Abstract
Clostridium difficile is the commonest cause of hospital-acquired infection in the United Kingdom. We characterized the abilities of 21 clinical isolates to form spores; to adhere to inorganic and organic surfaces, including stainless steel and human adenocarcinoma cells; and to germinate. The composition of culture media had a significant effect on spore formation, as significantly more spores were produced in brain heart infusion broth (Student's t test; P = 0.018). The spore surface relative hydrophobicity (RH) varied markedly (14 to 77%) and was correlated with the ability to adhere to stainless steel. We observed no correlation between the ribotype and the ability to adhere to steel. When the binding of hydrophobic (DS1813; ribotype 027; RH, 77%) and hydrophilic (DS1748; ribotype 002; RH, 14%) spores to human gut epithelial cells at different stages of cell development was examined, DS1813 spores adhered more strongly, suggesting the presence of surface properties that aid attachment to human cells. Electron microscopy studies revealed the presence of an exosporium surrounding DS1813 spores that was absent from spores of DS1748. Finally, the ability of spores to germinate was found to be strain and medium dependent. While the significance of these findings to the disease process has yet to be determined, this study has highlighted the importance of analyzing multiple isolates when attempting to characterize the behavior of a bacterial species.
INTRODUCTION
Clostridium difficile is an anaerobic, spore-forming, Gram-positive bacterium implicated as the primary cause of antibiotic-associated diarrhea in the United Kingdom (http://www.statistics.gov.uk/hub/index.html). Alterations in colonic microbiota, usually due to broad-spectrum antibiotic treatment, increase sensitivity to C. difficile infection and enable the vegetative organism to produce cytotoxins that destroy host intestinal epithelial cells (35). The subsequent increase in vascular permeability leads to the formation of pseudomembranes composed of neutrophils, fibrin, mucosa, blood, and cellular debris (28, 32). Bloody diarrhea ensues, resulting in the release of large numbers of spores into the environment.
Spores are formed when the vegetative organism is subjected to stress, such as nutrient limitation, and they allow the pathogen to survive in a dormant state outside the anaerobic environment of the gut until a new host is colonized (34). Differences in the abilities of isolates to form spores have been proposed as a contributing factor to the emergence of hypervirulent variants (e.g., B1/NAP1/027) of the pathogen (1, 32). The ability to adhere to surfaces commonly found in the health care environment, such as bed linen and stainless steel, is also thought to play a role (12, 30).
The attachment of bacterial spores to organic and inorganic surfaces is influenced by a number of factors, which include hydrophobicity and the presence of surface structures, such as appendages (7) and an outer spore layer known as the exosporium (9, 17, 19, 26, 34). This loose outer layer, which surrounds spores produced by some, but not all, Bacillus and Clostridium spp., is thought to play a key role in the attachment of spores to environmental and cellular surfaces (6, 9, 15, 16, 17). Furthermore, a correlation has been demonstrated between spore hydrophobicity and the ability of exosporium-positive pathogenic Bacillus cereus isolates to adhere to human colonic adenocarcinoma cells and to germinate (2). In a recent study of C. difficile spores, a similar correlation between spore hydrophobicity and cell surface binding to undifferentiated 5-day-old Caco-2 cells has been reported (24).
Once the spores have colonized a new host, they must germinate and express the pathogens' various virulence factors (18). The mechanisms by which spores determine that the local environmental conditions are conducive to growth are currently undetermined (5, 21, 29). While specific germination receptors have not been identified, studies have shown that germination is triggered in vitro by the amino acids glycine, histidine, and cysteine and the bile salt sodium taurocholate (10, 14, 35). As has been proposed for spore formation, variations in the abilities of strains to germinate in vivo could impact the ability to initiate infection.
In this study, we aimed to characterize the abilities of a diverse collection of hypervirulent (ribotype 027) and United Kingdom epidemic (ribotypes 001, 078, and 106) and endemic clinical isolates of C. difficile, selected to capture the genetic heterogeneity of the species, to form spores, to adhere to organic and inorganic surfaces, and to germinate (36). We specifically sought to determine if a relationship existed between spore surface properties and the ability to adhere to surfaces.
MATERIALS AND METHODS
Strains and growth conditions.
The clinical isolates of C. difficile used in this study are shown in Table 1 and were obtained from the National Anaerobic Reference Unit, Cardiff, Wales. Strain CD630 (33) was obtained from the National Collection of Type Cultures (NCTC), Health Protection Agency, United Kingdom. Bacillus atrophaeus ATCC 9372 was purchased from the American Type Culture Collection (ATCC), Manassas, VA. Unless otherwise stated, all organisms were stored as spores at 4°C. The following media were used to culture the organisms and to produce spores: Columbia agar and broth (Difco, Franklin Lakes, NJ) and brain heart infusion (BHI) agar and broth (Oxoid Ltd., Basingstoke, United Kingdom). To culture each organism, 10 μl of spore stock was used to inoculate the appropriate degassed agar plate and streaked to purity following incubation. Cultures were incubated at 37οC in a Bug Box Plus anaerobic workstation (Ruskinn Technology Ltd., Bridgend, United Kingdom) using an 85% nitrogen, 10% carbon dioxide, and 5% hydrogen gas mix and were examined at 48 h for the presence of characteristic colonies.
Table 1.
Strains of C. difficile used in this study
| Strain | PCR ribotype | Source |
|---|---|---|
| C. difficile | ||
| DS1759 | 001 | Maidstone |
| DS1747 | 001 | St. James, Leeds |
| R8652 | 001 | NCTC |
| DS1750 | 001 | St. James, Leeds |
| DS1813 | 027 | Hinchingbrooke |
| DS1801 | 027 | Leicester |
| R20291 | 027 | Stoke-Mandeville |
| DS1807 | 027 | Salford |
| R10459 | 106 | Dudley |
| DS1798 | 106 | Poole |
| DS1787 | 106 | Leicester |
| DS1771 | 106 | Bristol Southmead |
| DS1742 | 014 | Bristol Frenchay |
| DS1748 | 002 | Leeds |
| DS1721 | 005 | Leicester |
| DS1752 | 012 | Bradford |
| CD630 | 012 | NCTC |
| DS1723 | 078 | Leicester |
| DS1724 | 020 | Leicester |
| DS1684 | 010 | Brighton |
| DS1665 | 023 | Bath |
| Other species | ||
| B. atrophaeus ATCC 9372 | ATCC |
Medium composition.
Columbia agar medium (Difco) contained the following (approximate amount per liter): pancreatic digest of casein, 12.0 g; peptic digest of animal tissue, 5.0 g; yeast extract, 3.0 g; beef extract, 3.0 g; corn starch, 1.0 g; sodium chloride, 5.0 g; agar, 13.5 g; colistin, 10.0 mg; nalidixic acid, 10.0 mg.
BHI agar (Oxoid Ltd., Basingstoke, United Kingdom) contained the following (approximate amount per liter): calf brain infusion solids, 12.5 mg; beef heart infusion solids, 5.0 mg; protease peptone, 10.0 mg; sodium chloride, 5.0 mg; glucose, 2.0 mg; disodium phosphate, 2.5 mg; agar, 10.0 mg.
Spore production.
To produce spores, a single colony harvested from the appropriate agar plate was used to inoculate 10 ml of the corresponding degassed broth, which was then incubated for 10 days at 37οC in the anaerobic workstation (25). At the end of this period, the cultures were centrifuged (Heraeus Biofuge Primo R; Fisher, United Kingdom) at 5,000 × g for 15 min at room temperature, and the supernatant was discarded. The retained pellet was then resuspended in 10 ml sterile deionized water (SDW) and centrifuged at 5,000 × g again. This washing cycle was repeated a further two times, at the end of which the spore pellet was resuspended in 1 ml of SDW, heated to 80°C for 10 min to inactivate any remaining vegetative cells, and subsequently stored at 4°C.
Spore enumeration and viable-colony counts.
The number of spores produced following broth culture was determined using a drop count method based on that of Miles et al. (20). An aliquot of 20 μl of extracted spores was serially diluted in degassed sterile broth medium (180 μl) using a microtiter plate (Sterilin Ltd., Teddington, Middlesex, England) until a dilution down to 10−10 was achieved. A total of three 10-μl aliquots from each dilution were then spotted onto the surfaces of the appropriate degassed agar plates (neat to 10−10). To determine the effect of sodium taurocholate (Sigma-Aldrich, Dorset, England) on spore germination, plates with and without 1% sodium taurocholate were used to determine the presence of viable colonies. Once inoculated, the spots were allowed to dry at 25°C, and the plates were incubated anaerobically for 48 h at 37°C. CFU from each spot on the microtiter plate were then counted, and the mean number of CFU per milliliter was determined by multiplying the mean number of CFU by the dilution factor. The percent spore germination was calculated by dividing the number of spores germinated without taurocholate in the medium by the number of spores germinated with taurocholate in the medium. This value was then multiplied by 100 to obtain the percent germination.
Transmission electron microscopy (TEM) studies.
Spore samples (1 ml) were centrifuged at 2,000 × g for 4 min. The supernatant was removed, and the pelleted spores were fixed with 1 ml of a 2% glutaraldehyde and 2% osmium tetroxide mixture. This was left for 1 h at 22°C, after which the osmium tetroxide-spore mixture was centrifuged at 2,000 × g for 2 min. BHI agar was prepared (3 g to 50 ml water), and molten agar was added to the stained spore pellet and left to set. The extra agar around the spores was removed, and the agar-embedded spore pellets were dehydrated through an ethanol series from 50% ethanol to 100% alcohol for 10 min each. The 100% dehydration was repeated 3 times, and then the ethanol was replaced with propylene oxide (PO) for 15 min. Epoxy resin was composed of a mixture of araldite CY212, dodecyl succinic anhydride (DDSA), and benzyldimethylamine (BDMA) (Sigma-Aldrich, Dorset, England). An equal quantity of PO was added to the resin and used to cover each spore pellet. The pellet was left for 36 h to allow resin infiltration. The PO mixture was subsequently removed, and pure epoxy resin (Sigma-Aldrich, Dorset, England) was added to the samples, which were then embedded in a flat molded tray and left in an oven at 60°C to harden for 48 h. Transverse sections were cut on a microtome (Reichert-Jung, Depew, NY) and stained on nickel grids with uranyl acetate and lead citrate. Images were taken on a transmission electron microscope (Philips EM 208). Ten spores were viewed.
Negative-stain electron microscopy of whole spores.
Spore samples were diluted in sterile distilled water, placed on Formvar-coated grids, and examined after negative staining in 2% methylamine tungstate. After 15 to 30 s, the excess stain was withdrawn using filter paper. The grids were then washed twice with water and dried with filter paper. Samples were examined immediately using a Phillips EM 208 transmission electron microscope with an accelerating voltage of 80 kV, and magnifications between ×20,000 and ×55,000 were used.
MATH test.
To determine the hydrophobic characteristics of individual C. difficile spores from a range of isolates, we used a hexadecane hydrocarbon-based microbial adhesion to hydrocarbon (MATH) test (31). Thus, C. difficile spores of each isolate were suspended in 9 ml of SDW to achieve an optical density at 600 nm (OD600) between 0.500 and 0.600 (Ultrospec 1100 pro UV/Visible spectrophotometer; Biochrom, Cambridgeshire, United Kingdom). A 4-ml aliquot of spore suspension in a Universal container was vortex mixed with 0.4 ml of the hydrocarbon n-hexadecane (Sigma-Aldrich, Dorset, England) at full speed for 1 min. The mixture was incubated at room temperature (22°C) for 15 min to allow the different phases to partition settle, at which time the optical density of the lower aqueous phase was determined. Each assay was repeated twice. Changes in hydrophobicity were expressed as a percentage change by calculating the ratio between the original OD600 and the final OD600 after hexadecane exposure.
Contact angle measurements.
To ascertain the hydrophobic characteristics of the surfaces tested in this study, we employed a contact angle test on hospital grade stainless steel discs and agar. The angle of contact between the surface of the steel and the water was measured using a horizontal-projection technique. A droplet of water was put onto the stainless steel disc, and a light was used to project the image. The projection allowed a crude measurement of the contact angle between the droplet and the surface using a protractor, the hypothesis being that the contact angle between the water and a hydrophobic surface (steel) would be less than the contact angle between the water and a hydrophilic surface (agar).
Stainless steel plate transfer assay.
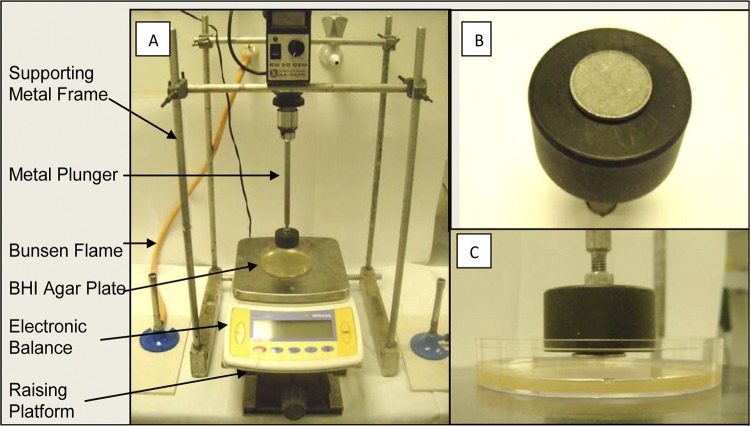
A transfer assay was employed to assess the transfer of spores from hospital grade stainless steel (hydrophobic surface) to the surface of a BHI agar plate supplemented with 0.1% sodium taurocholate (hydrophilic surface). A stainless steel disc 2 cm in diameter with a grade 2B finish (Goodfellows Cambridge Ltd., Huntingdon, United Kingdom) was inoculated with 10 μl of spore suspension (500 spores per 10 μl in units of CFU/μl) and left to dry at 25°C for 20 min. The inoculated steel discs were aseptically attached to a steel rod plunger and securely fastened into the supporting metal frame as shown in Fig. 1 (IKA, Labortechnik, Staufen, Germany). An electronic weighing balance was secured to a raising platform and positioned beneath the metal plunger. On either side of the platform, two Bunsen burners created a sterile vortex of air encompassing the platform area. A degassed BHI agar (Oxoid, Basingstoke, Hampshire, United Kingdom) plate was placed directly under the metal plunger on top of the electronic balance, which was subsequently tared. The platform and balance were raised until the steel disc compressed the agar surface with a force of 100 g (±5 g) and held for 10 s. The balance was lowered, the agar plate was replaced with another, and the compressing process was repeated a total of 16 times for each inoculated disc. The compressed agar plates were anaerobically incubated at 37°C for 48 h, and the number of CFU on each plate was determined. Sixteen strains of C. difficile were tested, with each strain being tested on two separate occasions.
Fig 1.
Plate transfer assay for spore transfer. Shown is the transfer apparatus used to assess spore transfer from stainless steel (38). (A) Apparatus set up for spore transfer. The plunger is attached to a supporting metal frame. To maintain a sterile environment, a Bunsen burner is placed on either side of an electronic balance with a BHI agar plate on it. The balance was tared with each agar plate, and the spores plunged at a force of 100 g/10 s. (B) Inoculated stainless steel disc fastened to the metal plunger. (C) BHI agar plate compressed aseptically with the inoculated steel disc.
Preparation of mammalian cell culture lines.
Two human epithelial colorectal adenocarcinoma cell lines, Caco-2 (ATCC HTB-37) and HT-29 (ATCC HTB-38), were grown as monolayers in continuous culture. The two intestinal cell lines were passaged in Eagle's minimum essential medium (EMEM) with Earle's balanced salt solution (EBSS) (Invitrogen, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, and 50 μg/ml penicillin-streptomycin solution (Invitrogen, Paisley, United Kingdom) and incubated at 37°C in a 5% CO2 atmosphere in 25-cm2 vented flasks. The flasks were subcultured after the cells reached full confluence (analyzed by phase-contrast microscopy according to cell density) every 6 or 7 days, at which point the cells were washed 2 times in PBS (pH 7.2), detached from the plastic flask using a trypsin-EDTA solution (Invitrogen, United Kingdom), and seeded into new flasks to a density of 1 to 5 cells per well for Caco-2 cells and 1 to 10 cells per well for HT-29 cells.
Ability of C. difficile spores to adhere to human gut epithelial cell lines.
To characterize the ability of spores of DS1813 and DS1748 to adhere to mammalian cells at different stages of cell differentiation, Caco-2 and HT-29 cells were seeded into 6-well plates at a density of 1 × 105 cells per well and incubated for 7 days and 15 days. These time points were selected for testing because Caco-2 cells require 15 days of culture to become fully differentiated, at which time they possess a microvillus brush border that is absent at day 7 (13, 22, 27). In contrast, HT-29 cells remain in an undifferentiated state but are documented to contain a small proportion (≤0.2%) of mucin-secreting goblet cells postconfluence, which produce mucus to which vegetative C. difficile has been shown to bind (8).
Following 7 and 15 days of incubation, Caco-2 cells (1.2 × 105 and 4.0 × 105 cells per ml) and HT-29 cells (1.3 × 106 and 6.0 × 106 cells per ml) were inoculated with 100 μl of 1.3 × 108 to 1.4 × 108 spores/ml of DS1813 and DS1748 in sterile deionized water and incubated for 100 min at 37°C in a Bug Box Plus anaerobic cabinet (Ruskinn Technology Ltd., Bridgend, United Kingdom). The ratio of spores to cells ranged from 108.3:1 and 116.7:1 for DS1813 and DS1748 against 7-day-old Caco-2 cells to 32.5:1 and 35:1 for 15-day-old Caco-2 cells. In the case of HT-29 cells, the ratios were 10:1 and 14:1 for DS1813 and DS1748 for 7-day-old and 2.16:1 to 2.33:1 for 15-day-old HT-29 cells. After incubation, the cell-spore mixture was washed in phosphate-buffered saline (PBS) buffer (Sigma-Aldrich, Dorset, England) via centrifugation at 800 × g to remove any unattached spores. This washing step was performed three times in total, and the supernatant from each wash step was collected for enumeration of any spores not adhering to the cells. The spores in the supernatant were enumerated according to the method of Miles et al. (20) on degassed BHI plus 0.1% sodium taurocholate. Controls included PBS incubated with spores and EMEM incubated with spores. All cell culture assays were performed in duplicate. The percentage of spore adherence was calculated by using the following formula: [(final CFU/ml)/(initial CFU/ml)] × 100.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Statistically significant differences were tested using one-way analysis of variance (ANOVA) at the 95% confidence interval in conjunction with Dunnett's posttest. Tests were also used where there were less than three variables to compare. A P value of <0.05 was considered significant (3). The Student t test was used to compare the means of samples against a standard reference value (3). Furthermore, a linear regression line analysis was performed to determine if a linear relationship existed between the x and y variables within a plot (3). The equation is defined as follows: y = mx + C, where C is the y intercept and m is the gradient of the slope (3).
RESULTS
Effect of medium composition on spore formation.
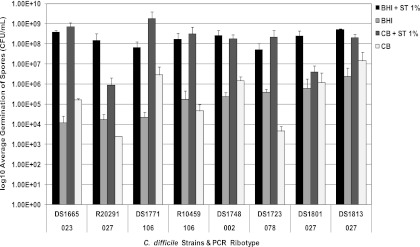
To determine if the ability of C. difficile isolates to form spores was influenced by the composition of the growth medium, the abilities of eight clinical isolates of C. difficile to form spores in BHI and Columbia media were determined. Following incubation for 10 days, heat-shocked cultures were diluted and plated onto either BHI or Columbia agar containing 0.1% sodium taurocholate to trigger germination. The abilities of isolates to form spores were affected by the composition of the media (Fig. 2). This was most evident for strains R20291 (ribotype 027; Stoke Mandeville isolate) and DS1801 (ribotype 027), which showed ∼3-log-unit and ∼2-log-unit differences in spore formation ability, with the higher number of spores being produced in BHI medium. Indeed, all of the strains showed significant differences in their abilities to form spores in BHI and Columbia media, as determined by Student's t test (P = 0.018).
Fig 2.
Effect of medium composition on spore formation. The abilities of eight clinical isolates of C. difficile to form spores in Columbia or BHI broth were determined. To determine the number of spores formed in each medium, viable counts were performed on the agar form of the medium in the presence and absence of 1% sodium taurocholate. A significant difference in the abilities of all strains to form spores in BHI and Columbia media was identified (Student's t test; P = 0.018; n = 4). The error bars indicate one standard deviation.
The effect of medium composition on spore germination with and without the cogerminant sodium taurocholate.
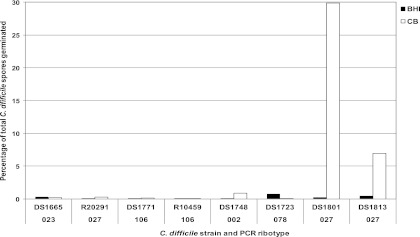
As can be seen from Fig. 2, isolates of C. difficile varied in their abilities to germinate in different growth media in the presence and absence of the cogerminant 1% sodium taurocholate. The percentage of spores that germinated in the absence of sodium taurocholate differed depending on the medium and the isolate (Fig. 3). Indeed, the results suggest that Columbia medium contains more essential cogerminants than BHI medium due to the observed variations in germination ability. Strains DS1801 and DS1813, both of which belong to the hypervirulent 027 ribotype, germinated more efficiently in the presence of Columbia medium, producing 30% and 7% more colonies on Columbia than on BHI medium (Fig. 3). The remaining strains showed no difference in spore germination efficiency on either Columbia or BHI medium.
Fig 3.
Effect of medium composition on spore germination with and without the cogerminant sodium taurocholate. The abilities of spores of eight clinical isolates of C. difficile to germinate on the corresponding agar form of the medium in the presence and absence of 1% sodium taurocholate were determined. The percentage of spore germination was calculated by dividing the number of spores germinated without taurocholate in the medium by the number of spores germinated with taurocholate in the medium. This value was then multiplied by 100 to obtain the percent germination. Each result is the mean of four repeats. CB, Columbia broth.
Hydrophobicity.
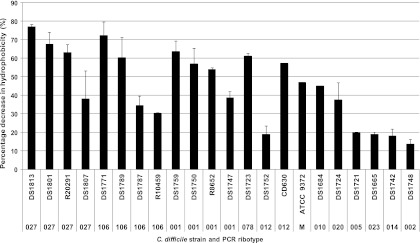
The ability of a spore to adhere to a surface is influenced by its hydrophobicity. To characterize the relative hydrophobicity (RH) of spores obtained from a range of clinical isolates, we employed a MATH test (31) with hexadecane as the organic solvent. Spores of B. atrophaeus ATCC 9372 (marker) were included as an internal control to enable comparisons to results from other studies (37). As expected, the RH value for the strain was similar to that reported by Wiencek et al. (37). As can be seen from Fig. 4, RH values ranged from 77% (DS1813; ribotype 027) to 14% (DS1748; ribotype 002). While the most hydrophobic isolates belonged to the hypervirulent (027) and United Kingdom epidemic (001, 078, and 106) ribotypes, there was no significant difference in the hydrophobicities of strains belonging to the same ribotype (Student's t test; 027, P = 0.005; 106, P = 0.016; 001, P = 0.0021; 078, P = 0.01), suggesting that there is no direct relationship between hydrophobicity and ribotype.
Fig 4.
Relative hydrophobicities of spores formed by different ribotypes of C. difficile. The relative hydrophobicities of 21 clinical isolates of C. difficile belonging to different ribotypes were determined using a microbial adhesion to hydrocarbon test in which hexadecane was the organic solvent. Each value is the mean of two independent tests. The error bars indicate one standard deviation. Spores of B. atrophaeus ATCC 9372 were included as an internal control.
Ability of C. difficile spores to adhere to stainless steel.
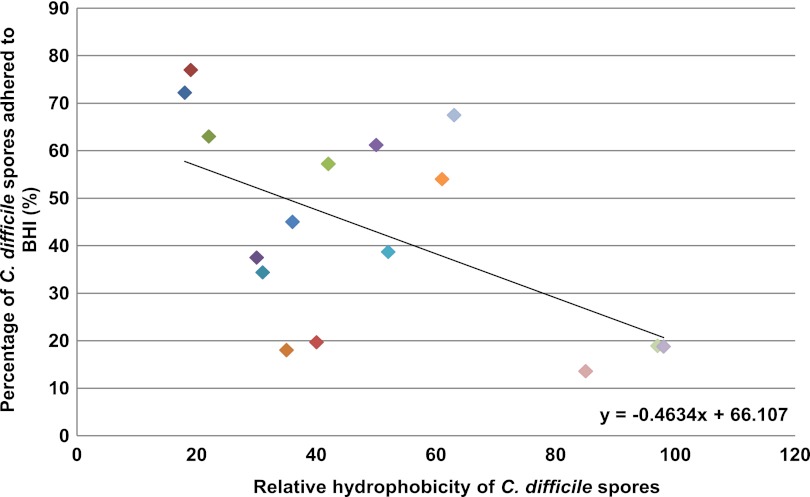
Stainless steel is a material commonly used in the hospital environment, and thus, we sought to determine if spores from a range of clinical isolates varied in their ability to adhere to this material. To achieve this, a plate transfer method was employed in which the percentage of spores that remained attached to the surface of the relatively hydrophobic (>45° contact angle) steel disc was determined following consecutive impressions on the surfaces of 16 hydrophilic (0° contact angle) BHI agar plates (Fig. 5). Regression analysis of the relationship between spore adherence and relative hydrophobicity revealed a significant correlation (P = 0.0247). The effect was most pronounced for spores of DS1813 (81% binding; RH, 77%), which adhered the most firmly to steel, compared to those of DS1748 (15% binding; RH, 14%), which were the least adherent. As was the case for the determination of RH, we observed no statistical correlation between spore ribotype and spore adherence to stainless steel.
Fig 5.
Ability of C. difficile spores to adhere to stainless steel. The percentages of C. difficile spores transferred from hydrophobic stainless steel to hydrophilic BHI agar were determined for 21 clinical isolates of C. difficile belonging to different ribotypes. Each assay was repeated twice. Regression analysis revealed a significant correlation (P = 0.0247) between spore adherence and relative hydrophobicity. There was no statistical correlation between spore ribotype and spore adherence to stainless steel. The slope is defined as y = −0.4634x + 66.107.
Adherence of C. difficile spores to human gut epithelial cell lines Caco-2 and HT-29.
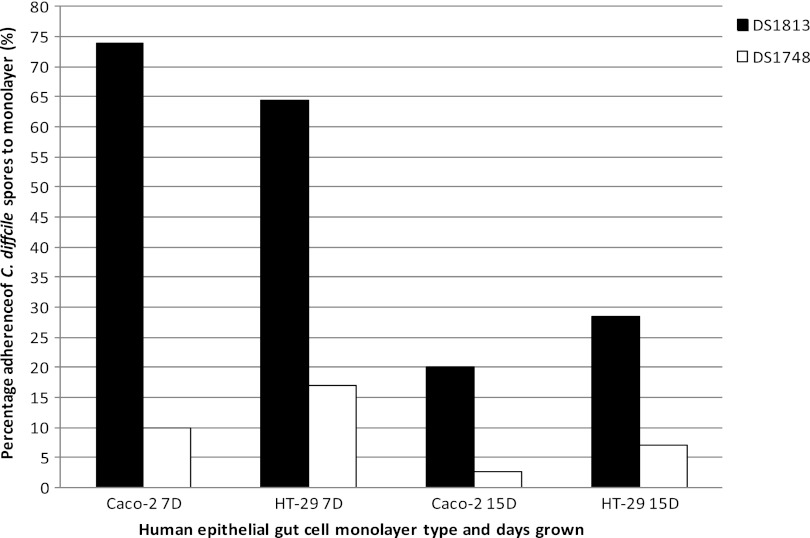
The abilities of spores that differed in surface hydrophobicity to adhere to human gut epithelial cells at different stages of cell development were determined (Fig. 6). While there was no significant difference in the ability of DS1813 and DS1748 spores to adhere to 7-day Caco-2 cells, DS1813 spores adhered significantly more strongly to 15-day-old Caco-2 cells than spores of DS1748 (one-way ANOVA; P = 0.0393). In the case of the HT-29 cell line, we observed a significant difference in the abilities of the spores of both strains to adhere to 7-day HT-29 cells, with the DS1813 spores adhering more strongly (P = 0.0079) than those produced by DS1748. These results suggest that spores of DS1813 have surface properties that allow better adhesion to the cell lines than spores of DS1748. There was no difference in spore adhesion to HT-29 cells that had been allowed to replicate for 15 days.
Fig 6.
Adherence of C. difficile spores to human gut epithelial cell monolayers. The spores of DS1813 (hydrophobic) and DS1748 (hydrophilic) were assessed for the ability to adhere to monolayers of the human colon adenocarcinoma grade II cell line HT-29 and the Caco-2 human colorectal cell line that had been incubated for 7 and 15 days. Spores were enumerated on 0.1% sodium taurocholate-supplemented BHI agar. The results are presented as percentages of the initial spore count. Each result represents the mean of two independent assays. D, days.
Spore surface characterization by electron microscopy.
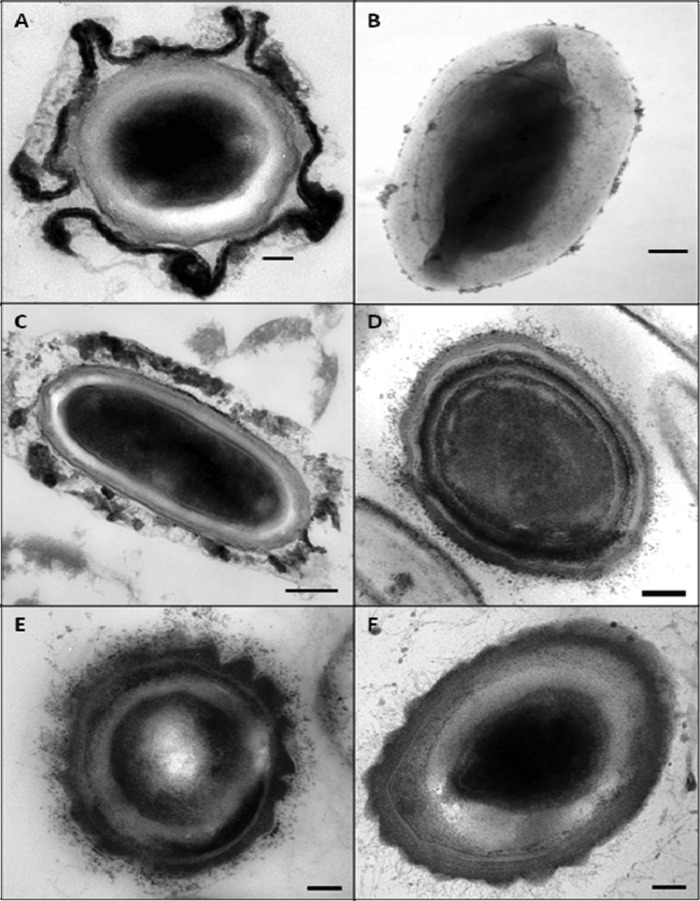
TEM was employed to characterize the surface architecture of spores of clinical isolates of C. difficile that differed in their hydrophobicity and in their ability to adhere to surfaces. While the spores of all the isolates share common structural features, such as spore coat layers, there were obvious differences (Fig. 7). The spores of the most hydrophobic isolate, DS1813 (RH, 77%), comprised an electron-dense cortex surrounded by concentric rings of peptidoglycan; a second, thinner coat layer; and an outer layer known as the exosporium (Fig. 7A, B, and C). In contrast, the exosporium was missing from spores of less hydrophobic isolates, such as DS1771 (RH, 72.2%), DS1684 (RH, 45%), and DS1748 (RH, 14%) (Fig. 7D, E, and F).
Fig 7.
Transmission electron microscopy of C. difficile spores. (A) TEM image of a cross section of a hydrophobic spore produced by C. difficile DS1813. The exosporial layer can be seen. (B) Negative-stain electron microscopy of an exosporium sac surrounding a spore of DS1813. (C) DS1813 with exosporium visible. Subsequent strains did not exhibit an exosporial layer. (D) Strain DS1771; the concentric layers of the spore can be seen, but no exosporial layer is visible. (E) Strain DS1684 does not exhibit an exosporium layer. (F) Strain DS1748 exhibits the concentric spore structure but does not appear to possess an exosporium. Scale bars = 100 nm.
DISCUSSION
To avoid the toxic effects of oxygen and to survive the transit to a new host, a pathogen forms spores (35). There is considerable debate as to whether clinical isolates, particularly hypervirulent type BI/NAP1/027 strains, differ in their abilities to form spores (1, 4). While we are unable to comment on the rate of spore formation in this study, we did observe that certain 027 strains, such as R20291, failed to achieve the same level of spore formation following 10 days of incubation as other isolates and that these differences were growth medium dependent. One can only speculate as to whether a similar difference in spore formation occurs in vivo.
Once a spore has been formed, it must escape from the infected individual and adopt strategies that enable it to survive and colonize an environment prior to finding a new host (35). We observed that spores produced by different clinical isolates in the same culture medium varied in their relative hydrophobicities and in their abilities to adhere to stainless steel, a material commonly found in the health care environment. While it is possible that spore surface properties are affected by the culture medium, the fact that a single medium was employed to determine RH and adherence suggests that the differences seen between strains are real.
While it is tempting to speculate that hypervirulent ribotypes are better adapted to colonize environmental surfaces and thereby increase their potential for person-to-person spread, we observed no significant correlation between hydrophobicity and ribotype. Indeed, while the most hydrophobic spores were predominantly United Kingdom epidemic ribotypes, this may simply reflect the relatively high number of these isolates in our study population (11).
In addition to mediating binding to steel, the surface of the spore also facilitates attachment to colonic cells (23). A recent study of the binding characteristics of C. difficile spores to undifferentiated 5-day-old Caco-2 cells revealed a correlation between spore hydrophobicity and cell surface binding (24). While we failed to observe any difference in the abilities of DS1813 and DS1748 spores to adhere to 7-day Caco-2 cells, we did see a marked difference with 15-day fully differentiated cells expressing the apical microvilli and brush border proteins (8). To give a more comprehensive picture of the cell populations encountered in the human gut, we also examined the ability of spores to adhere to HT-29 cells. In contrast to the results seen with Caco-2 cells, we observed a significant difference in the abilities of DS1813 and DS1748 spores to adhere to 7-day HT-29 cells but saw no difference with 15-day-old cells. Overall, these results suggest that spores of DS1813 have surface properties that allow better adhesion to gut cell lines than spores of DS1748 and that the ability of spores to bind is affected by the morphology and physiology of specific gut cells.
It had previously been observed that spores of C. difficile and Clostridium sporogenes attach to the apical microvilli of human epithelial cells (Caco-2 and MRC-5 cells) by means of an exosporium-like structure that, in addition to facilitating colonization, acted as an anchor during germination (22, 23). To determine if the observed differences in hydrophobicity and adherence were due to variations in spore structure, we employed electron microscopy to characterize the morphology of spores. The resulting TEM images were similar to those presented by others (19, 24). While spores of each isolate shared a number of common features, an exosporium-like structure was seen surrounding only DS1813 spores. It is thus tempting to speculate that the exosporium contributes to the hydrophobicity of the spore surface and plays a role in mediating adherence to gut cells. Indeed, it was recently reported that sonication of C. difficile spores altered the ultrastructure of the exosporium, which resulted in a significant reduction in spore hydrophobicity and adherence to Caco-2 cells (24).
While the precise factors that mediate spore adherence have yet to be defined, the results from this and other studies suggest that components of the spore surface play a central role in mediating attachment to inorganic (stainless steel) and organic (human adenocarcinoma cells) surfaces. By fully understanding the process by which spores attach to clinically relevant surfaces, we hope to be able to develop strategies that could inhibit attachment of spores and thus block the infection cycle.
Finally, once a spore has attached to the surface of a gut cell, it must transform into its biologically active vegetative form to initiate infection (11). While there appears to be an association between the 027 ribotype and the ability to germinate in Columbia medium in the absence of taurocholate, the limited number of isolates examined makes it impossible to draw any solid conclusions at this time. The results from this study suggest that further examination of strain DS1801 could aid our understanding of the factors that regulate germination.
In conclusion, we found that clinical isolates of C. difficile varied widely in their abilities to form spores, attach to clinically relevant surfaces, and subsequently germinate in vitro. While the significance of these findings for the disease process has yet to be determined, this study has highlighted the importance of analyzing multiple isolates when attempting to characterize the behavior of a bacterial species.
ACKNOWLEDGMENTS
We sincerely thank Jon Brazier and Val Hall for their kind donation of strains from the national anaerobic reference laboratory in Cardiff, United Kingdom. We also thank Anthony Hann from the Cardiff School of Biosciences Electron Microscopy Department for his assistance with electron microscopy.
We thank the Society for Applied Microbiology for funding this research.
Footnotes
Published ahead of print 24 August 2012
REFERENCES
- 1. Åkerlund DT, et al. 2008. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J. Clin. Microbiol. 46:1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson A, Granum PE, Ronner U. 1998. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 39:93–99 [DOI] [PubMed] [Google Scholar]
- 3. Bowker DW, Randerson PF. 2007. Practical data analysis workbook for biosciences, 3rd ed Pearson Education Ltd., Harlow, Essex, United Kingdom [Google Scholar]
- 4. Burns DA, Heap JT, Minton NP. 2010. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe 16:618–622 [DOI] [PubMed] [Google Scholar]
- 5. Burns DA, Heap JT, Minton NP. 2010. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J. Bacteriol. 192:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charlton S, Moir AJG, Baillie L, Moir A. 1999. Characterization of the exosporium of Bacillus cereus. J. Appl. Microbiol. 87:241–245 [DOI] [PubMed] [Google Scholar]
- 7. Doyle RJ, Rosenberg M. 1990. Microbial cell surface hydrophobicity. ASM Press, Washington, DC [Google Scholar]
- 8. Eveillard M, et al. 1993. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol. Microbiol. 7:371–381 [DOI] [PubMed] [Google Scholar]
- 9. Faille C, et al. 2002. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can. J. Microbiol. 48:728–738 [DOI] [PubMed] [Google Scholar]
- 10. Giel JL, Sorg JA, Sonenshein AL, Zhu J. 2010. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One 5:e8740 doi:10.1371/journal.pone.0008740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heeg D, Burns DA, Cartman ST, Minton NP. 2012. Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One 7:e32381 doi:10.1371/journal.pone.0032381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hellickson LA, Owens KL. 2007. Cross-contamination of Clostridium difficile spores on bed linen during laundering. Am. J. Infect. Control 35:32–33 [Google Scholar]
- 13. Howell S, Kenny AJ, Turner AJ. 1992. A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem. J. 284:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howerton A, Ramirez N, Abel-Santos E. 2011. Mapping interactions between germinants and Clostridium difficile spores. J. Bacteriol. 193:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Husmark U, Ronner U. 1992. The influence of hydrophobic, electrostatic and morphologic properties on the adhesion of Bacillus spores. Biofouling 5:335–344 [Google Scholar]
- 16. Klavenes A, Stalheim T, Sjovold O, Josefsen K, Granum PE. 2002. Attachment of Bacillus cereus spores with and without appendages to stainless steel surfaces. Food Bioproducts Proc. 80:312–318 [Google Scholar]
- 17. Koshikawa T, et al. 1989. Surface hydrophobicity of spores of Bacillus spp. Microbiology 135:2717. [DOI] [PubMed] [Google Scholar]
- 18. Kuehne SA, et al. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 19. Lawley TD, et al. 2009. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J. Bacteriol. 191:5377–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of the blood. J. Hyg. 38:732–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moir A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526–530 [DOI] [PubMed] [Google Scholar]
- 22. Panessa-Warren BJ, Tortora GT, Warren JB. 1997. Exosporial membrane plasticity of Clostridium sporogenes and Clostridium difficile. Tissue Cell 29:449–461 [DOI] [PubMed] [Google Scholar]
- 23. Panessa-Warren BJ, Tortora GT, Warren JB. 2007. High resolution FESEM and TEM reveal bacterial spore attachment. Microsc. Microanal. 13:251–266 [DOI] [PubMed] [Google Scholar]
- 24. Paredes-Sabja D, Sarker MR. 2012. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J. Med. Microbiol. 61:1208–1218 [DOI] [PubMed] [Google Scholar]
- 25. Perez J, Springthorpe VS, Sattar SA. 2005. Activity of selected oxidizing microbiocides against the spores of Clostridium difficile: relevance to environmental control. Am. J. Infect. Control 33:320–325 [DOI] [PubMed] [Google Scholar]
- 26. Pinto M, et al. 1982. Enterocytic differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol. Cell 44:193–196 [Google Scholar]
- 27. Pinto M, et al. 1983. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 47:323–330 [Google Scholar]
- 28. Poutanen SM, Simor AE. 2004. Clostridium difficile-associated diarrhea in adults. CMAJ. 171:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez N, Liggins M, Abel-Santos E. 2010. Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J. Bacteriol. 192:4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ronner U, Husmark U, Hendriksson A. 1990. Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Microbiol. 69:550–556 [DOI] [PubMed] [Google Scholar]
- 31. Rosenburg M, Gutnik D, Rosenberg E. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29–33 [Google Scholar]
- 32. Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536 [DOI] [PubMed] [Google Scholar]
- 33. Sebaihia M, et al. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779–786 [DOI] [PubMed] [Google Scholar]
- 34. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 35. Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as co-germinants for Clostridium difficile spores. J. Bacteriol. 190:2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stabler RA, et al. 2006. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 188:7297–7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiencek KM, Klapes NA, Foegeding PM. 1990. Hydrophobicity of Bacillus and Clostridium spores. Appl. Environ. Microbiol. 56:2600–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams GJ, Denyer SP, Hosein IK, Hill DW, Maillard JY. 2009. Limitations of the efficacy of surface disinfection in the healthcare setting. Infect. Control Hosp. Epidemiol. 30:570–573 [DOI] [PubMed] [Google Scholar]