Abstract
Many studies have demonstrated that intravenously administered bacteria can target and proliferate in solid tumors and then quickly be released from other organs. Here, we employed the tumor-targeting property of Escherichia coli Nissle 1917 to inhibit mouse B16 melanoma and 4T1 breast tumors through the expression of azurin protein. For this purpose, recombinant azurin-expressing E. coli Nissle 1917 was developed. The levels of in vitro and in vivo azurin secretion in the engineered bacterium were determined by immunochemistry. Our results demonstrated that B16 melanoma and orthotopic 4T1 breast tumor growth were remarkably restrained and pulmonary metastasis was prevented in immunocompetent mice. It is worth noting that this therapeutic effect partially resulted from the antitumor activity of neutrophils and lymphocytes due to inflammatory responses caused by bacterial infections. No toxicity was observed in the animal during the experiments. This study indicates that E. coli Nissle 1917 could be a potential carrier to deliver antitumor drugs effectively for cancer therapy.
INTRODUCTION
The use of bacteria for disease treatment has been under investigation for many years (1, 3, 10, 12). However, the detailed mechanisms of their clinical effects are still unknown. Previous studies clearly showed that some bacteria can target and proliferate in solid tumors, significantly inhibiting the growth of tumors (32, 34). So far, bacteria such as Clostridium (1, 4, 8), Salmonella (3, 19, 21), Bifidobacterium (24, 41) and Escherichia coli (6, 27) have been clinically employed for the delivery of drugs, RNA (35, 42), and immune factors (18, 36). Also, these bacteria are often used in combination with traditional methods such as radiation (4) and chemotherapy (25) for cancer therapy. Bacteria per se can stimulate immune responses (22) and secrete potential antitumor substances, DNA fragments, and other compounds that further improve the therapeutic effects (17, 26). Therefore, interaction between bacteria and tumor cells and their antitumor effects have been extensively studied in recent years (9, 12, 43).
An appropriate bacterial strain is crucial for efficient bacteriolytic cancer therapy. The widely used Salmonella enterica serovar Typhimurium had been placed in phase I clinical research in cancer treatment (30, 31). However, its tumor-targeting capability is lower than those of virulence-attenuated, icsA ius-deleted Shigella flexneri 21 SC602 and several E. coli strains, among which E. coli Nissle 1917 showed the highest tumor-targeting ability (27). This is probably because of the serum-sensitive lipopolysaccharide (LPS) structure of E. coli Nissle 1917, which ensures quick elimination from other organs (11). In addition, E. coli Nissle 1917 has been extensively used to treat acute diarrhea and some intestinal tract diseases in infants and toddlers (15, 16), as well as in daily health care products (7). Based on these advantages, we selected E. coli Nissle 1917 as the carrier to deliver azurin to solid cancers.
Azurin, a copper-containing redox protein with low molecular weight, is involved in electron transfer during denitrification by Pseudomonas aeruginosa (37). It can be efficiently internalized in order to initiate cancer cell apoptosis by raising the intracellular levels of p53 and Bax, resulting in the release of mitochondrial cytochrome c into the cytosol (38). Several studies showed that azurin can actively inhibit the growth of UISO-Mel-2 melanoma and MCF-7 human breast tumors (37–40). More interestingly, it can selectively kill carcinoma cells in vivo while sparing normal tissues (23, 29, 39). Several authors attribute this to the fact that azurin receptors are hyperexpressed on the surfaces of cancer cells relative to their expression on the surfaces of normal cells (39, 40). Also, amino acids 50 to 77 of azurin are responsible for the selective penetration in tumor cells (5, 40).
This study was aimed at testing the efficacy of E. coli Nissle 1917 as a new tumor-targeting carrier to deliver antitumor drug. Therefore, we employed E. coli Nissle 1917 to carry azurin to cure B16 mouse melanoma and 4T1 breast tumors. This engineered bacterium efficiently suppressed the growth of tumors and prevented pulmonary metastasis in vivo by releasing azurin and stimulating inflammatory responses. This work proposes a new tumor-targeting delivery system for cancer therapy.
MATERIALS AND METHODS
Bacteria and plasmids.
C-terminal DNA of azurin (128 amino acids) was amplified from P. aeruginosa strain PAO1 using PCR. pelB (ATGAAATACCTATTGCCTACGGCAGCCGCTGGATTGTTATTACTCGCTGCCCAACCAGCGATGGCT), from an Erwinia carotovora strain, was added in front of the mature azurin by using a 5′ primer. Then, the PCR products were inserted into the pSUM vector (modified from pSU2719) under the truncated promoter of lac, from the end of lacI to the beginning of lacZα (GCGCCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCATTAATGCAGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGAGCGCAACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCT), which resulted in constitutive gene expression. The engineered pSUMAzurin (2,714 bp) and pUC18-GFP plasmids were electroporated into E. coli Nissle 1917 separately. The bacteria were grown at 37°C in LB liquid medium to mid-logarithmic phase and prepared as previously described (27), and 2 × 107 live E. coli Nissle 1917 organisms with or without plasmid were injected into mice intravenously (i.v.) in a total volume of 100 μl phosphate-buffered saline (PBS) in all experiments. Immunoblotting was used to confirm the expression of azurin in both pellets and culture supernatants of E. coli Nissle 1917, E. coli Nissle 1917 bearing empty vector, or E. coli Nissle 1917 bearing azurin-expressing plasmids using rabbit anti-azurin polyclonal antibody.
Cell lines.
4T1 mouse breast tumor cells were purchased from the American Type Culture Collection (ATCC; CRL-2539), and B16 cancer cells were obtained from the Cell Bank of Type Culture Collection, Chinese Academy of Sciences. Both cell lines were cultured in RPMI 1640 medium supplemented with l-glutamine (Gibco) and 10% heat-inactivated fetal bovine (Hangzhou Sijiqing, Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Animal models.
All animal experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Hunan Normal University. Specific-pathogen-free (SPF) female BALB/c and C57BL/6 mice, 6 to 8 weeks old, were purchased from the SLRC Laboratory Animal Company in Hunan Province, China. Animals were bred and maintained under SPF conditions for at least 3 days before use. Tumors in the fourth mammary pads of female BALB/c mice were established with 1 × 105 4T1 breast tumor cells, and C57BL/6 mice were implanted with subcutaneous (s.c.) tumors through the injection of 1 × 105 B16 cells on the mid-right side. After the tumor volume reached ∼0.2 cm3, all experimental animals were randomly assigned to four groups and were observed every day. At a defined time, the mice were sacrificed by cervical dislocation. However, moribund animals exhibiting irregular respiration, tremors, absence of voluntary response to external stimuli, and coma before that time were killed for humane reasons and were considered to have died during the survival experiments. All animal experiments were repeated three times.
Fluorescence GFP imaging processing and bacterial distribution.
BALB/c mice bearing 4T1 breast tumors were injected i.v. with E. coli Nissle 1917 bearing pUC18-GFP. After 1 day, tissues were excised and fixed in TissueTek OCT medium (Sakura Finetechnical Co. Ltd., Tokyo, Japan) followed by snap-freezing in −180°C liquid nitrogen. Cryosections (18-μm thickness) were obtained using a Leica CM3050S cryostat (Leica, Nussloch, Germany) and stuck on glass slides. The availability of bacteria in tissues was appraised using a Nikon Eclipse E400 epifluorescence microscope (Nikon Corp., Tokyo, Japan) or a Leica MZ16 FA fluorescence stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany); BALB/c mice (n = 3) bearing 4T1 breast tumors were infected by i.v. administration of E. coli Nissle 1917. At 1, 3, 7, and 14 days after infection, tumors, livers, spleens, hearts, kidneys, and lungs were isolated and spread on LB plates, and the number of E. coli Nissle 1917 organisms in tissues were calculated.
Antitumor activity of azurin-expressing E. coli Nissle 1917 in vivo.
To test the inhibitory effects of azurin-expressing E. coli Nissle 1917 on primary tumors, BALB/c mice with orthotopic implantation of 4T1 breast tumors were given either PBS or E. coli Nissle 1917 carrying different plasmids after the tumor volume reached ∼0.2 cm3, and then they were treated weekly. Tumor volumes were measured every 2 days using Vernier calipers, and mice were monitored throughout the experiment. The experimental procedure for the determination of pulmonary metastases of 4T1 breast tumor was the same as that for testing the anticancer effects of azurin-expressing E. coli Nissle 1917 on primary tumors. At the end of treatment, mice were sacrificed and tumors and lungs were weighed. The lungs were fixed in Bouin's solution for 24 h, and the nodules on the surface were counted under a dissection microscope. Simultaneously, the syngeneic murine B16 melanoma model was also used to check the antitumor effect of this engineered bacterium, following the same treatment procedure as for 4T1 breast tumor mouse model.
Histological analysis.
In histological studies, mice were killed at a defined time. Tissues were fixed in 4% paraformaldehyde at 4°C overnight and then prepared for examination using standard hematoxylin and eosin (H&E) staining or immunohistochemistry procedures. The sections were observed and photographed under the microscope.
Host safety.
Healthy female BALB/c mice, 6 to 8 weeks old, were given E. coli Nissle 1917 i.v. weekly. The activity and weight of mice were monitored daily. At the end of the experiment, mice were sacrificed by cervical dislocation, and livers and spleens were weighed.
Statistical analysis.
Statistical significance for all experiment groups was determined by Student's t test. If P was <0.05, the results were considered significant.
RESULTS
Distribution of E. coli Nissle 1917 in vivo.
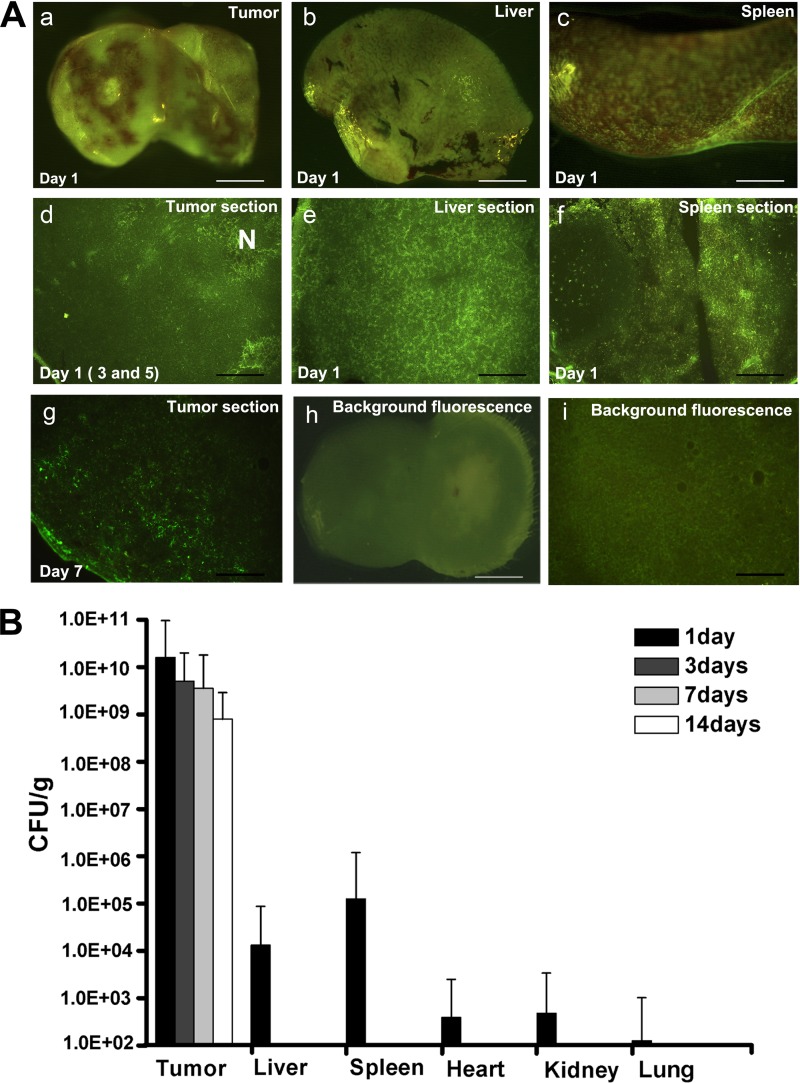
To depict the distribution of E. coli Nissle 1917 in vivo, green fluorescent protein (GFP)-bearing plasmids were electroporated into E. coli Nissle 1917, which was subsequently injected into the tail vein of BALB/c mice with 4T1 breast tumors. GFP plasmid-free E. coli Nissle 1917 and PBS were also administered to control groups (n = 3). The tumors, spleens, and livers were removed at 1, 3, 5, and 7 days after intravenous (i.v.) administration. Fluorescence microscopy was used to determine the bacterial distribution in these tissues. On day 1 after i.v. administration of GFP-bearing E. coli Nissle 1917, strong green fluorescence was detected in the tumors, spleens, and livers of three parallel mice using three-dimensional fluorescence microscopy (Fig. 1A, panels a, b, and c; all were obtained from one mouse). Furthermore, these tissues obtained from another group were snap-frozen, cut into 18-μm sections, and then observed by inverted fluorescence microscopy. The in vivo distribution characteristics of E. coli Nissle 1917 were the same as that observed by three-dimensional fluorescence microscopy. All three treated mice presented strong green fluorescence in the tissue sections (Fig. 1A, panels d, e, and f; all were obtained from one mouse). E. coli Nissle 1917 exhibited preferential accumulation in the necrotic tumor tissue and was evenly distributed in the liver and spleen. These data support the idea that most tumor-targeting bacteria proliferate only in necrotic areas because immune factors prevent the bacteria from reaching the viable part of a tumor. On days 3 and 5 postinfection, livers and spleens were clear of E. coli Nissle 1917, while the tumors still contained the bacteria (Fig. 1A, panel d). On day 7 after injection of GFP-expressing E. coli Nissle 1917, the fluorescence almost vanished in intact tumor and frozen tumor section, which could have resulted from plasmid loss (Fig. 1A, panel g).
Fig 1.
Bacterial distribution in vivo. (A) Bacterial growth in tumor, spleen, and liver tissues was observed using fluorescence microscopy after i.v. administration of PBS or 2 × 107 CFU of E. coli Nissle 1917 with or without a GFP-bearing plasmid. The distribution of E. coli Nissle 1917 in intact tissues (a, b, c, and h) and tissue cryosections (d, e, f, g, and i) was recorded on days 1, 3, 5, and 7 postinfection. N, necrotic regions in tumors. Intact tissue background fluorescence is shown in panel h; the background fluorescence of the cryosections is shown in panel i. Bars, 160 μm (a and i), 200 μm (b), 180 μm (c), and 100 μm (d, e, f, h, and j). (B) The retention of E. coli Nissle 1917 in the different tissues of BALB/c mice with 4T1 breast tumor was measured on days 1, 3, 7, and 14 after i.v. administration of 2 × 107 CFU E. coli Nissle 1917.
The preferential persistence of E. coli Nissle 1917 in the tumor, but not in normal tissues, is critical for its use as carrier to deliver anticancer agents for tumor therapy. After we noticed that E. coli Nissle 1917 successfully targeted the tumor site, the retention of E. coli Nissle 1917 in tumor was compared with that in other tissues in immunocompetent BALB/c mice by bacterial count (n = 3) after i.v. administration of 2 × 107 CFU of E. coli Nissle 1917. As observed by fluorescence imaging, the bacteria were quickly released from spleen and liver and were not found in hearts, kidneys, or lungs on day 3 postinjection. The number of bacteria in tumors reached 109 CFU/g on day 3 and remained at this level for 7 days. Interestingly, this number was maintained at 108 CFU/g in tumors even 14 days after i.v. injection (Fig. 1B). The results suggest that E. coli Nissle 1917 could be an ideal tumor-targeting candidate for the delivery of anticancer drugs.
Constitutive expression in vitro and in vivo of azurin.
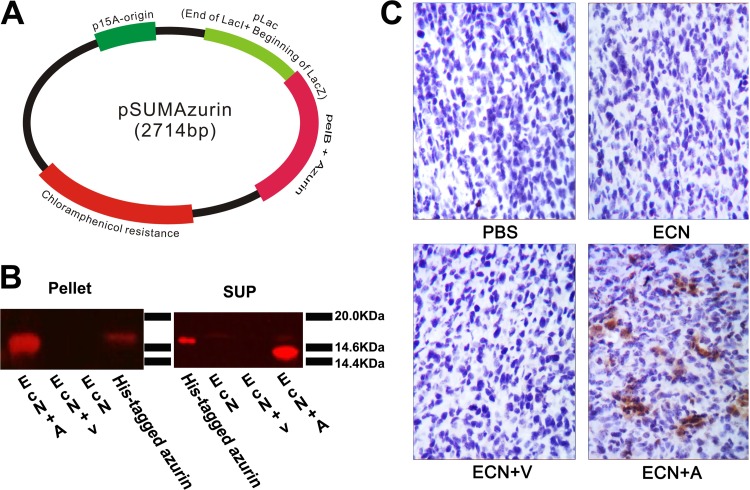
The continuous secretion of azurin by the engineered bacterium is important in eliciting its anticancer activity. Therefore, we constructed plasmid pSUMAzurin, in which the mature azurin gene was coexpressed with the pelB leader sequence, which then directs protein secretion under the control of a truncated lac promoter (Fig. 2A). This truncated lac worked as a constitutive promoter, leading to the continuous expression of azurin. The azurin-bearing and empty plasmids were then independently transferred into E. coli Nissle 1917 by electroporation. The bacteria were cultivated in LB medium overnight and collected by centrifugation. Equal numbers of bacteria and their culture supernatants from E. coli Nissle 1917 or E. coli Nissle 1917 with plasmids were tested for azurin expression by immunoblotting. The results showed that azurin protein was present in both cell pellets and supernatants of azurin-expressing E. coli Nissle 1917. However, it was not found in control E. coli Nissle 1917 with or without empty vector (Fig. 2B). Additionally, a plasmid with the full-length azurin gene was also constructed in this experiment, but azurin was not secreted in E. coli Nissle 1917 carrying this plasmid encoding full-length azurin, as it was in Pseudomonas aeruginosa strain PAO1 (data not shown).
Fig 2.
Azurin expression in vitro and in vivo. (A) Map of bacterial expression plasmid containing azurin gene (pSUMAzurin). (B) Azurin expression in cell lysates (Pellet) or culture supernatants (SUP) was detected by immunoblotting analysis of E. coli Nissle 1917 and E. coli Nissle 1917 bearing empty plasmid (EcN+V) or azurin-expressing plasmid (EcN+A). (C) BALB/c mice (n = 3) bearing 4T1 breast tumors were injected i.v. with either PBS or 2 × 107 CFU of E. coli Nissle 1917 (ECN), E. coli Nissle 1917 with empty plasmid (ECN+V), or azurin-expressing E. coli Nissle 1917 (ECN+A) on days 1 and 7. On day 3 after the last treatment, the presence of azurin in tumor sections was confirmed by immunohistochemistry.
In order to determine whether azurin could be expressed in vivo, BALB/c mice (n = 3) with s.c. 4T1 breast tumors were injected i.v. with either PBS or 2 × 107 CFU of various types of E. coli Nissle 1917 per mouse every week. On day 3 after the second round of treatment, tumor tissues were removed from BALB/c mice, fixed in 4% paraformaldehyde, and then embedded in paraffin. Azurin was confirmed in tumor tissue by immunohistochemistry using its specific antibody (Fig. 2C). Azurin clearly diffused into viable tumor regions. However, azurin was not found in livers, spleens, hearts, kidneys, or lungs (data not shown). This could be due to a limited amount of azurin produced in tumors and a short retention time of azurin-expressing E. coli Nissle 1917 in normal tissues.
Azurin-expressing E. coli Nissle 1917 inhibited primary and metastatic tumor growth in vivo.
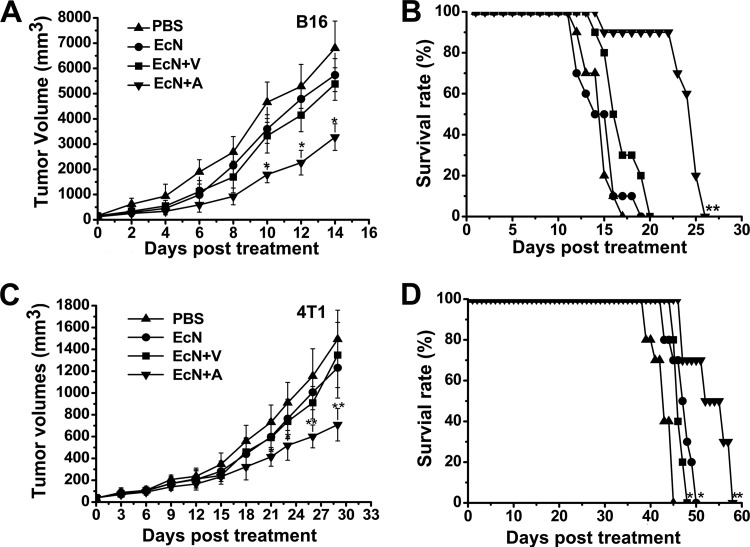
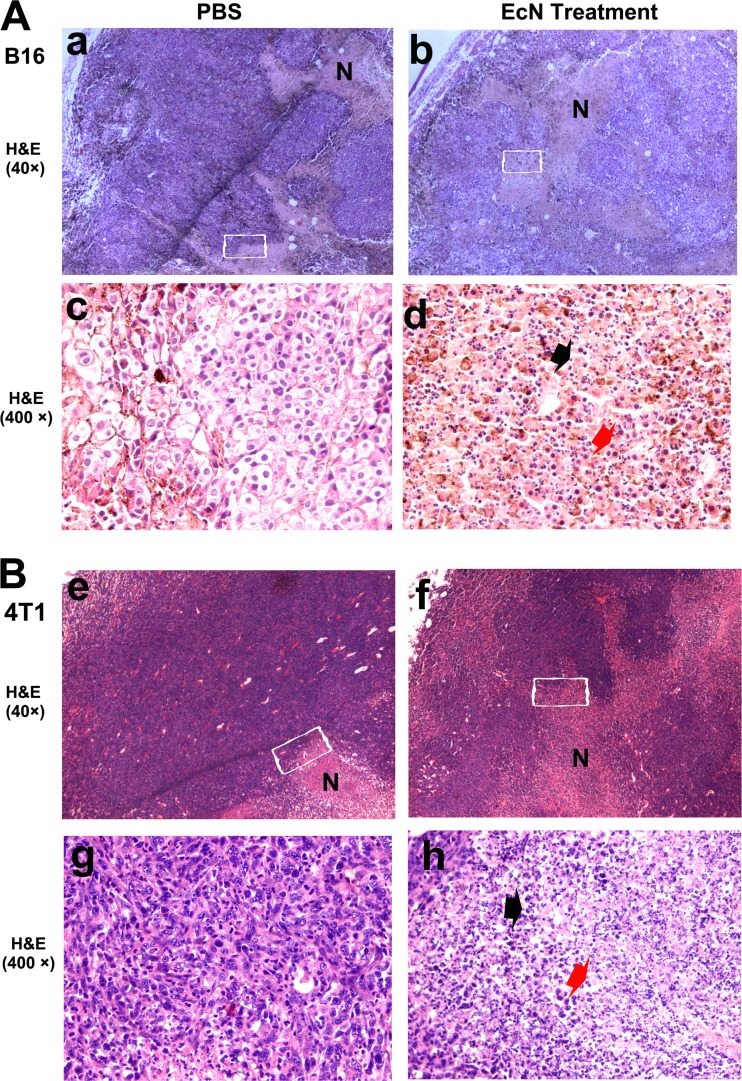
Syngeneic B16 melanoma and 4T1 breast tumor models were used to investigate the antitumor effects of azurin-expressing E. coli Nissle 1917. Soon after the tumor grew to ∼0.2 cm3, C57BL6 and BALB/c mice were given PBS, E. coli Nissle 1917, E. coli Nissle 1917 with empty vector, or E. coli Nissle 1917 with azurin-expressing plasmids by i.v. injection. E. coli Nissle 1917 and its variants were administered at a dose of 2 × 107 CFU per mouse. Bacteria levels of 2 × 107 CFU azurin-expressing E. coli Nissle 1917 notably delayed tumor growth and prolonged survival (Fig. 3). At sacrifice, the B16 melanomas and 4T1 breast tumors in the treatment group reached an average size of 3275 mm3 (Fig. 3A) and 709 mm3 (Fig. 3C), respectively. These corresponded to 51% (P < 0.05 for B16 melanoma) and 52% (P < 0.01 for 4T1 breast tumor) reductions compared to PBS control. However, groups that received empty-plasmid-bearing E. coli Nissle 1917 or E. coli Nissle 1917 alone had no notable differences from the PBS group in terms of tumor suppression, but these treatments slightly prolonged the survival of 4T1 breast tumor-bearing BALB/c mice (Fig. 3D). Nevertheless, this minor effect was not observed in mice with malignant B16 tumors (Fig. 3B) (n = 10; P < 0.05 versus PBS control). A possible explanation for this phenomenon could be that B16 tumors proliferated rapidly, and consequently, the anticancer effects of E. coli Nissle 1917 at the same dose may not have been sufficient to restrain tumor growth. Histological examination of tumors on day 3 after i.v. injection with E. coli Nissle 1917 revealed conspicuous necrosis extension and a massive infiltration of inflammatory cells (n = 3). These inflammatory cells mostly consist of neutrophils and lymphocytes, whereas fewer of these cells were found in the PBS-treated mice (Fig. 4). The inflammatory cells and cytokines of the immune system generated after stimulation by LPS and other secretion substrates of the infiltrating E. coli Nissle 1917 may directly contribute to the prevention of tumor growth. These effects may partially prolong the survival of tumor-bearing mice in the groups treated with E. coli Nissle 1917 and E. coli Nissle 1917 with empty vector (Fig. 3D).
Fig 3.
Therapeutic effect of azurin-expressing E. coli Nissle 1917 (EcN+A) on tumor growth and toxicity. Mice (n = 10) bearing B16 or 4T1 breast tumors were treated by i.v. administration of either PBS or 2 × 107 CFU of E. coli Nissle 1917 (EcN), E. coli Nissle 1917 bearing empty plasmid (EcN+V), or azurin-expressing E. coli Nissle 1917 (EcN+A) at weekly intervals. Tumor volumes (mm3) were estimated using external calipers (values are means ± standard deviations [SD]). B16 (A) and 4T1 (C) breast tumors were significantly inhibited by azurin-expressing E. coli Nissle 1917 (EcN+A) compared with the PBS control group. The survival of mice bearing B16 (B) and 4T1 (D) breast tumors was significantly prolonged in the azurin-expressing E. coli Nissle 1917 treatment group, while E. coli Nissle 1917 and E. coli Nissle 1917 bearing empty vector marginally extended the life of 4T1 breast tumor-bearing mice compared to the PBS control (D). *, P < 0.05; **, P < 0.01.
Fig 4.
E. coli Nissle 1917-induced tumor inflammation. After tumor volumes reached ∼0.2 cm3, mice (n = 3) were treated with PBS or 2 × 107 CFU E. coli Nissle 1917 by i.v. infection. The tumors were evaluated by H&E staining after 3 days of systemic injection. Enlargement of areas of necrosis (N) was found in both B16 melanoma (A) and 4T1 breast tumors (B), as indicated in panels b and f, compared with PBS treatment (a and e). The boxed areas in panels a, b, e, and f are magnified in panels c, d, g, and h, respectively. The H&E staining in panels d and h shows the number of neutrophils (red arrows) and lymphocytes (black arrows) that accumulated in necrosis, but few were found in the PBS group (c and g).
To further develop our understanding of the tumor-targeting property of azurin-expressing E. coli Nissle 1917, we used the 4T1 breast tumor, a very effective orthotopic metastatic mouse tumor model, to assess the ability of this bacterium to prevent pulmonary metastasis. After the tumor size reached ∼0.2 cm3, the mice were given either PBS or 2 × 107 CFU of E. coli Nissle 1917 carrying different plasmids. After 30 days, the mice were sacrificed by cervical dislocation, and then the white tumor nodes on the surfaces of the lungs were counted. The administration of azurin-expressing E. coli Nissle 1917 resulted in a significant reduction of metastatic tumor nodule and total tumor wet weight in the fourth mammary pads of BALB/c mice, i.e., 41% (n = 10; P < 0.05) and 56% (n = 10; P < 0.01), respectively, compared with PBS controls (Table 1). The groups receiving E. coli Nissle 1917 and E. coli Nissle 1917 with empty plasmid showed no significant differences from the PBS group. This finding reveals that this engineered bacterium strikingly restrained tumor growth and metastasis of 4T1 breast tumors from mammary glands to lungs. However, E. coli Nissle 1917 was insufficient to prevent tumor metastasis individually. In addition, the number of metastatic tumor nodules on the surfaces of lungs was statistically different between the experimental group and the control group, but lung mass showed no significant difference between them. This may be due to the limited number of nodules on the surface of lung in all experimental groups.
Table 1.
Inhibitory effect of azurin-expressing E. coli Nissle 1917 on the pulmonary metastasis of 4T1 breast tumorsa
| Treatment group | Tumor wet wt (g) | No. of metastatic lung nodules/mouse | Lung wet wt (g) |
|---|---|---|---|
| PBS | 3.00 ± 0.82 | 21.4 ± 6.50 | 0.32 ± 0.04 |
| E. coli N | 2.96 ± 0.74 | 18.6 ± 6.61 | 0.30 ± 0.05 |
| E. coli N + V | 2.75 ± 0.74 | 19.4 ± 7.40 | 0.31 ± 0.03 |
| E. coli N + A | 1.32 ± 0.58 (56%)** | 12.5 ± 5.35 (41%)* | 0.29 ± 0.04 |
Mice bearing 4T1 breast tumors (n = 10) were injected i.v. weekly with either PBS or 2 × 107 CFU of E. coli Nissle 1917 (E. coli N), E. coli Nissle 1917 with empty plasmid (E. coli N + V), or azurin-expressing E. coli Nissle 1917 (E. coli N + A) after tumor establishment. Mice were sacrificed after 30 days, and tumor and lung wet weights were measured. The metastatic lung nodules were counted under a dissection microscope. The differences in tumor wet weight and number of metastatic lung nodules between azurin-expressing E. coli Nissle 1917 and control group were significant (*, P < 0.05; **, P < 0.01). Values are means ± SD. The numbers in parentheses are rates of inhibition by azurin-expressing E. coli Nissle 1917.
Toxicity.
E. coli Nissle 1917 toxicity in healthy BALB/c mice (n = 10) was evaluated by weekly i.v. administration of 2 × 107 CFU E. coli Nissle 1917. Body weight was measured every 2 days for 50 days. At the end of the experiments, livers and spleens were excised and weighed. The weekly E. coli Nissle 1917 treatment had no effects on body, liver, and spleen weights, except for a symptom of nose scratching on the first day after injection (Fig. 5). No death occurred during the experiments, and E. coli Nissle 1917-treated animals behaved normally, as judged by comparison with the PBS-treated group. Also, no serious anaphylactic activity was recorded. Therefore, the results demonstrate that weekly systemic administration of E. coli Nissle 1917 did not have any noticeable pathogenic effect on the animals.
Fig 5.
Toxicity test of E. coli Nissle 1917. Normal mice (n = 10) were injected i.v. weekly with 2 × 107 CFU E. coli Nissle 1917 or PBS for 50 days. The body weights were recorded at 2-day intervals (means ± SD) (A). At the end of the experiment, liver and spleen were excised and weighed (means and SD) (B). There are no significant differences between PBS and E. coli Nissle 1917 treatment groups.
DISCUSSION
In this study, we assessed the systemic distribution of E. coli Nissle 1917 in immunocompetent mice and employed E. coli Nissle 1917 as a new drug carrier to deliver azurin for cancer therapy. We noted that E. coli Nissle 1917 proliferated to a high density of 109 CFU/g and efficiently secreted proteins in tumors. Moreover, it disappeared in the normal tissues 3 days after i.v. injection and preferentially remained within tumors, persisting at a density of 108 CFU/g even after 14 days. The typical immune regulation effects of E. coli Nissle 1917 may account for this result. E. coli Nissle 1917 can directly interact with adoptive immune system to regulate central T cell functions such as inducing γδ T cell apoptosis (13), lowering the expansion of newly recruited T cells (28) and downregulating IgG release from B cells (2). All these effects favor the growth of E. coli Nissle 1917 in tumors. Although E. coli Nissle 1917 can reduce inflammatory response in vivo, the modest levels of cytokines generated in the immune reaction participated in the killing of tumor cells (3, 18). In addition, the strong inhibitory effect of E. coli on pulmonary metastasis has already been confirmed (33). Also, the enhanced release of specific protein or CpG-rich extrachromosomal DNA by bacteria in response to cancer cells has been indicated for potential cancer therapy (20). However, these conclusions are not adequately supported by this study. Since E. coli Nissle 1917 proliferates at a high density in tumors, azurin with secretory signal peptide under the control of the modified lac promoter was continually released. Its low molecular weight makes azurin easily absorbed by tumor cells with inherent stability, which enables it to kill them through apoptosis induction (5, 37, 39). Our data show that azurin-expressing E. coli Nissle 1917 dramatically inhibited the growth of B16 melanoma and orthotopic 4T1 breast tumors as well as preventing pulmonary metastases, while the body and tissue weights of mice were not affected. This therapeutic effect probably resulted from the combined effects of E. coli Nissle 1917 and azurin.
This was the first attempt to use E. coli Nissle 1917 as a vector to carry an anticancer protein to tumors and depress proliferation of cancer in vivo. Additionally, we used GFP labeling and snap-frozen section technology to study the tumor-targeting functions of E. coli Nissle 1917, and hence a more vivid profile of bacterial distribution in tumors than is obtainable by other methods was achieved. Our results demonstrate that E. coli Nissle 1917 via i.v. injection not only targeted the tumors more efficiently than other well-known bacteria (27) but also served as a carrier to deliver antitumor substrates, which ultimately resulted in the inhibition of growth of solid tumors and pulmonary metastasis. Furthermore, an excellent peptide of exocytosis (14)—PelB—was introduced into E. coli Nissle 1917, which greatly increased the extracellular secretion of azurin. Our model provides an example of the potential application of numerous antitumor proteins with low molecular weights, such as azurin, for tumor-targeting therapy using the E. coli Nissle 1917 vector. The fact that azurin selectively targets tumor cells and does not induce apoptosis of nontumor cells (23, 37) makes it a prospective protein for cancer therapy in clinical studies. Since neither E. coli Nissle 1917 nor azurin is pathogenic, azurin-carrying E. coli Nissle 1917 could be administered orally in preclinical experiments (2, 15, 16).
In contrast to Salmonella and Clostridium novyi NT, which have been extensively investigated as vehicles for antitumor drugs (1, 21), E. coli Nissle 1917 has high tumor-targeting capability and can be rapidly released from normal organs when systemically administered. In addition, E. coli Nissle 1917—as an E. coli strain—has a comparatively simple genetic basis and therefore can be modified into an effective drug-producing machine more easily than other bacteria. In bacteriolytic cancer therapy, most studies focus on the use of cytokines (18, 36), prodrugs (6, 24), or bacteria per se (4, 43) for inhibiting tumor growth or metastasis. Here, we used the bacteria to express a secretory antitumor protein for cancer therapy. This novel strategy could outperform other approaches in bacteriolytic cancer therapy.
In this study, we tested the cancer therapeutics of azurin-carrying E. coli Nissle 1917 in murine models. The therapeutic efficacy of this engineered bacterium in human tumor models is not yet known. It is also necessary to investigate whether it can efficiently deliver other proteins and small molecular substrates to solid tumors. Moreover, it should be pointed out that E. coli Nissle 1917 requires some genetic modification to serve as a safe carrier for i.v. administration. Concerns regarding its safety could be alleviated if it were to be taken oral to treat some intestinal cancers. E. coli Nissle 1917 has been shown to have the ability to regulate immunoreactions and limit inflammation in the intestinal tract (2, 28), but whether this facilitates less-toxic colonization in tumors also needs to be studied. The mechanism by which E. coli Nissle 1917 targets tumor cells is another important and very interesting question.
In summary, we succeeded in our attempt to combine the tumor-targeting E. coli Nissle 1917 with the antitumor protein azurin to restrain the growth of primary cancers. This study opens up a new model for bacteriolytic cancer therapy. More importantly, E. coli Nissle 1917 is a probiotic and has been widely used in drugs and health care products (7, 15, 16). Therefore, E. coli Nissle 1917 can be genetically engineered to function as a carrier of various drugs for cancer therapy.
ACKNOWLEDGMENTS
This investigation was supported by the National Natural Science Foundation of China (30970066, 31070006, and 30900037), the National Key Basic Research and Development project (973) of China (2012CB722300) and the National High Technology Research and Development project (863) of China (2011AA10A203).
We thank Y. Q. Chen (Department of Respiratory Medicine, the First Affiliated Hospital, Guangxi Medical University) for P. aeruginosa strain PAO1, S. P. Li (Laboratory of Agricultural Environmental Microorganisms, Nanjing Agriculture University) for pUC18-GFP plasmids, Ya Cao (Cancer Research Institute, Xiangya School of Medicine, Central South University) for B16 cancer cells, and Xiong Liu and Edward D. Korn (Source Laboratory of Cell Biology, NHLBI, National Institutes of Health, Bethesda, Maryland) for advice during the writing of this paper.
Footnotes
Published ahead of print 24 August 2012
REFERENCES
- 1. Agrawal N, et al. 2004. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. U. S. A. 101:15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arribas B, et al. 2009. A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br. J. Pharmacol. 157:1024–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avogadri F, et al. 2005. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 65:3920–3927 [DOI] [PubMed] [Google Scholar]
- 4. Bettegowda C, et al. 2003. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc. Natl. Acad. Sci. U. S. A. 100:15083–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhari A, et al. 2007. Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry 46:1799–1810 [DOI] [PubMed] [Google Scholar]
- 6. Cheng CM, et al. 2008. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Ther. 15:393–401 [DOI] [PubMed] [Google Scholar]
- 7. de Vrese M, Schrezenmeir J. 2008. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 111:1–66 [DOI] [PubMed] [Google Scholar]
- 8. Diaz LA, Jr, et al. 2005. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. 88:562–575 [DOI] [PubMed] [Google Scholar]
- 9. Forbes NS, Munn LL, Fukumura D, Jain RK. 2003. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 63:5188–5193 [PubMed] [Google Scholar]
- 10. Fredriksen L, Mathiesen G, Sioud M, Eijsink VG. 2010. Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Appl. Environ. Microbiol. 76:7359–7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grozdanov L, et al. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J. Bacteriol. 184:5912–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guglielmetti S, et al. 2010. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl. Environ. Microbiol. 76:3948–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guzy C, et al. 2008. The probiotic Escherichia coli strain Nissle 1917 induces γδ T cell apoptosis via caspase- and FasL-dependent pathways. Int. Immunol. 20:829–840 [DOI] [PubMed] [Google Scholar]
- 14. Hemilä H, Pakkanen R, Heikinheimo R, Palva ET, Palva I. 1992. Expression of the Erwinia carotovora polygalacturonase-encoding gene in Bacillus subtilis: role of signal peptide fusions on production of a heterologous protein. Gene 116:27–33 [DOI] [PubMed] [Google Scholar]
- 15. Henker J, et al. 2008. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr. Infect. Dis. J. 27:494–499 [DOI] [PubMed] [Google Scholar]
- 16. Henker J, et al. 2007. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 166:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homburg S, Oswald E, Hacker J, Dobrindt U. 2007. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol. Lett. 275:255–262 [DOI] [PubMed] [Google Scholar]
- 18. Loeffler M, Le'Negrate G, Krajewska M, Reed JC. 2007. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc. Natl. Acad. Sci. U. S. A. 104:12879–12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loessner H, et al. 2007. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 9:1529–1537 [DOI] [PubMed] [Google Scholar]
- 20. Mahfouz M, Hashimoto W, Das Gupta TK, Chakrabarty AM. 2007. Bacterial proteins and CpG-rich extrachromosomal DNA in potential cancer therapy. Plasmid 57:4–17 [DOI] [PubMed] [Google Scholar]
- 21. Mengesha A, et al. 2006. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biol. Ther. 5:1120–1128 [DOI] [PubMed] [Google Scholar]
- 22. Presley LL, Wei B, Braun J, Borneman J. 2010. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 76:936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Punj V, et al. 2004. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 23:2367–2378 [DOI] [PubMed] [Google Scholar]
- 24. Sasaki T, et al. 2006. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 97:649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith AB, III, et al. 2005. Discodermolide analogues as the chemical component of combination bacteriolytic therapy. Bioorg. Med. Chem. Lett. 15:3623–3626 [DOI] [PubMed] [Google Scholar]
- 26. Stark FC, Sad S, Krishnan L. 2009. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 69:4327–4334 [DOI] [PubMed] [Google Scholar]
- 27. Stritzker J, et al. 2007. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int. J. Med. Microbiol. 297:151–162 [DOI] [PubMed] [Google Scholar]
- 28. Sturm A, et al. 2005. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via Toll-like receptor 2 signaling. Infect. Immun. 73:1452–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor BN, et al. 2009. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 69:537–546 [DOI] [PubMed] [Google Scholar]
- 30. Thamm DH, et al. 2005. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin. Cancer Res. 11:4827–4834 [DOI] [PubMed] [Google Scholar]
- 31. Toso JF, et al. 2002. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20:142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei MQ, Mengesha A, Good D, Anné J. 2008. Bacterial targeted tumor therapy-dawn of a new era. Cancer Lett. 259:16–27 [DOI] [PubMed] [Google Scholar]
- 33. Weibel S, Stritzker J, Eck M, Goebel W, Szalay AA. 2008. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol. 10:1235–1248 [DOI] [PubMed] [Google Scholar]
- 34. Wiemann B, Starnes CO. 1994. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol. Ther. 64:529–564 [DOI] [PubMed] [Google Scholar]
- 35. Xiang S, Fruehauf J, Li CJ. 2006. Short hairpin RNA-expressing bacteria elicit RNA interference in mammals. Nat. Biotechnol. 24:697–702 [DOI] [PubMed] [Google Scholar]
- 36. Xu C, et al. 2007. Construction of recombinant attenuated Salmonella typhimurium DNA vaccine expressing H pylori ureB and IL-2. World J. Gastroenterol. 13:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada T, Hiraoka Y, Das Gupta TK, Chakrabarty AM. 2004. Regulation of mammalian cell growth and death by bacterial redox proteins: relevance to ecology and cancer therapy. Cell Cycle 3:752–755 [PubMed] [Google Scholar]
- 38. Yamada T, et al. 2004. Apoptosis or growth arrest: modulation of tumor suppressor p53's specificity by bacterial redox protein azurin. Proc. Natl. Acad. Sci. U. S. A. 101:4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamada T, et al. 2005. Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol. 7:1418–1431 [DOI] [PubMed] [Google Scholar]
- 40. Yamada T, et al. 2009. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol. Cancer Ther. 8:2947–2958 [DOI] [PubMed] [Google Scholar]
- 41. Yazawa K, et al. 2001. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res. Treatment 66:165–170 [DOI] [PubMed] [Google Scholar]
- 42. Zhang L, et al. 2007. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res. 67:5859–5864 [DOI] [PubMed] [Google Scholar]
- 43. Zhao M, et al. 2006. Targeted therapy with a Salmonella Typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 66:7647–7652 [DOI] [PubMed] [Google Scholar]