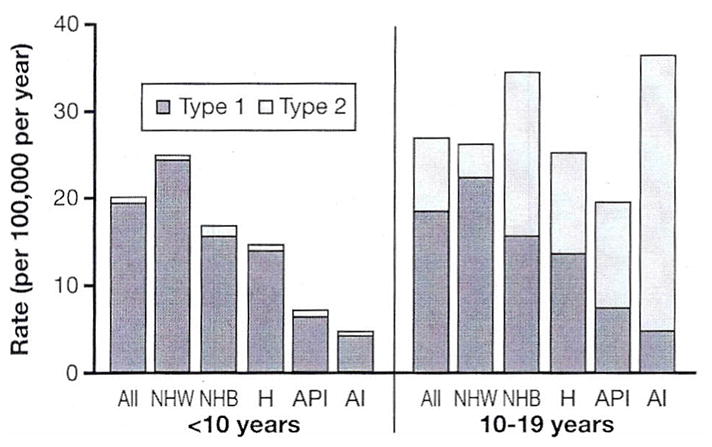
Rate of new cases of Type 1 and Type 2 diabetes among children, adolescents, and young adults <20 yr of age, by race/ethnicity, between 2002 and 2005. NHW: non-Hispanic whites; NHB: non-Hispanic blacks; H: Hispanics/Latinos; API: Asian/Pacific Islander Americans; Al: American Indians. Source: SEARCH for Diabetes in Youth Study (diabetes.niddk.nih.gov/dm/pubs/statistics/images/DMStats2011-Chart3.jpg&imgrefurl=http).
The value of diabetes registries cannot be overstated (8) since the recognition of geographic and temporal trends depends on the availability of large data sets. Such registries for Type 1 diabetes include the Diabetes Mondiale (DIAMOND) Project (9) worldwide and the EURODIAB study (7). In the US, national registries do not exist, instead local registries have provided important insights [e.g., the Allegheny County Type 1 Diabetes Registry (10)]. Since 2000, a wealth of knowledge in the field of pediatric Type 1 and Type 2 diabetes has been derived from SEARCH for Diabetes in Youth, a large, multicenter, epidemiologic study, conducted in 5 racial and ethnic groups (non-Hispanic whites, African-American, Hispanics, Asian/Pacific Islanders, and American Indians) at 6 sites (California, Colorado, Hawaii, Ohio, South Carolina, and Washington). SEARCH-based publications are summarized at http://www.searchfordiabetes.org/. Fortunately, funding for SEARCH has recently been renewed for an additional 5 years.
An excellent example for a global trend is the higher incidence of Type 1 diabetes in countries further away from the equator. This led to the ‘sunshine hypothesis’, which suggests that exposure to UV-B radiation, which converts 7-dehydrocholesterol to Vitamin D in the skin, is protective against autoimmunity in general, and Type 1 diabetes in particular (11). However, even in countries closer to the equator, more children are diagnosed with Type 1 diabetes, which in part may be related to local customs of protective clothing and prolonged time spent indoors leading to Vitamin D deficiency, but also to the fact that sufficient Vitamin D levels are not protective enough to ward off other etiological factors (12). In support of the ‘sunshine hypothesis’ are also data from NOD mice (13) and streptozotocin-treated rats (14) (two common animal models of Type 1 diabetes), in whom treatment with pharmacologic doses of Vitamin D helped lower the incidence of diabetes. To date, however, there is no good clinical evidence that Vitamin D supplementation prevents Type 1 diabetes in children.
Another explanation for the rising incidence of Type 1 diabetes is the ‘accelerator or overload hypothesis’ (15). Wilkin suggests that Type 1 and Type 2 diabetes are the same disorder of insulin resistance set against different genetic backgrounds. His theory does not argue against the presence of autoimmunity in Type 1 diabetes, only against its causative role. Other investigators may not support this view, but good evidence exists that faster linear growth (16), especially in the first year of life, and increasing body mass (17) contribute to Type 1 diabetes. Increased β-cell stress induced by hyperinsulinemia and decreased insulin sensitivity are plausible contributors to β-cell failure (18, 19). In individuals at risk for Type 1 diabetes, insulin resistance has been associated with progression to overt disease (20). Data from countries such as China, Japan, and Brazil are, however, somewhat difficult to reconcile with the clinical importance of this hypothesis because Type 1 diabetes has remained relatively rare despite the clearly rising incidence of childhood obesity (17).
A third explanation is the ‘hygiene hypothesis’ (21), which suggests that higher living standards associated with reduced exposure to microorganisms may lead to disordered regulation of the immune system. First supportive observations were made with allergic disorders (22) and were subsequently expanded to include autoimmune mediated illnesses. The originally formulated hypothesis had to be modified to accommodate the fact that both, the incidence of Th1- (e.g., Type 1 diabetes) and Th2-(e.g., asthma) mediated conditions increased in parallel. An alternative term ‘microbial deprivation hypothesis’ has been coined, which integrates the beneficial role of myriads of different non-pathogenic, commensal microorganisms, rather than infectious diseases, on proper development of the immune system (23). Specifically, the role of the intestinal microbiome in the pathogenesis of Type 1 diabetes is under intense investigation (24). Other investigators have focused on early infant nutrition (25) and additional environmental factors such as enteroviral infections (26). Knip recently challenged the diabetes research community to go beyond conventional epidemiology and add ‘etiological’ epidemiology in order to generate information on the causes of the rising incidence and prevalence trends (27).
INCIDENCE AND ETIOLOGY OF TYPE 2 DIABETES IN CHILDREN AND ADOLESCENTS
Similar to Type 1 diabetes, the nomenclature for Type 2 diabetes has changed. Terms like ‘adult onset’ or ‘non-insulin dependent diabetes mellitus (NIDDM)’ should be avoided for obvious reasons. Type 2 diabetes in youth was very rare until about two decades ago. According to the SEARCH study results, approximately 3000 children and adolescents in the US were diagnosed in 2002–2003 compared to about 1.4 million new cases in adults. Thus, describing its rising incidence as an ‘epidemic’ may still be an exaggeration, but this recent development is certainly of great concern (28).
In contrast to Type 1 diabetes, there is a paucity of ongoing interventional trials in pediatric Type 2 diabetes, which is mostly due to the disease’s recent emergence, but also due to a lack of an effective lobby [i.e., there is no equivalent to the Juvenile Diabetes Research Foundation (JDRF) for Type 2 diabetes]. Furthermore, there are many practical difficulties starting with recruitment (29). The largest and most important prospective, randomized interventional study is the Treatment Options for Type 2 Diabetes in Adolescents & Youth (TODAY) trial, comparing metformin alone, or metformin in combination with rosiglitazone or lifestyle modification (30). Recruitment took 2 years longer than expected, and study results are expected to be available in published form in the New England Journal of Medicine in June 2012.
The pathophysiologic components of insulin resistance and β-cell failure are probably similar in children and adults. In studies of offspring of parents with Type 2 diabetes, an impairment of the acute insulin response was frequently observed, which points to β-cell failure as the primary culprit (31). There are no prospective data available on the time course of β-cell failure in children before and after the diagnosis of Type 2 diabetes, in contrast to existing data in adults [e.g., from the United Kingdom Prospective Diabetes Study (UKPDS)]. Anecdotal reports support the notion that Type 2 diabetes in youth may be a more aggressive and faster progressing disease than in adults (32).
Knowledge of the genetic pathways leading to Type 2 diabetes in adults is rapidly growing (33) and analyses conducted in the TODAY Genetics Study are expected to provide more insights into the inherited predisposition of young individuals with Type 2 diabetes. Clinically, the strong genetic component is easily noticeable since approximately 75% of affected adolescents have first degree relatives with Type 2 diabetes (34). In concert with the genetic make-up, numerous environmental and epigenetic factors further contribute to the development of childhood Type 2 diabetes (Fig. 2).
Fig. 2.
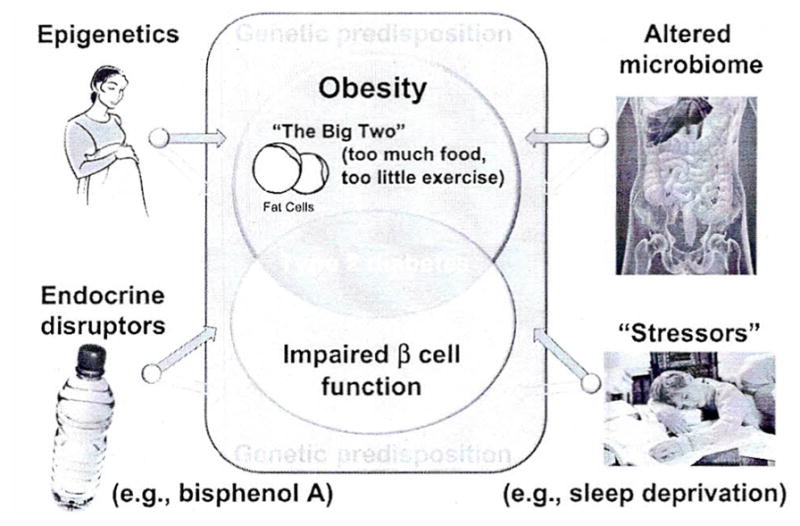
Genetic predisposition and a combination of obesity and β-cell failure lead to Type 2 diabetes. Several epigenetic and environmental factors contribute to both, weight gain and loss of β-cell function. Purple arrows indicate the contribution to obesity, green arrows to β-cell failure. The appropriate references can be found in the text.
Support for the role of epigenetic factors comes from studies in children born to mothers who had undergone bariatric surgery (35). Children who were born before their mothers had weight loss surgery, had a higher risk of obesity and insulin resistance than their siblings born to the same mothers after they had lost weight. Similarly, offspring of mothers with gestational diabetes have a higher risk of developing obesity and Type 2 diabetes compared to offspring of the same mothers born after an earlier pregnancy not yet complicated by gestational diabetes (36). The other side of the coin, represented by studies in offspring of mothers exposed to famine and in children born with intrauterine growth retardation of other/unknown etiology also clearly shows the increased incidence of insulin resistance and Type 2 diabetes later in life (37). Examples of plausible direct and indirect mechanisms creating a pro-inflammatory environment, insulin resistance, and ultimately β-cell exhaustion include changes in the human microbiome (38) and environmental/behavioral stressors such as chronic sleep deprivation (39).
CHILDHOOD DIABETES WITH CHARACTERISTICS OF TYPE 1 AND TYPE 2 DIABETES
Some obese children present with diabetic ketoacidosis, may have autoantibodies to islet cells and glutamic acid decarboxylase but maintain euglycemia without insulin therapy after resolution of the acute metabolic derangement. The period of insulin independence is typically rather short and most children require insulin within a few months. Clinicians have suggested treatment with met-formin in these children in the interim (40). In adults, the phenomenon of ketosis-prone diabetes, which has also been called atypical diabetes, or Type 1B diabetes, has been predominately described in African Americans. Several detailed studies have been undertaken (41), but the exact pathophysiology remains poorly understood. A more common scenario, however, is the presentation of typical features of Type 2 diabetes in a youth, who upon further examination turns out to have signs of β-cell autoimmunity. This occurred in about 10% of children and adolescents who were screened for the TODAY study (2). The term ‘double diabetes’ has been coined by Pozzilli and Buzzetti (42) and aptly describes the dilemma of categorizing this new type of diabetes. It appears reasonable to assume that ‘double diabetes’ represents the presence of autoimmune or Type 1 diabetes in an obese child or adolescent.
TREATMENT OF CHILDHOOD DIABETES
Special consideration of cardiovascular disease
With modem insulin treatment, mortality from Type 1 diabetes has decreased dramatically. Data from the Allegheny County Type 1 Diabetes Registry documented that patients diagnosed between 1965 and 1969 had 40% higher mortality ratios compared to individuals diagnosed a decade later (10). With improvements in management of acute diabetes complications, cardiovascular disease, the number one killer in the US, has become the leading cause of death in patients both with Type 1 diabetes (43) and Type 2 diabetes (44). While hyperglycemia is the single most important risk factor for microvascular disease, glycemia’s direct influence on cardiovascular disease is confounded by numerous other risk factors, including those important in the general population (e.g. hyperlipidemia and smoking), and those more specific to Type 1 diabetes, such as nephropathy (45). In addition, in Type 2 diabetes, the relationship between glycemia and cardiovascular disease appears to be non-linear, with recent major clinical trials demonstrating increased cardiovascular mortality in patients intensively treated to reach very low glycated hemoglobin targets (46).
Traditional risk factors for Type 2 diabetes, such as insulin resistance and subclinical inflammation, have recently also become the focus in the field of Type 1 diabetes research. They may contribute to the increased cardiovascular risk observed in both populations, and are certainly heightened by the increasing prevalence of overweight and obesity in all young diabetes patients (47). Investigators of the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study reported that individuals with Type 1 diabetes were half as insulin sensitive as body mass index-matched controls, and insulin resistance predicted the extent of the observed coronary artery calcifications in subjects with and without diabetes (48). Why insulin resistance in Type 1 diabetes is out of proportion to body fatness remains a subject of speculation.
Hyperinsulinemia, whether endogenous (in Type 2 diabetes) or exogenous (in both Type 1 and Type 2 diabetes) may be a major contributor to cardiovascular disease in diabetes. As discussed by Draznin in a recent editorial, insulin is a life-saving hormone, but in high concentrations it can substantially increase cellular mitogenic responsiveness to other growth factors, especially in the presence of insulin resistance (49). One pathway by which this growth-promoting effect occurs is excessive farnesylation and membrane association of Ras proteins, which may promote progression of cancer and vascular disease. The logical therapeutic consequence should be to provide as much insulin as necessary to achieve good blood glucose control, but to effectively improve insulin sensitivity and use insulin-sparing agents.
Beyond conventional pharmacotherapy
For patients with Type 1 and Type 2 diabetes, in addition to the obvious, but challenging choice of lifestyle modifications, numerous pharmacologic approaches have been tried. The insulin sensitizer metformin is the only approved oral agent for treatment of Type 2 diabetes in children (50), and is under study as an adjunct to insulin in children with clinical features of both Type 1 and Type 2 diabetes. Pramlintide, an analog of the β-cell hormone amylin, is Food and Drug Administration-approved in adults with both Type 1 and Type 2 diabetes, and results in improved post-prandial hyperglycemia with reduced insulin doses. Its effects are mostly due to slowed gastric emptying and less glucagon secretion. Numerous clinical trials with this agent are currently underway in pediatric patients. Incretin-based therapies, such the giucagon-like-peptide-1 analog exenatide are approved for treatment of Type 2 diabetes in adults, and are being studied in children with both Type 1 and Type 2 diabetes.
For patients with Type 1 diabetes, perhaps the most intriguing potential insulin-sparing agent is the adipocyte-derived hormone leptin, which was recently shown to normalize blood glucose in combination with low-dose insulin by markedly suppressing glucagon in diabetic mice (51). Leptin would only be expected to be beneficial in normal weight patients with Type 1 diabetes due to the existing leptin resistance in obese patients (thus excluding most individuals with Type 2 diabetes) (52). The recognition of leptin neutralizing antibodies associated with exogenous leptin therapy (in children with lipodystrophy) (53) and in combination with pramlintide in obese adults (54) may, however, prevent its broader application. Immunomodulatory interventions have so far been of limited effectiveness in the treatment of Type 1 diabetes (55) and recent results with the anti CD3 antibody teplizumab (56) and glutamic acid decarboxylase (57) vaccination therapy have been disappointing. Finally, the “artificial pancreas” (continuous blood glucose monitoring combined with a closed loop algorithm to automatically adjust insulin infusion rates in response to blood glucose) holds potential to reduce insulin doses, as well as hypo-and hyperglycemia, in Type 1 diabetes. However, unless insulin is infused directly into the portal vein, this technique will still be associated with peripheral hyperinsulinemia.
In adolescents with Type 2 diabetes, bariatric surgery appears to have similarly promising or even better results regarding improvement of cardiovascular risk factors compared to adults (58), in whom resolution of Type 2 diabetes has been reliably observed in about 80% of patients after gastric bypass (59). Modifications of existing surgical techniques or entirely new approaches, such as the endoscopic insertion of duodenal liners, are promising developments. Prospective clinical trials examining the long-term effects of bariatric surgery in young patients with Type 2 diabetes, such as Teen-Longitudinal Assessment of Bariatric Surgery (TEEN-LABS) (60), are much needed.
CHALLENGES IN THE FIELD OF CHILDHOOD DIABETES
The greatest challenge in the field of Type 1 diabetes is the identification of the initial process that sets off the immune system to attack ‘self’ – in this case pancreatic β-cells. Without this knowledge, it is unlikely that an effective prevention or an ultimate cure can be found. Even if we were capable of generating new β-cells with the help of regenerative medicine or transplanting ample numbers of islets, the underlying immune process will annihilate these efforts. The second greatest challenge is to change our treatment with its intrinsic dangerous acute and long-term effects. Recent JDRF Continuous Glucose Monitoring Study Group results (61) emphasize how commonly prolonged nocturnal hypoglycemia occurs, especially in young individuals, and that various strategies for changes in insulin administration may reduce its frequency. Improved algorithms and mathematical models combined with glucose sensors and insulin pump technology represent an enormous chance to improve quality of life and reduce complications. However, even with the advent of a well-functioning artificial pancreas the problem of non-physiologically high insulin levels in the periphery instead of in the portal vein is not resolved.
In Type 2 diabetes, effective strategies for both prevention and treatment have been well established in adults. Testing and translating these strategies into the pediatric population, however, has proved to be the fundamental challenge. The former surgeon general C. Everett Koop phrased it well: “Drugs don’t work in patients who don’t take them”. Similar to the patient described above, over half of adolescents with Type 2 diabetes fail to return for routine clinical follow-up over 2 years (62), and recruitment into clinical trials has proved equally challenging (29). The TODAY trial strikingly illustrates the medical, psychosocial, and socio-economical challenges faced by youths with Type 2 diabetes: poverty, low parental education, single-parent (or no parent) households, and strong genetic and cultural predisposition for obesity and diabetes (34). Effectively addressing these issues is crucial to optimizing medical management in this growing patient cohort.
The stage for a positive outlook toward the challenges ahead may be set with another quote: Theodore Roosevelt told us that ‘far and away the best prize that life has to offer is the chance to work hard at work worth doing’. Thus, let’s do it.
Acknowledgments
This research was supported by the Intramural Research Program of NIDDK, NIH.
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Klingensmith GJ, Pyle L, Arslanian S, et al. TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010;33:1970–5. doi: 10.2337/dc10-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu EH, Digon BJ, 3rd, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52:1369–80. doi: 10.1007/s00125-009-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–7. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–82. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 6.Brown RJ, Rother KI. Type 1 and Type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Curr Cardiovasc Risk Rep. 2010;4:175–7. [Google Scholar]
- 7.Patterson CC, Dahlquist GG, Gyürüs E, Green A Soltész G; EU-RODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 8.Dabelea D, Mayer-Davis EJ, Imperatore G. The value of national diabetes registries: SEARCH for Diabetes in Youth Study. Curr Diab Rep. 2010;10:362–9. doi: 10.1007/s11892-010-0135-1. [DOI] [PubMed] [Google Scholar]
- 9.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 10.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33:2573–9. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–46. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Borkar W, Devidayal, Verma S, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2010;11:345–50. doi: 10.1111/j.1399-5448.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–8. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 14.Del Pino-Montes J, Benito GE, Fernández-Salazar MP, et al. Calcitriol improves streptozotocin-induced diabetes and recovers bone mineral density in diabetic rats. Calcif Tissue Int. 2004;75:526–32. doi: 10.1007/s00223-004-0118-9. [DOI] [PubMed] [Google Scholar]
- 15.Wilkin TJ. The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes (Lond) 2009;33:716–26. doi: 10.1038/ijo.2009.97. [DOI] [PubMed] [Google Scholar]
- 16.Kharagjitsingh A, de Ridder M, Alizadeh B, et al. Genetic correlates of early accelerated infant growth associated with juvenile-onset type 1 diabetes. Pediatr Diabetes. 2011;13:266–71. doi: 10.1111/j.1399-5448.2011.00813.x. [DOI] [PubMed] [Google Scholar]
- 17.Verbeeten KC, Elks CE, Daneman D, Ong KK. Association between childhood obesity and subsequent Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2011;28:10–8. doi: 10.1111/j.1464-5491.2010.03160.x. [DOI] [PubMed] [Google Scholar]
- 18.Knip M, Reunanen A, Virtanen SM, Nuutinen M, Viikari J, Akerblom HK. Does the secular increase in body mass in children contribute to the increasing incidence of type 1 diabetes? Pediatr Diabetes. 2008;9:46–9. doi: 10.1111/j.1399-5448.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 19.Knerr I, Wolf J, Reinehr T, et al. DPV Scientific Initiative of Germany and Austria. The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia. 2005;48:2501–4. doi: 10.1007/s00125-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 20.Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP Diabetes Prevention Trial-Type 1 Study Group. Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care. 2007;30:2314–20. doi: 10.2337/dc06-2389. [DOI] [PubMed] [Google Scholar]
- 21.D’Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypotheses. Arch Pediatr Adolesc Med. 2010;164:732–8. doi: 10.1001/archpediatrics.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerrard JW, Geddes CA, Reggin PL, Gerrard CD, Home S. Serum IgE levels in white and metis communities in Saskatchewan. Ann Allergy. 1976;37:91–100. [PubMed] [Google Scholar]
- 23.Björkstén B. The hygiene hypothesis: do we still believe in it? Nestle Nutr Workshop Ser Pediatr Program. 2009;64:11–8. doi: 10.1159/000235780. discussion 18–22, 251–7. [DOI] [PubMed] [Google Scholar]
- 24.Neu J, Lorca G, Kingma SD, Triplett EW. The intestinal microbiome: relationship to type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:563–71. doi: 10.1016/j.ecl.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Knip M, Virtanen SM, Seppä K, et al. Finnish TRIGR Study Group. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–8. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6:279–89. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 27.Knip M. Descriptive epidemiology of type 1 diabetes-is it still in? Diabetologia. 2012;55:1227–30. doi: 10.1007/s00125-012-2522-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol. 2012;8:228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 29.Gemmill JA, Brown RJ, Nandagopal R, Rodriguez LM, Rother KI. Clinical trials in youth with type 2 diabetes. Pediatr Diabetes. 2011;12:50–7. doi: 10.1111/j.1399-5448.2010.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeitler P, Epstein L, Grey M, et al. TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogardus C, Tataranni PA. Reduced early insulin secretion in the etiology of type 2 diabetes mellitus in Pima Indians. Diabetes. 2002;51 (Suppl 1):S262–4. doi: 10.2337/diabetes.51.2007.s262. [DOI] [PubMed] [Google Scholar]
- 32.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638–44. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–50. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 34.Copeland KC, Zeitler P, Geffner M, et al. TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–67. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–83. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 36.Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care. 1993;16:310–4. doi: 10.2337/diacare.16.1.310. [DOI] [PubMed] [Google Scholar]
- 37.Pinney SE, Simmons RA. Epigenetic mechanisnjis in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21:223–9. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 39.Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:143–50. doi: 10.1210/jc.2009-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low JC, Felner EI, Muir AB, et al. Do obese children with diabetic ketoacidosis have type 1 or type 2 diabetes? Prim Care Diabetes. 2012;6:61–5. doi: 10.1016/j.pcd.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosmanov AR, Smiley D, Robalino G, et al. Effects of intravenous glucose load on insulin secretion in patients with ketosis-prone diabetes during near-normoglycemia remission. Diabetes Care. 2010;33:854–60. doi: 10.2337/dc09-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab. 2007;18:52–7. doi: 10.1016/j.tem.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Bosnyak Z, Nishimura R, Hagan Hughes M, et al. Excess mortality in Black compared with White patients with Type 1 diabetes: an examination of underlying causes. Diabet Med. 2005;22:1636–41. doi: 10.1111/j.1464-5491.2005.01671.x. [DOI] [PubMed] [Google Scholar]
- 44.Abi Khalil C, Roussel R, Mohammedi K, Danchin N, Marre M. Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Cardiovasc Prev Rehabil. 2011 Apr 28; doi: 10.1177/1741826711409324. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 46.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive giucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Vliet M, Van der Heyden JC, Diamant M, et al. Overweight is highly prevalent in children with type 1 diabetes and associates with cardiometabolic risk. J Pediatr. 2010;156:923–9. doi: 10.1016/j.jpeds.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–14. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draznin B. Mitogenic action of insulin: friend, foe or ‘frenemy’? Diabetologia. 2010;53:229–33. doi: 10.1007/s00125-009-1558-6. [DOI] [PubMed] [Google Scholar]
- 50.Flint A, Arslanian S. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34 (Suppl 2):S177–83. doi: 10.2337/dc11-s215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type 1 diabetes. Proc Natl Acad Sci USA. 2010;107:4813–9. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–7. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beltrand J, Lahlou N, Le Charpentier T, et al. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur J Endocrinol. 2010;162:1083–91. doi: 10.1530/EJE-09-1027. [DOI] [PubMed] [Google Scholar]
- 54.Amylin. r-MetHu leptin (A-100) Investigator’s Brochure.
- 55.Rother KI, Brown RJ, Morales MM, et al. Effect of ingested interferon-alpha on beta-cell function in children with new-onset type 1 diabetes. Diabetes Care. 2009;32:1250–5. doi: 10.2337/dc08-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacroGenics Inc, Eli Lilly and Company. MacroGenics and Lilly announce pivotal clinical trial of teplizumab did not meet primary efficacy endpoint. 2010 Oct 20; Available at: http://newsroom.lilly.com/releasedetail.cfm?releaseid=521014.
- 57.Diamyd Medical. Diamyd reports initial results from European Phase III trial in patients newly diagnosed with type 1 diabetes. 2011 May 9; Available at: http://www.cisionwinb.com/diamyd-medical/r/diamyd-reports-initial-results-from-european-phase-iii-trial-in-patients-newly-diagnosed-with-type-1-diabetes,c9117770.
- 58.Brandt ML, Harmon CM, Helmrath MA, Inge TH, McKay SV, Michalsky MP. Morbid obesity in pediatric diapetes mellitus: surgical options and outcomes. Nat Rev Endocrinol. 2010;6:637–45. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- 59.Nandagopal R, Brown RJ, Rother KI. Resolutioh of type 2 diabetes following bariatric surgery: implications for adults and adolescents. Diabetes Technol Ther. 2010;12:671–7. doi: 10.1089/dia.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42:1969–71. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabites Care. 2010;33:1004–8. doi: 10.2337/dc09-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R DPV-Wiss Study Group. Type 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centers. Horm Res. 2008;69:107–13. doi: 10.1159/000111814. [DOI] [PubMed] [Google Scholar]


