Abstract
An iterative combinatorial mutagenesis (ICM) strategy was used to engineer deacetoxycephalosporin C synthase of Streptomyces clavuligerus (scDAOCS) for improved activity toward penicillin G. Seven mutational sites were repeatedly combined onto a starter mutant (C155Y Y184H V275I C281Y) of scDAOCS. Eleven improved combinatorial mutants were identified from 24 mutants in four rounds of ICM.
TEXT
Cephalosporins are widely used in the treatment of bacterial infections. A large proportion of clinically used cephalosporins are derived from 7-aminodeacetoxycephalosporanic acid (7-ADCA). In China, 7-ADCA was commonly produced via a chemienzymatic process, involving chemical expansion of penicillin G into a six-membered dihydrothiazine ring product, phenylacetyl-7-ADCA (G-7-ADCA). This process needs to use a large amount of organic solvent and hence is harmful to the environment (15). The development of an alternative environmentally friendly method for the large-scale preparation of 7-ADCA is desirable.
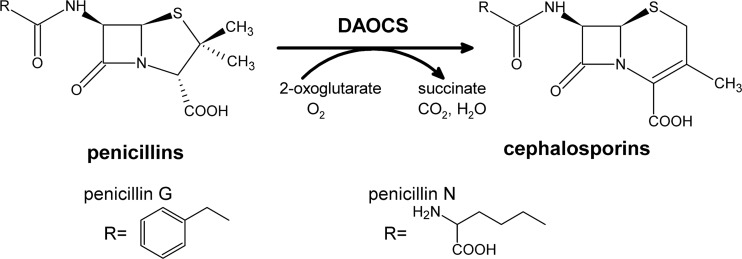
In Streptomyces clavuligerus, deacetoxycephalosporin C synthase (DAOCS) catalyzes the ring expansion of penicillin substrates into a ring-expanded product to eventually produce cephamycin C (Fig. 1) (2, 14). The natural substrate of S. clavuligerus DAOCS (scDAOCS) is penicillin N, which, as a pathway intermediate, is not available in large quantities (3). Therefore, an engineered scDAOCS capable of converting the cheap and easily available substrate penicillin G would be valuable for large-scale production of 7-ADCA. Both random and rational mutagenesis strategies have been attempted to evolve scDAOCS. Dozens of single mutations, such as M73T, G79E, T91A, C155Y, Y184H, M188V, H244Q, V275I, L277Q, C281Y, N304K, I305L, I305M, etc., and a few multiple mutations, such as C155Y Y184H V275I C281Y and FF8, were identified which show significantly improved catalytic efficiency toward penicillin G, ranging from 1.5- to 117.8-fold improvements (5, 7, 13, 16, 17). However, such practices are difficult to repeat, as the assay conditions for DAOCS are complex and delicate and extensive labor and human experience are needed to carry out large-scale screening of mutant enzymes. A more rational approach with targeted mutational site selection proved much more efficient in obtaining new beneficial mutations and improved enzymes (8).
Fig 1.
The reaction catalyzed by DAOCS in the conversion of penicillin G and N. “R” represents the side chain moieties on penicillins.
Despite the many single beneficial mutations of DAOCS reported, a combination of these mutations have not been systematically tested to further improve DAOCS activity. Some combinations of double mutations between V275I or C281Y and the C-terminal mutations N304X, I305M, R306L, and R307L were tested, and all mutants showed improved kcat/Km on ampicillin, indicating the potential of combinatorial mutagenesis in enzyme engineering. The C281Y N304R and C281Y I305M double mutants showed the greatest improvement in catalytic efficiency, with 101- and 68-fold increases in specific activities, respectively (6). Another two mutants with multiple mutations were obtained by Wei et al. by screening a random mutant library: they include YS67 (V275I I305M), with a 32-fold increase in kcat/Km, and YS81 (V275I C281Y I305M), with a 13-fold increase in specific activity toward penicillin G (17). However, generation and evaluation of all possible combinatorial mutants between known beneficial mutational sites is still a daunting task, as the number of combinations will increase exponentially with the number of potential mutational sites. Screening of a large mutant library is difficult without a facile and high-throughput screening method. In this study, we adopted a rational, systematic strategy, named iterative combinatorial mutagenesis (ICM), to direct the combination route. In ICM, targeted site-directed mutations at predefined mutational sites were introduced into a starter enzyme, and the most improved enzyme was selected as the starter for the next round of targeted mutations. The iterative strategy was first applied by Reetz et al. in combination with saturation mutagenesis (9–11). It helps to identify improved enzymes via iterative rounds of targeted mutations and high-throughput screening; such a strategy was successfully used to identify improved lipase from Bacillus subtilis (9, 12). The essence of ICM is to test the effects of different single mutations on a starter enzyme and then select the combinatorial mutant or mutants that showed the best performance as the next starter enzyme for the next round of single mutations. The cycle is repeated until no improved enzymes can be obtained. Our results demonstrate ICM is a highly efficient strategy to identify better combinatorial mutants as it can greatly reduce the number of mutant enzymes that need to be evaluated to identify the significantly improved combinatorial enzyme.
The chemicals, reagents, and sources of G-7-ADCA and the bacterial strains used in this study were described previously (8). The scDAOCS-encoding gene cefE was amplified from S. clavuligerus ATCC 27064 and inserted into pET30a to obtain pET-SC (8). Site-directed mutagenesis was performed with pET-SC as the template as described before (8). All mutant constructs were verified by DNA sequencing. The mutagenic primers are listed in Table 1. The mutant enzymes were expressed and purified by similar methods as described by Wu et al. (18). The ring expansion activity was assayed in a mixture containing 1 mM dithiothreitol (DTT), 1.8 mM FeSO4, 2.56 mM α-ketoglutarate, and 4 mM ascorbate with 5.6 mM penicillin G as the substrate in a final volume of 500 μl. The reaction mixture was analyzed by high-performance liquid chromatography (HPLC), and the separation and detection conditions were previously described (18). The kinetic parameters of purified wild-type (WT) and mutated enzymes for penicillin G conversion were determined using procedures similar to those described for the enzyme assays, except the reaction time was 30 min. The concentrations of penicillin G were 0.2, 0.5, 1.0, 1.4, 2.0, 3.0, 5.0, and 10.0 mM, while the amount of the enzyme was kept constant at 0.7 mg ml−1. The kinetic parameters were calculated via nonlinear curve fitting by Gnuplot 4.4.
Table 1.
Primers used for site-directed mutagenesis of scDAOCS
| Mutation | Direction | Sequence of mutagenic primera |
|---|---|---|
| C155Y | Forward | 5′-TCCTCGACTATGAGCCGCTGCTGC-3′ |
| Reverse | 5′-ATAGTCGAGGAATTCCTCGAC-3′ | |
| Y184H | Forward | 5′-CATCGACAGGTCCATGTGCGGCGCC-3′ |
| Reverse | 5′-ATGGACCTGTCGATGGTCACCCTC-3′ | |
| V275I | Forward | 5′-ACCTTCTCCATTCCGCTGGCGC-3′ |
| Reverse | 5′-AATGGAGAAGGTGAAGTCCGC-3′ | |
| C281Y | Forward | 5′-GCGCGCGAGTATGGCTTCGATG-3′ |
| Reverse | 5′-ATACTCGCGCGCCAGCGGGAC-3′ | |
| M73T | Forward | 5′-GTCCCCACCACCCGCCGCGGCTTC-3′ |
| Reverse | 5′-GGTGGTGGGGACGGGCGAGGTGAC-3′ | |
| N304K | Forward | 5′-CTACGTGAAAATCCGCCGCACATC-3′ |
| Reverse | 5′-TTTCACGTAGTTGCCCCCGATC-3′ | |
| I305M | Forward | 5′-CGTGAACATGCGCCGCACATC-3′ |
| Reverse | 5′-CATGTTCACGTAGTTGCCCCCG-3′ | |
| I305L | Forward | 5′-CGTGAACCTGCGCCGCACATC-3′ |
| Reverse | 5′-CAGGTTCACGTAGTTGCCCCCG-3′ | |
| S261A | Forward | 5′-GAGGAAGAACACTGCGGAGGTGCGG-3′ |
| Reverse | 5′-GCAGTGTTCTTCCTCCGTCCCAACG-3′ | |
| S261M | Forward | 5′-GAGGAAGAACACCATGGAGGTGCGG-3′ |
| Reverse | 5′-ATGGTGTTCTTCCTCCGTCCCAACG-3′ | |
| Q126M | Forward | 5′-CTTCGACCGCATGTACACCGCCTCCCG-3′ |
| Reverse | 5′-CATGCGGTCGAAGTACTGAGTCCAGATCC-3′ | |
| T213V | Forward | 5′-GGCGCGTTCGTGGACCTGCCCTAC-3′ |
| Reverse | 5′-CACGAACGCGCCGCCGACCTC-3′ |
The codons corresponding to the mutated residues are underlined.
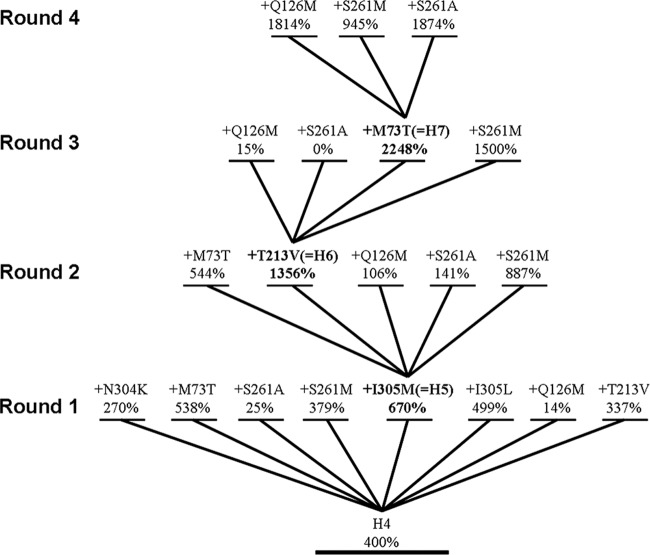
Two groups of mutational sites—together representing eight single beneficial mutations (i.e., N304K, I305M, I305L, M73T, Q126M, T213V, S261A, and S261M)—were selected for ICM. The first group includes most of the previously identified beneficial sites, such as M73, N304, and I305 (1, 16, 17), which are directly involved in substrate binding or in the C-terminal region that may influence product release. Another group includes several new sites recently identified by us, Q126, T213, and S261, which may influence DAOCS activity via indirect interactions (8). The quaternary mutant H4 (C155Y Y184H V275I C281Y) reported by Wei et al. was used as the starter enzyme. It has a 41-fold increase in kcat/Km relative to the WT enzyme (16). The selected mutational sites were repeatedly combined onto H4 by the ICM strategy illustrated in Fig. 2.
Fig 2.
Schematic illustration of the iterative combinatorial mutagenesis strategy used in this study. The H4 (C155Y Y184H V275I C281Y) mutant of scDAOCS was used as the starter enzyme. The relative specific activities shown are the means determined from at least three independent experiments; the activity of wild-type scDAOCS was defined as 100%. The specific activities of wild-type scDAOCS and H4 were 55.2 ± 2.6 and 221 ± 27 U · mg−1. One unit of activity is defined as the amount of DAOCS required to form 1 μg G-7-ADCA in a minute.
The WT scDAOCS and H4 were expressed and purified, and their specific activities toward penicillin G were analyzed. The activities of the H4 and WT scDAOCSs were determined at 5.6 mM penicillin G. Under our conditions, H4 showed activity of 399.84% relative to that of the WT for the conversion of penicillin G and a 37.84-fold increase in kcat/Km, a value which is comparable to that reported by Wei et al. (16) (41-fold increase in kcat/Km).
In the first round of ICM, eight mutants were generated: H4 N304K, H4 M73T, H4 I305M, H4 I305L, H4 S261A, H4 S261M, H4 Q126M, and H4 T213V. The mutants with the M73T, I305M, and I305L mutations introduced showed improved relative activities (Fig. 2). Mutant H4 I305M, which had the highest activity, was designated H5 and was used as the starter for a second round of ICM. In this round, five mutants were generated: H5 M73T, H5 Q126M, H5 T213V, H5 S261A, and H5 S261M. Only the T213V and S261M mutants showed improved activities (Fig. 2). The mutant showing the highest activity, H5 T213V, was designated H6 and was used in the next round of ICM. In this round, four mutants were generated: H6 M73T, H6 Q126M, H6 S261A, and H6 S261M. Two mutants, H6 M73T and H6 S261M, showed the greatest increase in relative activities. The mutant with a 22.5-fold increase in specific activity (H6 M73T, designated H7) is the most active enzyme toward penicillin G reported to date and was used in the fourth round of ICM. This time, three mutants (H7 Q126M, H7 S261A, and H7 S261M) were generated, but none showed improved activity.
The kinetic parameters for the conversion of penicillin G of several mutant scDAOCSs (including H4 and six other combinatorial mutants with improved specific activities [Table 2]) were determined. The WT scDAOCS was also included as a control. Mutant H4 showed apparent Km and kcat comparable to previous results obtained by Wei et al. (16). Mutants H4 I305M, H4 I305L, H5 T213V, H5 S261M, H6 M73T, and H6 S261M (containing different combinations of mutations) showed 46.37- to 87.24-fold increases in kcat/Km relative to that of the WT enzyme. Among these mutants, H5 S261M showed the highest catalytic efficiency for penicillin G (9.94-fold increase in kcat and 8.88-fold reduction in Km). It is interesting to note that the mutant with the best kcat/Km value (H5 S261M) didn't show the best conversion. These results demonstrate that although kcat and Km best describe the theoretical catalytic properties of an enzyme, the specific activity gives a better indication of the performance under specific assay conditions. For such a complex reaction catalyzed by DAOCS (7, 13, 16), both the kinetic parameters and specific activities need to be considered to select the enzyme with best potential for further development. Among the 5 mutations integrated into these improved mutants, the I305M and I305L mutants showed increased Km and kcat, suggesting accelerated catalytic rates but decreased substrate affinities. The other mutants, such as those with the mutations T213V, S261M, and M73T, showed similar kcat but decreased Km, indicating that better substrate binding was the main reason for their increased activities.
Table 2.
Kinetic parameters of wild-type and mutant scDAOCSs for penicillin G
| Enzyme | Km (mM)a | kcat (s−1)a | kcat/Km (M−1 s−1) | Relative activity (%) |
|---|---|---|---|---|
| scDAOCS | 14.38 ± 1.77 | 0.054 ± 0.012 | 3.8 | 100 |
| H4 (C155Y Y184H V275I C281Y) | 1.60 ± 0.06 | 0.230 ± 0.004 | 143.8 | 3,784 |
| H4 I305M (H5-3) | 3.58 ± 0.42 | 0.650 ± 0.067 | 181.6 | 4,779 |
| H4 I305L (H5-4) | 2.1 ± 0.23 | 0.370 ± 0.028 | 176.2 | 4,637 |
| H4 I305M T213V (H6-3) | 3.22 ± 0.21 | 0.654 ± 0.023 | 203.1 | 5,345 |
| H4 I305M S261M (H6-5) | 1.62 ± 0.16 | 0.537 ± 0.033 | 331.5 | 8,724 |
| H4 I305M T213V M73T (H7-1) | 1.92 ± 0.23 | 0.589 ± 0.035 | 306.8 | 8,074 |
| H4 I305M T213V S261M (H7-4) | 2.49 ± 0.46 | 0.668 ± 0.029 | 268.3 | 7,061 |
The values shown are means ± standard deviations from at least three independent experiments.
For scDAOCS (5), a method is needed to integrate many beneficial mutations into a superior enzyme. Although we did not model or calculate the probability of obtaining improved enzymes or try to predict the best combinations, we improvised a simple mutagenesis strategy to maximize the probability of identifying improved combinatorial mutants while minimizing workload. The ICM strategy yielded 11 new and improved combinatorial mutants from a pool of only 24 combinatorial mutants. If an ordinary strategy, such as random combinations, was used, depending on the number of mutational sites selected, the number of different combinations could soon reach a level that requires a huge amount of work. ICM significantly decreases the number of mutants that need to be screened. It is very useful in engineering hard-to-assay enzymes by efficiently identifying beneficial combinatorial mutations with minimum workload (9).
However, it is important to note that by selecting only the best mutant in each round, the local “best” candidate identified may not represent the theoretical maximum from the global fitness landscape. The rationale of ICM is similar to the “steepest ascent” algorithm commonly employed by computer search programs, which is a simple and practical way to quickly identify the local maximum. It has two disadvantages: (i) it is usually trapped in a local optimum not reaching the global optimum, and (ii) it is very sensitive to the start point. The latter means that one cannot predict the optimum of the best candidates from different start points. Nevertheless, ICM is the first simple and practical method to quickly identify beneficial combinatorial mutations. Before a better method is developed, it will be the strategy of choice to integrate beneficial mutations.
ACKNOWLEDGMENTS
This work was supported by National Major Special Project on New Varieties Cultivation for Transgenic Organisms (grant no. 2009ZX08009-130B) and the Fundamental Research Funds for the Central Universities of China University of Mining and Technology (Beijing) (grant no. 2010YH05).
Footnotes
Published ahead of print 24 August 2012
REFERENCES
- 1. Chin HS, Goo KS, Sim TS. 2004. A complete library of amino acid alterations at N304 in Streptomyces clavuligerus deacetoxycephalosporin C synthase elucidates the basis for enhanced penicillin analogue conversion. Appl. Environ. Microbiol. 70:607–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper RD. 1993. The enzymes involved in biosynthesis of penicillin and cephalosporin; their structure and function. Bioorg. Med. Chem. 1:1–17 [DOI] [PubMed] [Google Scholar]
- 3. Crawford L, et al. 1995. Production of cephalosporin intermediates by feeding adipic acid to recombinant Penicillium chrysogenum strains expressing ring expansion activity. Biotechnology 13:58–62 [DOI] [PubMed] [Google Scholar]
- 4. Reference deleted.
- 5. Goo KS, Chua CS, Sim TS. 2009. Directed evolution and rational approaches to improving Streptomyces clavuligerus deacetoxycephalosporin C synthase for cephalosporin production. J. Ind. Microbiol. Biotechnol. 36:619–633 [DOI] [PubMed] [Google Scholar]
- 6. Goo KS, Chua CS, Sim TS. 2008. Relevant double mutations in bioengineered Streptomyces clavuligerus deacetoxycephalosporin C synthase result in higher binding specificities which improve penicillin bioconversion. Appl. Environ. Microbiol. 74:1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu JS, et al. 2004. Family shuffling of expandase genes to enhance substrate specificity for penicillin G. Appl. Environ. Microbiol. 70:6257–6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ji J, Tian X, Fan K, Yang K. 2012. New strategy of site-directed mutagenesis identifies new sites to improve Streptomyces clavuligerus deacetoxycephalosporin C synthase activity toward penicillin G. Appl. Microbiol. Biotechnol. 93:2395–2401 [DOI] [PubMed] [Google Scholar]
- 9. Reetz MT, Carballeira JD. 2007. Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat. Protoc. 2:891–903 [DOI] [PubMed] [Google Scholar]
- 10. Reetz MT, Prasad S, Carballeira JD, Gumulya Y, Bocola M. 2010. Iterative saturation mutagenesis accelerates laboratory evolution of enzyme stereoselectivity: rigorous comparison with traditional methods. J. Am. Chem. Soc. 132:9144–9152 [DOI] [PubMed] [Google Scholar]
- 11. Reetz MT, et al. 2007. Learning from directed evolution: further lessons from theoretical investigations into cooperative mutations in lipase enantioselectivity. Chembiochem. 8:106–112 [DOI] [PubMed] [Google Scholar]
- 12. Reetz MT, Wang LW, Bocola M. 2006. Directed evolution of enantioselective enzymes: iterative cycles of CASTing for probing protein-sequence space. Angew. Chem. Int. Ed. Engl. 45:1236–1241 [DOI] [PubMed] [Google Scholar]
- 13. Valegard K, et al. 2004. The structural basis of cephalosporin formation in a mononuclear ferrous enzyme. Nat. Struct. Mol. Biol. 11:95–101 [DOI] [PubMed] [Google Scholar]
- 14. Valegard K, et al. 1998. Structure of a cephalosporin synthase. Nature 394:805–809 [DOI] [PubMed] [Google Scholar]
- 15. Velasco J, et al. 2000. Environmentally safe production of 7-aminodeacetoxycephalosporanic acid (7-ADCA) using recombinant strains of Acremonium chrysogenum. Nat. Biotechnol. 18:857–861 [DOI] [PubMed] [Google Scholar]
- 16. Wei CL, et al. 2005. Directed evolution of Streptomyces clavuligerus deacetoxycephalosporin C synthase for enhancement of penicillin G expansion. Appl. Environ. Microbiol. 71:8873–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei CL, et al. 2003. Engineering Streptomyces clavuligerus deacetoxycephalosporin C synthase for optimal ring expansion activity toward penicillin G. Appl. Environ. Microbiol. 69:2306–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu XB, et al. 2011. Saturation mutagenesis of Acremonium chrysogenum deacetoxy/deacetylcephalosporin C synthase R308 site confirms its role in controlling substrate specificity. Biotechnol. Lett. 33:805–812 [DOI] [PubMed] [Google Scholar]