Abstract
In this chapter we review the epidemiology of lung cancer incidence and mortality among never smokers/ nonsmokers and describe the never smoker lung cancer risk models used by CISNET modelers. Our review focuses on those influences likely to have measurable population impact on never smoker risk, such as secondhand smoke, even though the individual-level impact may be small. Occupational exposures may also contribute importantly to the population attributable risk of lung cancer. We examine the following risk factors in this chapter: age, environmental tobacco smoke, cooking fumes, ionizing radiation including radon gas, inherited genetic susceptibility, selected occupational exposures, preexisting lung disease, and oncogenic viruses. We also compare the prevalence of never smokers between the three CISNET smoking scenarios and present the corresponding lung cancer mortality estimates among never smokers as predicted by a typical CISNET model.
1. BACKGROUND: THE EPIDEMIOLOGY OF LUNG CANCER IN NEVER SMOKERS
Most of the papers in this volume focus on smoking rates or the role of smoking in lung cancer. Indeed, most lung cancer is attributable to smoking, with between 80% and 90% of lung cancer attributable to smoking(1). Nevertheless, that still leaves a large number of lung cancer victims who have never smoked. Indeed, of all cancer deaths, lung cancer deaths among never smokers have been estimated to be the 7th leading cause of cancer mortality(2). The purpose of this chapter is to consider those lung cancer deaths. We consider the reasons for lung cancer in never smokers and trends in lung cancer deaths among never smokers as suggested by the CISNET models presented in chapters 7–12(3–9) and based on findings from the published literature.
The following review of the epidemiology of lung cancer in never smokers relies on a literature that generally did not use biologically-validated definitions of nonsmokers. For the purposes of this chapter, we will accept the definition of never smoker as an adult who has never smoked as many as 100 cigarettes in a lifetime. This is consistent with World Health Organization nomenclature(10). This review was prompted in part by newer literature that does report biomarker-validated exposure to cigarette smoking, but critical new findings more often than not still relied exclusively on non-validated self-reports. All conclusions are therefore conditional on how well the authors minimized missclassification of never smokers in the individual studies cited. Moreover, much of the literature reported only current smoking status, not lifetime smoking status, in which case the term "nonsmoker" was used instead of "never smoker."
1.1. Literature Review on Causes of Lung Cancer
As a prelude to modeling lung cancer risks in never smokers, we updated earlier reviews by Subramanian and Govindan (2007)(11), Sun et al. (2007)(12), and Samet JM et al. (2009)(13) by reviewing below the following etiological influences on lung cancer incidence and mortality in never smokers: age, environmental tobacco smoke, cooking fumes, inherited genetic susceptibility, occupational and environmental exposures to carcinogens, hormonal factors, pre-existing lung disease, and oncogenic viruses. Lung cancer associations with hormonal factors remain speculative and were therefore not included in this review(14). In contrast, exposure to ionizing radiation is consistently associated with lung cancer risk and was therefore included here(15). The ISI Web of Science database and PubMed were searched for relevant abstracts for the period January, 2006 through June, 2010, to update previous reviews. Search terms used included: “lung cancer,” or “lung carcinoma” or “adenocarcinoma of the lung” or “non-small cell cancer” in conjunction with “nonsmok*,” or “never smok*.” Only articles with abstracts in English were considered.
1.2. Risk factors
1.2.1. Age
Perhaps because its influence on cancer risk is so pervasive and unavoidable, age is often given short shrift in discussions of risk factors for predicting cancer risk in never smokers. For most of the risk factors to be discussed below, duration of exposure is an implicit modifier. As a proxy for duration of exposure to lung carcinogens (e.g., cigarette smoke, radon gas, etc.), age is empirically an important predictor of lung cancer in both smokers and never smokers(16), and arguably the most important risk factor among never smokers. The strong influence of age on lung cancer among never smokers has been reported in a number of papers(17–19).
1.2.2. Environmental tobacco smoke (ETS)
Meta-analyses and reviews of the scientific literature have been commissioned by regulatory bodies such as the U.S. Environmental Protection Agency or the California Environmental Protection Agency, and more recently by the U.S. Surgeon General(20). These reviews have concluded on the basis of over 100 studies of nonsmokers chronically exposed to other people’s smoke that the evidence, although often weak at the level of the individual study, was sufficiently strong in the aggregate to support the conclusion that nonsmokers increase their risk of lung cancer mortality if they are chronically exposed to other people’s smoke. More recently, a meta-analysis of 55 recent studies of spousal smoking on the lung cancer risk of the nonsmoking spouse showed a pooled relative risk of 1.27 (95% CI: 1.17–1.37), with risk increasing monotonically with increasing exposure(21). This association was replicated in different populations across three continents: Asia, Europe and North America(21). In addition, downward trends in cotinine-validated self-reported exposure to secondhand smoke suggest that the contribution of environmental tobacco smoke exposure to nonsmokers’ risk of lung cancer should also decline if the association between exposure to secondhand smoke and lung cancer is causal(22,23) In the EPIC cohort, the authors reported a hazard rate of 1.65 (95% CI: 1.04 – 2.63) for lung cancer in nonsmokers exposed to ETS at work(24).
1.2.3. Cooking fumes
Especially in developing countries such as China, nonsmoking women chronically exposed to cooking fumes generated from burning wood or frying fat at high temperatures appear to be at increased risk of lung cancer, especially adenocarcinoma(25–27). The International Agency for Research on Cancer has classified emissions from burning wood or from high-temperature frying as ‘probably carcinogenic to humans (group 2A)’(28). The potential carcinogens implicated in these associations include polycyclic aromatic hydrocarbons(28) and trans, trans-2, 4-decadienal (high-fat frying)(29–31).
1.2.4. Ionizing radiation
The U.S. per-capita annual effective dose of ionizing radiation from medical procedures has increased about six-fold (0.5 mSv [1980] to 3.0 mSv [2006] over the last 25 years(32). The effective dose is defined as the sum of weighted equivalent doses in all the organs and tissues of the body Much of this increased exposure to radiation can be attributed to the twenty-fold increased usage of CT scans in the U.S. over the last 25 years(33). Medical exposures now represent, for the first time, more than half of the cumulative effective dose to which individuals in the US are exposed(32). While most diagnostic CT scans are associated with very favorable ratios of benefit to risk, there is increasing concern that too many CT studies are being performed in the United States and are contributing to an increased lung cancer risk in the U.S. population(33). Another source of ionizing radiation, namely the disintegration products of radon gas-222 is discussed below.
1.2.5. Radon gas
Exposure to short-lived radioactive disintegration products of the chemically inert gas radon-222, a natural product of the decay of uranium-238 ubiquitously present in rock and soil, was first shown to affect lung cancer rates in uranium miners(34–40). Hazelton et al. (2001) analyzed lung cancer mortality in a cohort of tin miners exposed to tobacco smoke (both cigarettes and Chinese pipes), arsenic and radon using the two-stage clonal expansion (TSCE) model(41), which is the principal exposure-response model used by the CISNET groups. While individual studies yield inconsistent results, meta-analyses suggest a linear exposure-response relationship among smokers. Results of a recent meta-analysis of 13 European studies examining the impact of residential radon gas exposure on lung cancer risk indicate a greater absolute hazard to cigarette smokers and recent ex-smokers than to lifelong non-smokers(42) This difference by smoking status was not confirmed in a meta-analysis of seven North American case-control studies of radon exposure and lung cancer risk, even though the meta-analysis did confirm a general increased lung cancer risk for residents exposed to higher levels of alpha-track detected levels of radon gas(43). Radon concentrations in homes tend to be higher in rural areas than in urban areas, in part because urban dwellers are more likely to live in upstairs apartments at least one floor removed from the foundation(44). The foundation is where the concentration of radon gas tends to be greatest. As population density rises, the proportion of the population living upstairs will increase, thereby likely decreasing the population average daily exposure to radon.
1.2.6. Asbestos
Although asbestos is known to cause cancer in humans, there are no reliable epidemiologic data to demonstrate an increased risk of asbestos-associated disease following average cumulative exposures of less than about 15 fibers per milliliter (f/mL) per year (f/mL-year). Most of the quantitative information on the risk of disease following exposure to asbestos is derived from epidemiological studies of cohorts of occupationally exposed workers. The most contemporary analyses of these cohorts have been undertaken by Berman and Crump (2008)(45). The average cumulative exposure in most of these cohorts is considerably higher than 15 fibers/mL- year. In addition to cohort studies, there are numerous case-control studies (e.g., Veglia et al., 2007(24)) of the association between asbestos exposure and lung cancer risk. The case-control studies have focused on exposure in specific industries or for specific job categories but do not have reliable quantitative information on either the intensity of exposure or the cumulative exposure. Asbestos fibers also interact with cigarette smoking in increasing the risk of lung cancer. The risk of lung cancer associated with asbestos exposure appears to depend on the type of asbestos fiber(46).
Cigarette smoke is a far stronger lung carcinogen than is asbestos, either amphibole or chrysotile. A smoker with a twenty pack-year habit increases his/her risk of lung cancer ten-fold or more(18,41,47). In contrast, heavy exposure to asbestos in never smokers (far higher than 100 f/mL-yr) is required just to double the risk(45,48–51). The relative risk for joint exposure to asbestos and smoking is generally taken to be multiplicative, often described as being “synergistic,” although there is some evidence to suggest that it may be less than multiplicative but greater than additive(52–54).
1.2.7. Inherited genetic susceptibility
Genome-wide association studies have identified several chromosome regions that may help explain variations in lung cancer risk(55). One region that has been repeatedly identified as a promising susceptibility locus across different genome-wide association studies (GWAS) has been region 5p15.33(55–59)(56). The locus 5p15.33 was confirmed to be a significant correlate of lung cancer risk, but only for the adenocarcinoma histological subtype. This locus has been associated with the telomerase gene TERT, a reverse transcriptase that is critical for telomere replication and stabilization by controlling telomere length(57).
Another susceptibility locus identified as possibly explaining family history of lung cancer incidence in Chinese nonsmoking women(60) is region 6p21. Underlying genetic susceptibility is suggested by studies demonstrating familial aggregation(61). A family linkage study of lung cancer has identified linkage of lung, laryngeal, and pharyngeal cancer in families to a region on chromosome 6q23–25(62). More recent confirmatory results identified a region of chromosome 6q that increases risk for lung cancer, particularly for never and light smokers(63).
1.2.8. Occupational and workplace environmental exposures to carcinogens
Workers in both the U.S.(64,65) and the UK(23) have been experiencing progressively lower exposure to secondhand smoke over the last 20 years as workplace restrictions on smoking increased. For example, data from 2504 CARDIA participants (Black and Whites, ages 18–30 when recruited in 1985–86) showed a reduction from 16.3 hours of exposure in 1985/86 to 2.3 hours in 2000/2001 among college-educated workers, a reduction of 86%. Various occupational lung diseases, such as beryllium disease, sarcoidosis, asbestosis, and silicosis are associated with higher lung cancer risk but these diseases are considered “rare” diseases by orphanet because they have a prevalence rate of less than 1/2000, (http://www.orpha.net) and therefore do not contribute importantly to population attributable lung cancer risk(66–70).
1.2.9. Pre-existing lung disease including chronic obstructive pulmonary disease (COPD)
The largest meta-analysis to date relating previous lung disease to lung cancer risk showed increasing risk of lung cancer with increasing history of pneumonia and tuberculosis in never smokers(71). The authors conjectured that the most likely explanation for the increased risk associated with these diseases was the inflammatory effects of these diseases on lung tissue. The recruitment and activation of inflammatory cells at a site of infection involves the orchestrated expression of leukocyte and vascular adhesion molecules, as well as the establishment of chemotactic gradients via the generation of proinflammatory cytokines and chemokines(72). In turn, evidence suggests that inflammation plays a pivotal role in the development of lung cancer(73–75), particularly among never smokers(71). Inflammation may increase the risk of cancer development as an initiator or promoter through a variety of processes, including increased genetic mutations, anti-apoptotic signaling and increased angiogenesis(76). Other worldwide research also supports an association between previous lung disease and increased lung cancer risk.(60) (77) Authors of a systematic review of 37 case-control and 4 cohort studies examining the link of tuberculosis infection and subsequent lung cancer risk concluded that there is a direct relation between tuberculosis infection and subsequent lung cancer risk, at least for adenocarcinoma(78). Another group reviewed the literature examining associations between asthma incidence and subsequent risk of lung cancer incidence(79) and concluded that the evidence supports a direct relation between asthma and lung cancer in never smokers. They conjectured that asthma may increase the risk of lung cancer by reducing the clearance of inhaled toxins and carcinogens that are found in the bronchoalveolar epithelium(79).
It is not just restrictive lung disease, such as asthma, that has been associated with increase risk of lung cancer incidence. Chronic obstructive lung disease (COPD) in women has been found to have precursors similar to those that predict lung cancer risk, such as exposure to environmental tobacco smoke and exposure to asbestos(80). COPD and restrictive lung disease were found to contribute equally to lung cancer risk in Swedish construction workers, both more often associated with squamous and small cell lung cancer, than with adenocarcinoma(81).
1.2.10. Oncogenic viruses
Observational studies have linked the presence of various types of the human papillomavirus (HPV) with increased risk of lung cancer(82). The authors of one meta-analysis of this literature concluded that the data suggest that HPV was an important contributor to lung cancer incidence(83).
1.3. Incidence and mortality
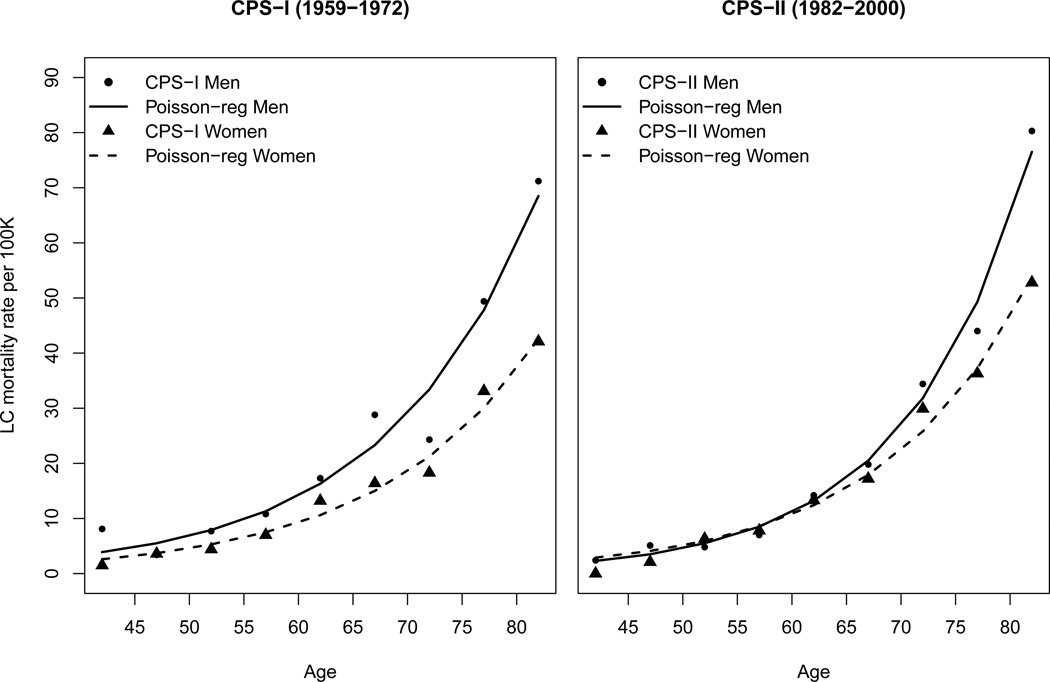
Approximately 10 to 15% of all lung cancers arise in never smokers, making lung cancer in never smokers about the seventh most important cause of cancer-related mortality after smoking(12,19,84). The earliest lung cancer incidence estimates were based on just a few large cohort studies, beginning with the British Doctors study(85), Prevention Study I of the American Cancer Society, and Prevention Study II of the ACS(86). U.S. incidence data for long-term trends (1975 to 2007) and data on lifetime probability of developing cancer were obtained from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, covering about 26% of the US population (National Cancer Institute, 2011). Recent incidence data (1995 to 2007) were obtained from cancer registries that participate in the SEER program or the Centers for Disease Control and Prevention (CDC)’s National Program of Cancer Registries (NPCR) through the North American Association of Central Cancer Registries (NAACCR)(84). Because of the historically high correlation between incidence and mortality, time trends have usually focused on mortality rates. Figure 1 shows gender- and age-specific trends in lung cancer mortality for each of the two Cancer Prevention Study cohorts of mostly white never smoker volunteers.
Figure 1.
Gender- and age-specific trends in lung cancer mortality among never smokers for the two Cancer Prevention Study I (CPSI) and II (CPSII) cohorts. Points represent the observed rates, and lines the rates smoothed by using Poisson regression (see Table 2 in Thun (2006)(19)).
1.4. Smoker versus never smoker differences in lung cancer survival
Survival in never smokers with lung cancer has been slightly better than survival in smokers with lung cancer, but mortality remains the typical result of diagnosis within five years(11,87).
Rudin and associates reviewed cohort studies of survival across stages in never smoker versus smoker lung cancer cases and found a 30% survival advantage for the never smokers(88). Postsurgical outcomes appeared to favor nonsmokers in two studies of surgically resected stage I nonsmall cell lung cancer(89,90), although the difference was small and disappeared when age, T stage, pleural invasion, and gender were included as covariates in a multivariate analysis(89). In two cancer treatment trials, the never smokers randomly assigned to the placebo arm had a statistically non-significant one-month survival advantage over lung cancer patients with a smoking history(91,92). In the active treatment arm of a related lung cancer treatment study, however, the survival advantage of the never smokers over former and current smokers was statistically significant, even after controlling for age, gender, stage, and performance status(93). Two other trials reported no difference in survival after lung cancer diagnosis between never smokers and those with a history of tobacco smoking(94,95) One possible resolution of these conflicting findings is the emerging clinical consensus that lung cancer patients with minimal or no history of tobacco use showed markedly better clinical outcomes when treated with getfitnib and erlotinib(88) than patients with substantial smoking history. Getfitnib and erolotinib are epidermal growth factor receptor (EGFR) small molecule tyrosine kinase inhibitors. Retrospective studies of patients have shown that compared with ever smokers, never smokers show more clinical benefit from treatment with an EGFR inhibitor including statistically significant higher responses rates, longer times to progression, and/or longer median overall survival(88). The better clinical response was seen especially in patients of East Asian ancestry, in women, and in tumors with adenocarcinoma histology(96).
1.5. Gender differences in lung cancer risk among never smokers
There are more than twice as many women as men age 60 years and older who have never smoked(19), which helps to explain why never smokers diagnosed with lung cancer are more likely to be women. Thun 2008, Thun 2006 and Meza 2008 report, contrary to earlier studies, that men never smokers have similar rates of lung cancer incidence as women never smokers(2,18,19). Wakelee et al. (2007) disagree, partly on the basis of data from the Nurses' Health Study and Health Professionals Follow-up Study(97). Freedman and associates also reported increased lung cancer incidence in women never smokers compared to men never smokers(98). Further confusing the picture is the advent of more sensitive screening technology, which may be detecting in women never smokers prevalent indolent lung cancers that would not have been detected previously and that would not have contributed to lung cancer mortality(13). Lung cancer detection has improved over time as new, more sensitive screening technology has become available. There is, as a result, no consensus yet on whether women and men never smokers differ with respect to risk of lung cancer incidence.
With respect to lung cancer survival, however, the evidence consistently favors women never smokers, both for small cell lung cancer(99) and for non-small cell lung cancer(100). Five year survival remains poor for both genders (< 50%), however, but particularly so for men (<40%)(100). The reasons for this survival advantage have not been identified. This advantage is seen in untreated lung cancer patients as well as in treated patients, suggesting that the advantage cannot be attributed to different treatment patterns or better response to treatment by women(100).
1.6. Ethnic, genetic differences in lung cancer risk
Subramanian (2007) and Thun (2008) noted the higher incidence of never smoker lung cancer in South East Asia compared to never smokers elsewhere(2,11). Subramanian conjectures that differences in genetics may explain discrepancies. Schwartz and colleagues discounted earlier conjectures about Blacks being at high risk of lung cancer susceptibility than Whites when they found no differences in incidence in a case control study in Detroit, Michigan, with the exception of Black men ages 40–54 having higher risk than White men of the same age(101). Thun and associates (2008) disagree, concluding from their review of the literature that African American women never smokers had significantly higher incidence rates from lung cancer than women of European descent who had never smoked (RR = 1.56, 95% CI = 1.1– 2.1)(2).
1.7. Lung cancer incidence among never smokers by histologic subtype
Never smokers have more adenocarcinomas(97) and are said to have tumors biologically distinct from those seen in smokers(11). Lung cancers in persons with a history of active cigarette smoking are proportionately more small cell and squamous cell lung cancers(102). A recent review of studies of lung cancer in never smokers concluded that lung cancers arising in never smokers have a distinct natural history, profile of oncogenic mutations, and response to targeted therapy(88). It’s not clear that the changing pattern of histologic subtypes has any relevance to changes in overall lung cancer mortality(103).
1.8. Nonsmoker lung cancer status and trends
Relative to other incident cancers, lung cancer among never-smokers would rank 11th among the 12 most common incident cancers in SEER areas of the US in 2004(2). Relative to other fatal cancers, if never smoker lung cancer rates were applied to the whole population, lung cancer mortality would rank as the 7th most common fatal cancer in 2004.(2). With respect to lung cancer time trends, Thun and associates (2008) concluded from their review that there was no discernible time trend and Thun (2006) said that there was a small, nearly negligible trend towards higher rates of lung cancer incidence in white women nonsmokers only(2,19). Wakelee and associates(97) made the point, after reviewing the results of six cohort studies, that not enough time has elapsed between the start of these cohort studies and the most recent data collection period to permit confident extrapolations about rate changes over time. More research is therefore needed to determine whether lung cancer rates among never smokers have remained constant over time.
2. LUNG CANCER IN NEVER SMOKERS - RISK MODELS
In this section we review some of the never smoker lung cancer risk models used by CISNET modelers and compare the number of never smokers by age and gender between the three CISNET smoking scenarios(104) and discuss the corresponding lung cancer mortality predictions among never smokers for a typical CISNET model.
As discussed in the previous sections, several risk factors have been associated with the lung cancer risk among never smokers. Nevertheless, among all of these factors, age remains as the most significant contributor to lung cancer risk in never smokers. For this reason and because many of these other risk factors have been identified only recently or because there is not much information about the historical exposure to these agents in the US, the lung cancer risk models used in CISNET at this point consider mainly age and gender as risk factors for never smokers.
2.1. TSCE Model
The two-stage clonal expansion (TSCE) model posits that cells initiated via a Poisson process undergo clonal expansion and malignant conversion via a birth-death-mutation process. The details of this model are presented in a number of publications(105,106). The model has the following parameters, the rate of initiation, μ0X, the rate of cell division, α, and cell death, β, of initiated cells and the rate of malignant conversion, μ1. Not all 4 parameters can be estimated from incidence data alone.
The hazard or age-specific mortality (incidence) function is the standard measure of cancer risk. The cancer hazard for the TSCE model can be expressed in terms of the model parameters as:
where p and q are the roots of a quadratic equation, with p + q = g = −(α − β − μ1) and −pq=αμ1. Note that g represents roughly the net rate of proliferation of initiated cells (since μ1 is a mutation rate and much smaller than α and β), q ~ μ1/(1 − β/α), and μ0X is what Fisher has called the index of diversity.
The TSCE model has been fitted to the lung cancer incidence and mortality in several US cohorts. Although most analyses have concentrated in estimating the effects of tobacco smoking on lung cancer risk, the background (never smoker) lung cancer risk as a function of age (hazard) is a byproduct of these kinds of analyses.
2.1.1. CPSI and CPSII
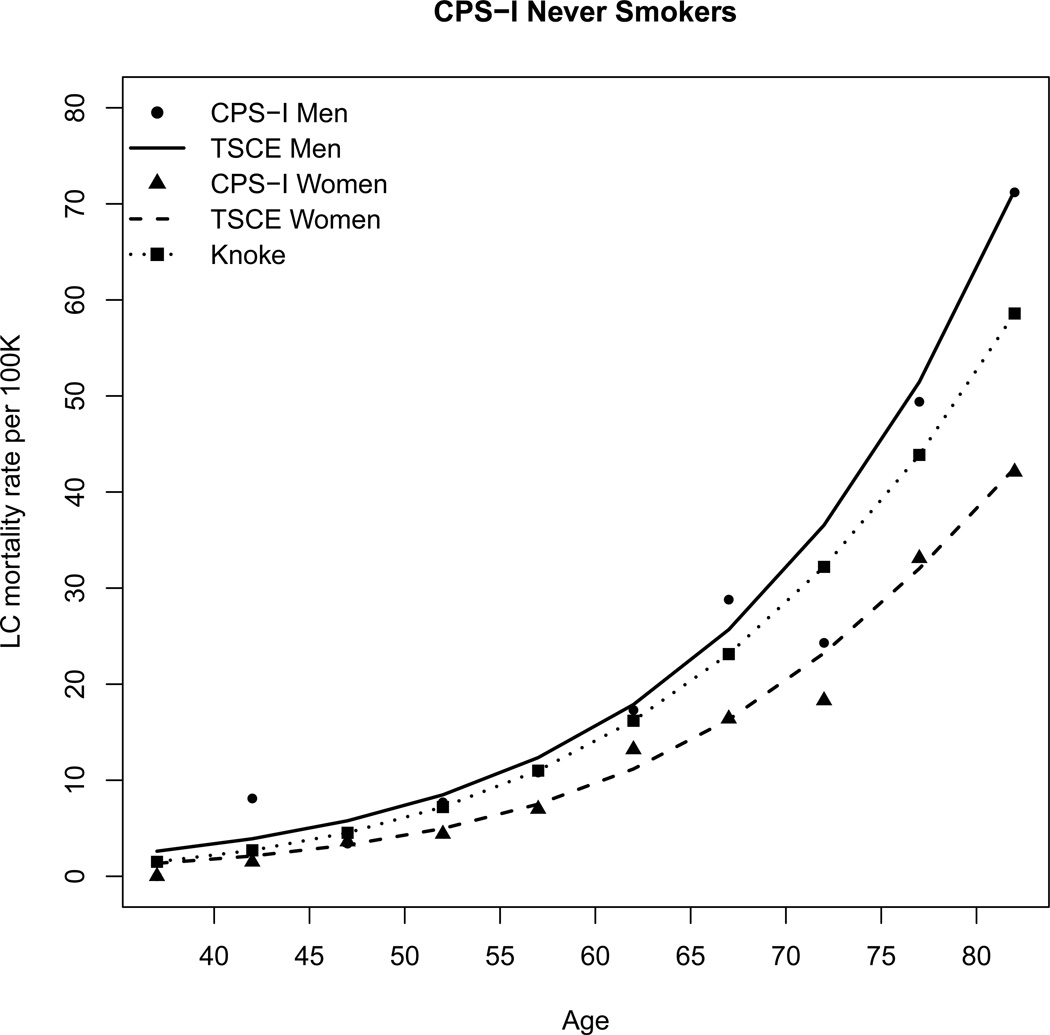
Hazelton et al. fitted the TSCE model to the lung cancer mortality in three cohorts, the ACS Cancer Prevention Study (CPS) I and CPSII cohorts and the UK British Doctors study(107). Table I shows the parameter estimates obtained by Hazelton et al. Figure 2 shows the CPS I lung cancer age-specific mortality rates among never smokers and the corresponding TSCE hazard functions.
Table I.
TSCE Model parameters
| CPSI Men LC Mortality |
CPSI Women LC Mortality |
CPSII Men LC Mortality |
CPSII Women LC Mortality |
NHS/HPFS LC Incidence |
HPFS LC Mortality |
NHS LC Mortality |
|
|---|---|---|---|---|---|---|---|
| μ0=μ1 | 1.40×10−7 | 8.93×10−8 | 7.16×10−8 | 1.07×10−7 | 8.14×10−8 | 7.55×10−8 | 1.15×10−7 |
| g | 0.075 | 0.086 | 0.090 | 0.071 | 0.096 | 0.091 | 0.075 |
| α | 22.65 | 71.56 | 7.70 | 15.82 | 3 | 5.52 | 5.09 |
Note. All models assume X=107 and a lagtime between cancer onset and cancer mortality or diagnosis (incidence) = 5.
Figure 2.
CPS I lung cancer age-specific mortality rates among never smokers by gender and the corresponding hazard functions from the TSCE and Knoke models.
2.1.2. NHS and HPFS
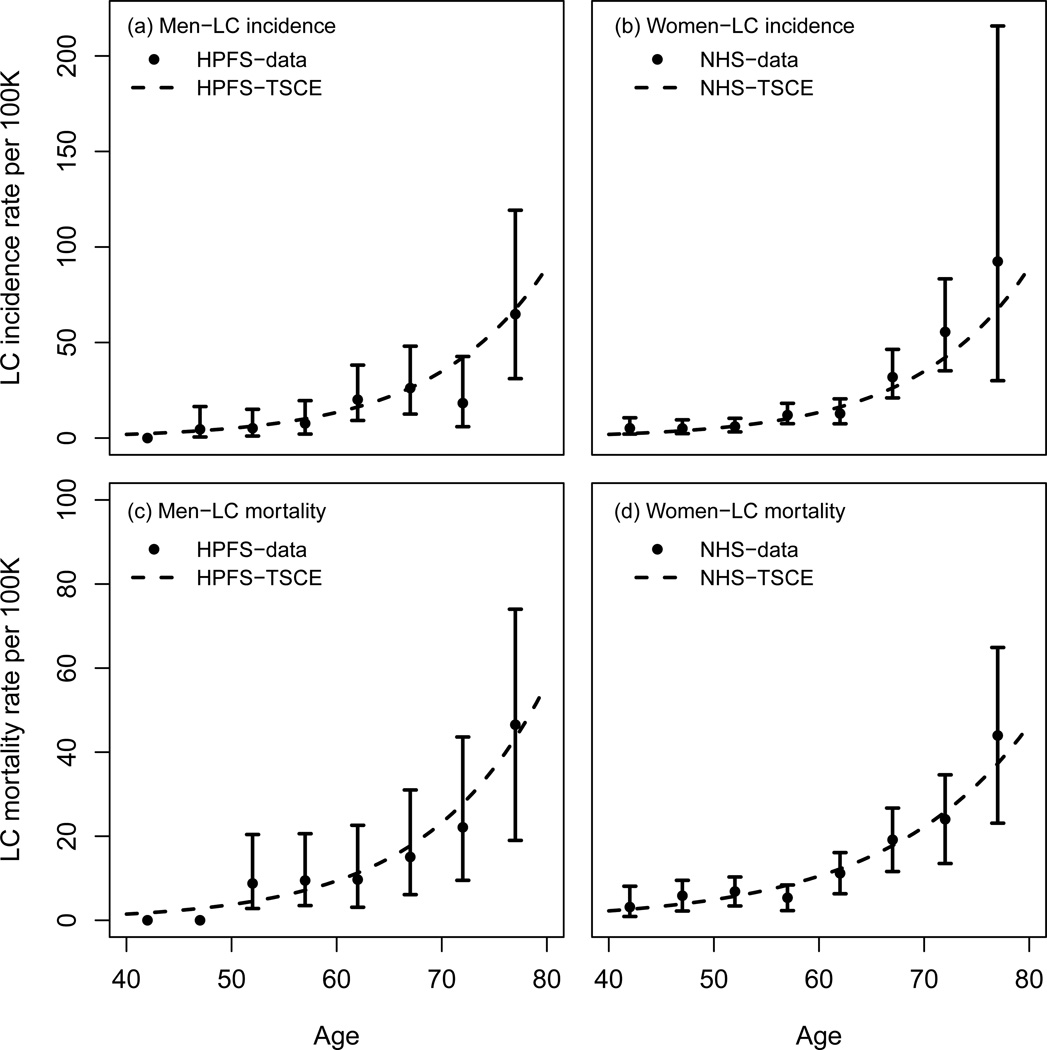
Meza et al. fitted the TSCE model to the lung cancer incidence in never smokers in the Nurses Health (NHS) and Health Professionals Follow-Up (HPFS) studies(18). From their analysis, Meza et al. concluded that the lung cancer incidence among never smokers was identical in both datasets, therefore deriving a common never smoker model for women and men. Table I shows the parameter estimates obtained by Meza et al. Figure 3 (upper panels) shows the lung cancer age-specific incidence rates among never smokers in the NHS and HPFS and the corresponding TSCE model hazard functions (data points and CI reproduced from Meza (2008)(18)).
Figure 3.
NHS and HPFS lung cancer age-specific incidence and mortality rates among never smokers and the corresponding TSCE model hazard functions. Notice the difference in scale between the top and bottom y-axis.
The lung cancer mortality data in NHS and HPFS have been analyzed using the TSCE model as part of the CISNET project. Table I shows parameter estimates obtained after fitting the TSCE hazard function to the NHS and HPFS lung cancer mortality rates in never smokers as reported in Thun (2008)(2). Figure 3 (lower panels) shows the corresponding hazard functions and the NHS and HPFS lung cancer age-specific mortality rates among never smokers (data points and CI as reported in Thun (2008)(2)).
Table II shows estimations of the lifetime lung cancer risk in never smokers with and without adjustment for other cause mortality. US decennial life tables for 1989–1991(108) were used to adjust for competing causes of mortality.
Table II.
Life-time probability (by age 85) of lung cancer (LC) incidence and mortality for never smokers with/without adjustment for death from other causes.
| Model1 | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| LC incidence | 1.57% | 1.57% | 0.93% | 1.17% |
| LC mortality | 1.02% | 0.93% | 0.61% | 0.71% |
: Life-time probabilities were calculated using the parameter estimates based on the analysis of NHS/HPFS data.
2.2. Knoke’s model
Knoke et al. fitted a power of age model (Armitage-Doll) to the lung cancer mortality among male nonsmokers in CPSI and found that the mortality rates increase with between the fourth and fifth power of age(109):
where α =9.21E-13 and β =4.60. This is consistent with previous estimates from the British Doctors study(85). Figure 2 shows the CPSI data and Knoke’s model hazard function.
2.3. Never smokers’ lung cancer risk in the three CISNET smoking scenarios
As discussed in chapters 2, 4, and 5 of this monograph(110–112), the CISNET modelers have investigated the consequences of existing tobacco control policies on lung cancer risk using three US smoking history scenarios. The Actual Tobacco Control (ATC) scenario reflects the actual smoking patterns observed in the US from 1890 until 2000. The Complete Tobacco Control (CTC) scenario simulates the US smoking histories assuming that everyone quit smoking in 1965 and that no one else picked up the habit afterwards. The No Tobacco Control (NTC) scenario simulates the US smoking rates assuming that the smoking behaviors of the US population were not affected by the discovery of the harmful effects of smoking on human health.
To simulate the three smoking scenarios, CISNET modelers relied on the smoking history generator (SHG)(112). The SHG is a CISNET-shared precursor model that produces cohort-specific smoking histories and related other cause death rates as inputs for the larger dose/response, survival generation models. For each smoking scenario (ATC, NTC, CTC), the SHG simulates detailed individual level smoking histories for individuals born between 1890 and 1985 over the period 1975–2000. For more details on the SHG please see chapter 5 in this monograph.
The central component of each CISNET lung model is a dose-response module that provides a quantitative description of the age-specific mortality of lung cancer among never smokers, and among continuing smokers and former smokers by detailed history of smoking. With the exception of one modeling group, which used logistic regression models, all CISNET groups used a version of the TSCE model described above as their underlying dose-response model (please see Chapters 7–13 for a detailed description of each CISNET lung model(3–9)).
For illustrative purposes we present estimations of the number of US lung cancer deaths for each CISNET smoking scenario using a typical CISNET model. To do this, we first used the SHG to simulate the population of never smokers in the US under the three CISNET smoking scenarios. We then applied one of the CISNET models, the FHCRC model (see Chapter 8 for model details), to produce estimations of the number of lung cancer deaths among never smokers by age, calendar year and gender for each CISNET smoking scenario.
3. RESULTS
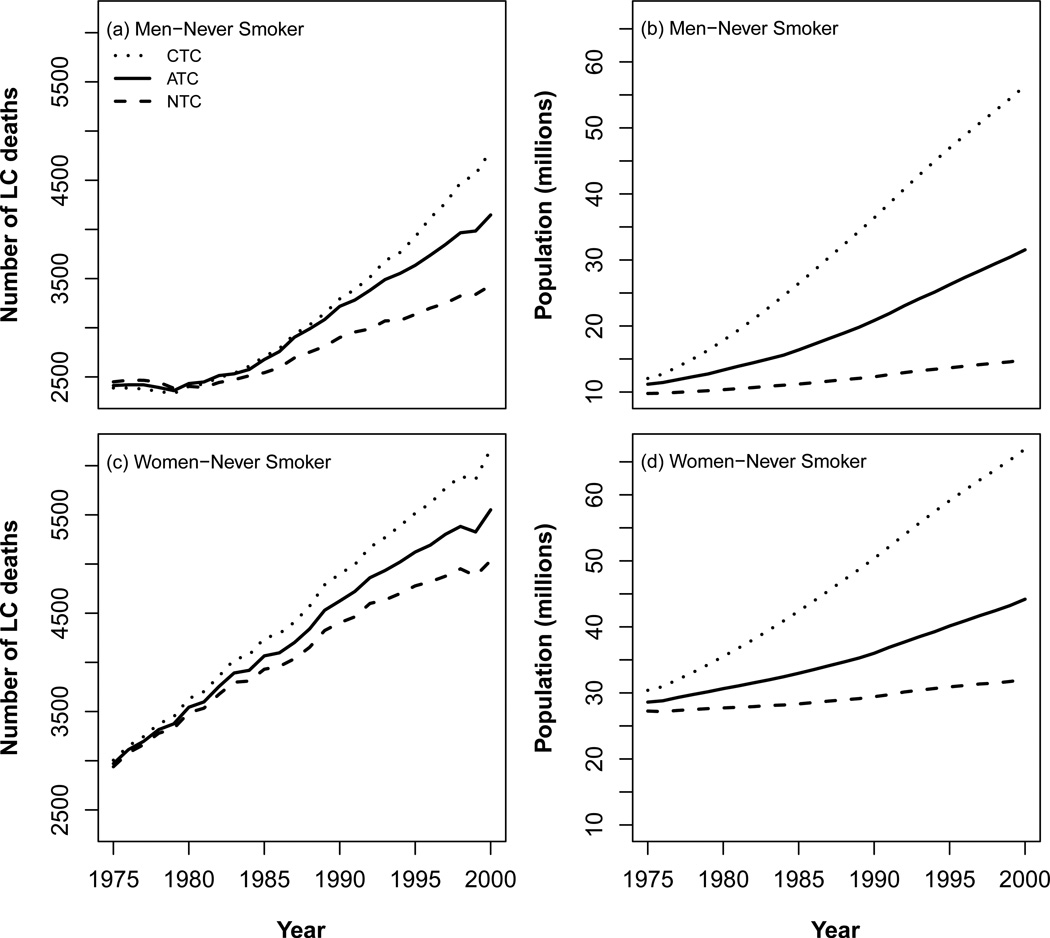
Figure 4 shows the predicted number of lung cancer deaths among never smokers by gender in the US (1975–2000) from a typical CISNET model for each of the three CISNET smoking scenarios (see Chapters 2 and 4). Although it would be ideal to compare the ATC prediction with the actual numbers of yearly LC deaths among never smokers in the US, there are no reliable statistics of lung cancer mortality in never smokers at the US population level. This highlights even further the value of the CISNET modeling predictions. Figure 4 also shows the simulated US never smoker populations for each CISNET smoking scenario (see Chapter 5). As shown in the figure the never smoker population increases with increasing calendar years in all CISNET smoking scenarios, yielding a commensurate increase in the corresponding number of predicted lung cancer deaths in never smokers. Clearly, the never smoker population is highest in the CTC scenario and lowest in the NTC scenario.
Figure 4.
Predicted lung cancer deaths among never smokers by gender in the US (1975–2000) from a typical CISNET model for each of the three CISNET smoking scenarios (ATC- Actual Tobacco Control; NTC – No Tobacco Control; CTC – Complete Tobacco Control).
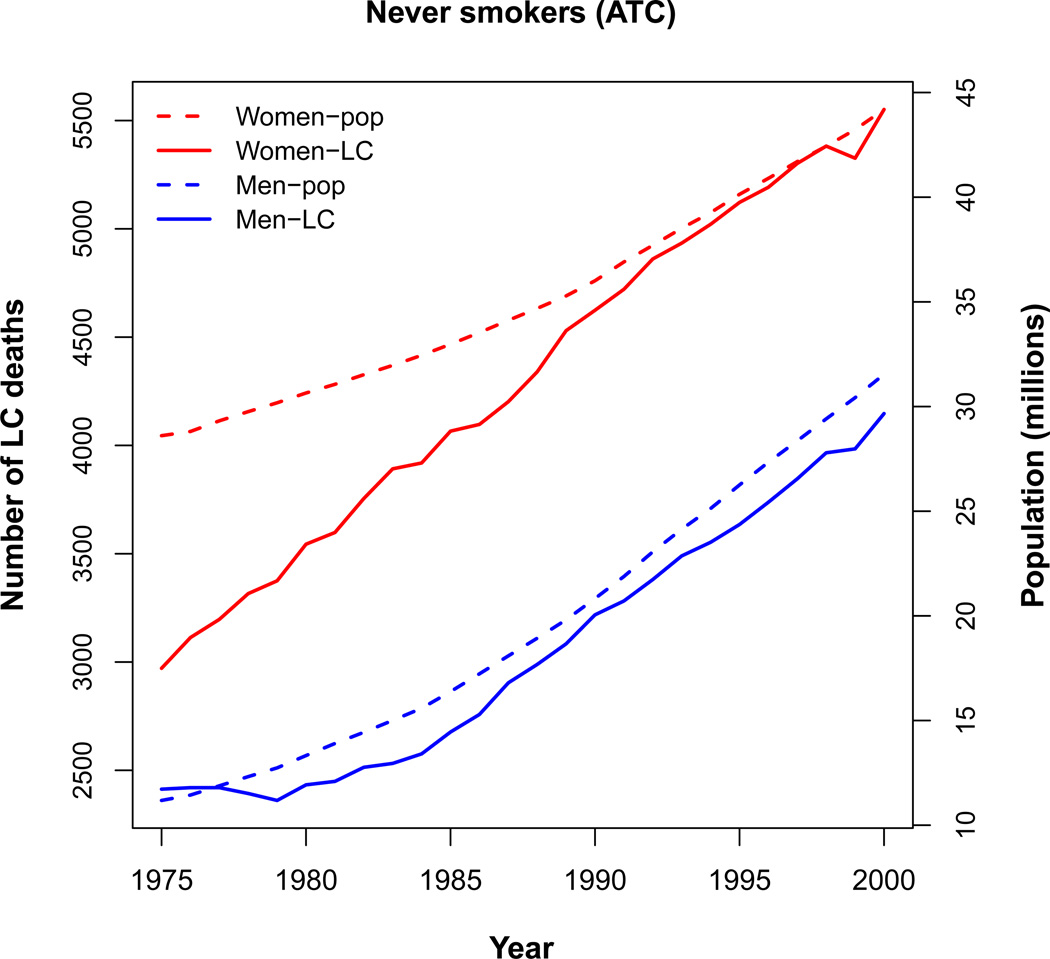
Figure 5 shows in a single panel the population of men and women never smokers in the ATC scenario and the corresponding lung cancer deaths by calendar year for a typical CISNET model. The figure shows that the population of never smokers is significantly higher in women than in men, but that the gap has been diminishing with increasing calendar year. Accordingly, the number of lung cancer deaths in never smokers is significantly higher in women, but the gap has started to diminish.
Figure 5.
Populations of men and women never smokers in the Actual Tobacco Control (ATC) scenario and the corresponding lung cancer deaths for a typical CISNET model.
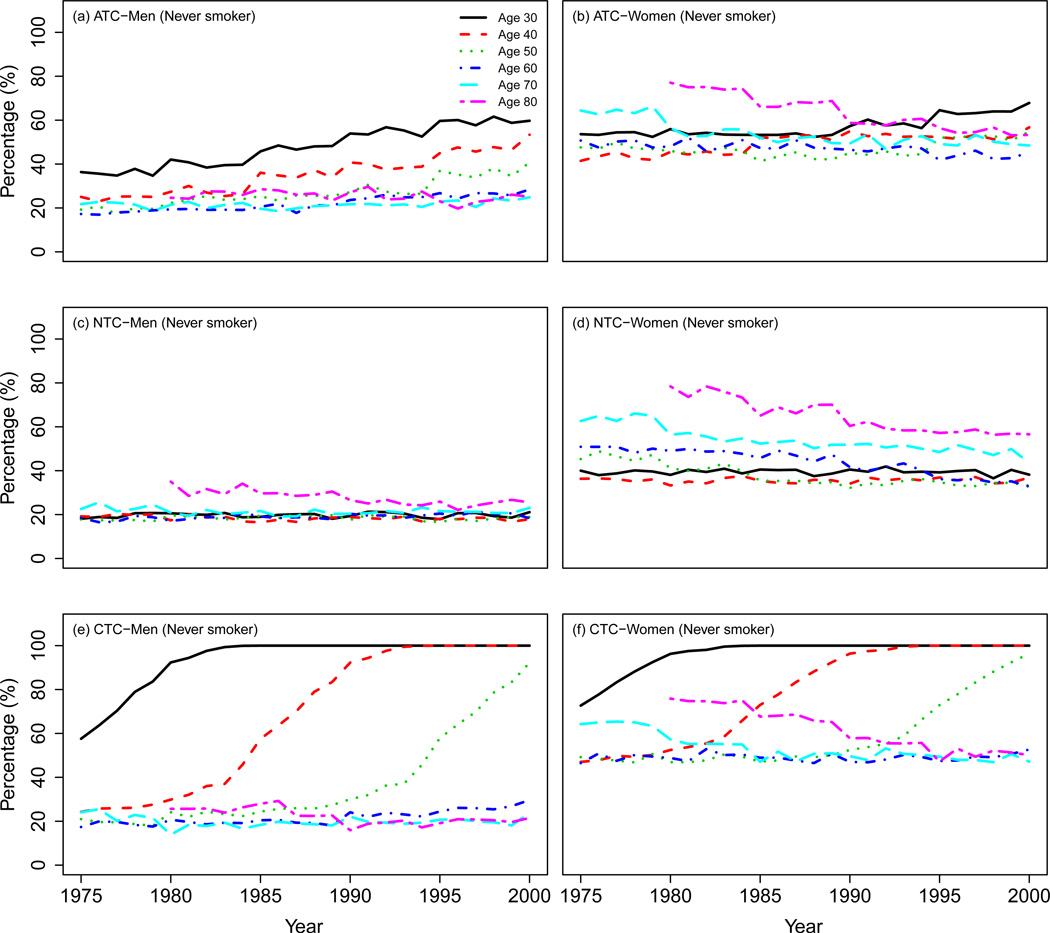
Figure 6 shows the percentage of never smokers by age and gender in the three CISNET smoking scenarios. The top panel shows that the percentage of male never smokers in the ATC scenario increases or remains constant across all ages during the period of analysis. In contrast, the percentage of female never smokers of ages ≥ 60 decreases steadily, reflecting the later onset of the tobacco epidemic in women. The medium panel shows that the percentage of male never smokers remains roughly constant for all ages in the NTC scenario, whereas the percentage of female never smokers decreases by calendar year particularly for older ages, reflecting again the later onset of the tobacco epidemic in women. The bottom panel shows that the percentage of never smokers of ages ≤ 50 in the CTC scenario increases steadily to 100% independently of gender. In contrast, the percentage of male never smokers of ages > 50 remains roughly constant while the percentage of female never smokers older than 50 decreases steadily during the period of analysis.
Figure 6.
Percentage of never smokers by gender and age in the three CISNET smoking scenarios.
4. DISCUSSION
Our review of the scientific literature on major influences on lung cancer risk in never smokers identified a range of influences, from environmental tobacco smoke, to radiation exposures, to asbestos. The review relied on studies that generally did not use biologically-validated definitions of non or never smokers. This literature spanned several decades, during which time cotinine-validated exposure to environmental tobacco smoke in the U.S. in self-reported nonsmokers dropped from nearly 100% exposure to 41% exposure(113). On the other hand, even though detectable cotinine levels in U.S. nonsmokers have fallen 70% since 1988, 45% of nonsmokers continue to show biological evidence of daily exposure to secondhand smoke(114)
Variations in serum cotinine, a well-accepted biomarker for exposure to cigarette smoke, have been shown to co-vary with self-reported exposure by self-described never smokers(115). Single measurements of serum cotinine have also been shown to provide stable estimates of smoking status over one year(116). Validation studies have confirmed the utility of serum cotinine in nonsmokers for reflecting nonsmokers’ exposure to secondhand smoke(117). In critically ill patients screened with these biomarkers, 96% of self-reported nonsmokers had biological evidence of recent exposure to cigarette smoke(118). Even today, therefore, differences between self-reported ETS-exposed nonsmokers and self-reported non-exposed nonsmokers are likely to be attenuated by misclassification of exposure status. A more recent, potentially more precise tobacco-specific biomarker highly correlated with cotinine (r = .92) is trans-3'-hydroxycotinine, urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butano (NNA) is more proximally related to cancer risk than cotinine and tends to be underpredicted by cotinine, especially in small children(119) Use of such tobacco-specific biomarkers of exposure are called for. Their use would permit more confident inferences about putative associations between exposure to environmental tobacco smoke and lung cancer risk(118). In any event, a large fraction of lung cancers occurring in never smokers cannot be definitively associated with established environmental risk factors, highlighting the need for additional epidemiologic research in this area(13).
In this Chapter we also reviewed some of the never smoker lung cancer risk models used in CISNET. We then compared the prevalence of never smokers between the three CISNET smoking scenarios and presented the corresponding never smoker lung cancer mortality predictions from a typical CISNET model.
The models used in CISNET did not incorporate many of the known or suspected risk factors described in this monograph, such as environmental tobacco smoke exposure, diet, radon exposure and air pollution that could have influenced trends in lung cancer mortality among never smokers in the U.S. However, age and gender remain the most significant contributors to lung cancer risk among never smokers, and any other risk factors have either just been recently identified or lack detailed information about their historical exposure patterns in the U.S. Nevertheless, to compensate for the absence of other lung cancer risk factors besides age, gender and smoking in their models, some CISNET groups chose to calibrate their models further to describe actual deaths in the U.S. population over the period 1975–2000 (see Chapters 7–13). However, since most of the current lung cancer risk in the population is due to smoking and since there is no information available on the number of yearly lung cancer deaths among never smokers in the US, it is not clear that these adjustments are directly applicable to never smokers. Even with these limitations, the CISNET models constitute an important tool that can help public health policy makers make predictions about the impact of changes in smoking prevalence on past, current and future lung cancer rates in US never smokers, predictions that would be otherwise very difficult or impossible to make(104).
As suggested by our analysis, even if the lung cancer risk among never smokers remains constant in time, the number of lung cancer deaths among never smokers would be expected to increase significantly in the near future as the prevalence of smoking continues to fade and as the population age-structure continues to shift to older ages. This implies that it is likely that lung cancer will continue to be one of the most prevalent cancers in the US in spite of the significant improvements in tobacco control.
Acknowledgments
The work reported in this paper was supported by National Cancer Institute grants
5U01CA097415 (Moolgavkar)
5U01CA097416 (de Koning)
Footnotes
The authors have no competing interests to report.
References
- 1.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States 1997–2001. MMWR Morb. Mortal. Wkly. Rep. 2005;54(25):625–628. [PubMed] [Google Scholar]
- 2.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, Flanders WD, Jee SH, Katanoda K, Kolonel LN, Lee IM, Marugame T, Palmer JR, Riboli E, Sobue T, Avila-Tang E, Wilkens LR, Samet JM. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz FW, Boer R, de Koning HJ. Description of MISCAN-lung, the Erasmus MC lung cancer model for evaluating cancer control interventions (Ch. 7 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazelton W, Jeon J, Meza R, Moolgavkar SH. FHCRC lung cancer model (Ch. 8 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon P, Kong CY, Johnson BE, Weinstein MC, Weeks JC, Tramontano AC, Cipriano LE, Bouzan C, Gazelle GS. The MGH ITA lung cancer policy model: Tobacco control versus screening (Ch. 9 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy D. A macro-model of smoking and lung cancer: Examining aggregate trends in lung cancer rates as they relate to CPS-I and CPS-II two-stage clonal expansion models (Ch. 10 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2012.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy M, Deng L, Spitz M, Gorlova O, Kimmel M. Rice-MD Anderson lung cancer model (Ch. 11 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 8.Holford TR, Ebisu K, McKay L, Oh C, Zheng T. Yale lung cancer model (Ch 12 of this monogrgaph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon P, Hazelton W, Kimmel M, Clarke L. CISNET lung models: Comparison of model assumptions and model structures (Ch. 13 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines for controlling and monitoring the tobacco epidemic: World Health Organization. 1998
- 11.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J. Clin. Oncol. 2007;25(5):561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat. Rev. Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 13.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009;15(18):5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce DA, Mendelsohn ML. A model for radiation-related cancer suggested by atomic bomb survivor data. Radiat. Res. 1999;152(6):642–654. [PubMed] [Google Scholar]
- 15.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br. J. Radiol. 2008;81(965):362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 16.Doll R. The age distribution of cancer: implications for models of carcinogenesis. Journal of the Royal Statistical Society.Series A (General) 1971;134(2):133–166. [Google Scholar]
- 17.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J. Epidemiol. Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meza R, Hazelton WD, Colditz GA, Moolgavkar SH. Analysis of lung cancer incidence in the Nurses' Health and the Health Professionals' Follow-Up Studies using a multistage carcinogenesis model. Cancer Causes Control. 2008;19(3):317–328. doi: 10.1007/s10552-007-9094-5. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J. Natl. Cancer Inst. 2006;98(10):691–699. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 20.USDHHS. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [Accessed on February 25, 2010]; Available at: http://www.surgeongeneral.gov/library/secondhandsmoke/report/fullreport.pdf.
- 21.Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int. J. Epidemiol. 2007;36(5):1048–1059. doi: 10.1093/ije/dym158. [DOI] [PubMed] [Google Scholar]
- 22.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke 1988–2002. Environ. Health Perspect. 2006;114(6):853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferis BJ, Thomson AG, Lennon LT, Feyerabend C, Doig M, McMeekin L, Wannamethee SG, Cook DG, Whincup PH. Changes in environmental tobacco smoke (ETS) exposure over a 20-year period: cross-sectional and longitudinal analyses. Addiction. 2009;104(3):496–503. doi: 10.1111/j.1360-0443.2008.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veglia F, Vineis P, Overvad K, Boeing H, Bergmann M, Trichopoulou A, Trichopoulos D, Palli D, Krogh V, Tumino R, Linseisen J, Steindorf K, Raaschou-Nielsen O, Tjonneland A, Gonzalez CA, Martinez C, Dorronsoro M, Barricarte A, Cirera L, Quiros JR, Day NE, Saracci R, Riboli E. Occupational exposures, environmental tobacco smoke, and lung cancer. Epidemiology. 2007;18(6):769–775. doi: 10.1097/ede.0b013e318142c8a1. [DOI] [PubMed] [Google Scholar]
- 25.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66(9):4961–4967. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 26.Wang XR, Chiu YL, Qiu H, Au JS, Yu IT. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Ann. Oncol. 2009;20(4):746–751. doi: 10.1093/annonc/mdn699. [DOI] [PubMed] [Google Scholar]
- 27.Chiu YL, Wang XR, Qiu H, Yu IT. Risk factors for lung cancer: a case-control study in Hong Kong women. Cancer Causes Control. 2010;21(5):777–785. doi: 10.1007/s10552-010-9506-9. [DOI] [PubMed] [Google Scholar]
- 28.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006;7(12):977–978. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 29.Young SC, Chang LW, Lee HL, Tsai LH, Liu YC, Lin P. DNA damages induced by trans, trans-2,4-decadienal (tt-DDE), a component of cooking oil fume, in human bronchial epithelial cells. Environ. Mol. Mutagen. 2010;51(4):315–321. doi: 10.1002/em.20550. [DOI] [PubMed] [Google Scholar]
- 30.Dung CH, Wu SC, Yen GC. Genotoxicity and oxidative stress of the mutagenic compounds formed in fumes of heated soybean oil, sunflower oil and lard. Toxicol. In. Vitro. 2006;20(4):439–447. doi: 10.1016/j.tiv.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Chang LW, Lo WS, Lin P. Trans, trans-2,4-decadienal, a product found in cooking oil fumes, induces cell proliferation and cytokine production due to reactive oxygen species in human bronchial epithelial cells. Toxicol. Sci. 2005;87(2):337–343. doi: 10.1093/toxsci/kfi258. [DOI] [PubMed] [Google Scholar]
- 32.Mettler FA, Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources-1950–2007. Radiology. 2009;253(2):520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 33.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N. Engl. J. Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 34.Lubin JH, Boice JD, Jr, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA, Morrison HI, Radford EP, Samet JM. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J. Natl. Cancer Inst. 1995;87(11):817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- 35.Qiao YL, Taylor PR, Yao SX, Schatzkin A, Mao BL, Lubin J, Rao JY, McAdams M, Xuan XZ, Li JY. Relation of radon exposure and tobacco use to lung cancer among tin miners in Yunnan Province, China. Am. J. Ind. Med. 1989;16(5):511–521. doi: 10.1002/ajim.4700160504. [DOI] [PubMed] [Google Scholar]
- 36.Radford EP, Renard KG. Lung cancer in Swedish iron miners exposed to low doses of radon daughters. N. Engl. J. Med. 1984;310(23):1485–1494. doi: 10.1056/NEJM198406073102302. [DOI] [PubMed] [Google Scholar]
- 37.Roscoe RJ, Steenland K, Halperin WE, Beaumont JJ, Waxweiler RJ. Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters. JAMA. 1989;262(5):629–633. [PubMed] [Google Scholar]
- 38.Tirmarche M, Raphalen A, Allin F, Chameaud J, Bredon P. Mortality of a cohort of French uranium miners exposed to relatively low radon concentrations. Br. J. Cancer. 1993;67(5):1090–1097. doi: 10.1038/bjc.1993.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasek L, Darby SC, Swerdlow AJ, Placek V, Kunz E. Radon exposure and cancers other than lung cancer among uranium miners in West Bohemia. Lancet. 1993;341(8850):919–923. doi: 10.1016/0140-6736(93)91212-5. [DOI] [PubMed] [Google Scholar]
- 40.Xuan XZ, Lubin JH, Li JY, Yang LF, Luo AS, Lan Y, Wang JZ, Blot WJ. A cohort study in southern China of tin miners exposed to radon and radon decay products. Health Phys. 1993;64(2):120–131. doi: 10.1097/00004032-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Hazelton WD, Luebeck EG, Heidenreich WF, Moolgavkar SH. Analysis of a historical cohort of Chinese tin miners with arsenic, radon, cigarette smoke, and pipe smoke exposures using the biologically based two-stage clonal expansion model. Radiat. Res. 2001;156(1):78–94. doi: 10.1667/0033-7587(2001)156[0078:aoahco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Makelainen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Rosario AS, Tirmarche M, Tomasek L, Whitley E, Wichmann HE, Doll R. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330(7485):223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, Klotz JB, Letourneau EG, Lynch CF, Lyon JL, Sandler DP, Schoenberg JB, Steck DJ, Stolwijk JA, Weinberg C, Wilcox HB. A combined analysis of North American case-control studies of residential radon and lung cancer. J. Toxicol. Environ. Health A. 2006;69(7):533–597. doi: 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- 44.Fisher EL, Field RW, Smith BJ, Lynch CF, Steck DJ, Neuberger JS. Spatial variation of residential radon concentrations: the Iowa Radon Lung Cancer Study. Health Phys. 1998;75(5):506–513. doi: 10.1097/00004032-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit. Rev. Toxicol. 2008;38(Suppl 1):49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- 46.Hodgson JT, Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000;44(8):565–601. [PubMed] [Google Scholar]
- 47.Moolgavkar SH, Luebeck EG, Krewski D, Zielinski JM. Radon, cigarette smoke, and lung cancer: a re-analysis of the Colorado Plateau uranium miners' data. Epidemiology. 1993;4(3):204–217. [PubMed] [Google Scholar]
- 48.Goodman M, Morgan RW, Ray R, Malloy CD, Zhao K. Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control. 1999;10(5):453–465. doi: 10.1023/a:1008980927434. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JC, Harris J, Armstrong B. Mortality in a cohort of vermiculite miners exposed to fibrous amphibole in Libby, Montana. Occup. Environ. Med. 2004;61(4):363–366. doi: 10.1136/oem.2003.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loomis D, Dement JM, Wolf SH, Richardson DB. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup. Environ. Med. 2009;66(8):535–542. doi: 10.1136/oem.2008.044362. [DOI] [PubMed] [Google Scholar]
- 51.Moolgavkar SH, Turim J, Alexander DD, Lau EC, Cushing CA. Potency factors for risk assessment at Libby, Montana. Risk Anal. 2010;30(8):1240–1248. doi: 10.1111/j.1539-6924.2010.01411.x. [DOI] [PubMed] [Google Scholar]
- 52.Wraith D, Mengersen K. Assessing the combined effect of asbestos exposure and smoking on lung cancer: a Bayesian approach. Stat. Med. 2007;26(5):1150–1169. doi: 10.1002/sim.2602. [DOI] [PubMed] [Google Scholar]
- 53.Liddell FD, Armstrong BG. The combination of effects on lung cancer of cigarette smoking and exposure in quebec chrysotile miners and millers. Ann. Occup. Hyg. 2002;46(1):5–13. doi: 10.1093/annhyg/mef008. [DOI] [PubMed] [Google Scholar]
- 54.Liddell FD. The interaction of asbestos and smoking in lung cancer. Ann. Occup. Hyg. 2001;45(5):341–356. [PubMed] [Google Scholar]
- 55.Truong T, Hung RJ, Amos CI, Wu X, Bickeboller H, Rosenberger A, Sauter W, Illig T, Wichmann HE, Risch A, Dienemann H, Kaaks R, Yang P, Jiang R, Wiencke JK, Wrensch M, Hansen H, Kelsey KT, Matsuo K, Tajima K, Schwartz AG, Wenzlaff A, Seow A, Ying C, Staratschek-Jox A, Nurnberg P, Stoelben E, Wolf J, Lazarus P, Muscat JE, Gallagher CJ, Zienolddiny S, Haugen A, van der Heijden HF, Kiemeney LA, Isla D, Mayordomo JI, Rafnar T, Stefansson K, Zhang ZF, Chang SC, Kim JH, Hong YC, Duell EJ, Andrew AS, Lejbkowicz F, Rennert G, Muller H, Brenner H, Le Marchand L, Benhamou S, Bouchardy C, Teare MD, Xue X, McLaughlin J, Liu G, McKay JD, Brennan P, Spitz MR. Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J. Natl. Cancer Inst. 2010;102(13):959–971. doi: 10.1093/jnci/djq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsiung CA, Lan Q, Hong YC, Chen CJ, Hosgood HD, Chang IS, Chatterjee N, Brennan P, Wu C, Zheng W, Chang GC, Wu T, Park JY, Hsiao CF, Kim YH, Shen H, Seow A, Yeager M, Tsai YH, Kim YT, Chow WH, Guo H, Wang WC, Sung SW, Hu Z, Chen KY, Kim JH, Chen Y, Huang L, Lee KM, Lo YL, Gao YT, Kim JH, Liu L, Huang MS, Jung TH, Jin G, Caporaso N, Yu D, Kim CH, Su WC, Shu XO, Xu P, Kim IS, Chen YM, Ma H, Shen M, Cha SI, Tan W, Chang CH, Sung JS, Zhang M, Yang TY, Park KH, Yuenger J, Wang CL, Ryu JS, Xiang Y, Deng Q, Hutchinson A, Kim JS, Cai Q, Landi MT, Yu CJ, Park JY, Tucker M, Hung JY, Lin CC, Perng RP, Boffetta P, Chen CY, Chen KC, Yang SY, Hu CY, Chang CK, Fraumeni JF, Jr, Chanock S, Yang PC, Rothman N, Lin D. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001051. e1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager M, Bergen AW, Li Q, Consonni D, Pesatori AC, Wacholder S, Thun M, Diver R, Oken M, Virtamo J, Albanes D, Wang Z, Burdette L, Doheny KF, Pugh EW, Laurie C, Brennan P, Hung R, Gaborieau V, McKay JD, Lathrop M, McLaughlin J, Wang Y, Tsao MS, Spitz MR, Wang Y, Krokan H, Vatten L, Skorpen F, Arnesen E, Benhamou S, Bouchard C, Metspalu A, Vooder T, Nelis M, Valk K, Field JK, Chen C, Goodman G, Sulem P, Thorleifsson G, Rafnar T, Eisen T, Sauter W, Rosenberger A, Bickeboller H, Risch A, Chang-Claude J, Wichmann HE, Stefansson K, Houlston R, Amos CI, Fraumeni JF, Jr, Savage SA, Bertazzi PA, Tucker MA, Chanock S, Caporaso NE. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am. J. Hum. Genet. 2009;85(5):679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin G, Xu L, Shu Y, Tian T, Liang J, Xu Y, Wang F, Chen J, Dai J, Hu Z, Shen H. Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis. 2009;30(6):987–990. doi: 10.1093/carcin/bgp090. [DOI] [PubMed] [Google Scholar]
- 59.McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, McLaughlin J, Shepherd F, Montpetit A, Narod S, Krokan HE, Skorpen F, Elvestad MB, Vatten L, Njolstad I, Axelsson T, Chen C, Goodman G, Barnett M, Loomis MM, Lubinski J, Matyjasik J, Lener M, Oszutowska D, Field J, Liloglou T, Xinarianos G, Cassidy A, Vineis P, Clavel-Chapelon F, Palli D, Tumino R, Krogh V, Panico S, Gonzalez CA, Ramon Quiros J, Martinez C, Navarro C, Ardanaz E, Larranaga N, Kham KT, Key T, Bueno-de-Mesquita HB, Peeters PH, Trichopoulou A, Linseisen J, Boeing H, Hallmans G, Overvad K, Tjonneland A, Kumle M, Riboli E, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P EPIC Study. Lung cancer susceptibility locus at 5p15.33. Nat. Genet. 2008;40(12):1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang XR, Yu IT, Chiu YL, Qiu H, Fu Z, Goggins W, Au JS, Tse LA, Wong TW. Previous pulmonary disease and family cancer history increase the risk of lung cancer among Hong Kong women. Cancer Causes Control. 2009;20(5):757–763. doi: 10.1007/s10552-008-9289-4. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Goldstein AM, Consonni D, Pesatori AC, Wacholder S, Tucker MA, Caporaso NE, Goldin L, Landi MT. Family history of cancer and nonmalignant lung diseases as risk factors for lung cancer. Int. J. Cancer. 2009;125(1):146–152. doi: 10.1002/ijc.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz AG, Ruckdeschel JC. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;173(1):16–22. doi: 10.1164/rccm.200502-235PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amos CI, Pinney SM, Li Y, Kupert E, Lee J, de Andrade MA, Yang P, Schwartz AG, Fain PR, Gazdar A, Minna J, Wiest JS, Zeng D, Rothschild H, Mandal D, You M, Coons T, Gaba C, Bailey-Wilson JE, Anderson MW. A susceptibility locus on chromosome 6q greatly increases lung cancer risk among light and never smokers. Cancer Res. 2010;70(6):2359–2367. doi: 10.1158/0008-5472.CAN-09-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arheart KL, Lee DJ, Dietz NA, Wilkinson JD, Clark JD, 3rd, LeBlanc WG, Serdar B, Fleming LE. Declining trends in serum cotinine levels in US worker groups: the power of policy. J. Occup. Environ. Med. 2008;50(1):57–63. doi: 10.1097/JOM.0b013e318158a486. [DOI] [PubMed] [Google Scholar]
- 65.Widome R, Jacobs DR, Jr, Schreiner PJ, Iribarren C. Passive smoke exposure trends and workplace policy in the Coronary Artery Risk Development in Young Adults (CARDIA) study (1985–2001) Prev. Med. 2007;44(6):490–495. doi: 10.1016/j.ypmed.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 1):1668–1672. doi: 10.1164/ajrccm.160.5.9904045. [DOI] [PubMed] [Google Scholar]
- 67.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr. Opin. Pulm. Med. 2005;11(5):431–437. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 68.Hollins DM, McKinley MA, Williams C, Wiman A, Fillos D, Chapman PS, Madl AK. Beryllium and lung cancer: a weight of evidence evaluation of the toxicological and epidemiological literature. Crit. Rev. Toxicol. 2009;39(Suppl 1):1–32. doi: 10.1080/10408440902837967. [DOI] [PubMed] [Google Scholar]
- 69.Sanderson WT, Ward EM, Steenland K, Petersen MR. Lung cancer case-control study of beryllium workers. Am. J. Ind. Med. 2001;39(2):133–144. doi: 10.1002/1097-0274(200102)39:2<133::aid-ajim1001>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 70.Steenland K, Sanderson W. Lung cancer among industrial sand workers exposed to crystalline silica. Am. J. Epidemiol. 2001;153(7):695–703. doi: 10.1093/aje/153.7.695. [DOI] [PubMed] [Google Scholar]
- 71.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6(3):e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol. Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 73.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, Chanock SJ, Goedert JJ, Engels EA. C-reactive protein and risk of lung cancer. J. Clin. Oncol. 2010;28(16):2719–2726. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int. Immunopharmacol. 2001;1(9–10):1651–1667. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 75.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat. Res. 2008;659(1–2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711,5. doi: 10.1136/bmj.313.7059.711. discussion 715–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He QC, Zhou BS. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int. J. Cancer. 2009;125(12):2936–2944. doi: 10.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- 79.Santillan AA, Camargo CA, Jr, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States) Cancer Causes Control. 2003;14(4):327–334. doi: 10.1023/a:1023982402137. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz AG, Cote ML, Wenzlaff AS, Van Dyke A, Chen W, Ruckdeschel JC, Gadgeel S, Soubani AO. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J. Thorac. Oncol. 2009;4(3):291–299. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax. 2007;62(1):51–56. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rezazadeh A, Laber DA, Ghim SJ, Jenson AB, Kloecker G. The role of human papilloma virus in lung cancer: a review of the evidence. Am. J. Med. Sci. 2009;338(1):64–67. doi: 10.1097/MAJ.0b013e3181a393ba. [DOI] [PubMed] [Google Scholar]
- 83.Klein F, Amin Kotb WF, Petersen I. Incidence of human papilloma virus in lung cancer. Lung Cancer. 2009;65(1):13–18. doi: 10.1016/j.lungcan.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer. J. Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 85.Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br. Med. J. 1976;2(6051):1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blot WJ, Fraumeni JF., Jr . Cancers of the Lung and Pleura. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. Oxford University Press; 1996. [Google Scholar]
- 87.Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126(2):347–351. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 88.Rudin CM, Avila-Tang E, Harris CC, Herman JG, Hirsch FR, Pao W, Schwartz AG, Vahakangas KH, Samet JM. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin. Cancer Res. 2009;15(18):5646–5661. doi: 10.1158/1078-0432.CCR-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujisawa T, Iizasa T, Saitoh Y, Sekine Y, Motohashi S, Yasukawa T, Shibuya K, Hiroshima K, Ohwada H. Smoking before surgery predicts poor long-term survival in patients with stage I non-small-cell lung carcinomas. J. Clin. Oncol. 1999;17(7):2086–2091. doi: 10.1200/JCO.1999.17.7.2086. [DOI] [PubMed] [Google Scholar]
- 90.Yoshino I, Kawano D, Oba T, Yamazaki K, Kometani T, Maehara Y. Smoking status as a prognostic factor in patients with stage I pulmonary adenocarcinoma. Ann. Thorac. Surg. 2006;81(4):1189–1193. doi: 10.1016/j.athoracsur.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 91.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 92.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 93.Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106(11):2428–2436. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 94.Subramanian J, Velcheti V, Gao F, Govindan R. Presentation and stage-specific outcomes of lifelong never-smokers with non-small cell lung cancer (NSCLC) J. Thorac. Oncol. 2007;2(9):827–830. doi: 10.1097/JTO.0b013e318145af79. [DOI] [PubMed] [Google Scholar]
- 95.Meguid RA, Hooker CM, Harris J, Xu L, Westra WH, Sherwood JT, Sussman M, Cattaneo SM, 2nd, Shin J, Cox S, Christensen J, Prints Y, Yuan N, Zhang J, Yang SC, Brock MV. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: comparing never smokers and current smokers. Chest. 2010;138(3):500–509. doi: 10.1378/chest.08-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, Krug LM, Pao W, Rizvi N, Pizzo B, Tyson L, Venkatraman E, Ben-Porat L, Memoli N, Zakowski M, Rusch V, Heelan RT. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2004;22(6):1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 97.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW. Lung cancer incidence in never smokers. J. Clin. Oncol. 2007;25(5):472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9(7):649–656. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 100.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J. Clin. Oncol. 2007;25(13):1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz AG, Swanson GM. Lung carcinoma in African Americans and whites. A population-based study in metropolitan Detroit, Michigan. Cancer. 1997;79(1):45–52. doi: 10.1002/(sici)1097-0142(19970101)79:1<45::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 102.Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S JPHC Study Group. Japanese Public Health Center. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: the JPHC study. Int. J. Cancer. 2002;99(2):245–251. doi: 10.1002/ijc.10308. [DOI] [PubMed] [Google Scholar]
- 103.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458(7239):719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feuer EJ, Levy DT, McCarthy WJ. Measuring the impact of the reduction in tobacco smoking on US lung cancer mortality 1975–2000: An introduction to the problem (Chapter 1 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moolgavkar SH, Venzon DJ. Two-event models for carcinogenesis: incidence curves for childhood and adult tumors. Math. Biosci. 1979;47(1):55–77. [Google Scholar]
- 106.Moolgavkar SH, Knudson AG., Jr Mutation and cancer: a model for human carcinogenesis. J. Natl. Cancer Inst. 1981;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- 107.Hazelton WD, Clements MS, Moolgavkar SH. Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol. Biomarkers Prev. 2005;14(5):1171–1181. doi: 10.1158/1055-9965.EPI-04-0756. [DOI] [PubMed] [Google Scholar]
- 108.National Center for Health and Statistic. vol 1. Hyattsville, Maryland: U.S. Department of Health and Human Services; 1997. U.S. Decennial Life Tables for 1989–91. [Google Scholar]
- 109.Knoke JD, Burns DM, Thun MJ. The change in excess risk of lung cancer attributable to smoking following smoking cessation: an examination of different analytic approaches using CPS-I data. Cancer Causes Control. 2008;19(2):207–219. doi: 10.1007/s10552-007-9086-5. [DOI] [PubMed] [Google Scholar]
- 110.Anderson CM, Burns DM, Dodd KW, Feuer EJ. Birth cohort specific estimates of smoking behaviors for the U.S. population (Ch. 2 in this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holford TR, Clarke L. Counterfactual smoking histories (Ch 4 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeon J, Meza R, Krapcho M, Clarke L, Byrne J, Levy D. Actual and counterfactual smoking prevalence rates in the U.S. population via microsimulation (Ch. 5 of this monograph) Risk Anal. doi: 10.1111/j.1539-6924.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol. Biomarkers Prev. 2010;19(11):2969–2977. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- 114.Ellis JA, Gwynn C, Garg RK, Philburn R, Aldous KM, Perl SB, Thorpe L, Frieden TR. Secondhand smoke exposure among nonsmokers nationally and in New York City. Nicotine Tob. Res. 2009;11(4):362–370. doi: 10.1093/ntr/ntp021. [DOI] [PubMed] [Google Scholar]
- 115.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey 1988 to 1991. JAMA. 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- 116.Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, Yoder AR, Carmella SG, Hecht SS. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers. 2010;15(4):345–352. doi: 10.3109/13547501003753881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bernert JT, Gordon SM, Jain RB, Brinkman MC, Sosnoff CS, Seyler TH, Xia Y, McGuffey JE, Ashley DL, Pirkle JL, Sampson EJ. Increases in tobacco exposure biomarkers measured in non-smokers exposed to sidestream cigarette smoke under controlled conditions. Biomarkers. 2009;14(2):82–93. doi: 10.1080/13547500902774613. [DOI] [PubMed] [Google Scholar]
- 118.Hsieh SJ, Ware LB, Eisner MD, Yu L, Jacob P, Jr, Havel C, Goniewicz ML, Matthay MA, Benowitz NL, Calfee CS. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Crit. Care Med. 2011;39(1):40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P, Benowitz NL. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob. Res. 2011;13(3):202–208. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]