Abstract
The most widely used animal model of attention-deficit/hyperactivity disorder (ADHD) is the spontaneously hypertensive rat (SHR/NCrl), which best represents the combined subtype (ADHD-C). Recent evidence has revealed that a progenitor strain, the Wistar Kyoto from Charles River Laboratories (WKY/NCrl), is useful as a model of the inattentive subtype (ADHD-PI) and the Wistar Kyoto from Harlan Laboratories (WKY/NHsd) and the Sprague Dawley (SD) have been suggested as controls. Dopamine (DA) dysfunction in the striatum (Str) and nucleus accumbens core (NAc) is thought to play a significant role in the pathophysiology of ADHD but data obtained with the SHR is equivocal. Using high-speed chronoamperometric recordings with carbon fiber microelectrodes, we found that the SHR/NCrl displayed decreased KCl-evoked DA release versus the WKY/NCrl model of ADHD-PI in the dorsal Str. The WKY/NCrl and the WKY/NHsd control did not differ from each other; however, the control SD released less DA than the WKY/NCrl model of ADHD-PI in the dorsal Str and less than the control WKY/NHsd in the intermediate Str. The SHR/NCrl had faster DA uptake in the ventral Str and NAc versus both control strains, while the WKY/NCrl model of ADHD-PI exhibited faster DA uptake in the NAc versus the SD control. These results suggest that increased surface expression of DA transporters may explain the more rapid uptake of DA in the Str and NAc of these rodent models of ADHD.
Keywords: spontaneously hypertensive rat, Wistar Kyoto rat, attention-deficit/hyperactivity disorder (ADHD), striatum, dopamine, dopamine uptake
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by impulsivity, hyperactivity, inattention and cognitive impairment. The DSM-IV-TR classifies ADHD into 3 subtypes: predominantly inattentive type (ADHD-PI), predominantly hyperactive-impulsive type (ADHD-HI) and the most common subtype – combined (ADHD-C). The seminal work of Terje Sagvolden (1945–2011) has pioneered the way for the spontaneously hypertensive rat (SHR) to be used as a model of ADHD-C. Sagvolden and colleagues found sustained attention deficits (Sagvolden 2000), motor impulsivity (Sagvolden et al. 1992; Wultz and Sagvolden 1992), and hyperactivity (Knardahl and Sagvolden 1979; Sagvolden 2000) in the SHR. The SHR is the most widely utilized animal model of ADHD (Russell 2011; Sagvolden and Johansen 2011); however, criticism of this strain lies in using the progenitor strain, the Wistar Kyoto (WKY), as a control (Alsop 2007). The NIH Animal Genetic Resource stock of the WKY was obtained in 1971 as outbred animals from the Kyoto School of Medicine in Japan. These animals were distributed to laboratories (i.e. Harlan and Charles River) before the F20 generation, resulting in multiple strains of the WKY (Sagvolden et al. 2009). Two commonly used WKY control strains, WKY/NCrl (Charles River) and WKY/NHsd (Harlan), display wide genetic divergence (Sagvolden et al. 2008). When contacted, both laboratories specified that inbred animals were subjected to single nucleotide polymorphism panels every quarter, thus the separate WKY strains should remain genetically different. Current behavioral evidence points to the SHR/NCrl being the most accurate animal model of ADHD-C (Russell 2011; Sagvolden et al. 2005) and the WKY/NHsd serves as the most appropriate control strain. The WKY/NCrl strain is best suited as a model of ADHD-PI because of its behavioral and neurochemical abnormalities (Roessner et al. 2010; Sagvolden et al. 2008; Sagvolden et al. 2009). It has been suggested that the outbred Sprague Dawley (SD) rat strain, used by some groups in the past, could be used as an additional control (Drolet et al. 2002; Sagvolden et al. 2009). Taken together, the SHR/NCrl and WKY/NCrl models of ADHD and their control strains, the inbred WKY/NHsd and outbred SD, seem to be valuable translational animal models to assess the neurobiological dysfunction associated with ADHD.
ADHD is thought to be caused by catecholamine dysfunction (Levy 1991; Solanto et al. 2001) in brain regions involved with attention and reward including the nucleus accumbens (NA) (Podet et al. 2010; Russell 2000) and striatum (Str) (Krause et al. 2003). Prior studies examining dopamine (DA) function in the SHR and WKY rat brains are equivocal and have been shown to range from no differences (Ferguson et al. 2003; Versteeg et al. 1976) to lower DA levels in the Str in vitro and in vivo in the SHR (Linthorst et al. 1991; Linthorst et al. 1990). Furthermore, a recent microdialysis study comparing the SHR to the SD determined that the SHR has 78% higher basal efflux of DA in the Str (Heal et al. 2008). Striatal uptake of DA in the SHR has been reported to be slower (Leo et al. 2003; Myers et al. 1981) or not different (Li et al. 2007; Linthorst et al. 1990) versus the WKY, yet a higher concentration of DA transporters (DAT) in the Str of the SHR was found (Roessner et al. 2010; Watanabe et al. 1997). Finally, it has been demonstrated that extracellular DA levels in the NA are higher in the SHR compared to the WKY (Carboni et al. 2003). Thus, there still remains controversy surrounding the regulation of DA release and uptake in the SHR.
One of the issues with the previously described studies is that the strains of the SHRs and WKYs were not properly defined, as the importance of the lineage of these strains was not yet understood. There might also be an issue with comparing these studies, as the techniques used to study DA regulation ranged from in vitro superfusion of brain slices to in vivo microdialysis. Microdialysis has been the dominant technique for in vivo measures in the SHR; however, this methodology varies across studies, with differing sampling times, flow rates, and probe sizes. Therefore, comparisons from prior studies can be compromised due to a variety of experimental variables. Furthermore, it has been shown that the microdialysis probes can cause extensive damage to the surrounding tissues (Clapp-Lilly et al. 1999; Rutherford et al. 2007), which can greatly affect neurotransmitter function. Recently, it was discovered that microdialysis probes significantly alter presynaptic dopaminergic dynamics in the rodent striatum (Wang and Michael 2012). Because of this, specialized techniques have been developed to evaluate DA dynamics in addition to microdialysis. These include electrochemical techniques such as fast-scan cyclic voltammetry, constant potential amperometry, and high-speed chronoamperometry (Lee et al. 2006; Littrell et al. 2012; Park et al. 2011; Zhang et al. 2011). Fast-scan cyclic voltammetry allows for high chemical and spatial resolution (Owesson-White et al. 2012; Robinson et al. 2003), but it has rarely been used to map dopaminergic nerve terminal density profiles in discrete brain regions in vivo (Chadchankar and Yavich 2011; Zhang et al. 2011). Also, this technique has rarely been used in conjunction with local application of chemicals from micropipettes placed adjacent to the microelectrodes in order to map the density of DA uptake and release from nerve terminals in a given brain area (Bergstrom et al. 2011; Howard et al. 2011; Owesson-White et al. 2012; Park et al. 2011; Sugam et al. 2012; Wang and Michael 2012). Constant potential amperometry has exceptional temporal and spatial resolution but is incapable of identifying the predominant contributors to the electrochemical response as with both fast-scan cyclic voltammetry and high-speed chronoamperometry (Lee et al. 2006; Schonfuss et al. 2001). Thus, researchers have begun to employ the power of high-speed chronoamperometry combined with local application of drugs from micropipettes to map the in vivo dynamics of release and uptake of dopamine in multiple sub-regions within specific brain regions, such as the striatum and nucleus accumbens (Littrell et al. 2012; Womersley et al. 2011).
In this study, the use of carbon fiber microelectrodes coupled to pressure-ejection of drugs allowed for the sub-regional mapping of DA nerve terminal properties with rapid temporal and spatial resolution. This technique allowed for better in vivo characterization of DA signaling closer to the synapse than with other techniques (Joyce et al. 2007; Littrell et al. 2012). In the present study, the information concerning the best control animals for the SHR and WKY models of ADHD was used to study DA release and uptake in sub-regions within the striatum and nucleus accumbens to better understand dopamine signaling and its regulation in animal models of ADHD-C and ADHD-PI.
2. Materials and methods
2.1 Animal Preparation for Acute Electrochemical Recordings
Male, 8–10 weeks old, spontaneously hypertensive rats (SHR/NCrl, average 225 g, average PND 60), Wistar Kyoto rats (WKY/NCrl, average 210 g, average PND 61), and Sprague Dawley rats (SD/NCrl, average 289 g, average PND 69) were obtained from Charles River Laboratories (NCrl), Wilmington, Massachusetts. A second group of Wistar Kyoto rats (WKY/NHsd, average 202 g, average PND 62) was obtained from Harlan Laboratories (NHsd), Indianapolis, Indiana. Animals were given access to food and water ad libitum and housed in a 12 hour light/dark cycle. Rats were anesthetized intraperitonealy (i.p.) using a 25% urethane solution (1.25 g/kg) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, California). A circulating heating pad (Gaymar Industries, Inc., Orchard Park, New York) was used to maintain body temperature. The skull overlying the striatum was removed bilaterally for recordings in the striatum (Str, AP +1.0, ML ±2.5, DV −4.0 to −6.5 in 0.5 mm increments) and the nucleus accumbens core (NAc, AP +1.0, ML ±2.5, DV −7.0 to −7.5 in 0.5 mm increments) – see Figure 1A (Paxinos and Watson 2009). A small hole remote from the site of surgery was drilled for placement of the miniature Ag/AgCl reference electrode. Protocols for animal use were approved by the Institutional Animal Care and Use Committee, which is Association for Assessment and Accreditation of Laboratory Animal Care International approved. All procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and all efforts were made to minimize animal suffering and to reduce the number of animals used.
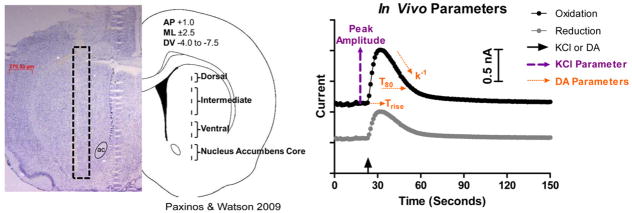
Figure 1.
Experimental methodology. A) Carbon fiber microelectrode placement within the rodent Str and NAc. The diagram on the right shows the approximate location of the tip of the carbon fiber microelectrode, and the left side shows the actual carbon fiber track (highlighted with the black box) from a 20 μm slice stained with cresyl violet. The carbon fiber microelectrode was lowered in 500 μm increments in both hemispheres (one hemisphere for KCl recordings and one for DA) and the average of these recordings was used to give one data point per sub-region. ac, anterior commissure. B) In Vivo Parameters. The arrow on the x axis indicates the time point of local application of KCl or DA. The KCl parameters used were the maximum amplitude of evoked DA release, represented by the purple dashed lines, and the ratio of the reduction/oxidation current (solid black line divided by the solid grey line). DA parameters included the time it took to clear 80% of the exogenous DA in reference to the maximum amplitude (T80), the curve fit based on first order fitting of the signal decay (k−1), and the amount of time it took to reach the peak amplitude (Trise), which are all represented by the orange dashed lines.
2.2 High-Speed Chronoamperometric Recordings of DA Release and Uptake
High-speed chronoamperometric measurements (1 Hz sampling rate, 200 ms total) were performed using the FAST16mkII recording system (Fast Analytical Sensing Technology, Quanteon, LLC, Nicholasville, Kentucky) as described previously (Gerhardt and Hoffman 2001; Littrell et al. 2012). Single carbon fiber electrodes (SF1A; 30 μm outer diameter × 150 μm length; Quanteon, LLC, Nicholasville, Kentucky) were coated with Nafion® (5% solution, 1–3 coats at 180°C, Aldrich Chemical Co., Milwaukee, Wisconsin) prior to an in vitro calibration used to determine selectivity, limit of detection, and slope before use in vivo (Gerhardt and Hoffman 2001). The average selectivity for all microelectrodes used in these experiments was 1877 ± 664 μM for DA vs. ascorbic acid. The average limit of detection for the measurement of DA was 0.028 ± 0.008 μM (S/N of 3). The average slope for the electrodes was −0.492 ± 0.111 nA/μM DA. The average red/ox ratio measured during the peak response of the potassium-evoked DA signals was 0.51 ± 0.11, which is indicative of the detection of predominantly DA (Gerhardt and Hoffman 2001; Joyce et al. 2007; Littrell et al. 2012). Finally, miniature Ag/AgCl reference electrodes were prepared as previously described (Littrell et al. 2012).
2.3 In Vivo Experimental Protocol
The carbon fiber microelectrode was affixed to a micropipette (10 μm inner diameter) which was positioned approximately 200 μm from the carbon fiber electrode tip using sticky wax (Kerr USA, Romulus, Michigan). The micropipette was filled with filtered isotonic KCl (120 mM KCl, 29 mM NaCl, 2.5 mM CaCl2·2H2O) solution (pH 7.2–7.4) using a 4 inch filling needle (Cadence Inc., Staunton, Virginia) and a 5 ml syringe. Experiments were initiated with the insertion of the micropipette/microelectrode assembly into a stereotactically selected region of the left or right hemisphere’s Str. The system was allowed to reach a stable baseline dorsal to the first recording site. The micropipette/microelectrode assembly was then lowered to the target recording depth where the signal was again allowed to stabilize for an average of 5 minutes before the effect of a single local application of KCl on DA release was determined (Joyce et al. 2007; Lundblad et al. 2009; Morris et al. 2011; Nevalainen et al. 2011; Thomas et al. 2007). The potassium solution was locally applied by pressure ejection (5–25 psi for 0.5 seconds). A single application of a set volume of KCl (75–125 nl) was delivered to each sub-region and was measured by determining the amount of fluid ejected from the micropipette using a dissection microscope fitted with an eyepiece reticule that was calibrated so that 1 mm of movement was equivalent to 25 nl of fluid ejected (Cass et al. 1992; Friedemann and Gerhardt 1992). If the volume was determined to be greater than or less than 75–125 nl, then that data point was excluded.
After the KCl studies, the micropipette/microelectrode assembly was filled with filtered isotonic 200 μM DA solution containing 100 μM ascorbic acid (an anti-oxidant) in 0.9% saline (pH 7.2–7.4). The micropipette/microelectrode assembly was then inserted stereotactically into the contralateral Str. Again, once a stable baseline was achieved in a location dorsal to the first recording site, the micropipette/microelectrode assembly was lowered to the target recording depth where the signal was again allowed to stabilize for an average of 5 minutes before the DA solution was locally applied by pressure ejection (10–30 psi for 0.5–10 s) to achieve a maximum amplitude ranging from 0.5 to 1.0 μM DA (Littrell et al. 2012). The maximum concentration of the DA in the extracellular space was measured by subtracting the apex of the recorded peak from the baseline recorded prior to the ejection. If the peak amplitude was greater than or less than 0.5 to 1.0 μM DA, then that data point was excluded.
2.4 Materials
Urethane, dopamine, ascorbic acid, sodium chloride, potassium chloride, calcium chloride and Nafion® were obtained from Sigma (St. Louis, MO). Carbon fiber microelectrodes (SF1A’s) were fabricated by the Center for Microelectrode Technology.
2.5 Histology
Brains were removed and processed (frozen) for histological evaluation of microelectrode recording tracks. Only data from histologically confirmed placement of microelectrodes into the Str and NAc were used for final data analysis. Based on histological analyses, no animals were excluded due to microelectrode placement errors (see Figure 1A).
2.6 Data Analyses
Collected data were processed using a custom Matlab®-based analysis package. For the potassium-evoked DA release portion of the study, maximum amplitude of the evoked DA peak was used as the major analysis parameter, with the volume of a single ejection of the KCl and the red/ox ratio – used to confirm the detection of DA by the Nafion®-coated microelectrodes – represented in Table 1. The volume of KCl applied was kept constant across depths and strains (75–125 nl). For DA uptake studies, the primary parameters used were the time to 80% decay of the DA signal (T80) and the first order rate constant of the signal (k−1), as well as the amount of time it took to reach the peak amplitude (Trise) – see Table 2. DA signals were amplitude matched (ranging from 0.5 to 1.0 μM DA) to ensure accurate measurement of DA uptake kinetics (Littrell et al. 2012). All data were averaged to one point per sub-region of the Str (dorsal, intermediate, and ventral) and the NAc – see Figure 1A. For a graphic representation of all parameters, see Figure 1B. Outliers were excluded via the Grubb’s test before averaging if the conditions for homogeneity of variance were met. To compare KCl-evoked DA release in the separate ADHD models to the control strains, as well as DA uptake, two-way repeated measures ANOVAs followed by Bonferroni post-hoc comparisons were used. Significance was set at p<0.05 (GraphPad Prism 5.0).
Table 1.
KCl-Evoked Dopamine Release.
| Sub-Region | SHR/NCrl | WKY/NCrl | WKY/NHsd | SD/NCrl | |
|---|---|---|---|---|---|
| ADHD-C | ADHD-PI | Control | Control | ||
|
|
|
|
|||
| Red/Ox | Dorsal Str | 0.58 ± 0.07 | 0.53 ± 0.11 | 0.47 ± 0.12 | 0.50 ± 0.08 |
| Intermediate Str | 0.47 ± 0.06 | 0.58 ± 0.11 | 0.51 ± 0.07 | 0.65 ± 0.05 | |
| Ventral Str | 0.45 ± 0.06 | 0.64 ± 0.20 | 0.55 ± 0.09 | 0.62 ± 0.10 | |
| NA Core | 0.52 ± 0.10 | 0.57 ± 0.07 | 0.51 ± 0.06 | 0.66 ± 0.07 | |
|
|
|
|
|||
| Volume (nl) | Dorsal Str | 92 ± 4 | 89 ± 4 | 95 ± 2 | 94 ± 2 |
| Intermediate Str | 92 ± 3 | 94 ± 3 | 93 ± 3 | 93 ± 2 | |
| Ventral Str | 92 ± 4 | 94 ± 4 | 95 ± 3 | 93 ± 3 | |
| NA Core | 97 ± 3 | 94 ± 5 | 98 ± 1 | 95 ± 1 | |
Similar reduction/oxidation ratios were obtained when the KCl was applied, all indicative for the presence of dopamine. The volumes of KCl applied between strains were not significantly different (mean ± SEM).
Table 2.
Dopamine Uptake.
| Sub-Region | SHR/NCrl | WKY/NCrl | WKY/NHsd | SD/NCrl | |
|---|---|---|---|---|---|
| ADHD-C | ADHD-PI | Control | Control | ||
|
|
|
|
|||
| k−1 (sec−1) | Dorsal Str | 0.007 ± 0.001 | 0.009 ± 0.003 | 0.014 ± 0.003 | 0.014 ± 0.003 |
| Intermediate Str | 0.011 ± 0.003 | 0.009 ± 0.004 | 0.013 ± 0.003 | 0.009 ± 0.001 | |
| Ventral Str | 0.010 ± 0.003 | 0.009 ± 0.004 | 0.011 ± 0.003 | 0.009 ± 0.001 | |
| NA Core | 0.008 ± 0.002 | 0.008 ± 0.003 | 0.006 ± 0.001 | 0.008 ± 0.001 | |
|
|
|
|
|||
| Trise (sec) | Dorsal Str | 7.95 ± 1.15 | 7.07 ± 0.94 | 7.85 ± 1.51 | 7.85 ± 1.51 |
| Intermediate Str | 5.71 ± 0.41 | 7.28 ± 0.75 | 8.24 ± 0.78 | 8.91 ± 3.38 | |
| Ventral Str | 6.71 ± 1.38 | 7.64 ± 0.79 | 9.43 ± 0.83 | 10.3 ± 2.73 | |
| NA Core | 9.25 ± 0.50 | 9.36 ± 0.85 | 12.9 ± 1.71 | 12.6 ± 3.5 | |
|
|
|
|
|||
| Peak Amplitude (μM) | Dorsal Str | 0.89 ± 0.04 | 0.83 ± 0.04 | 0.75 ± 0.04 | 0.73 ± 0.04 |
| Intermediate Str | 0.74 ± 0.03 | 0.79 ± 0.04 | 0.77 ± 0.04 | 0.73 ± 0.02 | |
| Ventral Str | 0.72 ± 0.04 | 0.74 ± 0.04 | 0.69 ± 0.03 | 0.82 ± 0.03 | |
| NA Core | 0.73 ± 0.06 | 0.79 ± 0.02 | 0.66 ± 0.04 | 0.74 ± 0.03 | |
k−1 values and the amount of time it took to reach the peak amplitude (Trise) did not vary between strains. Similar amplitudes of exogenous DA were applied to the Str and NAc and did not significantly differ (mean ± SEM).
3. Results
3.1 Evoked Dopamine Release
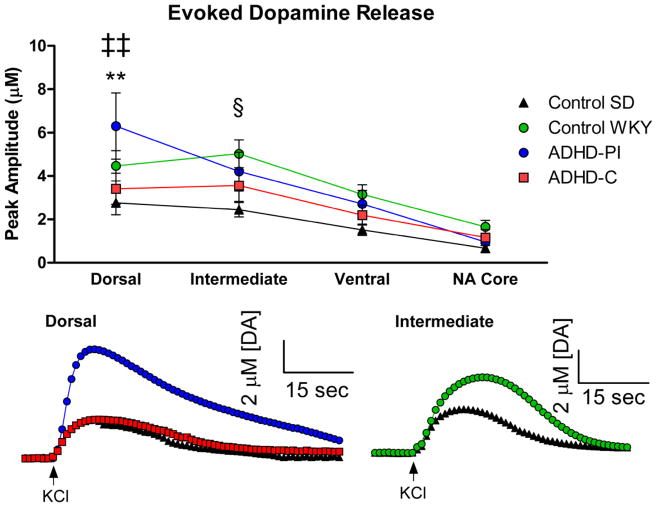
High-speed chronoamperometry coupled with carbon fiber microelectrodes was used to evaluate KCl-evoked DA release because of its capability to record DA release within sub-regions of the Str and the NAc (Littrell et al. 2012) using a local application of 75–125 nl KCl applied in 500 μm increments along the dorso-ventral axis. A two-way repeated measures ANOVA revealed a significant effect of depth (F3,27=33.03, p<0.0001) and interaction between strain and depth (F9,81=2.04, p<0.05) in the peak amplitude of KCl-evoked DA release. Bonferroni post-hoc comparisons revealed that the SHR model of ADHD-C (n=8) displayed significantly decreased KCl-evoked DA release versus the WKY model of ADHD-PI (n=8) in the dorsal Str (Figure 2A, 2B: p<0.01). Differences in KCl-evoked DA release were also observed between the control SD (n=7) and WKY model of ADHD-PI in the dorsal Str (Figure 2A, 2B: p<0.01), and the control SD and control WKY (n=8) strains in the intermediate region of the Str (Figure 2A, 2C: p<0.05). There were no significant differences in volumes applied between strains. In addition, there were no significant differences in the red/ox ratios (usually 0.4–0.6) throughout the Str and NAc in all strains, supporting the detection of predominantly DA in all sub-regions (Gerhardt and Hoffman 2001; Joyce et al. 2007; Littrell et al. 2012) – see Table 1. It should be noted that the amplitudes of the KCl-evoked signals decreased as the electrode moved ventrally in all strains, consistent with previous reports that the ventral Str has reduced vesicular-released concentrations of DA when compared to the dorsal Str (Gerhardt et al. 1986).
Figure 2.
Decreased KCl-evoked DA release in the SHR model of ADHD-C. A) The model of ADHD-C demonstrated significantly decreased KCl-evoked DA release versus the model of ADHD-PI in the dorsal Str (**p<0.01). The control SD also displayed decreased KCl-evoked DA versus the model of ADHD-PI in the dorsal Str (‡‡p<0.01). In the intermediate Str, it was observed that the control WKY displayed significantly increased KCl-evoked DA release versus the control SD strain (§p<0.05). Mean ± SEM. B) Representative tracing of the difference in KCl-evoked DA release in the dorsal Str between the model of ADHD-C, the model of ADHD-PI, and the control SD. C) Representative tracing of the difference in KCl-evoked DA release in the intermediate Str between the control SD and control WKY strains. Arrows indicate local application of KCl.
3.2 Dopamine Uptake
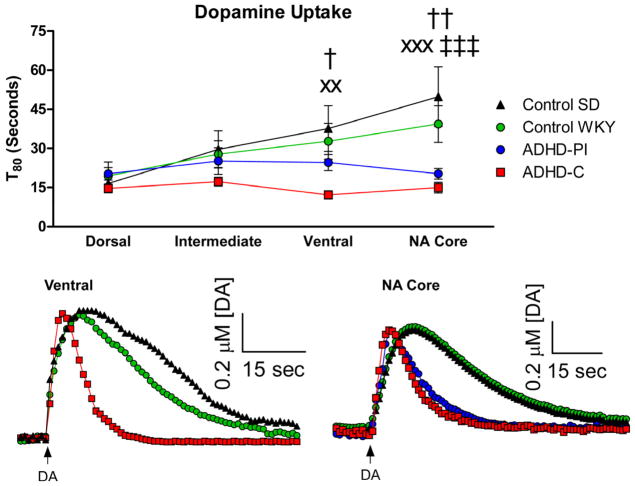
Local applications of exogenous DA were applied in the separate strains to study the functional properties of the dopamine transporter (Littrell et al. 2012). A two-way repeated measures ANOVA revealed a significant effect of depth (F3,30=7.49, p<0.001), strain (F3,30=3.93, p<0.05), and interaction between strain and depth (F9,90=3.16, p<0.01) in the length of time required to clear 80% of the locally applied exogenous DA (or T80). Bonferroni post-hoc comparisons revealed that the SHR model of ADHD-C (n=9) displayed significantly faster DA uptake in the ventral Str versus both the control WKY (n=8) (p<0.05) and control SD strains (Figures 3A, 3B: p<0.01). The SHR model of ADHD-C also displayed significantly faster uptake in the NAc versus both the control WKY (p<0.01) and control SD (Figure 3A, 3C: p<0.001). The WKY model of ADHD-PI was found to exhibit significantly faster DA uptake in the NAc versus the control SD (Figure 3A, 3C: p<0.001). In the control strains, the time to clear the locally applied exogenous DA was slower in the NAc when compared to the dorsal Str, supporting previous data that in the rodent, the NAc has lower concentrations of the dopamine transporter and therefore slower DA uptake rates (Calipari et al. 2012; Cass et al. 1993a; Cass et al. 1992; Cass et al. 1993b; Hebert et al. 1999; Mitch Taylor et al. 2012; Moquin and Michael 2011). Interestingly, the rodent models of ADHD did not display a dorsal-ventral change in dopamine uptake. There were no differences between the maximum amplitude achieved nor were there any differences in the k−1 values. Finally, there were no differences in the time required to reach peak amplitude (Trise) between the models of ADHD and control strains – see Table 2.
Figure 3.
DA uptake in the SHR model of ADHD-C, WKY model of ADHD-PI and control strains. A) A DA uptake depth profile through the Str and NA core revealed that B) in the ventral Str, the model of ADHD-C displayed faster DA uptake versus both the control SD (xxp<0.01) and the control WKY (†p<0.05). C) In the NAc, the model of ADHD-C exhibited faster DA uptake versus the control SD (xxxp<0.001) and the control WKY (††p<0.01). The model of ADHD-PI displayed faster DA uptake versus the control SD (‡‡‡p<0.001) in the NAc. Arrows indicate local applications of DA. Mean ± SEM.
4. Discussion
The SHR has been used for decades as a model of ADHD because of its various behavioral phenotypes that mimic the symptoms of ADHD. Its progenitor strain, the WKY, has been used as a control for the SHR in the past with no regard for where the strain originated. Recent evaluations of this control strain have revealed that not all WKY strains are identical, genetically or behaviorally. The NIH stock of the WKY strain was obtained in 1971 as outbred animals from the Kyoto School of Medicine. These animals were distributed to laboratories such as Harlan and Charles River before the F20 generation, which is considered to be the gold standard in obtaining a pure inbred animal (Sagvolden et al. 2009). It has recently been shown that two commonly used WKY strains, the WKY/NCrl from Charles River Laboratories and the WKY/NHsd from Harlan Laboratories, display wide genetic divergence (Sagvolden et al. 2008). When Harlan and Charles River Laboratories were contacted, both specified that all of their inbred animals, both at the USA and international laboratories, were subjected to single nucleotide polymorphism panels every quarter, thus these strains from the different laboratories will remain genetically separate. Although no behavioral data were gathered in this study, multiple studies have concluded that the WKY/NCrl from Charles River is not a valid control for the SHR due to its behavioral abnormalities (i.e. inattention) and is more appropriate as a model of ADHD inattentive type (Drolet et al. 2002; Roessner et al. 2010; Sagvolden et al. 2008; Sagvolden and Johansen 2011; Sagvolden et al. 2009). Terje Sagvolden (1945–2011), a leading researcher in the SHR/ADHD field, proposed this change and concluded that the most appropriate models of ADHD are the SHR/NCrl (ADHD-C) and the WKY/NCrl (ADHD-PI) (Roessner et al. 2010; Sagvolden et al. 2009). As for the most suitable control strain, Sagvolden and colleagues determined that the inbred WKY/NHsd strain from Harlan Laboratories, not the outbred Sprague Dawley (SD) (Sagvolden and Johansen 2011; Sagvolden et al. 2009), was the best fit control for the models of ADHD. However, the outbred SD strain from Charles River (SD/NCrl) was tested in this study as a potential control because some researchers have supported it as a way around the WKY control strain confusion (Drolet et al. 2002). Although the present study does not discount the many behavioral studies that have used the outbred SD as a control for the SHR, it does suggest that studies looking at neurochemical differences in ADHD should focus on the inbred WKY due to a difference observed in the evoked DA release parameter in the intermediate Str between these control strains. Based on this difference in DA regulation, this study proposes that the inbred SHR progenitor strain, the WKY/NHsd, is the most appropriate control for neurochemical investigations in the SHR.
A major purpose of this research was to help resolve the confusion over DA regulation in the SHR model of ADHD-C compared to the novel WKY model of ADHD-PI and the control strains by exploring DA release and uptake dynamics. The current experiments were performed using high-speed chronoamperometry coupled with Nafion®-coated carbon fiber microelectrodes. This technique has higher spatial and temporal resolution, decreased damage to surrounding tissue and samples a much smaller field of DA nerve terminals along the dorso-ventral axis compared to most techniques used in the past (e.g. in vivo microdialysis) (Joyce et al. 2007; Littrell et al. 2012). The reported neurochemical studies in the SHR prior to 2008 were unable to distinguish between sub-regions along the dorso-ventral axis in the Str due to the size of the microdialysis probes; however, the technology used in this study was able to provide a higher resolution representation of the DA dynamics within the heterogeneous Str (Gerfen et al. 1987a; Gerfen et al. 1987b; Lindvall and Bjorklund 1974; Veening et al. 1982).
To determine differences in DA release, a single local ejection of a set volume of potassium chloride was used to cause calcium-dependent vesicular release of DA. The results of this study reveal that the SHR model of ADHD-C exhibits decreased depolarization-evoked DA release in the dorsal Str versus the WKY model of ADHD-PI. This decrease in DA release could be due to a decrease in stored vesicular DA (Russell et al. 1998) because of a potential dysfunction of monoamine oxidase-B (MAO-B), an enzyme that catalyzes the oxidation of DA in DA-producing neurons. It was recently found that an MAO-B inhibitor, deprenyl, significantly improved ADHD symptoms (Feigin et al. 1996; Jankovic 1993; Mohammadi et al. 2004; Rubinstein et al. 2006). Moreover, it is possible that the vesicular monoamine transporter (VMAT) may be dysfunctional and as a result, DA may not be efficiently transported into the vesicles. It has been found that a common ADHD treatment, d-amphetamine, causes increased VMAT trafficking to the vesicular membrane within monoaminergic nerve terminals ex vivo (Riddle et al. 2007), thus supporting a possible role for VMAT dysfunction in ADHD.
Previous investigations have implicated the dopamine transporter (DAT) in the DA dysfunction of the SHR model of ADHD-C – see Table 3 (Leo et al. 2003; Roessner et al. 2010; Simchon et al. 2010; Viggiano et al. 2004; Watanabe et al. 1997). In the SHR brain, there have been reports of increased DAT expression (Roessner et al. 2010; Watanabe et al. 1997) and one likely explanation for the decreased DA release observed in the dorsal Str is over-abundance or over-activity of DAT. It has been shown that clearance of DA is primarily performed by DAT rather than metabolism or diffusion of DA (Cass et al. 1993b). In this study, potassium stimulation in the dorsal Str of the SHR produced a significantly smaller signal amplitude than in the WKY model of ADHD, suggesting that the DA was likely cleared through uptake by DAT before it reached our microelectrode. To the best of our knowledge, no study has been able to quantify levels of DAT in specific striatal sub-regions on the dorso-ventral axis; however, the ratio of DA to DAT to DA receptors is similar in the naïve rodent Str and NAc (Madras et al. 2005), supporting that DAT is the major regulator of DA neuron signaling strength and duration. However, when DA uptake was examined in the dorsal Str sub-region by locally applying exogenous DA, no differences in DA uptake were observed between these strains. It is worth noting that DA uptake was not studied directly after the local application of KCl because KCl stimulation allows for the direct examination of the release capacity output of the surrounding terminals. If the uptake dynamics of these signals were studied, changes in uptake may not be seen due to the overwhelming concentrations, or ‘ceiling effect,’ of the neurotransmitter and this effect would mask DAT productivity. In order to fully study DA regulation in these strains, exogenously applied DA was used to examine uptake dynamics. While no differences in uptake were found in the dorsal Str, it is possible that exogenously applied DA may not be as efficiently removed by DAT as the DA released inside the synapse. In addition, KCl depolarizes the DA nerve terminal membrane causing synaptic release of DA, creating the possibility that the action potential could potentiate some of the differences observed in DA uptake. The DAT is known to be electrogenic and depolarization causes the DAT to exist in a different state than the basal state (El Ayadi et al. 2001; Hoffman et al. 1999; Kandasamy 2000; Reith et al. 1991; Zahniser et al. 1998). By testing with local applications of DA rather than by using depolarization alone, the current study was able to prevent the activation of DAT to fully focus on the uptake capability of the protein, which showed no differences in the SHR versus the other strains in the dorsal Str. While this KCl-evoked difference was not observed in the other striatal sub-regions in our study, DAT location is not homogenous throughout the Str (Hebert et al. 1999) and the possibility exists that when the KCl-evoked DA is released from the nerve terminals in the dorsal Str of the SHR model of ADHD-C, the DAT is the primary mechanism to clear the released DA.
Table 3.
Dopamine Transporter Function in the SHR.
| DAT Function | Study |
|---|---|
| ↑ | Current |
| ↑ | Roessner, Sagvolden et al. 2010 |
| ↑ | Watanabe, Fujita et al. 1997 |
| = | Li, Lu et al. 2007 |
| = | Linthorst, Van den Buuse et al. 1990 |
| ↓ | Leo, Sorrentino et al. 2003 |
Present and past data on dopamine transporter function in the SHR model of ADHD reveal conflicting results versus control, likely due to unknown strains and variable techniques.
Interestingly, in the ventral striatum and nucleus accumbens core (NAc), it was discovered that the SHR model of ADHD-C demonstrated faster DA uptake compared to both of the control strains, both of which are areas highly implicated in impulsive ADHD behaviors (Basar et al. 2010). These results may provide neurochemical evidence for this ADHD-like behavior observed in the SHR (Johansen and Sagvolden 2004; Russell 2011; Sagvolden 2000; Sagvolden et al. 1992; Wultz and Sagvolden 1992). As mentioned previously, clearance of DA is primarily performed by DAT rather than metabolism or diffusion (Cass et al. 1993b). Therefore, it is reasonable to assume that the faster uptake observed in the rodent model of ADHD-C is due to differences in DAT expression, activity, and/or affinity for dopamine. Michaelis-Menten kinetics dictates that two variables affect an enzyme’s productivity, affinity and velocity. The k−1 parameter (the first-order rate constant) is a measure of velocity and remained unchanged in all strains. This suggests that the affinity of DAT for DA is increased in the ventral Str and NAc of the rodent model of ADHD-C. Increased affinity of DAT for DA could be due to increased concentrations of the DAT at the synaptic membrane resulting from increased DAT trafficking, which, to our knowledge, has not been investigated in specific striatal sub-regions. Because the SHR has been found to possess increased DAT expression (Roessner et al. 2010; Wallis 2010; Watanabe et al. 1997), the results from this study suggest that increased DAT expression is coupled to increased DAT function at a synaptic level. No strain differences were observed in depolarization-evoked DA release in the NAc; however, the faster DA uptake when exogenous DA was applied supports that the DA is cleared by an amplified number of DATs or enhanced DAT activity. Future studies should focus on quantifying the levels of DAT in the separate striatal sub-regions to obtain more precise evidence of the role of DAT in the rodent models of ADHD.
Finally, in the dorsal Str, the WKY model of ADHD-PI displayed increased evoked DA release as well as faster DA uptake in the NAc when compared to the control SD strain. These findings are in agreement with a previous study which showed that behavioral activity in running wheels was negatively correlated with in vitro K+-stimulated DA release in rat NAc and dorsal Str but not in the shell division of the NA (Tarr et al. 2004).
The present study uses a neurochemical technique to complement the established behavioral evidence indicating that the SHR/NCrl is a useful rodent model of ADHD-C. The decreased KCl-evoked DA release observed in the dorsal sub-region of the Str may aid in the explanation of why the SHRs express some behavioral aspects of ADHD, as the dorsal Str of the rat is akin to the human putamen (Grahn et al. 2008) and is believed to play a role in motor activity (Grahn et al. 2008). The results from this study reveal that the hyperactive SHR has DA dysfunction in this region compared to the less active WKY/NCrl. The findings of DA dysfunction in the ventral Str and NAc of the SHR is consistent with the understanding that the more ventral Str is associated with impulsive behaviors (Basar et al. 2010). Finally, the NAc may play a role in the inattention observed in individuals with ADHD (Volkow et al. 2009; Volkow et al. 2011) and the differences in DA uptake between the SHR/NCrl and WKY/NCrl models of ADHD and the control strains provide preliminary neurochemical evidence for the inattentive behavior observed in these strains; however, more evidence is needed to confirm the speculation that DAT is altered in discrete sub-regions of the Str and NAc in the SHR/NCrl and WKY/NCrl.
In conclusion, the results from this study demonstrate that the SHR/NCrl model of ADHD-C and the WKY/NCrl model of ADHD-PI have distinct differences in the regulation of DA release and uptake in the Str and NAc compared to each other as well as compared to the WKY and SD control strains. The results from this study further reveal that the SD strain may not be a useful control when investigating neurochemical changes in the rodent models of ADHD. However, because of increasing evidence that ADHD is not limited to purely DA dysfunction, it will be valuable to study other neurotransmitter systems in the future, such as norepinephrine and glutamate (Heal et al. 2008; Kotecha et al. 2002; Madras et al. 2005; Russell 2001; 2002), and how they relate to the SHR and WKY rodent models of ADHD.
Highlights.
Differences in dopamine release exist between ADHD models in dorsal striatum.
Dopamine uptake differences between ADHD-C model and controls in ventral striatum.
Dopamine uptake differences between ADHD models and controls in nucleus accumbens.
Acknowledgments
The authors would like to thank Ofelia Meagan Littrell and Kurt Myers for their technical support, and Jason Hinzman, Kelli Frueh, and Seth Batten for helpful editing of this manuscript. This study was supported by USPHS grants MH070840, AG13494, and 5T32AG000242-13, NSF EEC-0310723, and DARPA N66001-09-C-2080.
Footnotes
5. Disclosure/Conflict of Interest
The authors disclose that Greg A. Gerhardt is the owner of Quanteon Limited Liability Company (Nicholasville, KY). Quanteon developed the FAST system utilized for these studies. No financial support was provided on behalf of Quanteon. The authors, therefore, agree that none have financial support, compensation, and personal financial holdings that could be perceived as constituting a potential conflict of interest. All authors have full control of the primary data and thus agree to allow the journal to review data if requested.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin M. Miller, Email: emmo222@uky.edu.
Francois Pomerleau, Email: francois.pomerleau@uky.edu.
Peter Huettl, Email: pfhuet2@uky.edu.
Vivienne A. Russell, Email: vivienne.Russell@uct.ac.za.
Greg A. Gerhardt, Email: gregg@uky.edu.
Paul E.A. Glaser, Email: pglas0@uky.edu.
References
- Alsop B. Problems with spontaneously hypertensive rats (SHR) as a model of attention-deficit/hyperactivity disorder (AD/HD) J Neurosci Methods. 2007;162:42–8. doi: 10.1016/j.jneumeth.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–57. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bergstrom BP, Sanberg SG, Andersson M, Mithyantha J, Carroll FI, Garris PA. Functional reorganization of the presynaptic dopaminergic terminal in parkinsonism. Neuroscience. 2011;193:310–22. doi: 10.1016/j.neuroscience.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Huggins KN, Mathews TA, Jones SR. Conserved dorsal-ventral gradient of dopamine release and uptake rate in mice, rats and rhesus macaques. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Valentini V, Di Chiara G. Effect of amphetamine, cocaine and depolarization by high potassium on extracellular dopamine in the nucleus accumbens shell of SHR rats. An in vivo microdyalisis study. Neurosci Biobehav Rev. 2003;27:653–9. doi: 10.1016/j.neubiorev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced clearance of exogenous dopamine in rat nucleus accumbens, but not in dorsal striatum, following cocaine challenge in rats withdrawn from repeated cocaine administration. J Neurochem. 1993a;61:273–83. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59:259–66. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J Neurochem. 1993b;61:2269–78. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Chadchankar H, Yavich L. Sub-regional differences and mechanisms of the short-term plasticity of dopamine overflow in striatum in mice lacking alpha-synuclein. Brain Res. 2011;1423:67–76. doi: 10.1016/j.brainres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–42. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Drolet G, Proulx K, Pearson D, Rochford J, Deschepper CF. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar rat strains. Neuropsychopharmacology. 2002;27:400–9. doi: 10.1016/S0893-133X(02)00303-2. [DOI] [PubMed] [Google Scholar]
- El Ayadi A, Afailal I, Errami M. Effects of voltage-sensitive calcium channel blockers on extracellular dopamine levels in rat striatum. Metab Brain Dis. 2001;16:121–31. doi: 10.1023/a:1012549225235. [DOI] [PubMed] [Google Scholar]
- Feigin A, Kurlan R, McDermott MP, Beach J, Dimitsopulos T, Brower CA, Chapieski L, Trinidad K, Como P, Jankovic J. A controlled trial of deprenyl in children with Tourette’s syndrome and attention deficit hyperactivity disorder. Neurology. 1996;46:965–8. doi: 10.1212/wnl.46.4.965. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Gough BJ, Cada AM. In vivo basal and amphetamine-induced striatal dopamine and metabolite levels are similar in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley male rats. Physiol Behav. 2003;80:109–14. doi: 10.1016/s0031-9384(03)00214-2. [DOI] [PubMed] [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–32. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci. 1987a;7:3935–44. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987b;7:3915–34. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Hoffman AF. Effects of recording media composition on the responses of Nafion-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J Neurosci Methods. 2001;109:13–21. doi: 10.1016/s0165-0270(01)00396-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Rose GM, Hoffer BJ. Release of monoamines from striatum of rat and mouse evoked by local application of potassium: evaluation of a new in vivo electrochemical technique. J Neurochem. 1986;46:842–50. doi: 10.1111/j.1471-4159.1986.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav. 2008;90:184–97. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Larson GA, Zahniser NR, Gerhardt GA. Age-related reductions in [3H]WIN 35,428 binding to the dopamine transporter in nigrostriatal and mesolimbic brain regions of the fischer 344 rat. J Pharmacol Exp Ther. 1999;288:1334–9. [PubMed] [Google Scholar]
- Hoffman AF, Zahniser NR, Lupica CR, Gerhardt GA. Voltage-dependency of the dopamine transporter in the rat substantia nigra. Neurosci Lett. 1999;260:105–8. doi: 10.1016/s0304-3940(98)00951-3. [DOI] [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem. 2011;118:668–76. doi: 10.1111/j.1471-4159.2011.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Deprenyl in attention deficit associated with Tourette’s syndrome. Arch Neurol. 1993;50:286–8. doi: 10.1001/archneur.1993.00540030052014. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T. Response disinhibition may be explained as an extinction deficit in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2004;149:183–96. doi: 10.1016/s0166-4328(03)00229-8. [DOI] [PubMed] [Google Scholar]
- Joyce BM, Glaser PE, Gerhardt GA. Adderall produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers. Psychopharmacology (Berl) 2007;191:669–77. doi: 10.1007/s00213-006-0550-9. [DOI] [PubMed] [Google Scholar]
- Kandasamy SB. Possible involvement of L-type voltage-gated calcium channels in release of dopamine in the striatum of irradiated rats. Radiat Res. 2000;154:39–43. doi: 10.1667/0033-7587(2000)154[0039:pioltv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Knardahl S, Sagvolden T. Open-field behavior of spontaneously hypertensive rats. Behav Neural Biol. 1979;27:187–200. doi: 10.1016/s0163-1047(79)91801-6. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–22. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, la Fougere C, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:605–13. doi: 10.1016/j.neubiorev.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson’s disease. Eur J Neurosci. 2006;23:1005–14. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- Leo D, Sorrentino E, Volpicelli F, Eyman M, Greco D, Viggiano D, di Porzio U, Perrone-Capano C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci Biobehav Rev. 2003;27:661–9. doi: 10.1016/j.neubiorev.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Aust N Z J Psychiatry. 1991;25:277–83. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu G, Antonio GE, Mak YT, Rudd JA, Fan M, Yew DT. The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem Int. 2007;50:848–57. doi: 10.1016/j.neuint.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- Linthorst AC, De Lang H, De Jong W, Versteeg DH. Effect of the dopamine D2 receptor agonist quinpirole on the in vivo release of dopamine in the caudate nucleus of hypertensive rats. Eur J Pharmacol. 1991;201:125–33. doi: 10.1016/0014-2999(91)90335-n. [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Van den Buuse M, De Jong W, Versteeg DH. Electrically stimulated [3H]dopamine and [14C]acetylcholine release from nucleus caudatus slices: differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Brain Res. 1990;509:266–72. doi: 10.1016/0006-8993(90)90551-l. [DOI] [PubMed] [Google Scholar]
- Littrell OM, Pomerleau F, Huettl P, Surgener S, McGinty JF, Middaugh LD, Granholm AC, Gerhardt GA, Boger HA. Enhanced dopamine transporter activity in middle-aged Gdnf heterozygous mice. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, af Bjerken S, Cenci MA, Pomerleau F, Gerhardt GA, Stromberg I. Chronic intermittent L-DOPA treatment induces changes in dopamine release. J Neurochem. 2009;108:998–1008. doi: 10.1111/j.1471-4159.2008.05848.x. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Mitch Taylor I, Jaquins-Gerstl A, Sesack SR, Michael AC. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi MR, Ghanizadeh A, Alaghband-Rad J, Tehranidoost M, Mesgarpour B, Soori H. Selegiline in comparison with methylphenidate in attention deficit hyperactivity disorder children and adolescents in a double-blind, randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14:418–25. doi: 10.1089/cap.2004.14.418. [DOI] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. An inverse correlation between the apparent rate of dopamine clearance and tonic autoinhibition in subdomains of the rat striatum: a possible role of transporter-mediated dopamine efflux. J Neurochem. 2011;117:133–42. doi: 10.1111/j.1471-4159.2011.07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, Brooks WM, Gerhardt GA, Geiger PC, Stanford JA. Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MM, Whittemore SR, Hendley ED. Changes in catecholamine neuronal uptake and receptor binding in the brains of spontaneously hypertensive rats (SHR) Brain Res. 1981;220:325–38. doi: 10.1016/0006-8993(81)91221-x. [DOI] [PubMed] [Google Scholar]
- Nevalainen N, Af Bjerken S, Lundblad M, Gerhardt GA, Stromberg I. Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J Neurochem. 2011;118:12–23. doi: 10.1111/j.1471-4159.2011.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White C, Roitman M, Sombers L, Belle A, Keithley R, Peele J, Carelli R, Wightman R. Sources Contributing to the Average Extracellular Concentration of Dopamine in the Nucleus Accumbens. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–44. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press/Elsevier; Amsterdam; Boston: 2009. [Google Scholar]
- Podet A, Lee MJ, Swann AC, Dafny N. Nucleus accumbens lesions modulate the effects of methylphenidate. Brain Res Bull. 2010;82:293–301. doi: 10.1016/j.brainresbull.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Jacobson AE, Rice KC, Benuck M, Zimanyi I. Effect of metaphit on dopaminergic neurotransmission in rat striatal slices: involvement of the dopamine transporter and voltage-dependent sodium channel. J Pharmacol Exp Ther. 1991;259:1188–96. [PubMed] [Google Scholar]
- Riddle EL, Hanson GR, Fleckenstein AE. Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur J Pharmacol. 2007;571:25–8. doi: 10.1016/j.ejphar.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–91. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Rubinstein S, Malone MA, Roberts W, Logan WJ. Placebo-controlled study examining effects of selegiline in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16:404–15. doi: 10.1089/cap.2006.16.404. [DOI] [PubMed] [Google Scholar]
- Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Differences between electrically-, ritalin- and D-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of Attention-Deficit Hyperactivity Disorder. Behav Brain Res. 1998;94:163–71. doi: 10.1016/s0166-4328(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Russell VA. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat as studied in vitro by the superfusion slice technique. Neurosci Biobehav Rev. 2000;24:133–6. doi: 10.1016/s0149-7634(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Russell VA. Increased AMPA receptor function in slices containing the prefrontal cortex of spontaneously hypertensive rats. Metab Brain Dis. 2001;16:143–9. doi: 10.1023/a:1012584826144. [DOI] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2002;130:191–6. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD) Curr Protoc Neurosci. 2011;Chapter 9(Unit9):35. doi: 10.1002/0471142301.ns0935s54. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102:712–22. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Dasbanerjee T, Zhang-James Y, Middleton F, Faraone S. Behavioral and genetic evidence for a novel animal model of Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype. Behav Brain Funct. 2008;4:56. doi: 10.1186/1744-9081-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB. Rat Models of ADHD. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_126. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Woien G, Walaas SI, Storm-Mathisen J, Bergersen LH, Hvalby O, Jensen V, Aase H, Russell VA, Killeen PR, Dasbanerjee T, Middleton FA, Faraone SV. The spontaneously hypertensive rat model of ADHD--the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–26. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–12. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–47. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schonfuss D, Reum T, Olshausen P, Fischer T, Morgenstern R. Modelling constant potential amperometry for investigations of dopaminergic neurotransmission kinetics in vivo. J Neurosci Methods. 2001;112:163–72. doi: 10.1016/s0165-0270(01)00465-4. [DOI] [PubMed] [Google Scholar]
- Simchon Y, Weizman A, Rehavi M. The effect of chronic methylphenidate administration on presynaptic dopaminergic parameters in a rat model for ADHD. Eur Neuropsychopharmacol. 2010;20:714–20. doi: 10.1016/j.euroneuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Arnsten AFT, Castellanos FX. Stimulant drugs and ADHD: basic and clinical neuroscience. Oxford University Press; New York: 2001. [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol Psychiatry. 2012;71:199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr BA, Kellaway LA, St Clair Gibson A, Russell VA. Voluntary running distance is negatively correlated with striatal dopamine release in untrained rats. Behav Brain Res. 2004;154:493–9. doi: 10.1016/j.bbr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Kruzich PJ, Joyce BM, Gash CR, Suchland K, Surgener SP, Rutherford EC, Grandy DK, Gerhardt GA, Glaser PE. Dopamine D4 receptor knockout mice exhibit neurochemical changes consistent with decreased dopamine release. J Neurosci Methods. 2007;166:306–14. doi: 10.1016/j.jneumeth.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Cowan WM, Nieuwenhuys R, Geeraedts LM. The medial forebrain bundle of the rat. II. An autoradiographic study of the topography of the major descending and ascending components. J Comp Neurol. 1982;206:82–108. doi: 10.1002/cne.902060107. [DOI] [PubMed] [Google Scholar]
- Versteeg DH, Van Der Gugten J, De Jong W, Palkovits M. Regional concentrations of noradrenaline and dopamine in rat brain. Brain Res. 1976;113:563–74. doi: 10.1016/0006-8993(76)90057-3. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11:97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–54. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. The search for biomarkers for attention deficit/hyperactivity disorder. Drug News Perspect. 2010;23:438–49. doi: 10.1358/dnp.2010.23.7.1472296. [DOI] [PubMed] [Google Scholar]
- Wang Y, Michael AC. Microdialysis probes alter presynaptic regulation of dopamine terminals in rat striatum. J Neurosci Methods. 2012;208:34–9. doi: 10.1016/j.jneumeth.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Fujita M, Ito Y, Okada T, Kusuoka H, Nishimura T. Brain dopamine transporter in spontaneously hypertensive rats. J Nucl Med. 1997;38:470–4. [PubMed] [Google Scholar]
- Womersley JS, Hsieh JH, Kellaway LA, Gerhardt GA, Russell VA. Maternal separation affects dopamine transporter function in the Spontaneously Hypertensive Rat: An in vivo electrochemical study. Behav Brain Funct. 2011;7:49. doi: 10.1186/1744-9081-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wultz B, Sagvolden T. The hyperactive spontaneously hypertensive rat learns to sit still, but not to stop bursts of responses with short interresponse times. Behav Genet. 1992;22:415–33. doi: 10.1007/BF01066613. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Gerhardt GA, Hoffman AF, Lupica CR. Voltage-dependency of the dopamine transporter in rat brain. Adv Pharmacol. 1998;42:195–8. doi: 10.1016/s1054-3589(08)60726-7. [DOI] [PubMed] [Google Scholar]
- Zhang B, Heien ML, Santillo MF, Mellander L, Ewing AG. Temporal resolution in electrochemical imaging on single PC12 cells using amperometry and voltammetry at microelectrode arrays. Anal Chem. 2011;83:571–7. doi: 10.1021/ac102502g. [DOI] [PMC free article] [PubMed] [Google Scholar]