Abstract
A local melanocortin system is active during tissue injury and inflammation. Thus far this system has been described as autocrine in nature where local production of pro-opiomelanocortin (POMC) peptides by leukocytes feeds back on melanocortin receptor (MC-R) expressing immune cells to quell inflammatory cytokine production. Here we present evidence that POMC peptides may generate extracellular matrix (ECM) changes by inducing matrix production by cells of the mesenchymal lineage through activation of the MC2-R. Using immunoblot, we determined that mouse aorta-derived mesenchymal progenitor cells express both MC2-R and MC3-R. These progenitors respond to treatment with ACTH by increasing collagen matrix synthesis as assessed by picrosirius red stain and 3H-proline incorporation. ACTH also induces transient increases in intracellular calcium ([Ca2+]i) as assessed using the fluorescent Ca2+ indicator, fura- 2. The ACTH-induced changes in [Ca2+]i are consistent with MC2-R signaling and consist of both an intracellular release and an extracellular influx of Ca2+. Both mouse aortic mesenchymal progenitors and mouse macrophage cells express POMC and the prohormone convertase 1/3 (PC1/3) indicating they have the potential to contribute to the local production of POMC peptides. These data demonstrate functional MC2-R expression in mouse aorta-derived mesenchymal progenitors and implicate both macrophage and mesenchymal cells as relevant sources of local POMC peptides.
Keywords: proopiomelanocortin, ACTH, paracrine, mesenchymal, macrophage
1. Introduction
Pro-opiomelanocortin (POMC) is the precursor peptide to the melanocortin family of endocrine peptides. POMC is traditionally known as an endocrine prohormone secreted and processed by corticotropes of the anterior pituitary in response to hypothalamic corticotrophin releasing hormone. POMC is processed by prohormone convertases (PCs) into the melanocortin peptides. Cleavage via PC1/3 produces ACTH and β-lipotropin, whereas PC2 is required for the generation of α-melanocyte-stimulating hormone (α-MSH), β-MSH, γ-MSH and the endorphins (Bicknell 2008; Catania 2007; Cone 2006). The receptors for these peptides are a family of five G-protein coupled receptors, collectively known as melanocortin receptors (MC-R). MC1-R in melanocytes regulates the eumelanin-pheomelanin switch (Le Pape, et al. 2008). MC2-R regulates adrenocortical sterodiogeneisis when stimulated by ACTH (Cone 2006). MC3-R and MC4-R are highly expressed by hypothalamic neurons and are known to play a role in satiety and energy balance (Butler 2006; Cone 2006; Sutton, et al. 2006). The MC5-R is the most ubiquitously expressed melanocortin receptor and primarily known for its functional role in exocrine tissues (Cone 2006; van der Kraan, et al. 1998).
Recently, there has been an increased focus on the role the melanocortin system has in the resolution of inflammation. This area of investigation encompasses anti-inflammatory effects in the brain, gouty arthritis and during reperfusion injury (Catania 2007; Catania, et al. 2010; Gatti, et al. 2010; Getting, et al. 2001; Holloway, et al. 2011; Leoni, et al. 2010; Montero-Melendez, et al. 2011; Muceniece and Dambrova 2010; Patel, et al. 2010). Leukocytes produce POMC and melanocortin peptides, i.e. ACTH and α-MSH, in response to inflammatory mediators which then act in an autocrine fashion to reduce inflammatory cytokine production and leukocyte trafficking during inflammation (Catania 2007; Getting et al. 2001; Leoni et al. 2010; Patel et al. 2010). The MC1-R, MC3-R and MC5-R have been implicated as the mediators of these actions in immune cells.
The expression of MC-R in mesenchymal cell populations and mesenchymal progenitor cell populations is also well documented. The MC3-R is expressed in bone marrow- and calvariae-derived MSC populations (Evans, et al. 2004; Evans, et al. 2005). Expression of the MC2-R has been detected in murine MSC, human osteoblasts and human MSC (Zaidi, et al. 2010; Zhong, et al. 2005) and adipocytes express the MC2-R and MC5-R (Jun, et al. 2010). Evidence points to a role for the melanocortin system in the regulation of matrix synthesis by cells of the mesenchymal lineage. ACTH is a novel regulator of bone mass and enhances collagen production by osteoblasts and human mesenchymal cells reportedly through the MC2-R (Isales, et al. 2010; Zaidi et al. 2010). It also increases the expression of chondrogenic matrix components in chondrogenic cells and mesenchymal progenitor cell populations in an MC3-R dependent manner (Evans et al. 2004; Evans et al. 2005).
Obesity and diabetes are associated with chronic inflammation as well as a hyperactive hypothalamic-pituitary-adrenal (HPA) axis. This along with leukocyte infiltration of an inflamed vasculature can lead to significant local exposure of melanocortin peptides. Studies also indicate that selective depletion of macrophage cells reduces collagen production in the atherosclerotic plaque (Martinet and De Meyer 2007; Stoneman, et al. 2007). Therefore, we have hypothesized that after the initial endothelial damage and during the pathophysiological progression of the atherosclerotic plaque, ACTH, both systemically and locally produced, is involved in the regulation of matrix synthesis by cells of the mesenchymal lineage.
In this initial investigation, we hypothesized that in order to respond to ACTH, mouse-aorta-derived mesenchymal progenitors express MC-R and calcium flux is a potential mechanism through which MC-R modulate matrix synthesis and secretion. Therefore we examined MC-R expression in mouse-aorta-derived mesenchymal progenitor cultures. We also used specific MC-R agonists and the known agonist profiles of the MC-R to determine the receptor responsible for changes in intracellular calcium. Additionally, we identified mesenchymal progenitors and macrophage cells as potential local sources of ACTH by examining their expression of POMC and PC1/3.
2. Methods
2.1. Materials
All cell culture media, trypsin, FBS, charcoal/dextran treated FBS and antibiotic/antimycotic solutions were obtained from Invitrogen (Carlsbad, CA). The POMC and PC1/3 antibodies were from Novus Biologicals (Littleton, CO). The integrin β1, vimentin, fibronectin and MC-R polyclonal primary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The monoclonal Na+/K+ ATPase α-1 antibody (clone C464.6) was from Millipore (Temeculae, CA) and the β-Actin monoclonal antibody from SIGMA-Aldrich (USA). The α-MSH and γ2-MSH peptides were from BACHEM (Torrance, CA). The ACTH (1–39) porcine pituitary-derived peptide and all other chemicals were from SIGMA Aldrich (USA) unless otherwise specified.
2.2. Animals
All animal protocols were approved by the Institutions Animal Care and Use Committee and adhere to the regulations outlined by the National Institutes of Health. C57BL/6 mice were purchased from Hilltop Animal Labs and Wistar-Kyoto rats were obtained from Taconic, North America. Animals were housed under local vivarium conditions (12 h light-dark cycle) and allowed to acclimate for at least 7 days prior to experimentation. Mice and rats were euthanized under CO2 at 8–12 weeks of age and hind limbs were removed in preparation for bone marrow isolation and where indicated aortas were removed for isolation of mesenchymal progenitors.
2.3. Cell isolation
2.3.1. Aortic mesenchymal progenitor cell lines
Mouse aortic progenitor lines were derived using the method of da Silva Mierelles (da Silva Meirelles, et al. 2006) with slight modification. Briefly, descending aorta were dissected free of connective tissue, rinsed several times in DMEM supplemented with 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B, denuded and minced. Minced aorta pieces were subjected to digestion with collagense type IV (0.5 mg/ml in DMEM) for 30 min to 3 h. After 30 min of digestion, the first cell fraction was discarded and the remaining vessel fragments rinsed with DMEM and placed in fresh collagenase solution. Cell fractions were collected every 30 min thereafter and pooled. At the end of 2.5 h very few visible vessel fragments remained. The pooled supernatants were plated in growth medium (DMEM, high glucose, supplemented with 10% FBS, 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B).
For subculture, cells were treated with 0.25% trypsin/0.9 mM EDTA balanced salt solution and split ratios determined empirically. Initial cultures were split 1:2 or 1:3 with subsequent split ratios reaching 1:6 when cells reached maximum growth kinetics. Cells were subcultured no more than twice per week and cells at passage 17 or greater were used for experiments and termed mAo. The clonal cell line, C8, was created by low density plating of mAo cells at passage 10. Highly proliferative colonies were isolated and subsequently cultured. The C8 colony continued to proliferate and was subcultured and used at passage 7–10 for experiments.
2.3.2. Mouse bone marrow macrophage
Bone marrow from the hind limbs of the C57BL/6 mice was isolated as previously described (Yeh, et al. 1999). After creating a single cell suspension in growth medium, mature macrophage were adherence depleted by plating in 100 mm non-cell-culture treated Petri dishes for 4 h. Non-adherent populations were then plated again in Petri dishes in the presence 25 ng/ml M-CSF. Only monocytes will adhere to the non-treated plastic and stromal mesenchymal populations were depleted with every medium change. Cells were subcultured by gentle scraping. For experiments, monocyte/macrophage were plated at a density of 2 × 104 cells per well in 6-well tissue-culture plates and allowed to grow in basal medium (α-MEM without phenol red supplemented with 10% FBS, 15 mM glucose, 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, 0.25 µg/ml amphotericin B) and 25 ng/ml recombinant mouse M-CSF for 7 days prior to treatment with LPS from E.coli and recombinant mouse IL-4 (R&D systems, Minneapolis, MN).
2.3.3. Wistar-Kyoto (WKY) rat bone marrow mesenchymal cells
Bone marrow from the hind limbs of the WKY rat was isolated as previously described (Yeh et al. 1999). After creating a single-cell suspension, nucleated cells were counted using 3% acetic acid/trypan blue exclusion and plated in basal medium at 107 cells per 100 mm dish. Cultures were depleted of mature macrophage by adherence depletion. Non-adherent cells were then re-plated into 100 mm dishes and allowed to adhere for 3–4 days before the first medium change. The medium was changed every 2–3 days thereafter. At 80% confluence cells are split at a ratio of 1:3 to 1:4. Cultures at passage 3 or 4 were used to induce adipogenesis medium was changed with fresh medium containing 1 uM dexamethasone, 10 mg/ml insulin, and 450 µM IBMX and cells incubated for 4 days. Medium was then replaced with basal medium supplemented with 10 mg/ml insulin and cultures incubated for 4–5 more days. Crude membrane fractions were prepared as described in section 2.9.
2.4. Lineage differentiation
mAo or C8 cultures were established at a density of 3 × 104 cells per well in 12-well plates in basal medium until confluent. To induce adipogenesis medium was changed with fresh medium containing 1 uM dexamethasone, 10 mg/ml insulin, and 450 µM IBMX and cells incubated for 4 days. Medium was then replaced with basal medium supplemented with 10 mg/ml insulin and cultures incubated for 4–5 more days. This procedure was repeated twice more before cells were stained with oil-red-O to delineate adipocytes. To induce osteogenesis, basal medium was supplemented with 10 mM β-glycerophosphate, 50 ug/ml ascorbate-2-phosphate and 10 nM dexamethasone. Cells were maintained in this medium for 30–35 days before staining with von Kossa.
2.5. Fluorescent Immunocytochemistry
Aortic cell lines were initiated in 8-well chamber slides at a density of 2500 cells per well in basal medium. After 4 days of growth, cells were fixed in 2% PFA, rinsed in PBS and blocked using 10% horse serum in PBS with 0.3% Triton x-100. Primary and appropriate secondary antibodies were diluted at 1:100 and 1:50 respectively, in a solution containing 1% BSA, 1% horse serum, 0.3% Triton x-100 and 0.01% sodium azide. Normal goat IgG was used as a control. Washes were carried out with 0.1%BSA in PBS. Slides were mounted using Vectashield mounting medium containing DAPI (Vector Labs, Burlingame, CA).
2.6. Proliferation
Proliferation was assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were plated in basal medium at 7500 cells per well in 96-well plates. After 24 h (day 0) MTT, diluted in serum –free α-MEM, was added at 500 µg/ ml and incubated for 3 h. Precipitated formazan was solubilized with 50% DMSO in EtOH and absorbance read at 550 nm. On day 0, additional 96-well plates were treated with and without ACTH (1–39) at 10 nM, 100 nM and 1 µM. Medium was changed every 2–3 days and during the last 3 h of incubation on days 1, 4 and 7, MTT was added at 500 µg/ ml, incubated for 3 h and precipitated formazan was solubilized with 50% DMSO in EtOH and absorbance read at 550 nm. Data were expressed as fold change OD from day 0.
2.7. Picrosirius red stain
mAo cells in 24-well plates were cultured in basal medium with medium changes every 2–3 days. At confluence, cells were treated with and without ACTH (1–39) at 10 nM, 100 nM and 1 µM at every medium change. On day 14, cultures were fixed with phosphate buffered 10% formalin for 30 min at room temperature and stained with picorsirius red for 1 h. Cultures were washed several times with dH2O and representative photomicrographs were taken of each treatment. Photomicrographs were analyzed using threshold intensity feature of the MetaMorph morphometry program.
2.8. [3H]-proline incorporation
The incorporation of 3H-proline provides an estimate of total collagen production therefore this assay was used to estimate collagen production by mAo cultures treated with or without ACTH. Cells were grown as described for picrosirius red staining. At day 14, the medium was replaced with basal medium containing 1.0 µCi/ml of [L-2,3-3H] -proline (Perkin Elmer, MA, USA) with or without ACTH (1–39). After 24 h incubation, cells were washed with 4X with cold PBS and lysed with 1N NaOH for 2 h at 37°C. Equal aliquots of solubilized lysate were subjected to scintillation counting. Representative culture wells from each treatment group were subject to cell count. Cells were treated with 0.5% trypsin/0.9 mM EDTA balanced salt solution followed by a 0.5 mg/ml collagenase in Hank’s balanced salt solution with Ca2+ and Mg2+ to create a single cell suspension. Cells were counted using a hemocytometer. Data was expressed as dpm/104 cells.
2.9. Immunoblotting
Crude membrane fractions were prepared from mAo and C8 aortic cell lines as well as rat bone marrow MSC induced to form adipocytes. After growing to confluence in basal medium, mAo and C8 cell lines were changed to α-MEM supplemented with 5% charcoal/dextran treated FBS, 15 mM glucose, 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B. Cells were lysed in HES buffer (0.25 M sucrose, 5 mM EDTA, 2 mM EGTA, 20 mM HEPES) with 1 X protease inhibitor cocktail (SIGMA-Aldrich, USA, #P8340) and homogenized with a Teflon on glass homogenizer. The homogenate was then centrifuged at 760 g for 5 min and the supernatant centrifuged at 25,000 rpm for 1 h. The pellet was dissolved in modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40 (w/v), 0.25% Na-deoxycholate (w/v), 150 mM NaCl, 1 mM EDTA and 1X protease inhibitor cocktail) and protein content determined via bicinchroninic acid assay (BCA) assay (Thermo Fisher Scientific). Protein samples, 50 µg per lane were separated by SDS-PAGE and transferred to PVDF membrane. After blocking in 5% milk in Tris-buffered saline with 0.1% tween-20 (TBST), membranes were incubated overnight with MC-R primary antibodies diluted 1:500 with 5% BSA in TBST. After washing in TBST, membranes were then incubated for 1 h with the appropriate horseradish peroxidase-tagged secondary antibody diluted with 5% milk in TBST. After washing, bands were visualized using enhanced chemiluminescence (ECL). To normalize for protein loading, membranes were also probed for Na+/K+ ATPase expression.
Whole lysates were prepared from macrophage cultures that had either been left untreated, treated with LPS (100 ng/ml) or treated with IL-4 (50 ng/ml) for 24 h. Protein concentrations were determined by BCA assay. Protein samples were separated by SDS-PAGE and transferred to PVDF membrane. After blocking, membranes were incubated with either POMC or PC1/3 primary antibodies. After incubation with appropriate horseradish peroxidase-tagged secondary antibody, bands were visualized using ECL. To normalize protein loading, blots were also probed for β-actin expression.
2.10 Calcium Measurements
Transient elevations in calcium in response to melanocortin peptides were measured as previously described (Evans et al. 2005). Briefly, at confluence mAo or C8 cultures were incubated in α-MEM supplemented with 5% charcoal/dextran treated FBS, 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B. Cultures were maintained in this medium for at least 5–7 days. Before assay some cultures were treated with 10 nM dexamethasone for at least 48 h. Single-cell suspensions were created using trypsin and collagenase, centrifuged and re-suspended in calcium loading buffer with Ca2+ (Hanks; balanced salt solution plus Ca2+ and Mg2+ supplemented with 0.05% BSA) and loaded with 1.5 mM fura-2 at room temperature. For measurements made in the absence of extracellular calcium, calcium loading buffer without Ca2+ (Hank’s balanced salt solution without Ca2+ and Mg2+ supplemented with 0.05% BSA and 80 mM MgCl) was used. All peptides were first diluted in sterile water at a stock concentration of 5 mM and stored in small aliquots at −20° C. On the day of use, stocks were further diluted by 10X and 100X in calcium loading buffer without Ca2+ and used in experiments.
2.11 Statistical Analysis
Data were analyzed using Student’s t-test or one-way ANOVA. Two-way ANOVA (DEX x dose) was used to analyze calcium flux studies. Post hoc test P values were adjusted using the Bonferroni correction. All tests were two-tailed and a nominal significance level of 0.05 was used.
3. Results
3.1. Similar to bone marrow derived mesenchymal cells, clonal and late passage populations of mouse aortic cells are capable of mineralizing and accumulating lipid
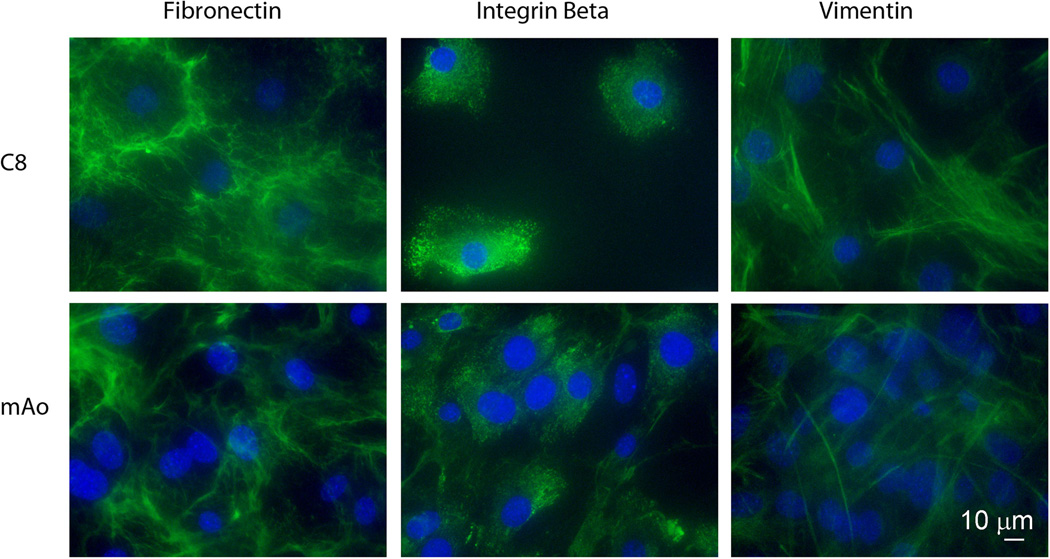
Mesenchymal progenitor cell populations are present throughout the vasculature (da Silva Meirelles et al. 2006). Our initial goal was to establish aorta-derived mesenchymal progenitor populations using the method of da Silva Mierelles (da Silva Meirelles et al. 2006). Two aorta-derived mesenchymal progenitor cell lines were established; one late passage mouse aortic cell line (mAo) and one penultimate mouse aortic cell line (C8) named after its clone number. In order to confirm we were working with homogeneous mesenchymal populations we examined the expression of integrinβ1, fibronectin and vimentin in these cell lines using fluorescent immunocytochemistry. The late passage cell line, mAo, and the clonal aortic cell line (C8) were greater than 97% positive for mesenchymal cell markers (Fig. 1).
Fig. 1.
Immunofluorescent detection of mesenchymal cell markers in aorta-derived cell lines. The clonal cell line (C8) and the late passage (mAo) cell lines are positive for fibronectin, integrin β1 and vimentin. Digital multi-channel fluorescent images were taken at a 400× magnification using a Nikon A1 confocal microscope, bar = 10 µM.
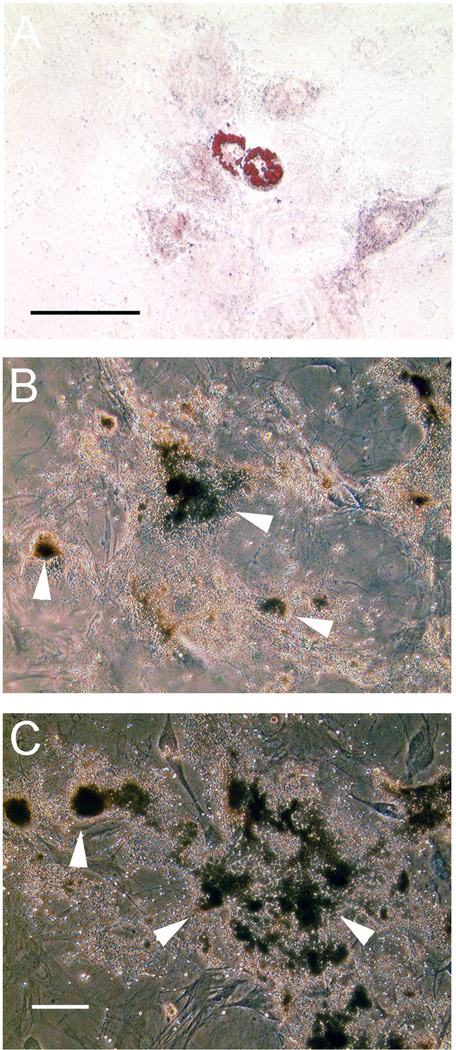
To establish that these aortic cell lines were progenitor populations, we tested their capacity to mineralize and accumulate lipid. Similar to bone marrow derived mesenchymal cells; the mAo cell line accumulated lipid after adipogenic induction and mineralized under osteogenic conditions (Fig. 2A & B). Also like bone marrow derived cultures, mAo culture underwent spontaneous adipogenesis under osteogenic condtions (not shown). The C8 clonal cells were capable only of mineralization under osteogenic conditions (Fig. 2C) and did not accumulate lipid after adipogenic induction (not shown).
Fig. 2.
Adipogenic and osteogenic lineage differentiation of aortic mesenchymal progenitor cell lines. The mAo late passage cell line was capable of adipogenic differentiation with induction (A) as assessed by oil-red-O stain. Both the mAo (B) and the C8 clonal cell line (C) were capable of mineralization as assessed by von Kossa stain. Bars = 100 µM
3.2. ACTH reduces proliferation and increases matrix production in aortic mesenchymal cell populations
ACTH increases matrix synthesis in rat bone marrow- and calvariae-derived mesenchymal cell populations, and collagen production in osteoblasts and human mesenchymal cells (Evans et al. 2004; Isales et al. 2010; Zaidi et al. 2010). We hypothesized that ACTH can also affect changes in proliferation and matrix synthesis in aorta-derived progenitors. Using the MTT proliferation assay, we determined that ACTH dose-dependently reduces proliferation over time in both mAo MSC and the C8 clonal cultures (Fig. 3A & B). By day 7 of treatment, proliferation in mAo cultures was reduced by 8.63% at 10 nM, 12.35% at 100 nM and 17.97% at 1000 nM ACTH. C8 cells were not as sensitive to ACTH, showing a 1.37% reduction at 10 nM, 4.72% reduction at 100 nM and 12.94% reduction at 1000 nM after 7 days of treatment. Results were analyzed using two-way ANOVA with time and dose of ACTH as factors. With both cell types, there was a significant interaction effect between time and dose, mAo [F(6,60) = 61.32, P < 0.0001] and C8 [F(6,84) = 118.77, P < 0.0001] confirming a significant reduction in proliferation with ACTH treatment over time and with increasing dose of ACTH.
Fig. 3.
ACTH reduces proliferation and increases proline incorporation in aorta-derived mesenchymal progenitor cell lines. Proliferation was assessed using MTT assay in mAo (A) and C8 cultures (B). Cells were plated at 7500 cells per well in 96-well plates. After 24 h (day 0), MTT was added and after 3 h and precipitated formazan was solubilized and absorbance read at 550 nm. On day 0, additional 96-well plates were treated with and without ACTH (1–39) at 10 nM, 100 nM and 1 µM. Medium was changed every 2–3 days and during the last 3 h of incubation on days 1, 4 and 7, MTT was added, incubated for 3 h and precipitated formazan was solubilized and absorbance read at 550 nm. Data were expressed as fold change in OD from day 0. Results are presented as mean ± SD, n=6–12.
The mAo cell line was capable of both osteogenic and adipogenic differentiation and therefore represents an earlier stage progenitor than the C8 line. Thus we used the mAo cell line to determine the effects of ACTH treatment on matrix synthesis. Picrosirius red stain and 3H-proline incorporation were used to evaluate quantitative changes in collagen production in response to ACTH Treatment of mAo cultures with ACTH increased the intensity of pricorsirius red stain (Fig. 4A & B) and 3H-proline incorporation in a dose dependent manner (Fig. 4C).
Fig. 4.
Collagen synthesis is increased in mAo MSC after exposure to ACTH. Collagen production was examined by picrosirius red stain (A & B) and by 3H-proline incorporation (C). For quantitation of picrosirius red stain (B) the threshold intensity used was ≥80% the highest intensity in the untreated control (0) and 4 fields per well analyzed, n = 4. For 3H-proline incorporation (C), data are presented as mean ± SD, n = 3 wells. Statistically significant differences between individual treatments were determined after a significant one-way ANOVA using the Bonferonni correction with a P < 0.05 considered significant. *- significantly different from 0. Data are representative of 3 separate experiments with similar results.
3.3. The MC2-R and MC3-R are expressed by aortic mesenchymal progenitor cells
Many previous studies document the expression of MC-R in mesenchymal cell populations and mesenchymal progenitor cell populations. Using crude membrane fraction preparations, we detected expression of the MC2-R (Fig 5A) and MC3-R (Fig. 5B) protein but not the MC5-R (Fig. 5C) in the mAo and C8 cell lines. The MC4-R was not expressed by these cells (not shown).
Fig. 5.
Immunoblot results showing MC2-R and MC3-R expression in aorta-derived mesenchymal cell lines. (A) A representative blot showing MC2-R detection in crude membrane fractions of mouse aorta cells mAo) untreated (−) or treated (+) with 10 nM Dex; the clonal mouse aorta-derived cell line (C8) untreated (−) or treated (+) with 10 nM Dex; and rat bone marrow derived MSC induced to form adipocytes (Adip) as a control (Adip con). (B) A representative blot showing detection of MC3-R in crude membrane fractions of mAo cells untreated (−) or treated (+) with 10 nM Dex and C8 cells untreated (−) or treated (+) with 10 nM Dex. Adip were used as a negative control (neg con). (C) A representative blot showing the absence of MC5-R in crude membrane fractions of mAo cells untreated (lane 1) or treated with 10 nM Dex (lane 2) and C8 cells untreated untreated (−) or treated (+) with 10 nM Dex Adip were used as a positive control (pos con). SDS-PAGE gels were loaded with 50 µg protein crude membrane fraction per lane. Detection of Na+/K+ ATPase was used as a control for gel loading. (D) Densitometric analysis of MC2-R and MC3-R immunoblots. Data are presented as mean % expression ± SD of untreated (− Dex) cultures, n = 3–4.
Rat bone marrow mesenchymal stem cell (MSC) preparations induced to form adipocytes were used as a positive control for MC2-R and MC5-R expression and a negative control for MC3-R expression. Rat bone marrow MSC were used in lieu of mouse bone marrow because in the latter, cells of the hematopoietic lineage predominate. This confounds the production of a homogeneous MSC population.
The MC2-R was detected as a 50 kDa band which indicates the receptor is expressed in its mature glycosylated form (Sebag and Hinkle 2007) in the mAo and C8 cell lines, and in induced bone marrow adipocytes (Fig. 5A). In addition, the 33 kDa immature MC2-R as well as the mature glycosylated MC2-R is expressed by C8 cells. Both forms of the MC2-R are up-regulated in C8 cells with dexamethasone treatment (Fig. 5A & D).
3.4. Aortic mesenchymal progenitors respond to ACTH treatment with transient increases in intracellular calcium ([Ca2+]i)
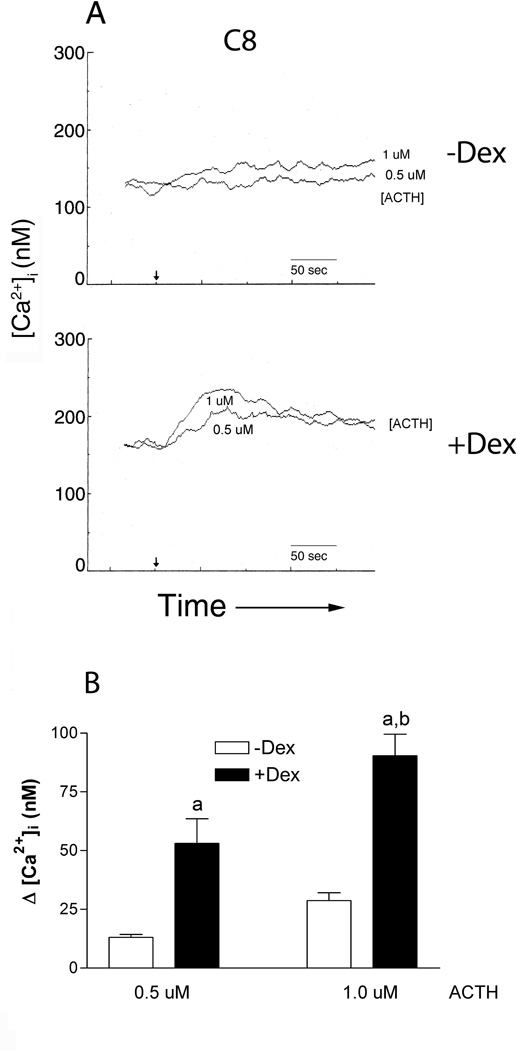
Down-stream effects of activated MC-R’s are modulated through the induction of cAMP accumulation or the elevation of intracellular calcium ([Ca2+]i) (Evans et al. 2005; Gallo-Payet and Payet 2003; Hoogduijn, et al. 2002; Yang 2011). These cascades are initiated by the MC-R through activation of the G-protein alpha subunit; activation of Gαs for the most part leads to cAMP accumulation while activation of Gαq is known to result in transient elevations in [Ca2+]i (Neves, et al. 2002). Transient increases in intracellular calcium are associated with ACTH-enhanced matrix synthesis in chondrogenic cells (Evans et al. 2005). Therefore we determined whether ACTH can evoke calcium flux in aorta-derived mesenchymal progenitors to provide a potential mechanism for the matrix associated changes. Like chondrogenic cells, C8 and mAo cell lines respond to ACTH with transient increases in intracellular calcium (Fig. 6 and 7, respectively) but only in the clonal cell line are these increases enhanced with dexamethasone priming (Fig 6A & B). Augmenting the dose from 0.5 µM ACTH to 1.0 µM ACTH in C8 cells results in a 2.2-fold increase in calcium flux. After dexamethasone treatment, calcium flux at 0.5 uM ACTH increases by 4.1 fold and at 1.0 µM increases by 3.15 fold. Two-way ANOVA (DEX X dose) confirm a dose dependent effect, [F(2,16) = 22.34, P < 0.0001] and a significant dexamethasone effect [F(1,16) = 62.57, P < 0.0001] but not an interaction effect of dexamethasone and dose in the C8 cell line.
Fig. 6.
The clonal aorta-derived mesenchymal progenitor cell line, C8, responds to ACTH with transient increases in intracellular calcium ([Ca2+]i) and these increases are significantly enhanced with dexamethasone priming. (A) Representative fluorimetric traces of calcium flux are shown in cultures left untreated (−Dex) or treated with 10 nM dexamethasone (+Dex) for 24 h. The arrow shows the time of ACTH addition and dose is presented under the corresponding trace. (B) The quantitative comparison of calcium flux shown in A. Clear bars: −DEX, Solid bars: +DEX. Statistically significant differences between individual treatments were determined after a significant two-way ANOVA using the Bonferonni correction with a P < 0.05 considered significant, n = 3–4, a- significantly different from −DEX counterpart, b- significantly different from 500 nM ACTH counterpart.
Fig. 7.
Mouse aorta-derived mesenchymal progenitor cells, mAo cells, respond to ACTH with transient increases in intracellular calcium ([Ca2+]i) in a dose-dependent manner (A & B) and these increases have both an intracellular and extracellular contribution (C & D). In (A) representative fluorimetric traces of the dose-dependent, ACTH-induced transient elevations in [Ca2+]i in the presence of extracellular calcium are shown. The arrow shows the time of ACTH addition and the dose is presented under or to the right of the corresponding trace. In (B) the quantitative comparison of calcium flux represented in A is shown. Data are presented as the mean change in [Ca2+]i from baseline ± SD, n = 4–5. In (C) representative traces of ACTH-induced transient elevations in [Ca2+]i in the absence of extracellular calcium are shown along with the influx of calcium after the re-addition of 1.8 mM CaCl2 to the medium. The arrows show the times of ACTH and CaCl2 addition. The initial dose of ACTH ([ACTH]init) is shown to the right of the corresponding trace. In (D) the quantitative comparison of calcium flux represented in C is shown. Clear bars represent mean ± SD of intracellular calcium release (IC) and the solid bars represent the mean ± SD of the extracellular influx (EC), n = 4. a -significantly different from 10 and 0 nM counterpart, b -significantly different from 100, 10 and 0 nM counterpart.
The results of the calcium flux experiments were consistent with MC2-R protein expression patterns suggesting an MC2-R mediated signaling pathway. In C8 cells dexamethasone treatment increases both MC2-R expression (Fig. 5) and ACTH-induced calcium flux (Fig. 6). In mAo cells, MC2-R expression and ACTH-induced transient elevations in [Ca2+]i are increased compared to C8 cells (Fig. 6 & 7). Further, there is no difference in MC2-R expression or calcium flux with dexamethasone treatment in mAo cells (Fig. 5 & 7).
To confirm a MC2-R mediated effect in both cell lines, we tested the response to MC3-R specific agonists, γ2-MSH and MTII. Neither the γ2-MSH nor the MTII melanocortin peptides elicited transient elevations in [Ca2+]i in either the mAo or C8 cell lines (Fig. 8) which rules out MC3-R-mediated ACTH signaling.
Fig. 8.
MC3-R agonists do not induce calcium flux in aorta-derived mesenchymal cell lines. The C8 and mAo cell lines were loaded with the fura-2 Ca2+ indicator and responses to γ2-MSH (1 µM) and MTII (1 µM) were measured in the presence of extracellular calcium. The arrows show the time of peptide addition.
3.5. In the mAo cell line, ACTH-induced transient increases in [Ca2+]i consist of both an intracellular and extracellular component
Extracellular calcium entry mechanisms are often associated with intracellular calcium release. Using the more sensitive mAo cell line we investigated the source of calcium during ACTH-induced transient elevations in [Ca2+]i. Measurements of calcium flux were made with and without calcium in the extracellular medium (Fig. 7). Removing calcium from the extracellular medium allows the isolation of the intracellular release from the extracellular influx. After the initial ACTH-initiated intracellular release, calcium (1.8 mM CaCl2) is added back to the medium to demonstrate the extracellular component of the flux (Fig. 7C & D). In the mAo cell line, with calcium present in the extracellular medium, ACTH induces transient increases in [Ca2+]i (Fig. 7A & B). This calcium flux consists of an intracellular release as well as an extracellular influx of calcium (Fig. 7C & D) both following a dose-dependent pattern with a sensitivity of 10 nM.
3.6. POMC and PC1/3 expression in aortic progenitor cells and bone marrow derived macrophage populations
It is well-known that the systemic source of ACTH is the pituitary. Under psychological as well as physiological stress such as obesity and diabetes, production and release of ACTH is elevated. The range of ACTH used in these studies was 10 nM to 1000 nM. At the lowest end of this range there was a detectable increase in 3H-proline incorporation and a significant increase in [Ca2+]i. However the lower end of this range is about 100-fold above what would be found with obesity and diabetes. We therefore looked for potential local sources of ACTH by examining the expression of its precursor peptide, POMC as well as its cleavage enzyme PC1/3 in mesenchymal progenitors as well as macrophage cells. We confirmed that POMC is expressed in aortic progenitor cells as well as murine monocyte/macrophage cells (Fig. 9). PC1/3, the cleavage enzyme that produces ACTH and α-MSH from POMC, is activated by proteolytic cleavage of its C-terminal tail (Lee, et al. 2004). We were able to detect the expression of the partially active 84 kD and the inactive 97 kD form in aortic mesenchymal progenitors and the highly active 66 kD form of PC1/3 in the murine macrophage (Fig. 9).
Fig. 9.
PC1/3 and POMC expression in aorta-derived mesenchymal cell lines and activation of murine macrophage regulates PC1/3 and POMC expression. Twenty-five micrograms of total protein lysate from mAo, C8 and mouse macrophage (mMa) cells were analyzed for POMC and PC1/3 expression via immunoblot.
4. Discussion
Our aorta-derived mesenchymal progenitor cell lines expressed both the MC2-R and MC3-R. The MC2-R is unique among MC-R in that it is the only receptor with sensitivity to a single melanocortin peptide ligand, ACTH (Veo, et al. 2011). Our cell lines responded to ACTH with [Ca2+]i flux in a manner that was consistent with their MC2-R expression patterns but they did not respond to the strong agonists of MC3-R, MTII and γ2-MSH, indicating a functional MC2-R. Additionally, our murine bone marrow derived macrophage cultures express the melanocortin peptide precursor, POMC, and the highly active form of its cleavage enzyme, PC1/3. This supports earlier reports of the production of POMC-derived peptides by immune cells (Harbour, et al. 1987; Rajora, et al. 1996; Star, et al. 1995). In addition, we have shown that aortic mesenchymal progenitors also highly express POMC. This along with the fact that these cells express the inactive and partially active form of the PC1/3 enzyme also gives them the potential to contribute to local melanocortin peptide levels. The precise conditions under which this would occur have yet to be determined.
Traditionally the MC2-R is best known for its role in the regulation of adrenal steroidogenesis (Cone 2006). Its role in tissues other than the adrenal cortex is less well-defined. However it is expressed by human MSC during adipogenic induction (Smith, et al. 2003) as well as by 3T3-L1 adipocytes during their differentiation (Noon, et al. 2004). In adipose the MC2-R responds to ACTH by initiating lipolysis and inhibiting leptin production (Boston 1999). More recently, MC2-R expression has been described in human and murine osteoblast cell lines and plays a putative role in murine osteoblast differentiation through production of VEGF (Zaidi et al. 2010). In light of these data the expression of this receptor by aorta-derived mesenchymal progenitors is not surprising however its role in this tissue remains to be fully elucidated. Our initial studies have shown that it increases collagen production which suggests it could play a role in the maintenance and repair of the vascular extracellular matrix.
On the other hand, expression of this receptor by vascular mesenchymal progenitors could also contribute to vascular pathologies such as atherosclerosis. Diabetes and obesity have been described as a chronic inflammatory state and hypertension under these conditions can lead to endothelial damage. After this damage, both vascular SMC and vascular mesenchymal progenitors would be in direct contact with circulating hormones and infiltrating macrophage cells. This has been confirmed by immunohistochemical and electron microscope analysis of human atherosclerotic plaques (Stary, et al. 1994). Further, da Silva Mierelles et al. have postulated that mesenchymal stem cells (MSC) are located just below the endothelial lining (da Silva Meirelles et al. 2006). Therefore, in the developing atherosclerotic plaque of the diabetic or obese individual, vascular SMC and MSC are exposed to elevated levels of ACTH both from a hyperactive stress axis and increased local levels of ACTH produced by macrophage cells. We have shown that aorta-derived mesenchymal progenitors express MC2-R and when exposed to ACTH increase collagen matrix synthesis. These data suggest that both a hyperactive HPA- axis and paracrine signaling between macrophage and vascular mesenchymal cell may contribute to matrix changes during atherosclerotic plaque formation.
An investigation of the specific ACTH-mediated changes in collagen matrix production by vascular MSC may shed light on whether these changes have a role in repair or contribute to the atherosclerotic pathology. While the fibrillar Type I and III collagens promote SMC stabilization and quiescence, type VIII collagen is associated with increased production of MMP2 and MMP9 and cellular proliferation and migration. (Adiguzel, et al. 2009). In both atherosclerotic plaque formation and restenosis, type VIII collagen expression is increased and this can overcome the strong adhesion between SMC and polymerized collagen (Adiguzel, et al. 2006). Previous studies have shown that ACTH strongly induces type VIII collagen transcription in a chondroprogenitor cell line. Production of this non-fibrillar collagen by MSC of the damaged or atherosclerotic vessel would therefore contribute to SMC proliferation and migration. More data are necessary to delineate specific ACTH-induced changes in collagen production by aorta-derived MSC.
The majority of works investigating MC-R mechanisms focus on adenylyl cyclase activation and the subsequent accumulation of cAMP. However these receptors are also capable of initiating calcium-mediated second messenger pathways. Here we show that ACTH can elicit transient elevations in [Ca2+]i and increase basal [Ca2+]i in aorta-derived mesenchymal progenitors. In osteoblasts and chondrocytes elevations in [Ca2+]i are linked to increased matrix synthesis and differentiation (Evans et al. 2005; Shin, et al. 2008; Wang, et al. 1998). Additionally the inhibition of calcium-calmodulin-dependent protein kinase II (CaMKII), a major mediator of intracellular Ca2+ changes, suppresses ECM secretion in cardiac fibroblasts (Zhang, et al.). These data suggest that this may also be the mechanism through which ACTH induces matrix changes in aorta-derived mesenchymal progenitors. More studies are needed to delineate ACTH initiated signaling mechanisms in these cells.
Glucocorticoid levels are also elevated as part of a hyperactive stress axis in the obese and diabetic therefore we tested the ACTH-induced calcium flux response in both aorta-derived cell lines after priming with dexamethasone. The C8 cell line behaved similarly to the resting chondrocytes of previous studies (Fig. 6) where pre-treatment with dexamethasone significantly enhances transient elevations in [Ca2+]i (Evans et al. 2005) while there was no effect in the mAo cultures (Fig. 7). These data likely reflect dexamethasone-induced changes in the expression of proteins along the MC-R signaling cascade. Consistent with this, MC2-R expression was increased after dexamethasone treatment in the C8 cultures. The fact that the mAo cell line was capable of both adipogenic and osteogenic differentiation suggests it’s an earlier stage progenitor compared with the C8 cell line. This is also supported by the fact that the rate of proliferation was greater in the mAo cultures compared to the C8 cultures (Fig. 3). These data indicate that MC-R expression patterns could coincide with lineage stage where MC2-R expression decreases with differentiation and that exposure to glucocorticoid can reiterate its expression.
Previous works have established that the MC2-R is expressed in an immature and mature form (Sebag and Hinkle 2007). The mAo cell line, like the adipocytes in our studies, expressed the 50 kD glycosylated form of the receptor while in the C8 cell line, the 33 kD immature form predominated. However with dexamethasone treatment expression of both the 50 kD and 33 kD band was increased (Fig. 5). These data suggest that dexamethasone increases both the translation and processing of the MC2-R receptor in these cells. The increase in sensitivity to ACTH after dexamethasone treatment in C8 cells, as assessed by [Ca2+]i, flux supports this hypothesis. Our membrane preparations were crude fractions intended to concentrate membrane proteins; detection of the 33 kD form of the receptor in these lysates likely reflect its co-isolation with endoplasmic reticulum membranes.
Although the levels of ACTH used in these studies were high relative to those reported as circulating levels in the diabetic and obese, local vascular exposure is likely increased due to autocrine and paracrine production by leukocytes and possibly mesenchymal cells. In addition the ACTH precursors POMC and pro-ACTH are present in the human circulation at concentrations 5-fold greater than that of ACTH (Talbot, et al. 2003) and these peptides have the potential to be processed peripherally (Stewart, et al. 1994). Moreover, glucagon-like-peptide-1 (GLP-1), released in response to food intake, and exendin-4, a GLP-1 analog used to treat diabetic patients, increase the activity of the HPA in rodents and humans (Gil-Lozano, et al. 2010). Therefore, the ingestion of a meal and bouts of hyperphagia also elevate circulating ACTH levels.
Additionally, contrasts in exposure should be considered when evaluating the in vitro results. In vivo, mammals experience a continual systemic exposure to ACTH with periodic spikes or pulses. In humans this ACTH secretion has a 24 h mean pulse frequency of 18 in men and 10 in women, with a mean peak amplitude of 3.7 and 2.3 pmol/L, respectively (Horrocks, et al. 1990). With diabetes these values can increase 10-fold at the diurnal peak (Murakami, et al. 2010) in addition to increases that occur after ingestion of a meal. In this study cells were exposed to one pulse of ACTH, the lowest effective dose tested being 10 nM, every two to three days. Therefore, although the dose of ACTH was relatively high, the frequency of exposure was much lower in our in vitro studies.
Overall, we have demonstrated that mouse aorta-derived mesenchymal progenitor cells express functional MC2-R and when exposed to ACTH, transiently elevate [Ca2+]i and increase their collagen matrix synthesis. These data represent the foundations for a local melanocortin signaling in the vasculature. Future studies that investigate the role of this system during inflammatory responses are warranted.
> The melanocortin system is active during inflammation. > Melanocortin peptides may induce extracellular matrix changes by mesenchymal progenitors. > ACTH increases collagen synthesis by mesenchymal progenitors. > Macrophage and mesenchymal cells are relevant sources of local melanocortin peptides. > Functional melanocortin-2 receptors are expressed by aorta-derived mesenchymal progenitors
Acknowledgments
Funding
This work was supported by a grant from the NIH/NHLBI, K99HL091116-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors have no conflicts of interest.
Contributor Information
Jodi F. Evans, Email: jevans@winthrop.org.
Anne Fernando, Email: afernando@winthrop.org.
Louis Ragolia, Email: lragolia@winthrop.org.
References
- Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14:73–89. doi: 10.1177/1358863X08094801. [DOI] [PubMed] [Google Scholar]
- Adiguzel E, Hou G, Mulholland D, Hopfer U, Fukai N, Olsen B, Bendeck M. Migration and growth are attenuated in vascular smooth muscle cells with type VIII collagen-null alleles. Arterioscler Thromb Vasc Biol. 2006;26:56–61. doi: 10.1161/01.ATV.0000194155.96456.b7. [DOI] [PubMed] [Google Scholar]
- Bicknell AB. The tissue-specific processing of pro-opiomelanocortin. J Neuroendocrinol. 2008;20:692–699. doi: 10.1111/j.1365-2826.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- Boston BA. The role of melanocortins in adipocyte function. Ann N Y Acad Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Evans JF, Niu QT, Canas JA, Shen CL, Aloia JF, Yeh JK. ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes. Bone. 2004;35:96–107. doi: 10.1016/j.bone.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Evans JF, Shen CL, Pollack S, Aloia JF, Yeh JK. Adrenocorticotropin evokes transient elevations in intracellular free calcium ([Ca2+]i) and increases basal [Ca2+]i in resting chondrocytes through a phospholipase C-dependent mechanism. Endocrinology. 2005;146:3123–3132. doi: 10.1210/en.2004-1612. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Payet MD. Mechanism of action of ACTH: beyond cAMP. Microsc Res Tech. 2003;61:275–287. doi: 10.1002/jemt.10337. [DOI] [PubMed] [Google Scholar]
- Gatti S, Lonati C, Sordi A, Catania A. Protective effects of melanocortins in systemic host reactions. Adv Exp Med Biol. 2010;681:117–125. doi: 10.1007/978-1-4419-6354-3_9. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. 2001;69:98–104. [PubMed] [Google Scholar]
- Gil-Lozano M, Perez-Tilve D, Alvarez-Crespo M, Martis A, Fernandez AM, Catalina PA, Gonzalez-Matias LC, Mallo F. GLP-1(7-36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology. 2010;151:2629–2640. doi: 10.1210/en.2009-0915. [DOI] [PubMed] [Google Scholar]
- Harbour DV, Smith EM, Blalock JE. Novel processing pathway for proopiomelanocortin in lymphocytes: endotoxin induction of a new prohormone-cleaving enzyme. J Neurosci Res. 1987;18:95–101. doi: 10.1002/jnr.490180116. [DOI] [PubMed] [Google Scholar]
- Holloway PM, Smith HK, Renshaw D, Flower RJ, Getting SJ, Gavins FN. Targeting the melanocortin receptor system for anti-stroke therapy. Trends Pharmacol Sci. 2011;32:90–98. doi: 10.1016/j.tips.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, McGurk S, Smit NP, Nibbering PH, Ancans J, van der Laarse A, Thody AJ. Ligand-dependent activation of the melanocortin 5 receptor: cAMP production and ryanodine receptor-dependent elevations of [Ca(2+)](I) Biochem Biophys Res Commun. 2002;290:844–850. doi: 10.1006/bbrc.2001.6283. [DOI] [PubMed] [Google Scholar]
- Horrocks PM, Jones AF, Ratcliffe WA, Holder G, White A, Holder R, Ratcliffe JG, London DR. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf) 1990;32:127–134. doi: 10.1111/j.1365-2265.1990.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- Jun DJ, Na KY, Kim W, Kwak D, Kwon EJ, Yoon JH, Yea K, Lee H, Kim J, Suh PG, et al. Melanocortins induce interleukin 6 gene expression and secretion through melanocortin receptors 2 and 5 in 3T3-L1 adipocytes. J Mol Endocrinol. 2010;44:225–236. doi: 10.1677/JME-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pape E, Wakamatsu K, Ito S, Wolber R, Hearing VJ. Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigment Cell Melanoma Res. 2008;21:477–486. doi: 10.1111/j.1755-148X.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SN, Prodhomme E, Lindberg I. Prohormone convertase 1 (PC1) processing and sorting: effect of PC1 propeptide and proSAAS. J Endocrinol. 2004;182:353–364. doi: 10.1677/joe.0.1820353. [DOI] [PubMed] [Google Scholar]
- Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin MC(1) receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010;160:171–180. doi: 10.1111/j.1476-5381.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR. Selective depletion of macrophages in atherosclerotic plaques: myth, hype, or reality? Circ Res. 2007;100:751–753. doi: 10.1161/01.RES.0000263397.14481.96. [DOI] [PubMed] [Google Scholar]
- Montero-Melendez T, Patel HB, Perretti M. Role of melanocortin receptors in the regulation of gouty inflammation. Curr Rheumatol Rep. 2011;13:138–145. doi: 10.1007/s11926-011-0163-0. [DOI] [PubMed] [Google Scholar]
- Muceniece R, Dambrova M. Melanocortins in brain inflammation: the role of melanocortin receptor subtypes. Adv Exp Med Biol. 2010;681:61–70. doi: 10.1007/978-1-4419-6354-3_5. [DOI] [PubMed] [Google Scholar]
- Murakami H, Nigawara T, Sakihara S, Kageyama K, Yamashita M, Matsuki K, Tanabe J, Matsui J, Tamasawa N, Suda T. The frequency of type 2 diabetic patients who meet the endocrinological screening criteria of subclinical Cushing's disease. Endocr J. 2010;57:267–272. doi: 10.1507/endocrj.k09e-352. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Noon LA, Clark AJ, King PJ. A peroxisome proliferator-response element in the murine mc2-r promoter regulates its transcriptional activation during differentiation of 3T3-L1 adipocytes. J Biol Chem. 2004;279:22803–22808. doi: 10.1074/jbc.M401861200. [DOI] [PubMed] [Google Scholar]
- Patel HB, Leoni G, Melendez TM, Sampaio AL, Perretti M. Melanocortin control of cell trafficking in vascular inflammation. Adv Exp Med Biol. 2010;681:88–106. doi: 10.1007/978-1-4419-6354-3_7. [DOI] [PubMed] [Google Scholar]
- Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM. alpha-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. 1996;59:248–253. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci U S A. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Kim MK, Bae YS, Jo I, Lee SJ, Chung CP, Park YJ, Min do S. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell Signal. 2008;20:613–624. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Smith SR, Gawronska-Kozak B, Janderova L, Nguyen T, Murrell A, Stephens JM, Mynatt RL. Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes. 2003;52:2914–2922. doi: 10.2337/diabetes.52.12.2914. [DOI] [PubMed] [Google Scholar]
- Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1995;92:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr., Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Gibson S, Crosby SR, Penn R, Holder R, Ferry D, Thatcher N, Phillips P, London DR, White A. ACTH precursors characterize the ectopic ACTH syndrome. Clin Endocrinol (Oxf) 1994;40:199–204. doi: 10.1111/j.1365-2265.1994.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Dietgenotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JA, Kane JW, White A. Analytical and clinical aspects of adrenocorticotrophin determination. Ann Clin Biochem. 2003;40:453–471. doi: 10.1258/000456303322326371. [DOI] [PubMed] [Google Scholar]
- van der Kraan M, Adan RA, Entwistle ML, Gispen WH, Burbach JP, Tatro JB. Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology. 1998;139:2348–2355. doi: 10.1210/endo.139.5.6008. [DOI] [PubMed] [Google Scholar]
- Veo K, Reinick C, Liang L, Moser E, Angleson JK, Dores RM. Observations on the ligand selectivity of the melanocortin 2 receptor. Gen Comp Endocrinol. 2011;172:3–9. doi: 10.1016/j.ygcen.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhong S, Ouyang J, Jiang L, Zhang Z, Xie Y, Luo S. Osteogenesis of electrically stimulated bone cells mediated in part by calcium ions. Clin Orthop Relat Res. 1998:259–268. [PubMed] [Google Scholar]
- Yang Y. Structure, function and regulation of the melanocortin receptors. Eur J Pharmacol. 2011;660:125–130. doi: 10.1016/j.ejphar.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JK, Evans JF, Chen MM, Aloia JF. Effect of hypophysectomy on the proliferation and differentiation of rat bone marrow stromal cells. Am J Physiol. 1999;276:E34–E42. doi: 10.1152/ajpendo.1999.276.1.E34. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen DQ, Qi F, Wang J, Xiao WY, Zhu WZ. Inhibition of calcium-calmodulin-dependent kinase II suppresses cardiac fibroblast proliferation and extracellular matrix secretion. J Cardiovasc Pharmacol. 55:96–105. doi: 10.1097/FJC.0b013e3181c9548b. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36:820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]