Abstract
Resistance to the quaternary ammonium disinfectant benzalkonium chloride (BC) may be an important contributor to the ability of Listeria spp. to persist in the processing plant environment. Although a plasmid-borne disinfectant resistance cassette (bcrABC) has been identified in Listeria monocytogenes, horizontal transfer of these genes has not been characterized. Nonpathogenic Listeria spp. such as L. innocua and L. welshimeri are more common than L. monocytogenes in food processing environments and may contribute to the dissemination of disinfectant resistance genes in listeriae, including L. monocytogenes. In this study, we investigated conjugative transfer of resistance to BC and to cadmium from nonpathogenic Listeria spp. to other nonpathogenic listeriae, as well as to L. monocytogenes. BC-resistant L. welshimeri and L. innocua harboring bcrABC, along with the cadmium resistance determinant cadA2, were able to transfer resistance to other nonpathogenic listeriae as well as to L. monocytogenes of diverse serotypes, including strains from the 2011 cantaloupe outbreak. Transfer among nonpathogenic Listeria spp. was noticeably higher at 25°C than at 37°C, whereas acquisition of resistance by L. monocytogenes was equally efficient at 25 and 37°C. When the nonpathogenic donors were resistant to both BC and cadmium, acquisition of cadmium resistance was an effective surrogate for transfer of resistance to BC, suggesting coselection between these resistance attributes. The results suggest that nonpathogenic Listeria spp. may behave as reservoirs for disinfectant and heavy metal resistance genes for other listeriae, including the pathogenic species L. monocytogenes.
INTRODUCTION
Listeria spp. are Gram-positive bacteria commonly found in the environment. The single human pathogen in this genus, Listeria monocytogenes, continues to be associated with significant disease burden due to its high morbidity and mortality toward vulnerable populations such as the elderly, pregnant women and their fetuses, neonates, and immunocompromised patients (10, 21, 34, 46). L. monocytogenes remains largely susceptible to antibiotics, with a common course for treatment being a combination of ampicillin and gentamicin (20). However, reported cases of listeriosis involving strains with multidrug resistance (6, 17, 38, 40, 52) suggest the potential for enhanced resistance through horizontal gene transfer with accompanying increases in public health concerns associated with this pathogen.
Colonization of the processing plant environment by Listeria spp. is a major contributor to contamination of processed, ready-to-eat foods (23, 53). Exposure to disinfectants commonly used in food processing plants, such as the quaternary ammonium compound benzalkonium chloride (BC), may provide selective pressures for disinfectant resistance with an accompanying increase in fitness within the processing plant environment. Several studies have investigated prevalence and mechanisms of BC resistance in L. monocytogenes (12, 31, 43, 50). However, less is known about the behavior and response of nonpathogenic Listeria spp. to selection pressures in food processing plants and other environments.
The processing plant environment may present many opportunities for nonpathogenic Listeria spp. to interact with L. monocytogenes. Nonpathogenic species such as L. innocua and L. welshimeri have been found to be more common than L. monocytogenes in food processing environments and in foods and to grow faster than L. monocytogenes in foods and other media (2, 8, 19, 30, 36, 47, 49, 54). These Listeria spp. are also more likely than L. monocytogenes to exhibit antibiotic resistance and to harbor plasmids (1, 6, 9, 13, 14, 35, 42, 44). Such findings suggest the potential for horizontal gene transfer among listeriae, with nonpathogenic strains serving as reservoirs for resistance determinants, potentially altering the fitness of the pathogenic species, L. monocytogenes.
In L. monocytogenes the efflux system encoded by bcrABC confers high-level resistance to BC and other quaternary ammonium compounds (12). The bcrABC cassette was first identified on a large plasmid (pLM80) harbored by strains implicated in the 1998–1999 hotdog outbreak of listeriosis. The cassette on these plasmids appears to be part of a composite transposon that also includes genes conferring resistance to cadmium (12, 24, 33).
Further evidence for the association between BC resistance and resistance to cadmium was obtained from characterization of L. monocytogenes from turkey processing plants. All BC-resistant strains were found to be also resistant to cadmium, leading to the speculation that BC resistance determinants (e.g., bcrABC) were acquired by plasmids that already harbored cadmium resistance determinants (31, 32).
Such findings suggest the need to characterize horizontal transfer of bcrABC in Listeria spp., including transfer from nonpathogenic species that may act as reservoirs. However, efforts to assess the conjugative transfer of BC resistance in Listeria spp. can be thwarted by the frequent occurrence of spontaneous mutants exhibiting high-level resistance to BC (41, 51; M. Rakic-Martinez and S. Kathariou, unpublished data). On the other hand, spontaneous mutants with high levels of resistance to cadmium appear to be extremely rare (S. Katharios-Lanwermeyer and M. Rakic-Martinez, unpublished data). Therefore, we hypothesized that BC-resistant, cadmium-resistant strains of L. innocua and L. welshimeri harbored the corresponding resistance determinants on plasmids (similarly to L. monocytogenes strains with pLM80 and related plasmids, described above), and we used cadmium resistance transfer as a surrogate for assessments of conjugative transfer of BC resistance. We examined the transfer of such resistance among L. innocua and L. welshimeri, as well as from these nonpathogenic species to L. monocytogenes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Listeria spp. strains used in the present study are listed in Table 1. Five BC-resistant strains of nonpathogenic Listeria spp. (four strains of L. welshimeri and L. innocua L1221) were used as potential donors of BC resistance to other nonpathogenic listeriae (L. welshimeri L1316S, L. welshimeri L0927S, and L. innocua L1206S). All BC-resistant donor strains were also resistant to cadmium (Table 1). To further assess conjugative transfer of resistance we used as potential donors of cadmium resistance six L. innocua strains resistant to cadmium but susceptible to BC (Table 1). With the exception of the reference strain L. innocua CLIP 11262 (16), the nonpathogenic Listeria spp. strains were isolated from the environment of turkey processing plants in the United States between 2003 and 2005. Isolation and characterization of Listeria spp. from these processing plants will be described elsewhere.
Table 1.
Bacterial strains used in this study
| Strain | Resistancea | Genotypeb |
Source or reference | ||
|---|---|---|---|---|---|
| cadA1 | cadA2 | bcrABC | |||
| Donor strains | |||||
| L. innocua | |||||
| L0910 | Cdr | – | – | – | This study |
| L1214B | Cdr | + | – | – | This study |
| L1306A | Cdr | + | – | – | This study |
| CLIP 11262 | Cdr | – | + | – | 16 |
| L1221 | Cdr BCr | – | + | + | This study |
| L1333a | Cdr | – | + | – | This study |
| L0921 | Cdr | + | – | – | This study |
| L. welshimeri | |||||
| L0725 | Cdr BCr | – | + | + | This study |
| L1325 | Cdr BCr | – | + | + | This study |
| L0918 | Cdr BCr | – | + | + | This study |
| L0926 | Cdr BCr | – | + | + | This study |
| Recipient strains | |||||
| L. innocua | |||||
| L1206S | Strr | – | – | – | This study |
| L. welshimeri | |||||
| L1316S | Strr | – | – | – | This study |
| L0927S | Strr | – | – | – | This study |
| L. monocytogenes (serotype) | |||||
| 1/2a3 (1/2a) | Strr | – | – | – | 22 |
| 10403S (1/2a) | Strr | – | – | – | 37 |
| 2381L (4b) | Strr | – | – | – | 39 |
| 2857S (1/2a) | Strr | – | – | – | This study |
| 2858S (1/2b) | Strr | – | – | – | This study |
Cdr, BCr, and Strr indicate resistance to cadmium, BC, and streptomycin, respectively, determined as described in Materials and Methods.
Determined by PCR with the respective primers as described in Materials and Methods.
Nonpathogenic Listeria spp. used as recipients were streptomycin-resistant derivatives of L. welshimeri and L. innocua (Table 1). Spontaneous mutants with resistance to streptomycin (MIC > 600 μg/ml) were isolated on brain heart infusion agar (BHIA) plates (BHI broth [BHI; Becton Dickinson, Sparks, MD] plus 1.2% Bacto agar [Becton Dickinson]) with streptomycin sulfate (600 μg/ml; Sigma, St. Louis, MO). In addition, five L. monocytogenes strains of diverse serotypes were used as potential recipients. The strains included the serotype 1/2a laboratory reference strains 10403S and 1/2a3 (22, 25, 37), serotype 4b strain 2381L, a streptomycin-resistant derivative of a strain from the 1985 California outbreak (39), and strains 2857S and 2858S of serotypes 1/2a and 1/2b, respectively, streptomycin-resistant derivatives of two strains from the 2011 cantaloupe outbreak (5) (Table 1). These streptomycin-resistant derivatives (streptomycin MIC > 600 μg/ml) were obtained as described above. Bacteria were grown in BHI or on BHIA and preserved at −80°C as described previously (12).
Cadmium and BC susceptibility and determinations of MIC.
BC and cadmium susceptibility assessments were performed as described previously (31). Strains were considered resistant to cadmium when they yielded confluent growth in the presence of 35 μg of cadmium chloride anhydrous (Sigma)/ml after incubation at 37°C for 48 h. For cadmium MIC determinations, 10 μl of an overnight culture was spotted onto BHIA plates with variable concentrations of cadmium chloride (2.5, 5, 10, 20, 35, 70, 140, and 200 μg/ml), and the plates were incubated at 25°C and observed daily for 5 days. The MIC was defined as the lowest concentration of cadmium that prevented visible growth. MIC of BC (Acros, New Jersey) was determined as described previously (31) using variable concentrations of BC (0.1, 0.5, 2.5, 5, 10, 20, 35, and 40 μg/ml) and after incubation of the plates at 37°C for 48 h.
Conjugations.
Cultures of recipient and donor strains were grown overnight (18 h) at 37°C and mixed in a 1:10 donor/recipient ratio for conjugations. Filter matings were done as described previously (22). Briefly, the mixture (100 μl of donor and 900 μl of recipient) was centrifuged (6,000 rpm, 3 min) and resuspended in 100 μl of BHI, which was then spotted onto sterile membrane filters (0.45-μm pore size; Millipore Corp., Bedford, MA), followed by incubation at the indicated temperature for 24 h. For agar matings, after centrifugation and resuspension in BHI (100 μl), the mixture was spotted (50 μl) onto BHIA and incubated at the indicated temperature for 24 h. To isolate transconjugants, mating mixtures were rinsed off the membrane filter or, for agar matings, removed from the surfaces of the agar plates with a sterile glass rod and plated on double-selective medium (BHIA with 600 μg of streptomycin/ml and 35 μg of cadmium chloride/ml), incubated at 25°C, and observed for up to 96 h. Controls included each of the parental strains plated on the double-selective medium and incubated similarly. The conjugation frequency was determined as the ratio of the number of transconjugants over CFU of the recipient strain at the end of the conjugation period. CFU were determined by plating dilutions on BHIA with streptomycin (600 μg/ml) and incubation at 37°C for 36 h. Experiments were performed in duplicate and in at least three independent trials.
PCR.
Primers used for PCR are listed in Table S1 in the supplemental material. Identification of the three different cadA determinants used the primers cadA-Tn5422F and cadA-Tn5422R for cadA1, associated with Tn5422, the primers cadA-pLM80F and cadA-pLM80R for cadA2, harbored on pLM80, and the primers cadA-EGDeF and cadA-EGDeR for cadA3, harbored by EGDe (32). Primers BcF and BcR were used to produce a PCR fragment containing the entire bcrABC cassette along with the ∼800-nucleotide (nt) upstream intergenic region (12). PCR for hly, encoding the L. monocytogenes virulence determinant listeriolysin O, used the primers hlyAF and hlyAR (15) (see Table S1 in the supplemental material). L. welshimeri was differentiated from L. innocua using the primers Lw_0908_F and Lw_0908_R (L. welshimeri-specific), as well as the primers Li_0558_F and Li_0558_R (L. innocua specific) (Table S1), derived from genome sequences specific to the corresponding species (16, 18). In addition to cadA2 and bcrABC (harbored on pLM80), we used a panel of several additional primer pairs to screen for other pLM80 open reading frames (ORFs) representing diverse locations on both fragments of the plasmid (see Table S1 in the supplemental material) (33). PCR was performed as previously described (12, 31) using an ExTaq kit (TaKaRa, Madison, WI).
RESULTS
PCR analysis of the strains used as donors revealed that all BC-resistant strains, regardless of species (L. welshimeri or L. innocua) harbored bcrABC as well as the plasmid-associated cadmium resistance determinant cadA2. The cadA2 determinant was also harbored by L. innocua 1333a and L. innocua CLIP 11262, which were resistant to cadmium but susceptible to BC and lacking bcrABC. Three strains of L. innocua harbored an alternative plasmid-associated cadmium resistance determinant, cadA1, and one cadmium-resistant strain (L. innocua L0910) was found to be negative for both cadA1 and cadA2 (Fig. 1 and Table 1). None of the strains harbored the chromosomal cadmium resistance determinant cadA3 (data not shown). The strains used as recipients (L. welshimeri L1316S, L. welshimeri L0927S, and L. innocua L1206S) were susceptible to BC and cadmium and lacked bcrABC, cadA1, cadA2, or cadA3 (Table 1).
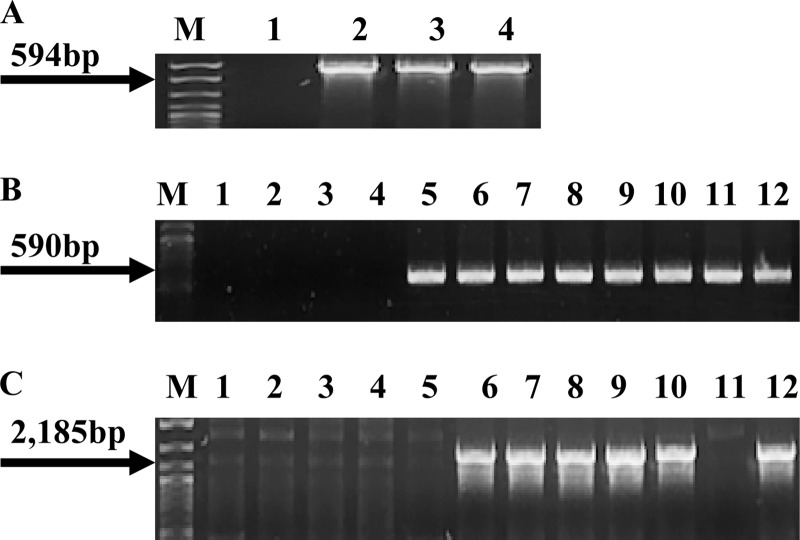
Fig 1.
Presence of cadA1, cadA2, and bcrABC in nonpathogenic donor strains. (A) PCR-based detection of cadA1 using the primers cadA-Tn5422F and cadA-Tn5422R. Lanes 1, 2, 3, and 4, L. innocua strains L0910, L0921, L1214B, and L1306A, respectively; lane M, DNA molecular marker XIV (Roche, Indianapolis, IN). The arrow points to the expected cadA1 PCR product. (B) PCR-based detection of cadA2 using the primers cadA-pLM80F and cadA-pLM80R. Lanes 1, 2, 3, and 4, L. innocua strains L0910, L0921, L1214B, and L1306A, respectively; lanes 5, 10, and 11, L. innocua strains CLIP 11262, L1221, and L1333a, respectively; lanes 6, 7, 8, and 9, L. welshimeri strains L0725, L1325, L0918, and L0926, respectively. Lane 12, L. monocytogenes H7550, used as a positive control (12). Lane M, same as in panel A. Arrow points to the expected cadA2 PCR product. (C) PCR of bcrABC using primers BcF and BcR. Lanes are as defined in panel B. With the exception of L. innocua strains CLIP 11262 and L1333a in lanes 5 and 11, respectively, all donors harboring cadA2 also contained bcrABC. Lane M, same as in panel A. Arrow points to the expected bcrABC product.
L. welshimeri and L. innocua harboring cadA2 can readily transfer cadmium resistance to other nonpathogenic listeriae.
Results from matings performed at 25°C on filter membranes and on agar were comparable, but the frequency of transfer was generally higher and more consistent for the latter (data not shown). The transfer frequency averaged between 10−7 and 10−8 (Table 2). Selected putative L. welshimeri transconjugants obtained using L. innocua strains as donors were evaluated with PCR using species-specific primers. The putative transconjugants were positive with the L. welshimeri-specific primers but negative for those specific for L. innocua (Fig. 2 and data not shown), confirming that they were derivatives of the recipient strain and not spontaneous streptomycin-resistant mutants of the donor.
Table 2.
Transfer frequency of cadmium resistance in conjugations between indicated donor and recipient strainsa
| Donor | Transfer frequency (recipient) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
L. welshimeri |
L. innocua |
L. monocytogenes |
||||||
| L0927S | L1316S | L1206 | 1/2a3 | 2381L | 10403S | 2857S | 2858S | |
| L. welshimeri | ||||||||
| L0725 | 5.8 × 10−7 | 1.4 × 10−7 | <1.0 × 10−9 | 1.8 × 10−7 | 2.8 × 10−8 | 1.8 × 10−8 | 6.7 × 10−7 | 1.6 × 10−7 |
| L1325 | 3.7 × 10−6 | 5.6 × 10−7 | <1.0 × 10−9 | 1.5 × 10−7 | 2.0 × 10−8 | 1.1 × 10−8 | 6.7 × 10−7 | 2.1 × 10−7 |
| L0918 | 2.6 × 10−6 | 1.5 × 10−7 | <1.0 × 10−9 | 1.5 × 10−7 | 1.4 × 10−8 | 7.4 × 10−9 | 5.5 × 10−8 | 6.1 × 10−8 |
| L0926 | 3.3 × 10−7 | 1.6 × 10−7 | <1.0 × 10−9 | 1.3 × 10−7 | 5.5 × 10−8 | 1.9 × 10−8 | 4.0 × 10−7 | 1.5 × 10−7 |
| L. innocua | ||||||||
| L0910 | <1.0 × 10−9 | <1.0 × 10−9 | <1.0 × 10−9 | <2.8 × 10−9 | <3.5 × 10−9 | <2.4 × 10−9 | <2.3 × 10−9 | <2.4 × 10−9 |
| L1214B | <1.0 × 10−9 | <1.0 × 10−9 | <1.0 × 10−9 | <3.3 × 10−9 | <4.6 × 10−9 | <2.0 × 10−9 | <1.8 × 10−9 | <2.3 × 10−9 |
| L0921 | <1.0 × 10−9 | <1.0 × 10−9 | <1.0 × 10−9 | <3.0 × 10−9 | <3.7 × 10−9 | <2.8 × 10−9 | <1.8 × 10−9 | <16 × 10−9 |
| L1306A | <1.0 × 10−9 | <1.0 × 10−9 | <1.0 × 10−9 | <4.6 × 10−9 | <5.4 × 10−9 | <3.1 × 10−9 | <2.6 × 10−9 | <3.5 × 10−9 |
| CLIP 11262 | <4.9 × 10−10 | <2.3 × 10−10 | <4.9 × 10−10 | 5.1 × 10−9 | 7.9 × 10−9 | 5.6 × 10−9 | 9.7 × 10−9 | 4.1 × 10−8 |
| L1221 | 6.1 × 10−8 | 2.7 × 10−7 | <4.3 × 10−9 | 1.5 × 10−7 | 8.2 × 10−8 | 1.1 × 10−8 | 3.4 × 10−8 | 6.6 × 10−9 |
| L1333a | 1.3 × 10−8 | 2.8 × 10−8 | <4.1 × 10−9 | 6.7 × 10−8 | 1.3 × 10−8 | 1.6 × 10−7 | 3.8 × 10−7 | 3.6 × 10−7 |
Results are from one representative experiment, and all experiments were performed in at least three independent trials.
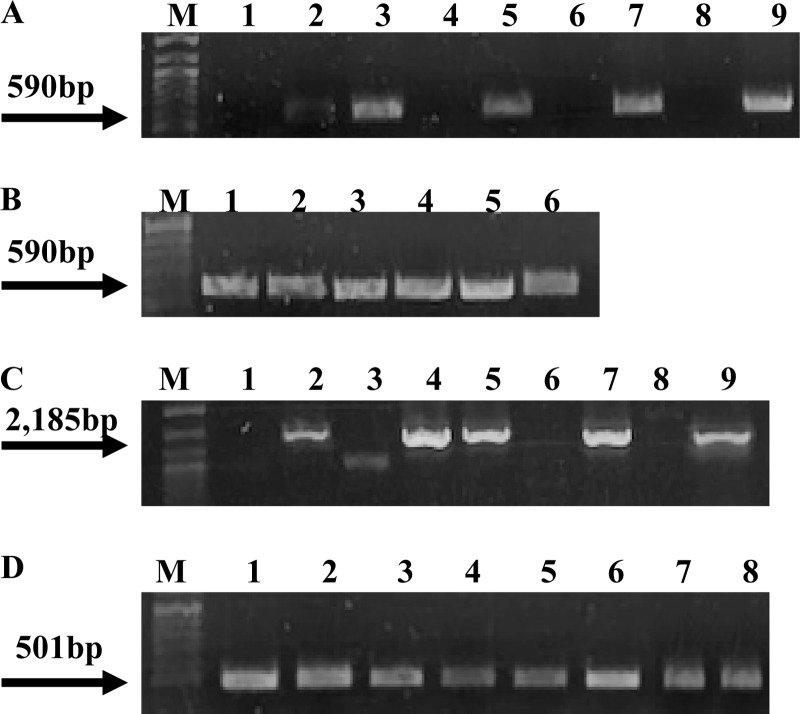
Fig 2.
Identification of L. welshimeri transconjugants from conjugations between L. welshimeri as recipient and L. innocua as donor. PCR used the L. welshimeri-specific primers Lw_0908_F and Lw_0908_R. Lanes 1 and 2, L. welshimeri strains L0725 and L1316S, respectively; lanes 3 and 4, L. innocua strains L1221 and L1333a, respectively; lanes 5, 6, 7, and 8, transconjugants from conjugations of L. welshimeri L1316S as the recipient with the donors L. innocua 1221 (lanes 5 and 6) and L. innocua 1333a (lanes 7 and 8). Arrow points to the expected PCR product.
The efficiency of conjugative transfer depended markedly on the strains used as donors as well as recipients. Few or no transconjugants were obtained from certain L. innocua donors (L. innocua strains L0910, L1214B, L1306A, and L0921), regardless of the strain that was used as a potential recipient. Furthermore, one of the strains used as recipient, L. innocua L1206S, failed to yield transconjugants with any of the donors (Table 2).
Based on PCR analysis of the resistance determinants, all donor strains that could efficiently transfer resistance harbored cadA2. With the exception of L. innocua strains 1333a and CLIP 11262, these strains were also resistant to BC and harbored bcrABC (Table 1). In contrast, of the four other potential donors that failed to yield transconjugants three harbored cadA1, and one (L. innocua L0910) lacked cadA1 or cadA2 (Table 1).
The BC resistance determinant bcrABC is cotransferred with the cadmium resistance determinant cadA2.
As mentioned above, most (five of seven) of the cadA2-harboring cadmium-resistant strains used as donors also harbored the BC resistance cassette bcrABC. PCR analysis of selected transconjugants obtained using these strains as donors revealed that, in addition to cadA2, they also harbored bcrABC (Fig. 3A and C). The transconjugants exhibited elevated MICs not only for cadmium (200 μg/ml) but also for BC (35 to 40 μg/ml), similar to the cadmium and BC MICs for the donor strains. In contrast, for strains used as recipients the MICs for both cadmium and BC were 10 μg/ml.
Fig 3.
Presence of cadA2 and bcrABC in L. welshimeri and L. monocytogenes transconjugants. (A) PCR-based detection of cadA2 using the primers cadA-pLM80F and cadA-pLM80R. Lanes 1, 4, 6, and 8, strains used as recipients (L. welshimeri strains L0927S and L1316S [lanes 1 and 4, respectively] and L. monocytogenes 1/2a3 and L. innocua L1206 [lanes 6 and 8, respectively]); lanes 2, 3, and 5, transconjugants from conjugations between L. welshimeri L0927S and L. innocua L1221 (lane 2), L. welshimeri L1316S and L. welshimeri L0918 (lane 3), L1316S with L. innocua L1221 (lane 5); lane 7, transconjugant from conjugation between L. monocytogenes 1/2a3 (recipient) and L. welshimeri L1325 (donor). Lane 9, L. monocytogenes H7550, used as a positive control (12). Lane M, DNA molecular marker XIV (Roche). The arrow points to the expected cadA2 PCR product. (B) PCR-based detection of cadA2 in transconjugants from conjugations between L. monocytogenes 2381L (recipient) and L. welshimeri L1325 (donor). Lane M, same as in panel A. The arrow points to the expected cadA2 PCR product. (C) PCR-based detection of bcrABC using BcF and BcR primers. Lanes 1, 3, 6, and 8, strains used as recipients (L. welshimeri strains L0927S and L1316S [lanes 1 and 3, respectively, with unspecific PCR product in lane 3] and L. monocytogenes strains 1/2a3 and L. innocua L1206 [lanes 6 and 8, respectively]); lanes 2, 4, and 5, transconjugants from conjugations between L. welshimeri L1316S (recipient) and L. welshimeri L1325 (donor); lane 7, transconjugant from conjugation between L. monocytogenes 1/2a3 (recipient) and L. welshimeri L1325 (donor); lane 9, L. monocytogenes H7550, used as a positive control (12). Lane M, same as in panel A. The arrow points to the expected bcrABC PCR product. Weak band in lane 3 is unspecific PCR product. (D) PCR-based detection of hly in L. monocytogenes transconjugants using the primers hlyAF and hlyAR. Lanes 1 and 2, transconjugants from conjugations between L. monocytogenes 1/2a3 with L. innocua L1221 (lane 1) and L. innocua L1333a (lane 2); lanes 3 to 7, transconjugants from conjugations between L. monocytogenes 2381L with L. welshimeri strains L0725 (lane 3), L1325 (lane 4), and L0926 (lane 5) and with L. innocua strains L1221 (lane 6) and L1333a (lane 7); lane 8 is L. monocytogenes 2381L. Lane M, same as in panel A. The arrow points to the expected hly PCR product.
Nonpathogenic Listeria spp. can effectively mediate the conjugative cotransfer of BC and cadmium resistance to L. monocytogenes.
To assess the ability of nonpathogenic Listeria spp. to serve as donors of BC resistance to L. monocytogenes, we used as potential recipients five L. monocytogenes strains of the three serotypes predominant in human listeriosis (1/2a, 1/2b, and 4b) (Table 1). All L. monocytogenes strains used as potential recipients were susceptible to cadmium and BC (MICs of 10 μg/ml for both compounds) (Table 1).
Regardless of the serotype of the L. monocytogenes strains used as recipients, conjugative transfer of resistance from nonpathogenic listeriae to L. monocytogenes generally exhibited the same dependence on donor strains that was observed with conjugations among nonpathogenic listeriae. Generally, the L. innocua strains that failed to donate cadmium resistance to other nonpathogenic listeriae also failed to transfer such resistance to L. monocytogenes (Table 2). On the other hand, the nonpathogenic Listeria spp. donor strains that efficiently transferred BC and cadmium resistance to other nonpathogenic listeriae also transferred these resistance determinants to L. monocytogenes. When these strains were used as donors, transconjugants were readily obtained from all L. monocytogenes strains in the panel, including the two strains associated with the recent cantaloupe outbreak (Table 2).
Acquisition of cadA2 by L. monocytogenes was confirmed by PCR (Fig. 3A and B and data not shown). Furthermore, when the donors also harbored bcrABC, this cassette was detected in the cadmium-resistant transconjugants as well (Fig. 3C and data not shown). Transconjugants were also tested for hemolytic activity on blood agar plates and by PCR with primers specific for hly, encoding the L. monocytogenes virulence determinant listeriolysin O and absent from L. innocua or L. welshimeri. All tested transconjugants were hemolytic and produced the hly amplicon (Fig. 3D and data not shown), confirming that they were derived from the L. monocytogenes recipients and were not spontaneous streptomycin-resistant derivatives of the donors. As noted above with transconjugants from conjugations between nonpathogenic Listeria spp., the L. monocytogenes transconjugants exhibited elevated MICs for cadmium and BC (200 μg/ml and 35 to 40 μg/ml, respectively), similar to those of the donor strains.
Temperature affects the transfer of BC and cadmium resistance to nonpathogenic listeriae, while the transfer to L. monocytogenes is equally efficient at 25 and 37°C.
Nonpathogenic Listeria spp. donor-recipient combinations that failed to yield transconjugants at 25°C were similarly negative at 37°C (data not shown). However, for successful conjugations the frequency of transfer of BC and cadmium resistance among nonpathogenic listeriae was higher when conjugations were done at 25 than at 37°C. The impact of temperature was dependent on the recipient strain, being noticeably stronger for L. welshimeri L0927S than for L. welshimeri L1316S (Fig. 4). Interestingly, the efficiency of the transfer of resistance from these same donors to L. monocytogenes was not affected by temperature. Transfer was equally efficient at 25 and 37°C for all L. monocytogenes strains in the panel, regardless of serotype (data not shown).
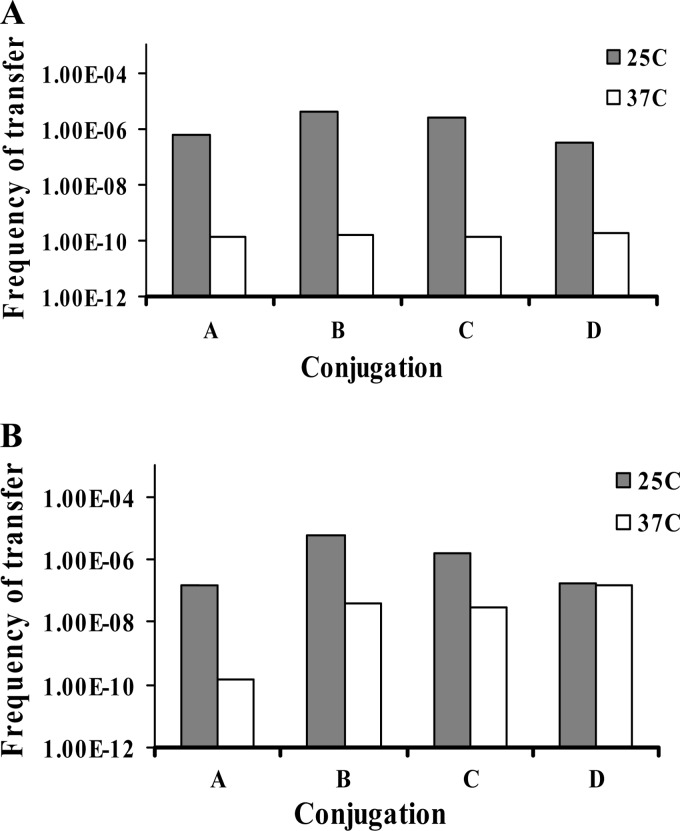
Fig 4.
Impact of conjugation temperature (25°C versus 37°C) on the frequency of transfer of cadmium resistance in conjugations among nonpathogenic Listeria spp. (A) A, B, C, and D, transfer frequency from conjugations between L. welshimeri L0927S as recipient and L. welshimeri strains L0725, L1325, L0918, and L0926, respectively, as donors. (B) A, B, C, and D, transfer frequency from conjugations between L. welshimeri L1316S as recipient and L. welshimeri strains L0725, L1325, L0918, and L0926, respectively, as donors. Conjugations were done on agar plates at 25°C (gray bars) or 37°C (white bars), and the transfer frequency was determined as described in Materials and Methods. The results are from one representative experiment, and experiments were performed in at least three independent trials.
Evidence of pLM80-like plasmids in BC- and cadmium-resistant nonpathogenic donors.
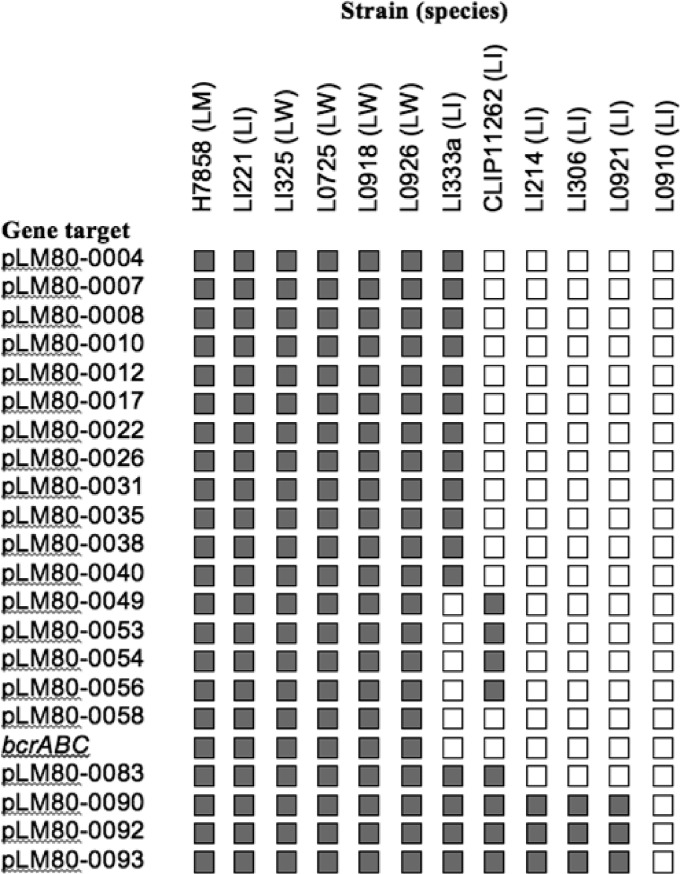
To determine whether the L. innocua and L. welshimeri strains used as donors harbored plasmids similar to pLM80, these strains were tested by PCR with primers derived from a panel of ORFs at different sites of pLM80 (see Table S1 in the supplemental material). The findings suggested that strains positive for both bcrABC and cadA2 (L. innocua 1221 and all four L. welshimeri donors) harbored plasmids highly similar to pLM80, with all primer pairs in the panel yielding the expected amplicons. In contrast, strains harboring cadA2 but lacking bcrABC (L. innocua strains 1333a and CLIP 11262) failed to produce several of the expected amplicons (Fig. 5). Strains harboring cadA1 yielded amplicons with only three primer pairs in the panel, two of which were derived from plasmid replication-associated genes, whereas no amplicons were obtained using L. innocua L0910 (cadmium-resistant but lacking cadA1 or cadA2) (Fig. 5). It is noteworthy that all donors consistently involved in high-efficiency transfer (L. welshimeri L0725, L1225, L0918, and L0926 and L. innocua L1333a and L1221) harbored LMOh7858_pLM80_0022, encoding a putative TraG/TraD family protein that may be involved in facilitating plasmid transfer (Fig. 5).
Fig 5.
Detection of pLM80 genes in nonpathogenic Listeria spp. used as donors. Shaded or white boxes indicate the presence or absence, respectively, of the expected PCR product. LW, LI, and LM in strain (species) designations refers to L. welshimeri, L. innocua, and L. monocytogenes, respectively. L. monocytogenes H7858 harbors pLM80 (33) and is included as a positive control. PCR-based detection of the indicated genes was performed using the pLM80-derived primers listed in Table S1 in the supplemental material as described in Materials and Methods.
DISCUSSION
In this study we provide evidence that BC resistance mediated by bcrABC can be effectively transferred among certain strains of nonpathogenic Listeria spp. that harbored both bcrABC and the cadmium resistance determinant cadA2. We also showed that resistance could be transferred from nonpathogenic Listeria spp. to L. monocytogenes strains of all three serotypes primarily associated with human listeriosis (1/2a, 1/2b, and 4b). The findings indicated that cadmium resistance transfer can be effectively used as a surrogate for transfer of resistance to BC, since transconjugants selected on cadmium were resistant to both cadmium and to BC.
Transfer of resistance was not indiscriminate: even though all tested L. monocytogenes strains yielded transconjugants with at least some of the nonpathogenic donors, one of the nonpathogenic strains used as recipient failed to yield any transconjugants. Furthermore, transconjugants were produced with markedly higher frequency when nonpathogenic donors harbored cadA2 (often while also harboring bcrABC) than when they harbored the alternative cadmium resistance determinant cadA1.
Nonpathogenic Listeria spp. have potential to serve as reservoirs for resistance genes and transfer them among themselves as well as to L. monocytogenes inhabiting the same environments, but data on such transfers remain scarce. Thus, far only one study reported conjugative transfer of disinfectant resistance attributes among Listeria spp. (29). That study used only two strains of nonpathogenic listeriae (L. innocua in both cases), and these exhibited resistance to the dye ethidium bromide but not to cadmium (29). In contrast, strains used as donors in the present study were resistant to cadmium but susceptible to ethidium bromide (M. Rakic-Martinez, unpublished findings). Thus, it is hard to compare data from this earlier study with the current findings.
In the case of nonpathogenic recipients, transfer was found to be more efficient at 25°C than at 37°C, suggesting that it may represent an environmental adaptation. In contrast, no difference in transfer frequency between 25 and 37°C was noted for L. monocytogenes, regardless of serotype, suggesting the potential for such transfers to be taking place not only in the environment but in vivo as well, e.g., in the mammalian gastrointestinal tract.
In the present study conjugations were on agar or on membrane filters overlaid on solid media. Only limited information is currently available on horizontal gene transfer in listeriae in foods and other complex systems such as the gastrointestinal tract (6, 11). Further studies are needed to determine whether conjugative transfer frequency is impacted by the presence of the conjugation partners on surfaces relevant to food processing environments (e.g., stainless steel and food) and within polymicrobial biofilms.
Although direct evidence of nonpathogenic Listeria spp. behaving as resistance gene reservoirs for L. monocytogenes is still lacking, coselection and disinfectant resistance gene distribution in other pathogens perhaps illustrate analogous processes at work. For instance, Ciric et al. have shown the presence of a Streptococcus oralis Tn916-like conjugative transposon, Tn6087, that confers resistance to cetrimide bromide, a disinfectant in the QAC family, as well as tetracyclines, potentially providing a mechanism for coselection of disinfectant and antibiotic resistance (7). In food-derived staphylococci, the presence of BC resistance genes linked to antibiotic resistance genes suggested the potential for similar coselection (48). Bjorland et al. demonstrated the widespread distribution of QAC resistance genes in bovine and equine coagulase-negative staphylococci (3). The wide distribution of such genes and the potential of increased fitness through coselection have important public health implications (45).
L. monocytogenes plasmids (e.g., pLM80) harboring both bcrABC and cadA2 have been characterized in the course of genome sequencing investigations (12, 24, 33). Genome sequence data of the nonpathogenic listeriae used as donors in the present study are currently not available. Nonetheless, when donors harbored both bcrABC and cadA2, the transconjugants acquired both of these determinants, even though selection was only for cadmium resistance, suggesting plasmid-associated resistance genes similar to those harbored by pLM80. Further evidence for pLM80-like plasmids was provided by detection of all other tested pLM80-associated genes in these donor strains. Such data provide compelling reasons to further elucidate the sequence content of the plasmids of these L. welshimeri and L. innocua donor strains harboring bcrABC and cadA2.
Our PCR data suggest that different plasmids were harbored by strains containing cadA1. However, it was intriguing that such strains failed to serve as efficient donors to other listeriae. Plasmids harboring cadA1 have been extensively described in L. monocytogenes (4, 24, 26, 27), and the conjugative transfer of one such plasmid was demonstrated in an earlier study. A single donor-recipient strain combination was examined in that study, and nonpathogenic strains were not included (28). Further studies are needed to determine whether our findings reflect differences in the plasmids or in the types of strains used in the conjugations.
In conclusion, we have demonstrated the potential for resistance to BC and to cadmium to be conjugatively transferred among nonpathogenic listeriae and from these strains to L. monocytogenes of diverse serotypes. Our findings also demonstrate coselection of resistance to BC and cadmium when the nonpathogenic Listeria spp. donors were resistant to both of these agents: transconjugants selected in the presence of cadmium were also resistant to BC. Coselection between cadmium resistance and BC resistance has not been documented before in Listeria or other bacteria. Future work should examine additional factors in food processing and other dynamic environments that may affect the efficacy of resistance transfer, such as varied growth surfaces and the presence of mixed and single-species biofilms. Further study of conjugative dissemination of BC and cadmium resistance in Listeria spp. would provide the opportunity to assess impact on fitness in disinfectant-abundant environments such as food processing plants and health care settings. The data from such studies would be needed to characterize potential impacts of such resistance determinant acquisitions on additional attributes, including those associated with virulence.
Supplementary Material
ACKNOWLEDGMENTS
This study was partially supported by a grant from the American Meat Institute Foundation and USDA grant 2011-2012-67017-30218.
We thank J. Eifert and C. Tarr for strains used in this study. We are grateful to R. M. Siletzky, M. Thompson, and V. Dutta for assistance, and we thank all of the other members of our laboratories for their support and encouragement.
Footnotes
Published ahead of print 17 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Antunes P, Réu C, Sousa JC, Pestana N, Peixe L. 2002. Incidence and susceptibility to antimicrobial agents of Listeria spp. and Listeria monocytogenes isolated from poultry carcasses in Porto, Portugal. J. Food Prot. 65:1888–1893 [DOI] [PubMed] [Google Scholar]
- 2. Barbalho TCF, Almeida PE, Almeida RCC, Hofer E. 2005. Prevalence of Listeria spp. at a poultry processing plant in Brazil and a phage test for rapid confirmation of suspect colonies. Food Contr. 16:211–216 [Google Scholar]
- 3. Bjorland J, et al. 2005. Widespread distribution of disinfectant resistance genes among staphylococci of bovine and caprine origin in Norway. J. Clin. Microbiol. 43:4363–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canchaya C, Giubellini V, Ventura M, de los Reyes-Gavilán CG, Margolles A. 2010. Mosaic-like sequences containing transposon, phage, and plasmid elements among Listeria monocytogenes plasmids. Appl. Environ. Microbiol. 76:4851–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC 2011. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August–September 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1357–1358 [PubMed] [Google Scholar]
- 6. Charpentier E, Courvalin P. 1999. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 43:2103–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciric L, Mullany P, Roberts A. 2011. Antibiotic and antiseptic resistance genes are linked on a novel mobile genetic element: Tn6087. J. Antimicrob. Chemother. 66:2235–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curiale MS, Lewus C. 1994. Detection of Listeria monocytogenes in samples containing Listeria innocua. J. Food Prot. 57:1043–1051 [DOI] [PubMed] [Google Scholar]
- 9. Davis JA, Jackson CR. 2009. Comparative antimicrobial susceptibility of Listeria monocytogenes, L. innocua, and L. welshimeri. Microb. Drug Resist. 15:27–32 [DOI] [PubMed] [Google Scholar]
- 10. den Bakker HC, Fortes ED, Wiedmann M. 2010. Multilocus sequence typing of outbreak-associated Listeria monocytogenes isolates to identify epidemic clones. Foodborne Pathog. Dis. 7:257–265 [DOI] [PubMed] [Google Scholar]
- 11. Doucet-Populaire F, Trieu-Cuot P, Dosbaa I, Andremont A, Courvalin P. 1991. Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob. Agents Chemother. 35:185–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elhanafi D, Dutta D, Kathariou S. 2010. Genetic characterization of plasmid-associated benzalkonium chloride resistance determinants in a Listeria monocytogenes strain from the 1998–1999 outbreak. Appl. Environ. Microbiol. 76:8231–8238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Facinelli B, Giovanetti Varaldo PE, Casolari C, Fabio U. 1991. Antibiotic resistance in foodborne listeria. Lancet 338:1272. [DOI] [PubMed] [Google Scholar]
- 14. Facinelli B, et al. 1993. Genetic basis of tetracycline resistance in food-borne isolates of Listeria innocua. Appl. Environ. Microbiol. 59:614–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furrer B, Candrian U, Hofelein C, Luthy J. 1991. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J. Appl. Bacteriol. 70:372–379 [DOI] [PubMed] [Google Scholar]
- 16. Glaser P, et al. 2001. Comparative genomics of Listeria species. Science 294:849–852 [DOI] [PubMed] [Google Scholar]
- 17. Hadorn K, Hächler H, Schaffner A, Kayser FH. 1993. Genetic characterization of plasmid-encoded multiple antibiotic resistance in a strain of Listeria monocytogenes causing endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 12:928–937 [DOI] [PubMed] [Google Scholar]
- 18. Hain T, et al. 2006. Whole-genome sequence of Listeria welshimeri reveals common steps in genome reduction with Listeria innocua as compared to Listeria monocytogenes. J. Bacteriol. 188:7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofer E, Ribeiro R, Feitosa DP. 2000. Species and serovars of the genus Listeria isolated from different sources in Brazil from 1971 to 1997. Mem. Inst. Oswaldo Cruz 95:615–620 [DOI] [PubMed] [Google Scholar]
- 20. Jones EM, MacGowan AP. 1995. Antimicrobial chemotherapy of human infection due to Listeria monocytogenes. Eur. J. Clin. Microbiol. Infect. Dis. 14:165–175 [DOI] [PubMed] [Google Scholar]
- 21. Kathariou S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811–1829 [DOI] [PubMed] [Google Scholar]
- 22. Kathariou S, Metz P, Hof H, Goebel W. 1987. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J. Bacteriol. 169:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kornacki JL, Gurtler JB. 2007. Incidence and control of Listeria in food processing facilities, p 681–766 In Ryser ET, Marth EH. (ed), Listeria, listeriosis, and food safety, 3rd ed CRC Press, Inc, Boca Raton, FL [Google Scholar]
- 24. Kuenne C, et al. 2010. Comparative analysis of plasmids in the genus Listeria. PLoS One 5:e12511 doi:10.1371/journal.pone.0012511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauer P, Man YNC, Loessner MJ, Portnoy DA. 2003. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 185:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lebrun M, Audurier A, Cossart P. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J. Bacteriol. 176:3049–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lebrun M, Audurier A, Cossart P. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are similar to cadA and cadC of Staphylococcus aureus and are induced by cadmium. J. Bacteriol. 176:3040–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lebrun M, Loulergue J, Chaslus-Dancla E, Audurier A. 1992. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl. Environ. Microbiol. 58:3183–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemaitre JP, Echchannaoui H, Michaut G, Divies C, Rousset A. 1998. Plasmid-mediated resistance to antimicrobial agents among listeriae. J. Food Prot. 61:1459–1464 [DOI] [PubMed] [Google Scholar]
- 30. MacDonald F, Sutherland AD. 1994. Important differences between the generation times of Listeria monocytogenes and List. innocua in two Listeria enrichment broths. J. Dairy Res. 61:433–436 [DOI] [PubMed] [Google Scholar]
- 31. Mullapudi S, Siletzky RM, Kathariou S. 2008. Heavy-metal and benzalkonium chloride resistance of Listeria monocytogenes isolates from the environment of turkey-processing plants. Appl. Environ. Microbiol. 74:1464–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mullapudi S, Siletzky RM, Kathariou S. 2010. Diverse cadmium resistance determinants in Listeria monocytogenes isolates from the turkey processing plant environment. Appl. Environ. Microbiol. 76:627–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson KE, et al. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Painter J, Slutsker L. 2007. Listeriosis in humans, p 85–109 In Ryser ET, Marth EH. (ed), Listeria, listeriosis, and food safety, 3rd ed CRC Press, Inc, Boca Raton, FL [Google Scholar]
- 35. Peterkin PI, Gardiner MA, Malik N, Idziak ES. 1992. Plasmids in Listeria monocytogenes and other Listeria species. Can. J. Microbiol. 38:161–164 [DOI] [PubMed] [Google Scholar]
- 36. Petran RL, Swanson KMJ. 1993. Simultaneous growth of Listeria monocytogenes and Listeria innocua. J. Food Prot. 56:616–618 [DOI] [PubMed] [Google Scholar]
- 37. Portnoy DA, Jacks PS, Hinrichs DJ. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poyart-Salmeron C, Carlier C, Trieu-Cuot P, Courtieu AL, Courvalin P. 1990. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 335:1422–1426 [DOI] [PubMed] [Google Scholar]
- 39. Promadej NF, Fiedler P, Cossart S, Dramsi Kathariou S. 1999. Cell wall teichoic acid glycosylation in Listeria monocytogenes serotype 4b requires gtcA, a novel, serogroup-specific gene. J. Bacteriol. 181:418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quentin C, Thibaut MC, Horovitz J, Bebear C. 1990. Multiresistant strain of Listeria monocytogenes in septic abortion. Lancet 336:375. [DOI] [PubMed] [Google Scholar]
- 41. Rakic-Martinez M, Drevets DA, Dutta V, Katic V, Kathariou S. 2011. Listeria monocytogenes strains selected on ciprofloxacin or the disinfectant benzalkonium chloride exhibit reduced susceptibility to ciprofloxacin, gentamicin, benzalkonium chloride, and other toxic compounds. Appl. Environ. Microbiol. 77:8714–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts MC, Facinelli B, Giovanetti E, Varaldo PE. 1996. Transferable erythromycin resistance in Listeria spp. isolated from food. Appl. Environ. Microbiol. 62:269–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romanova N, Favrin S, Griffiths MW. 2002. Sensitivity of Listeria monocytogenes to sanitizers used in the meat processing industry. Appl. Environ. Microbiol. 68:6405–6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rota C, et al. 1996. High prevalence of multiple resistance to antibiotics in 144 Listeria isolates from Spanish dairy and meat products. J. Food Prot. 59:938–943 [DOI] [PubMed] [Google Scholar]
- 45. Russell AD. 2002. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 92(Suppl):121S–135S [PubMed] [Google Scholar]
- 46. Scallan E, et al. 2011. Foodborne illness acquired in the United States: major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sheridan JJ, Duffy G, McDowell DA, Blair IS. 1994. The occurrence and initial numbers of Listeria in Irish meat and fish products and the recovery of injured cells from frozen products. Int. J. Food Microbiol. 22:105–113 [DOI] [PubMed] [Google Scholar]
- 48. Sidhu MS, Heir E, Sørum H, Holck A. 2001. Genetic linkage between resistance to quaternary ammonium compounds and beta-lactam antibiotics in food-related Staphylococcus spp. Microb. Drug Resist. 7:363–371 [DOI] [PubMed] [Google Scholar]
- 49. Skovgaard N, Morgen CA. 1988. Detection of Listeria spp. in faeces from animals, in feeds, and in raw foods of animal origin. Int. J. Food Microbiol. 6:229–242 [DOI] [PubMed] [Google Scholar]
- 50. Soumet C, Ragimbeau C, Maris P. 2005. Screening of benzalkonium chloride resistance in Listeria monocytogenes strains isolated during cold smoked fish production. Lett. Appl. Microbiol. 41:291–296 [DOI] [PubMed] [Google Scholar]
- 51. To MS, Favrin S, Romanova N, Griffiths MW. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 68:5258–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsakaris A, Papa A, Douboyas J, Antoniadis A. 1997. Neonatal meningitis due to multi-resistant Listeria monocytogenes. J. Antimicrob. Chemother. 39:553–554 [DOI] [PubMed] [Google Scholar]
- 53. Wagner M, MacLauchlin J. 2008. Biology, p 3–26 In Liu D. (ed), Handbook of Listeria monocytogenes. CRC Press, Inc, Boca Raton, FL [Google Scholar]
- 54. Walsh D, Duffy G, Sheridan JJ, Blair IS, McDowell DA. 1998. Comparison of selective and non-selective media for the isolation of Listeria species from retail foods. J. Food Safety 18:85–89 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.