Abstract
Summary: Sepsis is among the most common causes of death in hospitals. It arises from the host response to infection. Currently, diagnosis relies on nonspecific physiological criteria and culture-based pathogen detection. This results in diagnostic uncertainty, therapeutic delays, the mis- and overuse of antibiotics, and the failure to identify patients who might benefit from immunomodulatory therapies. There is a need for new sepsis biomarkers that can aid in therapeutic decision making and add information about screening, diagnosis, risk stratification, and monitoring of the response to therapy. The host response involves hundreds of mediators and single molecules, many of which have been proposed as biomarkers. It is, however, unlikely that one single biomarker is able to satisfy all the needs and expectations for sepsis research and management. Among biomarkers that are measurable by assays approved for clinical use, procalcitonin (PCT) has shown some usefulness as an infection marker and for antibiotic stewardship. Other possible new approaches consist of molecular strategies to improve pathogen detection and molecular diagnostics and prognostics based on transcriptomic, proteomic, or metabolic profiling. Novel approaches to sepsis promise to transform sepsis from a physiologic syndrome into a group of distinct biochemical disorders and help in the development of better diagnostic tools and effective adjunctive sepsis therapies.
INTRODUCTION
Sepsis is among the most common causes of death in hospitalized patients. Its death toll is in the same range as that of myocardial infarction (8). In the United States, the rate of hospitalization of patients with sepsis or septicemia increased by 70% from 221 (in 2001) to 377 (in 2008) per 100,000 population (100), and the incidence of severe postoperative sepsis trebled from 0.3% to 0.9% (16). Sepsis is especially common in the elderly and is likely to increase substantially as the population ages (8, 100). Nearly 90% of people in North America are not familiar with the term “sepsis,” and of those who are, most are not aware that sepsis is a leading cause of death (232).
Sepsis has been called a hidden public health disaster (7). Patients who survive sepsis bear an underrecognized risk of physical and cognitive impairment (119) and suffer a more-than-doubled risk of dying in the following 5 years compared with hospitalized controls (213). According to the Centers for Disease Control and Prevention, in 2008, an estimated $14.6 billion was spent on hospitalizations for sepsis in the United States, and from 1997 to 2008, the inflation-adjusted aggregate costs of the treatment of patients hospitalized for this condition increased on average annually by 11.9% (100).
Sepsis arises from the host response to infection, which is directed to kill the invading pathogens. For this reason, patient outcomes from sepsis are determined not only by the viability of the invading pathogen, which can be directly toxic and destructive to tissue, but also even more so by the host response, which may be exuberant and result in collateral organ and tissue damage (Fig. 1), because the highly potent effectors do not discriminate between microbial and host targets (190). Over the last decades, the understanding of pathogen-host interactions and inflammation has increased considerably (177, 281). Basic and clinician scientists hoped for a therapeutic breakthrough, and billions of dollars were invested by the pharmaceutical industry in the development of innovative, adjunctive sepsis therapies. However, so far, none of the numerous interventions that achieved a modulation of the host response and led to improved survival in animal models of sepsis were successful in the clinical setting (57). Drotrecogin alfa (activated protein C), the only approved drug specifically indicated for the treatment of severe sepsis, was withdrawn from the market in 2011 (6), because the positive findings of improved patient outcomes from earlier trials (21) could not be confirmed by follow-up studies.
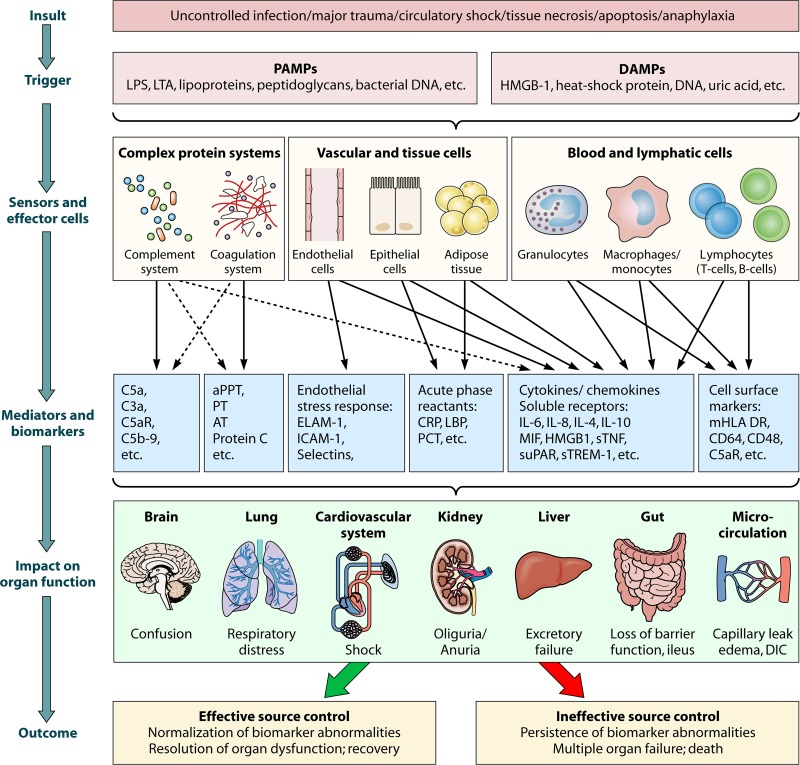
Fig 1.
The inflammatory response. This simplified overview shows the course of the inflammatory response. An insult triggers the release of PAMPs (pathogen-associated molecular patterns) and/or DAMPs (danger-associated molecular patterns), which are sensed by pattern recognition mechanisms such as receptors (pattern recognition receptors (PRRs) on the cell surface or within the cytosol or nucleus of sensor cells as well as by pattern-recognizing complex systems such as the complement system and others. Therefore, sensors can be different types of cells, tissues/organs, or proteins/other molecules, which themselves may function as effectors to modulate the immune response through various different pro- or anti-inflammatory mediators or biomarkers. As a result, the underlying insult can be cleared or not, and organ function may be temporarily or permanently impaired. LPS, lipopolysaccharide (part of the membrane of Gram-negative bacteria); LTA, lipoteichoic acid (part of the cell wall of Gram-positive bacteria); HMGB1, high-mobility-group protein B1; C5a and C3a, complement components 5a and 3a; C5aR, C5a receptor protein; C5b-9, terminal complement complex; aPPT, activated partial thromboplastin time; PT, prothrombin time; AT, antithrombin; ELAM-1, endothelial leukocyte adhesion molecule 1; ICAM-1, intercellular adhesion molecule 1; CRP, C-reactive protein; LBP, LPS-binding protein; PCT, procalcitonin; IL-6, interleukin-6; MIF, macrophage migration inhibitory factor; sTNF, soluble tumor necrosis factor; suPAR, soluble urokinase-type plasminogen activator receptor; sTREM-1, soluble triggering receptor expressed on myeloid cells 1; mHLA-DR, monocytic human leukocyte antigen DR; CD64 and CD48, integral membrane glycoproteins; DIC, disseminated intravascular coagulation.
Sepsis is still defined and diagnosed by nonspecific alterations in physiology, including changes in temperature and heart and respiration rates, and not by the specific cellular processes that would be amenable to specific interventions. This also makes it difficult to identify appropriate patients for the evaluation of such innovative interventions. The disconnect between the identification of new therapeutic targets and the shortcomings of the currently available diagnostic tools was elegantly criticized previously by pointing to the fact that “it makes no sense to use twenty-first century technology to develop drugs targeted at specific infections whose diagnosis is delayed by nineteenth-century methods” (190). Moreover, the diagnostic uncertainty may contribute to delays in the initiation of lifesaving standard therapies such as the administration of appropriate antibiotics (135) and may further increase the mis- and overuse of antimicrobial agents.
Therefore, the development of sepsis-specific biomarkers and molecular diagnostics for the assessment of the host response and for pathogen detection is expected to foster both drug development and the improved clinical management of sepsis. Because the complex pathophysiology of sepsis involves almost all cell types, tissues, and organ systems, it is not surprising that a recent systematic search identified nearly 180 distinct molecules that have been proposed as potential biological markers of sepsis (207). However, only 20% of these biomarkers have been assessed specifically in appropriate studies for use in the diagnosis of sepsis (207). Here, we discuss how the understanding of sepsis has evolved over time and to which degree currently available sepsis biomarkers may help to overcome the present diagnostic uncertainty. We address how new insights into the pathogenesis of sepsis may help in the development of sepsis-specific biomarkers and how this may also impact the identification and development of new therapeutic targets.
HISTORICAL DEVELOPMENT OF THE UNDERSTANDING OF SEPSIS
The word “sepsis” was derived from the ancient Greek for rotten flesh and putrefaction. Without using the term, the Greek physician Hippocrates (460 to 370 BC) was probably the first to describe the clinical course of septic shock (“when continuing fever is present, it is dangerous if the outer parts are cold, but the inner parts are burning hot”) (99). The Florentine philosopher Niccolò Machiavelli (1469 to 1527) described the difficulty in the diagnosis and treatment of sepsis as follows: “as the physicians say it happens in hectic fever, that in the beginning of the malady it is easy to cure but difficult to detect, but in the course of time, not having been either detected or treated in the beginning, it becomes easy to detect but difficult to cure” (164). In 1546, the Italian physician Girolamo Fracastoro formulated the concept of contagion by proposing that epidemic diseases could be communicated by direct or indirect contact and from a distance through the air, the causative agents being invisible seeds or germs (257). When microscopic observations became possible, Anthony van Leeuwenhoek was the first to publish illustrations of bacteria scraped from human teeth (1683) (257). In 1847, Hungarian physician Ignaz Semmelweis, following the observation of increased puerperal fever in parturients treated by obstetricians who took part in autopsies, introduced antiseptic practices before patient exams. The washing of hands with a solution of lime reduced the mortality rate of puerperal fever from 18% to 3% (105). This likely represented the first clinical trial in infectious diseases ever performed. In the second half of the 19th century, the germ theory was confirmed by Robert Koch and Louis Pasteur, and in 1879, Louis Pasteur announced to the French Academy that Streptococcus causes puerperal sepsis. He also proposed preventing the entry of microorganisms into the human body, leading Joseph Lister to develop antiseptic methods in surgery (154). It was only fully understood during the last century that the “Black Death,” one of the most devastating pandemics in human history, which began its move from western Asia through Europe in the 14th to 15th centuries, was caused by septicemia due to Yersinia pestis (78). Richard Pfeiffer (1858 to 1945), working with Robert Koch in Berlin, Germany, intellectually and experimentally conceived the concept of endotoxin as a heat-stable bacterial poison responsible for the pathophysiological consequences of certain infectious diseases. In 1909, L. Jacob published the first 12 cases of patients with Gram-negative sepsis caused by Escherichia coli, 50% of whom died (120). In 1914, Hugo Schottmüller provided the first scientific definition of sepsis: “sepsis is a state caused by microbial invasion from a local infectious source into the bloodstream which leads to signs of systemic illness in remote organs” (241). According to this definition, bacteremia was a conditio sine qua non to the diagnosis of sepsis. This notion did not change significantly over the years. Sepsis and septicemia were considered to refer to a number of ill-defined clinical conditions in addition to bacteremia, and in practice, the terms were often used interchangeably. However, fewer than one-half of the patients who have signs and symptoms of sepsis have positive blood culture (BC) results or other microbiological proof of an infectious focus (42, 81, 239, 288). William Osler (1849 to 1919) was the first to recognize the important role of the host response in sepsis: “except on few occasions, the patient appears to die from the body's response to infection rather than from the infection.” This insight represented an important milestone in the modern understanding of the role of the host response to an infection.
EVOLUTION OF THE UNDERSTANDING OF SEPSIS IN THE INTENSIVE CARE ERA
Sepsis is the most common cause of multiple-organ failure. It is a syndrome that emerged only after intensive care units (ICUs) that delivered lifesaving organ support, such as mechanical ventilation or renal replacement therapy, were established. In the pre-ICU era, most patients with acute sepsis and septic shock ultimately died from irreversible shock within short time periods, without any chance for the development of progressive multiple-organ failure. In 1975, a classic editorial by A. Baue, entitled Multiple, Progressive or Sequential Systems Failure, a Syndrome of the 1970s (17), described a new clinical syndrome which was unknown in the pre-intensive-care era. Several terms were coined thereafter, such as multiple-organ failure, multiple-system organ failure, and multiple-organ system failure, to describe this evolving clinical syndrome of otherwise unexplained progressive physiological failure of several interdependent organ systems (86). More recently, the term multiple-organ dysfunction syndrome (MODS) was proposed as a more appropriate description (148, 168). The increasing incidence of Gram-positive sepsis and fungal sepsis during this era led to the understanding that not only endotoxin but also other exogenous factors, such as peptidoglycan and lipoteichoic acid, play an important role in the host response to sepsis (27).
The discovery that the host response to infection was both necessary and sufficient to recapitulate septic shock in Gram-negative bacteremia led to the development of a novel generation of adjunctive therapies (274). This also rekindled an interest in the definition of sepsis, because in order to assess the efficacy of novel immunomodulatory sepsis therapies, it became necessary to stratify and enroll defined cohorts of patients in placebo-controlled clinical trials. Driven by the need to define adequate patient populations to evaluate the role of high-dose corticosteroids, R. C. Bone et al. (30, 31) proposed the term “sepsis syndrome” in 1989 for critically ill patients with clinical and laboratory signs of severe infection irrespective of blood culture results and microbiological confirmation of infection. The presence of systemic inflammation as one prerequisite for the definition of “sepsis syndrome” required the presence of at least two of the following signs: elevated heart rate, elevated respiration rate, decreased blood pressure, hyper- or hypothermia, and leukocytosis or leukopenia. These criteria later defined the “systemic inflammatory response syndrome” (SIRS) and were called SIRS criteria. R. C. Bone et al. demonstrated that the clinical phenotype and mortality rates for patient cohorts with microbiologically proven infection or positive blood cultures and patients with only a clinical suspicion of sepsis and signs of systemic inflammation were similar (29).
In 1992, the American College of Chest Physicians and the Society of Critical Care Medicine convened a consensus panel to further develop the concept of sepsis and systemic inflammatory response syndrome, to stage sepsis, and to differentiate infectious and sterile noninfectious causes of systemic inflammation (29). The results can be summarized as follows. There is a continuum from infection, sepsis, and severe sepsis to septic shock. SIRS may follow a variety of clinical insults, including infection, pancreatitis, ischemia, multiple trauma, tissue injury, hemorrhagic shock, or immune-mediated organ injury. Sepsis is a systemic response to infection. This is identical to SIRS except that it must result from infection. The definition of sepsis requires the presence of infection and at least two signs of systemic inflammation. Severe sepsis is defined when sepsis results in dysfunction of at least one remote organ function. Septic shock is defined as sepsis with hypotension (systolic blood pressure [BP] of <90 mm Hg or a reduction of 40 mm Hg from baseline) despite adequate fluid resuscitation. Concomitant organ dysfunction or perfusion abnormalities (e.g., lactic acidosis, oliguria, or coma) are present in the absence of other known causes.
These categorical definitions of sepsis and the SIRS concept have major limitations in the clinical and also the research settings. Despite this, they are widely used today in epidemiological and clinical studies for the evaluation of sepsis-specific therapies and may have contributed considerably to the failure of previous sepsis trials. These definitions were also instrumental for the new ICD-9-CM codes for sepsis (ICD-9-CM 995.91) and severe sepsis (code 995.92), which were added to this coding system to make it possible to distinguish between septicemia and sepsis (100).
When it became evident not only that the initial intense inflammatory response, or “cytokine storm,” consists of proinflammatory cytokines but also that the body mounts an anti-inflammatory response as a reaction to the inciting event, R. C. Bone dubbed this phenomenon “compensatory anti-inflammatory response syndrome” (CARS) (28). There is increasing evidence that patients who survive early sepsis often develop nosocomial infections with organisms not typically pathogenic in immunocompetent hosts and may suffer a reactivation of latent viruses (130, 151, 197). These observations have led to the hypothesis that the early hyperinflammatory state evolves to a subsequent hypoinflammatory state with significant immunosuppression. The term “immunoparalysis” was coined. Immunoparalysis in patients with sepsis was further characterized by an association between low levels of monocytic HLA-DR (mHLA-DR) surface expression and immune cell dysfunctions (289, 301). This immunosuppression caused by sepsis is manifested by the loss of a delayed-type hypersensitivity response to positive-control antigens, a failure to clear the primary infection, and the development of new secondary infections (111). This concept is supported by the fact that patients who die from sepsis have biochemical, flow cytometric, and immunohistochemical findings consistent with immunosuppression (32, 113).
CURRENT DEFICITS AND THE NEED FOR NEW DIAGNOSTIC APPROACHES FOR SEPSIS
The accurate and timely detection of sepsis remains a challenge. As described above, current methods of detection still rely on nonspecific clinical and laboratory signs, whereas other medical fields have successfully implemented biomarkers for rapid diagnosis, such as D-dimers for pulmonary embolism, natriuretic peptides for acute heart failure, and troponin for myocardial infarction (2, 141).
The main problem is that the clinical phenotype of a patient with sepsis may be similar to that of a patient with a systematic inflammatory response caused by sterile inflammation, such as pancreatitis, trauma, burn, or intoxication (29). Over the last decades, it has become evident that the immune system is concerned more with entities that do damage than with those that are foreign (172), referred to as endogenous alarmins and danger signals or danger-associated molecular patterns (DAMPs). Recent research confirmed that cellular necrosis from major physical injury and trauma releases mitochondrial DNA into the circulation, where it is capable of eliciting inflammatory signals (309). Microbial pathogen-associated molecular patterns (PAMPs) activate innate immunocytes through pattern recognition receptors (PRRs). Hence, DAMPS stimulate an acute-phase response which is biologically concordant with PAMPs released during infection (Fig. 1). This explains why it is difficult to distinguish infectious from noninfectious SIRS or to identify single molecules or molecular patterns of the host response that allow this distinction.
The treating physician at the bedside is often confronted with questions such as, Is the patient infected or displaying signs of “sterile inflammation”? Which antimicrobials should I choose for initial empirical therapy? Are antimicrobial therapy and other measures of source control effective? When microbiology results become available, do they indicate infection or colonization? When should I discontinue antimicrobial therapy? The SIRS criteria are of limited value for addressing these questions, because they may be triggered by many factors and noninfectious diseases (287). Other conventional laboratory signs of sepsis, such as lactate, blood glucose, or thrombocyte counts, which are sensitive and easy to measure, are also very nonspecific (148). For example, the commonly used laboratory parameter of leukocytosis has very low sensitivity and specificity for the diagnosis of infection, with a likelihood ratio of 1.5; band counts have a similarly low diagnostic accuracy (25, 66, 187). The diagnostic uncertainty may explain why physicians find it difficult to define the disease and to communicate about it with patients and relatives (232).
Moreover, in one-third of sepsis patients, the causative pathogens cannot be identified (31). Appropriate therapy is therefore often delayed. Empirical antimicrobial therapy has to be started as soon as sepsis is presumed, before results from blood culture become available, and this diagnostic uncertainty is compensated for by the liberal use of broad-spectrum antibiotics, contributing to the increasing resistance of antimicrobial drugs—a growing public health problem (298).
In general, a biomarker has been defined as the “quantifiable measurement(s) of biological homeostasis that define(s) what is ‘normal'; therefore providing a frame of reference for predicting or detecting what is ‘abnormal’ (65) or as “an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (11). Moreover, a biomarker should provide timely information beyond that which is readily available from routine physiological data. The potential of biomarkers in the field of sepsis was first addressed systematically in the context of a colloquium convened by the International Sepsis Forum in 2005 (170). In this report, it was concluded that, “First, sepsis is a concept—that of disease arising from the host response to infection—rather than a measurable pathological process. Second, that concept is a complex one that hinges on documentation of both infection and a response. Third, that response is nonspecific, defined by consensus criteria that emphasize physiologic changes in vital parameters that are common to a number of disparate processes,” and that “biomarkers promise to transform sepsis from a physiologic syndrome to a group of distinct biochemical disorders” (170).
By definition, sepsis biomarkers should reflect the biology of sepsis, as evidenced by the biochemical changes that are characterized as the host response to infection at the cellular and the subcellular levels. Inflammatory mediators can be classified generally into seven groups according to their biochemical properties: vasoactive amines, vasoactive peptides, fragments of complement components, lipid mediators, cytokines, chemokines, and proteolytic enzymes, all of which comprise hundreds of distinct, single molecules (136, 165). The host inflammatory response to infection may involve most if not all of these elements. The search for sepsis biomarkers is focused primarily on the biochemical changes at the plasma level (complement system, coagulation system, and kallikrein-kinin system) and the indicators of the activation or downregulation of cellular elements (neutrophils, monocytes/macrophages, and endothelial cells), which may lead to the release of a number of mediators and molecules (cytokines, chemokines, and acute-phase proteins) (127) (Fig. 1). The host response to sepsis involves hundreds of mediators and single molecules, many of which have been proposed to be sepsis biomarkers (171, 207). It is unlikely that it will be possible to identify one single biomarker that is able to satisfy all the existing needs and expectations in sepsis research and management.
In the absence of an objective pathological gold standard for sepsis, currently proposed sepsis biomarkers must be defined and evaluated with reference to the clinical decision for which a given marker might provide information that is not available by using conventional clinical and laboratory signs.
One important requirement for sepsis biomarkers is the time benefit that they should offer for the detection of a systemic inflammatory response to an infection before clinical signs and organ damage become apparent. Ultimately, such biomarkers could facilitate earlier supportive treatment and should lower sepsis mortality rates. It would also be helpful to have biomarkers that allow the monitoring of the immune status, thereby identifying patients who might benefit from a certain immunomodulatory intervention and ruling out those who would not. The concept of personalized medicine, for instance, through companion diagnostics, has already been successfully applied in other fields (203). A biomarker that can rapidly detect elevated levels of a specific target of an adjunctive treatment or reduced levels of a critical factor for replacement therapy is a prerequisite for drug development and the evaluation of novel and specific sepsis therapies.
MONITORING THE INFLAMMATORY RESPONSE: ASPECTS TO BE CONSIDERED
In the broad majority of immunocompetent individuals with sepsis as well as in experimental sepsis models, the inflammatory response will involve a more or less pronounced proinflammatory phase, dominated by the activation of innate immune weapons such as leukocytes, monocytes, macrophages, complement and coagulation systems, endothelial and epithelial cell responses, and others (Fig. 1). The latter will result in the typical signs of systemic inflammation (fever, leukocytosis, elevated heart and breathing rates, elevated cardiac index, organ dysfunction, and edema through vascular leakage) (Fig. 1). This phase may or may not then be followed by an anergic phase, where such mechanisms cease to function and in which a great susceptibility to secondary infectious hits may exist (197, 225). R. S. Hotchkiss and I. E. Karl pointed out the importance of taking into account the individual responses of various groups of patients to clinical sepsis (112). The concept of late-phase immunosuppression has recently been strengthened by a study which examined postmortem findings for patients who had died during late-stage sepsis disease. Those authors found an extensive depletion of splenic CD4, CD8, and HLA-DR cells and the expression of ligands for inhibitory receptors on lung epithelial cells (32), and it was proposed that the potential of proinflammatory therapy in late-stage sepsis should be evaluated in greater detail (113).
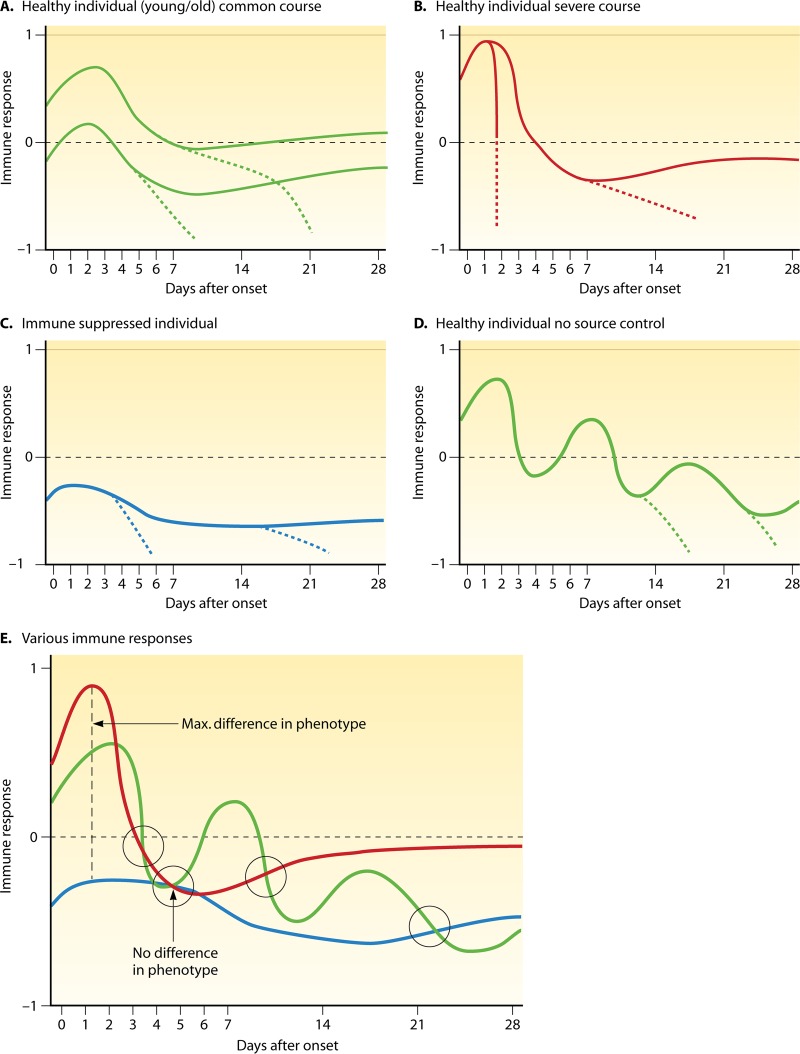
Obviously, the potential for an inflammatory response will vary from patient to patient, and in addition, the individual response potential may be influenced by the current situation such that the clinician in the ICU is challenged with a complicated situation. Figure 2A to D illustrate various possible inflammatory responses of different patient populations. While the vast majority of sepsis patients will likely fall into category 2A (Fig. 2) and may display more or less pronounced pro- and anti-inflammatory responses, there are other patients in which the course may be much more dramatic, such as Waterhouse-Friderichsen syndrome-like cases (Fig. 2B), in which an immunosuppression phase is not seen before death. Other cases may already start with a severe suppression of the immune response (e.g., transplant patients on immunosuppressive therapy) and may not display proinflammatory responses at all (Fig. 2C). Patients for whom no surgical source control can be established often display several proinflammatory courses of sepsis, followed by more and more pronounced immunosuppression (Fig. 2D). When several such courses (Fig. 2E) are overlaid, it becomes clear that a technically difficult and time-consuming snapshot analysis of the inflammatory response at a given point in time may not be helpful, because the same snapshot picture might describe patients with dramatically different response features and very different outcomes. The fact that almost all immunomodulatory agents that were tested in the past were given in fixed doses over fixed periods of time during the first 24 to 96 h after the onset of sepsis, without any knowledge of the immune status of the patient, may have largely contributed to the failure of these trials.
Fig 2.
Various possible courses of the immune response to severe sepsis and septic shock over 28 days. (A to D) Immune responses are displayed, with 1 being maximally proinflammatory and −1 being maximally anti-inflammatory. Dotted lines indicate a course leading to death. (E) Overlay of various possible immune response courses during sepsis and resulting aspects with respect to differences in phenotypes of the inflammatory response at various hypothetical time points.
Some biomarkers have already been used for patient stratification in clinical studies, but conflicting study results might conceivably be due to an inability to properly assess the patient's immune response at the time of enrollment and during the course of the studies. Paradoxically, trials which assessed the efficacy of immune-enhancing strategies used the same nonspecific SIRS criteria for patient enrollment as those used by the numerous trials that addressed downregulating immunomodulatory strategies. It was proposed that these limitations might be overcome by using reduced mHLA-DR or other more specific indicators of sepsis-associated immunosuppression for the assessment of novel antiapoptotic, immunostimulatory approaches, such as the cytokines interleukin-7 (IL-7) and interleukin-15 which have shown efficacy in sepsis models (113, 185). In one single-center study, targeted immune-enhancing therapy guided by HLA-DR measurements with gamma interferon (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) resulted in improved patient outcomes and a restoration of monocyte function (74, 178). On the other hand, results from recent meta-analyses that included all randomized controlled trials (RCTs) that evaluated immune-enhancing therapies with granulocyte colony-stimulating factor (G-CSF) and GM-CSF found no beneficial effects (24).
Other examples concern data from a phase II trial that investigated an antibody to tumor necrosis factor (TNF), suggesting that patients with severe sepsis with IL-6 levels of >1,000 pg/ml (223, 224) would benefit more from this therapy. Accordingly, a large multicenter study was performed on patients with severe sepsis by using an anti-TNF antibody and stratifying patients on the basis of baseline levels of IL-6, which was considered a surrogate marker for TNF (200). Although the impact on mortality was statistically significant in an adjusted analysis of IL-6-positive patients, the incremental benefit over IL-6-negative patients was minimal. The selected cutoff of 1,000 pg/ml may have been too low: an analysis of the treatment effect over a range of IL-6 values revealed a greater separation of the mortality curves of placebo- and anti-TNF-treated patients at higher levels of IL-6 monoclonal antibody (170). Although IL-6 is a good prognostic marker for sepsis and also for trauma patients (223), it may be inadequate as a surrogate marker for TNF. Furthermore, circulating cytokine levels may not correlate adequately with cytokine levels in tissues and organs. Another hypothesis concerning the state of relative adrenal insufficiency, identified on the basis of the response to an adrenocorticotropic hormone stimulation test, to define a high-risk population that would benefit from treatment with exogenous corticosteroids (9) has not been confirmed either (259).
Therefore, the challenge of monitoring the inflammatory response is enormous. Regardless of whether early anti-inflammatory or later proinflammatory therapy should be applied, a critical precondition would be to define the status of the patient in order to not cause harm.
BIOMARKERS AND MOLECULAR DIAGNOSTICS IN CLINICAL MANAGEMENT
Given that adjunctive sepsis therapy is not progressing, antimicrobial therapy and other measures of source control together with supportive measures such as fluid resuscitation, vasoactive drugs, and organ support remain the mainstays for the therapy of sepsis (70). Growing evidence from clinical studies suggests that a delay in the initiation of appropriate therapy costs lives and that survival can be increased by the systematic application of evidence-based clinical practice guidelines (85, 147). The elements of bundled care for septic shock that contributed most to improved patient outcomes were a shortening of the time to the administration of antibiotics, the appropriateness of the antibiotics, and early fluid resuscitation with crystalloids (15, 85, 147, 226). This underscores the necessity for the early and accurate detection of sepsis.
In the following section, we discuss the degree to which some of the most commonly proposed sepsis and infection biomarkers may help to answer some of the difficult questions that arise in the management of patients with severe sepsis. Unfortunately, almost none of the numerous proposed sepsis or infection biomarkers have been validated adequately in prospective clinical trials to answer the question of to which degree biomarker-directed decision making has an impact on relevant clinical outcomes for patients. Furthermore, it is important to note that an adequate evaluation of clinical utility is possible only if the assays that are used in this context for biomarker measurement provide reliable and reproducible results (170). For example, endotoxin from Gram-negative bacteria plays a crucial role in the pathophysiology of sepsis and may be present in the circulation of critically ill patients (116, 169). Endotoxin could therefore be an interesting sepsis biomarker; however, there are some issues with endotoxin assays. The classic Limulus amebocyte lysate assay (146) is not specific for endotoxin and can also be activated by other microbial products, particularly components of fungal cell walls (182). As plasma proteins inhibit the reaction, the reliability of the assay using protein-containing biological fluids is also reduced (231). Meanwhile, a bioassay based on the priming of neutrophil respiratory burst activity by complexes of endotoxin and antiendotoxin antibody has become available for clinical use. This test is currently used to stratify patients in the context of studies that investigate the potential of antiendotoxin strategies, and its utility and limitations still have to be established (169).
In the following section, we therefore discuss primarily those biomarkers that are measurable by assays which have been approved for clinical use, keeping in mind that this does not mean that the biomarkers themselves have been adequately proven to be clinically useful.
Acute-Phase Protein Biomarkers
C-reactive protein.
C-reactive protein (CRP) is an acute-phase protein released by the liver after the onset of inflammation or tissue damage. During infections, CRP has both proinflammatory and anti-inflammatory effects. CRP may recognize and adhere to pathogens and to damaged cells and mediate their elimination through interactions with inflammatory cells and mediators. However, C-reactive protein also prevents the adhesion of neutrophils to endothelial cells, inhibits superoxide production, and increases IL-1 receptor antagonist (IL-1ra) production. Although IL-6 is the prototypical stimulus for the induction of CRP, other cytokines also play a role in its production (87). C-reactive protein is a clinical marker frequently used to assess the presence of infection and sepsis (280) and therefore is often used as a comparator in diagnostic studies (Tables 1 and 2). Studies with cancer patients support C-reactive protein as a marker of infection or of sepsis (80, 211). Povoa et al. also found that the CRP course was not influenced by the presence or absence of neutropenia in septic cancer patients (211).
Table 1.
Cohorts studies of biomarkers and biomarker panelsa
| Setting and study design (reference) | Biomarker | Specificity (%) | Sensitivity (%) | Cutoffd | AUC | Direction of effect | Clinical relevance | Verification of infection (diagnostic reference standard) |
|---|---|---|---|---|---|---|---|---|
| Correlation of PCT, CRP, and IL-6 levels with infection likelihood or septicemia in emergency department patients with suspected sepsis (276) | PCT | 63–97 (infection likelihood), 38–90 (septicemia) | 68–18 (infection likelihood), 91–47 (septicemia) | 0.1–3.0 ng/ml | 0.72 (infection likelihood), 0.79 (septicemia) | ↑ | PCT, IL-6, and CRP highly correlate with several infection parameters but are inadequately discriminating to be used independently as diagnostic tools | Determined retrospectively from patient files by specialists blinded to biomarker data |
| IL-6 | 67–96 (infection likelihood), 34–89 (septicemia) | 58–14 (infection likelihood), 90–27 (septicemia) | 40–500 pg/ml | 0.69 (infection likelihood), 0.70 (septicemia) | ↑ | |||
| CRP | 33–88 (infection likelihood), 39–88 (septicemia) | 90–43 (infection likelihood), 82–31 (septicemia) | 40–100 mg/dl | 0.75 (infection likelihood), 0.67 (septicemia) | ↑ | |||
| Correlation of PCT, IL-6, and IL-8 with sepsis in 78 critically ill patients with SIRS and suspected infection (102) | PCT | 78 | 97 | 1.1 ng/ml | 0.92 | ↑ | Addition of PCT to the standard workup of critically ill patients with suspected sepsis could increase diagnostic certainty | Determined retrospectively from patient files by specialists blinded to biomarker data |
| IL-6 | 72 | 67 | 200 pg/ml | 0.75 | ↑ | |||
| IL-8 | 78 | 63 | 30 pg/ml | 0.71 | ↑ | |||
| Cohort study of 32 ICU patients undergoing cardiac surgery with cardiopulmonary bypass, 11 of these patients with SIRS and 6 of these with sepsis (69) | BPW | 93 | 100 | 0.465%T/s | 0.95 | BPW has potential clinical applications in sepsis diagnosis in the postoperative period following cardiac surgery under CPB | Sepsis was defined as SIRS associated with a documented infection, determined by 2 experts taking into account complete medical data | |
| PCT | 0.70 | ↑ | ||||||
| CRP | 0.66 | ↑ | ||||||
| Cohort study of 200 ICU patients to detect sepsis at admission and sepsis during ICU stay (308) | BPW | 92 (sepsis at admission), 76 (sepsis during ICU stay) | 79 (sepsis at admission), 81 (sepsis during ICU stay) | 0.075%T/s | 0.83 | ↑ | BPW may be useful as a simple diagnostic marker of sepsis; specificity can be increased by combing BPW with PCT; these tests may be more useful to rule out sepsis | Diagnosis of sepsis made by treating clinician (SIRS and documented or suspected infection), blinded to biomarker data |
| PCT | 79 (sepsis at admission), 75 (sepsis during ICU stay) | 83 (sepsis at admission), 83 (sepsis during ICU stay) | 1 ng/ml | ↑ | ||||
| BPW plus PCT | 95 (sepsis at admission), 94 (sepsis during ICU stay) | 79 (sepsis at admission), 81 (sepsis during ICU stay) | ↑ | |||||
| Cohort study of 161 patients with at least 2 SIRS criteria presenting to the medical emergency department and department of infectious diseases suspected of community-acquired infection (129) | suPAR | 0.67 | 0.35 | 2.7 μg/liter | 0.50 | ↑ | Measurements of suPAR, sTREM-1, and MIF had limited value as single markers, whereas PCT and CRP exhibited acceptable diagnostic characteristics; combined information from several markers improves diagnostic accuracy | Diagnosis of infection determined retrospectively by 2 infectious disease specialists based on clinical and laboratory findings, response to treatments, radiographic and other imaging procedures, and both positive and negative bacteriological, viral, and parasitic findings during the first 7 days of admission |
| sTREM-1 | 0.40 | 0.82 | 3.5 μg/liter | 0.61 | ↑ | |||
| MIF | 0.47 | 0.80 | 0.81 μg/liter | 0.63 | ↑ | |||
| PCT | 0.58 | 0.80 | 0.28 μg/liter | 0.72 | ↑ | |||
| Neutrophil count | 0.74 | 0.64 | 8.5 × 109 cells/liter | 0.74 | ↑ | |||
| CRP | 0.60 | 0.86 | 59 mg/liter | 0.81 | ↑ | |||
| Composite 6-marker test | 0.78 | 0.88 | 0.88 | ↑ | ||||
| Prospective study including inceptive and validation cohorts of unselected ICU patients to test performance of a combination biomarker (“bioscore”)b with sTREM-1, PCT, and PMN CD64 index to diagnose sepsis (91) | sTREM-1 | 86.3 | 53.2 | 755 pg/ml | 0.73 | ↑ | A bioscore of 0 excluded the presence of sepsis (101 of 105 [96.2%] patients); 129 of the 134 patients (96.3%) with a bioscore of 2 or 3 (44.6% of the cohort) were septic; bioscore of 1 was not helpful (61 patients [20.3%]) | Diagnosis of sepsis made retrospectively by 2 intensivists blinded to biomarker data |
| PCT | 84.9 | 83.1 | 1.55 ng/ml | 0.91 | ↑ | |||
| CD64 index | 95.2 | 84.4 | 1.62 | 0.95 | ↑ | |||
| Bioscore (derived from the 3 markers) | 0 = sepsis unlikely; 2 or 3 = sepsis likely | 0.95 | ↑ | |||||
| Cohort study of 293 medical ICU patients (98) | CD64 index | 89 | 63 | 2.2 | 0.8 | ↑ | As a result of its weak sensitivity, the CD64 index may not be practically recommended but may be useful in combination with a more sensitive biological marker | Bacterial infection |
| Cohort study of 109 critically ill neonates (118) | CD64 index | 88.7 | 94.6 | 1.19 | 0.94 | ↑ | The CD64 index is a useful and inexpensive test for improving the diagnosis and management of hospital patients with bacterial infection | Clinical diagnosis of sepsis and/or positive blood cultures |
| Prospective cohort study with 60 infants with 104 episodes to detect probable clinical sepsis, septicemia, or NEC (191) | ApoSAA scorec | 76 | 96 | 0.199 | 0.93 | ↑ | The ApoSAA score could potentially allow early and accurate diagnosis of sepsis/NEC and helps to guide antibiotic therapy | Clinical diagnosis of sepsis and/or positive blood cultures |
AUC, area under the concentration-time curve; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; BPW, biphasic waveform derived from activated partial thromboplastin time; suPAR, soluble urokinase-type plasminogen activator receptor; sTREM-1, soluble triggering receptor expressed on myeloid cells 1; MIF, macrophage migration inhibitory factor; CD64 index, immunoglobulin Fc fragment receptor I (FcγRI) CD64 on neutrophils.
The bioscore ranged between 0 (all three markers below their respective thresholds) and 3 (all three markers above their thresholds).
The “ApoSAA score” is a biomarker panel identified through a proteomic approach. The ApoSAA score is computed from plasma concentrations of proapolipoprotein CII (Apo) and a desarginine variant of serum amyloid A (SAA).
%T/s, transmittance percentage per second.
Table 2.
Meta-analyses of biomarker performance to detect sepsisa
| Setting and study design (reference) | Biomarker | Specificity (%) | Sensitivity (%) | Cutoff | AUC | Clinical relevance | Verification of infection (diagnostic reference standard) |
|---|---|---|---|---|---|---|---|
| MA of 12 studies of hospitalized patients (46 neonates, 638 children, and 702 adults) on the accuracy of PCT and CRP to diagnose bacterial infection (255) | PCT | 83 | 85 | 0.5–6.1 ng/ml | Graph provided | Diagnostic accuracy of PCT markers was higher than that of CRP markers | Microbiologically proven infection |
| CRP | 60 | 78 | 6–100 mg/liter | ||||
| MA of 17 studies with 2,008 patients in the emergency department on the accuracy of PCT to diagnose bacteremia (124) | PCT | 70 | 76 | 0.4–0.5 ng/ml | 0.84 | Diagnostic performance of the PCT test for identifying bacteremia in emergency department patients is judged as moderate | Positive blood culture |
| MA of 33 studies with 3,943 critically ill adults or patients after trauma or surgery on the accuracy of PCT alone or compared with that with CRP to diagnose sepsis, severe sepsis, or septic shock (280) | PCT | 48–100 | 42–100 | 0.6–5 ng/ml | Graph provided | Procalcitonin represents a good biological diagnostic marker and is superior to C-reactive protein | Clinical and/or microbiological criteria defined by those authors |
| CRP | 18–85 | 35–100 | 39–180 mg/liter | ||||
| MA of 18 studies with 2,097 critically ill patients from ICU, emergency department, and hospital wards on the accuracy of PCT to diagnose sepsis (267) | PCT | Mean joint values of both sensitivity and specificity of 71 (95% CI, 67–76) | 0.2–20 ng/ml | 0.78 | Diagnostic performance of PCT was upwardly biased in smaller studies but moving toward a null effect in larger studies | Microbiologically proven infection | |
| MA of 24 studies on the value of PCT as a marker of development of severe acute pancreatitis and infected pancreatic necrosis (183) | PCT | 0.86 (severe acute pancreatitis), 0.91 (infected pancreatic necrosis) | 0.72 (severe acute pancreatitis), 0.80 (infected pancreatic necrosis) | No significant heterogeneity in studies using a cutoff of >0.5 ng/ml | 0.87 (severe acute pancreatitis), 0.91 (infected pancreatic necrosis) | PCT may be valuable in predicting the severity of acute pancreatitis and the risk of developing infected pancreatic necrosis | Confirmed diagnosis of acute pancreatitis |
| MA of 13 studies with 980 patients on the accuracy of the sTREM-1 as a diagnostic test for bacterial infection (122) | sTREM-1 | 0.86 | 0.82 | 5–374 pg/ml | 0.91 | sTREM-1 represents a reliable biological marker of bacterial infection | Bacterial infection |
| MA of 16 studies with 1,959 neonates on the accuracy of PCT to diagnose sepsis in comparison with other conditions (292) | PCT | 79 | 81 | 0.87 | PCT has very good diagnostic accuracy for the diagnosis of neonatal sepsis | Culture-proven or clinically diagnosed sepsis |
MA, meta-analysis; CI, confidence interval.
C-reactive protein has been found to differentiate patients with pneumonia from those with endotracheal infections (71), to aid in the diagnosis of appendicitis (82), or to differentiate bacterial and viral infections (251). Studies of critically ill patients showed that elevated plasma concentrations of CRP were correlated with an increased risk of organ failure and/or death (131, 156, 199). However, in another prospective study of postoperative sepsis, where the courses of procalcitonin (PCT), interleukin-6 (IL-6), and CRP were evaluated as a percent decrease from the baseline in order to predict survival at day 28 after the onset of sepsis, PCT and IL-6 levels significantly decreased in survivors from days 1 to 14, whereas CRP levels did not (278). Likewise, a study that evaluated the predictive value of CRP for hospital mortality in 60 surgical patients at the onset of sepsis revealed no significant difference between survivors and nonsurvivors (279). After pancreatic resections, CRP levels peaked on the third postoperative day and gradually decreased thereafter in unproblematic cases, whereas they remained elevated in cases with postoperative complications (297). In postoperative esophagectomy patients admitted to the ICU, CRP levels were significantly higher at day 2 and day 3 in patients who developed postoperative complications (282). In patients with community-acquired sepsis, the course of CRP levels was associated with the resolution of pneumonia (212). Conflicting data exist regarding whether absolute CRP concentrations at ICU discharge represent an independent predictor of in-hospital mortality after discharge from an ICU (4, 108, 155, 218, 254).
In the ICU setting, CRP could discriminate only between patients with severe sepsis, those with sepsis, and those without sepsis in the first 2 days after admission but not during the later course in the ICU (236). One reason for this may be that plasma levels of CRP increase with a delay of up to 24 h compared to cytokines or procalcitonin (184). Second, plasma concentrations of C-reactive protein may increase during minor infections and do not adequately reflect the severity of the infection. Third, plasma levels remain elevated for up to several days, even when infection is eliminated (180). Finally, the CRP level is also elevated during inflammatory states of noninfectious etiologies, e.g., with autoimmune and rheumatic disorders (79), myocardial infarction, or malignant tumors or after surgery (179).
Lipopolysaccharide-binding protein.
Lipopolysaccharide (LPS)-binding protein (LBP) is an acute-phase reactant that binds the LPS of Gram-negative bacteria to form an LPS-LBP complex (273, 305). The LPS-LBP complex binds to CD14 and to Toll-like receptors (TLRs) to initiate signal transduction, which leads to the activation of the mitogen-activated protein kinase and nuclear factor κB pathways. In humans, LBP is constitutively present in serum at a concentration of 5 to 10 μg/ml, and levels increase during the acute-phase reaction, reaching peak levels of up to 200 μg/ml (272). Several studies have reported that LBP may be a useful marker of infection and a potential marker of severity and outcome (88, 196). However, this could not be confirmed in a more recent study (236). That analysis found that LBP discriminated patients without infection from patients with severe sepsis in a surgical ICU only moderately and failed in patients with sepsis without organ dysfunction. LBP concentrations did not distinguish between Gram-positive and Gram-negative infections, and the correlation of LBP concentrations with disease severity and outcome was weak compared with other markers. Those authors concluded that the use of LBP as a biomarker is not warranted in this patient population.
Procalcitonin.
The use of procalcitonin (PCT) as a potential biomarker for sepsis and infection was first described in 1993 (10). PCT is the prohormone of calcitonin, but the induction of the prohormone during sepsis and infection is regulated differently than for hormonal activities of the mature hormone (19, 26, 249). The biological role of PCT is not yet fully elucidated. It involves a modulation of inducible nitric oxide synthase and cytokine induction; furthermore, the protein interferes with the receptor binding of other peptide hormones that play a role in the modulation of the intravascular fluid and vascular tone (110, 153). PCT is produced ubiquitously in response to endotoxin or to mediators released in response to bacterial infections (that is, IL-1β, TNF-α, and IL-6) and strongly correlates with the extent and severity of bacterial infections (96). To date, second to C-reactive protein, PCT has become the most widely used biomarker in the management of infection and sepsis in Europe and meanwhile has also become available in most parts of the world.
PCT shows a more favorable kinetic profile than CRP and cytokines: its levels increase within 4 to 12 h upon stimulation, and circulating PCT levels halve daily when the infection is controlled by the host immune system or antibiotic therapy (20, 45). Compared to any other currently available sepsis marker, PCT seems to have some potential to discriminate between infectious and noninfectious systemic inflammation (44, 102, 186) (Table 1), also in low-acuity patients (152). The levels of PCT correlate with the severity of the bacterial infection and bacterial load. In patients with community-acquired pneumonia (CAP) and in patients with urinary tract infections, PCT at a cutoff of 0.1 μg/liter had a very high sensitivity to exclude bacteremia (188, 283). Accordingly, the course of PCT may have prognostic implications (102, 134, 187, 283). PCT also seems to have some potential to differentiate between viral and bacterial infections and may indicate the presence of bacterial superinfection in patients with viral diseases (3, 52, 64, 247). A meta-analysis that included 24 studies found a sensitivity and a specificity of PCT of 0.80 and 0.91, respectively, for the prediction of infected necrosis in patients with pancreatitis (183) (Table 2). A recent study of emergency department patients, however, found that PCT, IL-6, or CRP only moderately discriminated infected from noninfected patients (276) (Table 1).
The clinical utility of PCT in patients with febrile neutropenia remains to be elucidated. In a recent systematic review, we identified 30 articles on the topic and concluded that PCT may have some value as a diagnostic and prognostic tool in patients with febrile neutropenia, but firm conclusions are not possible due to differences in patient populations and the quality of the studies (238). Interestingly, PCT seems not to be attenuated by corticosteroids (67), and PCT production does not depend on white blood cells (216).
Six meta-analyses have meanwhile been performed on the diagnostic accuracy of PCT to detect infection in different patient populations (Table 2). Four of these meta-analyses identified PCT as being helpful for the diagnosis of clinically or microbiologically documented infection (183, 255, 280, 292), whereas one meta-analysis identified only a moderate benefit in the detection of bacteremia (124), and another found the benefit moving toward a null effect in larger studies (267) (Table 1). Recent guidelines issued by the Infectious Diseases Society of America and the American College of Critical Care Medicine recommended the use PCT as an adjunctive diagnostic marker to differentiate sepsis from systemic inflammatory response syndromes of a noninfectious origin (194).
PCT for antibiotic stewardship.
Antimicrobial resistance has emerged as a major factor affecting patient outcomes and overall resources. This calls for more stringent efforts to reduce antibiotic overuse (229). The potential of PCT to assist in decisions about the initiation and/or duration of antibiotic therapy (antibiotic stewardship) was prospectively evaluated in 13 randomized controlled studies in which 4,395 patients were enrolled (245). Depending on the setting, this resulted in a reduction in the prescription of antibiotics of between 74% and 11% and a reduction of days on antibiotics by between 13% and 55% (245). All published studies on antibiotic stewardship used similar clinical algorithms, with recommendations for or against antibiotic treatment based on PCT cutoff ranges. For moderate-risk patients with respiratory tract infections in the emergency department, algorithms recommended the initiation and discontinuation of antibiotic therapy based on four different cutoff ranges. For high-risk patients in the ICU setting, algorithms focused on the discontinuation of antibiotic therapy if a patient showed a clinical recovery and PCT levels decreased to “normal” levels, or by at least 80% to 90% (244). Most of those studies were performed with patients with proven or suspected respiratory tract infections (47, 54, 133, 159, 160, 246, 260, 261). One of those studies was performed in the emergency department (54), and one was performed in the primary care setting (41). A small proof-of-concept study (193) found a 4-day reduction in the duration of antibiotic therapy in patients with severe sepsis but only in the per-protocol analysis. One larger multicenter study and two smaller monocentric studies of postsurgical patients also confirmed a reduction of antibiotic exposure by procalcitonin use in the ICU setting (35, 109, 242). The most recent meta-analysis by Schuetz et al. also addressed the question of whether the reduction of antibiotic exposure has an impact on patient outcomes (245). In that analysis, the researchers included an additional RCT that did not address the impact of the use of a procalcitonin algorithm on antibiotic prescription but on the timing of reinterventions in septic patients after multiple trauma or major surgery (265). Overall, a total of 4,467 patients were included in the 14 RCTs (i.e., 2,240 in the control group and 2,227 in the PCT group). Those authors found no significant difference in mortality between procalcitonin-treated and control patients overall (odds ratio, 0.91; 95% confidence interval, 0.73 to 1.14) or in a primary care setting (odds ratio, 0.13; 95% confidence interval, 0 to 6.64), in the emergency department (odds ratio, 0.95; 95% confidence interval, 0.67 to 1.36), or the intensive care unit (odds ratio, 0.89; 95% confidence interval, 0.66 to 1.20) in particular. Another meta-analysis focused on adult and neonatal ICU patients including seven randomized controlled studies with 1,131 patients (1,010 adults and 121 neonates). In comparison with routine practice, the implementation of procalcitonin-guided algorithms decreased the duration of antibiotic therapy for the first episode of infection by approximately 2 days and the total duration of antibiotic treatment by 4 days (132) (Table 2). Of note, a recent multicenter RCT of intensive care patients with an algorithm to substantially increase antibiotic therapy if procalcitonin levels of ≥1.0 ng/ml were not decreasing at least 10% from the previous day resulted in the escalation of antibiotic use and the prolongation of antimicrobial therapy by 2 days, did not improve survival, and led to worse secondary outcomes in the PCT-guided patient group (121).
A recent meta-analysis and systematic review of procalcitonin-guided therapy in respiratory tract infections included eight studies that randomized 3,431 patients and came to the conclusion that this approach “appears to reduce antibiotic use without affecting overall mortality or length of stay in the hospital” (149). Updated international sepsis guidelines suggest that procalcitonin may be used for the discontinuation of antibiotic therapy in patients with respiratory tract infections (70a).
Despite these encouraging results, the use of PCT for the discontinuation of antibiotics in patients with severe sepsis and ICU patients awaits further evaluation before definitive conclusions can be drawn for these patient cohorts. There was a trend toward a higher mortality rates in the PCT group of the PRORATA trial, which was performed in the intensive care setting (35). To exclude a true excess mortality of 4%, similar to that seen in the trial, a study would require about 4,220 patients; a study to exclude an excess mortality of 2% would need to be powered by 16,500 patients (270). In their response to this criticism, those authors pointed to the fact that random allocation at ICU admission had resulted in slight imbalances in terms of higher disease severity scores for the PCT group (34). There are currently different ongoing trials focusing on this patient population, for example, the Placebo Controlled Trial of Sodium Selenite and Procalcitonin Guided Antimicrobial Therapy in Severe Sepsis (SISPCT) (trial number NCT00832039), Safety and Efficacy of Procalcitonin Guided Antibiotic Therapy in Adult Intensive Care Units (trial number NCT01139489), Procalcitonin To Shorten Antibiotics Duration in ICU Patients (trial number NCT01379547), Diagnostic and Prognostic Value of Serial Procalcitonin (PCT) Measurements in Critically Ill Patients (trial number NCT01362920), Serum Procalcitonin Study in the Management of Ventilated Patients (trial number NCT00726167), Duration of Antibiotic Therapy in the Treatment of Severe Postoperative Peritonitis Admitted in ICU (trial number NCT01311765), and the Neonatal Procalcitonin Intervention Study (trial number NCT00854932) (all accessible at www.clinicaltrials.gov).
Limitations of PCT.
A number of limitations of PCT as an infection and sepsis marker must been taken into account. Nonspecific elevations of PCT levels in the absence of a bacterial infection can occur in situations of massive stress, such as after severe trauma and surgery (258), or in patients after cardiac shock (243). This is the reason why the power of PCT to discriminate between sepsis and sterile inflammation is better for medical than for surgical patients (60). Also, various other causes of nonbacterial systemic inflammation have been reported, such as birth stress in newborns, heat shock, and acute graft-versus-host disease as well as different types of immunotherapy, such as granulocyte transfusions, the administration of antilymphocyte globulin or anti-CD3 antibody, or therapy with cytokines or related antibodies (alemtuzumab, IL-2, or TNF-α). Some autoimmune diseases (Kawasaki disease or different types of vasculitis) and paraneoplastic syndromes can also be associated with elevated PCT levels (222).
Only sparse and conflicting results regarding the value of PCT in systemic fungal infections have been provided up to now (59, 77, 115, 264). Candida-related severe sepsis or septic shock does not necessarily elicit a substantial increase in serum PCT levels. A retrospective comparison of episodes of bacteremia or candidemia in nonneutropenic patients with sepsis showed that PCT levels were significantly lower or near normal in patients with candidemia (53). Thus, the value of PCT for the diagnosis of fungal infection and sepsis is poor. The 2012 update of the SSC (Surviving Sepsis Campaign) guidelines consequently suggests the use of the fungal cell wall components 1,3-β-glucan and mannan as well as mannan antibodies as biomarkers for fungal sepsis (Dellinger et al., personal communication).
In conclusion, the use of PCT—like any other biomarker—should be considered within the context of the clinical workup and should take into account all patient- and therapy-related factors that may interfere with the initial magnitude and the course of this parameter.
Pentraxin.
Pentraxins are a superfamily of proteins involved in acute immunological responses. They act as pattern recognition receptors (PRRs). The classic “short” pentraxins include serum amyloid P component (SAP) and C-reactive protein (CRP), which is produced in the liver following inflammatory signals. Like the classical short pentraxins, the “long” pentraxin 3 (PTX3) is secreted by various cells, including leukocytes and endothelial cells. It binds to specific patterns of fungi, bacteria, and viruses and induces phagocytosis by its binding to complement component C1q (33). In patients with severe sepsis and septic shock, persisting high levels of circulating PTX3 over the first days from the onset of sepsis were associated with mortality; furthermore, PTX3 correlated with the severity of sepsis and with sepsis-associated coagulation/fibrinolysis dysfunction (173). In febrile patients presenting to the emergency department, PTX3 levels were significantly higher in patients admitted to intensive/intermediate care units than in patients referred to normal wards, and PTX3 was proven to be predictive for patients with positive blood cultures (68). In bacteremic patients, maximum PTX3 levels between days 1 and 4 were markedly higher in nonsurvivors than in survivors (117); in hematologic patients with febrile neutropenia after chemotherapy, high pentraxin 3 levels predicted septic shock and bacteremia at the onset of febrile neutropenia (284). Studies of the potential of PTX3 for differentiation between noninfectious SIRS and severe sepsis and septic shock are currently missing.
Other acute-phase reactants.
Levels of other acute-phase reactants, such as serum amyloid A, ceruloplasmin, alpha 1 acid glycoprotein, and hepcidin have been found to be elevated in cases sepsis and to be higher in patients with poor outcomes (207).
Cytokine/Chemokine Biomarkers
Cytokines.
Cytokines are immune-modulating agents that may be derived from virtually all nucleated cells. Endothelial/epithelial cells and resident macrophages in particular are potent producers of proinflammatory and anti-inflammatory cytokines (38, 235). In septic patients, the secretion of both pro- and anti-inflammatory cytokines occurs in a simultaneous manner from the very first moments of infection (266). Mean serum levels of cytokines are higher in septic than in nonseptic patients. Cytokines have therefore been proposed to be sepsis biomarkers in cases of neonatal and adult sepsis (80, 253).
Persistently high or increasing levels of pro- and anti-inflammatory cytokines are found mostly in nonsurvivors, whereas low and decreasing levels are found in survivors of sepsis (106, 200). Despite their important role in the pathogenesis of sepsis, however, the role of cytokines as sepsis biomarkers remains to be established. They may be of limited value as sepsis biomarkers, because they can be induced by numerous noninfectious diseases as well. Studies comparing the performance of IL-6 or IL-8 to those of PCT and CRP in the detection of infection have found them to be of inadequate discriminative value (102, 276) (Table 1). Serum levels of the cytokines IL-6 and IL-8 are closely related to the severity and outcome of sepsis in patients (101), as are TNF-α and biomarkers of the anti-inflammatory, endothelial, and apoptotic aspects of systemic inflammation, such as interleukin-1 receptor antagonist (IL-1ra) (227). In neutropenic patients, levels of IL-8 and IL-6, but not C-reactive protein, were significantly different between patients with microbiologically documented infections and patients with unexplained fever (80). In neonates, increased plasma levels of both IL-6 and IL-8 could predict an early onset of sepsis with high sensitivity and specificity (22, 192). Serum interleukin-8 levels obtained within 24 h of admission had a 95% negative predictive value for mortality in children with septic shock (304) However, plasma interleukin-8 levels were not effective as a risk stratification tool in older adults with septic shock (50). Furthermore, IL-8 plasma levels were unable to discriminate between infected and noninfected pancreatic necrosis (220). Of note, IL-6 and IL-8 can also be induced to a variable degree after major surgery and major trauma (189, 252), during acute exacerbations of autoimmune disorders (137, 228), during viral infections, and after transplant rejection (90, 166). IL-10 levels have been found to be higher in patients with septic shock than in patients with sepsis and to distinguish between survivors and nonsurvivors at 28 days (106, 294). IL-10 is a broadly immunosuppressive cytokine and was proposed to contribute to compensatory anti-inflammatory response syndrome (CARS) (1).
A combined cytokine score that comprised IL-6, IL-8, and the anti-inflammatory cytokine IL-10 was associated with a worse outcome for patients with sepsis; in this small observational study, the predictive value of the combined interleukin score for mortality was highly superior to those of CRP and PCT (5).
Macrophage migration inhibitory factor.
Macrophage migration inhibitory factor (MIF) is a regulator of innate immunity (49), the level of which is elevated in septic shock. MIF distinguished between survivors and nonsurvivors (39) but failed to differentiate infectious from noninfectious causes of inflammation (144).
High-mobility-group box 1.
High-mobility-group box 1 (HMGB1) is a 30-kDa nuclear and cytosolic protein known because of its properties as a transcription and growth factor. It is also a cytokine mediator of local inflammation as well as lethal systemic inflammation (e.g., endotoxemia and sepsis). It is released by activated macrophages, and during endotoxemia and sepsis, serum levels increase significantly. However, it reacts more slowly than tumor necrosis factor (TNF) and interleukin-1β. HMGB1 plays a role in the activation of macrophages/monocytes to release proinflammatory cytokines, the upregulation of endothelial adhesion molecules, the stimulation of epithelial cell barrier failure, and the mediation of fever and anorexia (295). Because of its delayed kinetics, it has been investigated for its value as a prognostic marker. In a prospective randomized multicenter study, serum HMGB1 concentrations were elevated in patients with severe sepsis but did not differ between survivors and nonsurvivors and did not predict hospital mortality (126).
Cytokine/chemokine biomarker summary.
In summary, pro- and anti-inflammatory cytokines and chemokines have some value in the assessment of the inflammatory response; however, they lack discriminative power to differentiate between infectious and noninfectious systemic inflammation. Low IL-8 levels have a high negative predictive value for sepsis. Whether IL-6 can be used as a very early predictor of infection or of sepsis deserves further investigation.
Coagulation Biomarkers
The host response that is induced by severe infections and sepsis is often accompanied by disseminated intravascular coagulation, which is triggered by several proinflammatory cytokines, such as interleukin-6 and TNF-α (145). The pathogenic mechanisms that are involved in the interplay between coagulation and inflammation are very complex, and it is known that changes in coagulation factors such as thrombocyte counts, antithrombin, protein C and S, activated partial thromboplastin time, D-dimers, fibrin, plasminogen activator inhibitor, and thrombomodulin are appropriate markers to gauge the severity of the host response and that the course of these markers has prognostic implications (43, 72, 206, 207, 237) Interestingly, protein C serum concentrations in neutropenic patients were decreased significantly before the clinical signs of severe sepsis and septic shock were apparent (181).
There is no added value from the use of antithrombin or protein C measurements for predictions of outcome. The receiver-operator characteristic curves for intensive care unit mortality predictions were almost identical between protein C and the acute physiology and chronic health evaluation II (APACHE II) score as well as simplified acute physiology score II (SAPS II) (43). The prognostic power of antithrombin for mortality compared to these severity scores was even lower (237).
Biphasic waveform (BPW) analysis is a new biological test derived from the activated partial thromboplastin time that has recently been proposed for the diagnosis of sepsis. The atypical biphasic transmittance waveform is due to decreased light transmission and is thought to derive from the formation and precipitation of a calcium-dependent complex involving CRP and very-low-density lipoprotein (VLDL) in serum (308). In an observational study including 16 patients with SIRS, 5 of whom were classified as sepsis patients, the BPW, in contrast to CRP or PCT, was significantly higher in the sepsis group than in the nonseptic SIRS group (median of 0.57 and interquartile range of 0.54 to 0.78 versus median of 0.19 and interquartile range of 0.14 to 0.29%T/s; P < 0.01) (69) (Table 1). The major limitation of coagulation parameters as sepsis biomarkers results from the fact that disseminated intravascular coagulation may also be triggered by a number of other disease states, such as trauma, obstetrical disorders, or cancer (145), and thus, coagulation parameters are not helpful to establish the diagnosis of sepsis. Nevertheless, they might be used as part of the inclusion criteria for sepsis trials that aim to evaluate the efficacy of anticoagulants in cases of severe sepsis.
Soluble Receptor, Cell Surface, and Other Markers
sTREM-1.
Triggering receptor expressed on myeloid cells 1 (TREM-1) is a recently discovered member of the immunoglobulin superfamily. Studies have shown that the expression of TREM-1 is greatly upregulated in the presence of bacteria or fungi in cell culture, peritoneal lavage fluid, and tissue samples from patients infected with these microorganisms (36, 63). TREM-1 is not upregulated in patients with noninfectious inflammatory conditions (37); TREM-1 expression is associated with the release of soluble TREM-1 (sTREM-1). Studies by Gibot et al. suggested that the value of plasma sTREM-1 levels as an indicator of sepsis was superior to those of CRP and PCT (92–95). A meta-analysis found that the sensitivity of sTREM-1 for the diagnosis of bacterial infection was 0.82 and that the specificity was 0.86. However, it was neither sufficiently sensitive nor specific for the diagnosis of urinary tract infections (122) (Table 2). Other studies reported that the value of sTREM-1 for the diagnosis of sepsis from community-acquired infections is inferior to those of CRP and PCT (14, 129, 139). The authors of a recent study that compared sTREM-1 to other proposed sepsis biomarkers in postoperative patients with suspected sepsis concluded that “sTREM-1 levels could differentiate sepsis from SIRS and reflected sepsis severity noninferior to TNF-α, PCT, IL-6 and CRP. sTREM-1 levels were also an effective prognostic indicator in critically ill patients. The clinical application of sTREM-1 as a diagnostic and prognostic marker still requires larger studies for further elucidation” (150).
suPAR.
The urokinase-type plasminogen activator (uPA) system consists of a protease, a uPA receptor (uPAR), and inhibitors (230). In 1991, the soluble form of uPAR (suPAR) was identified (209). uPAR is expressed on various cell types, including neutrophils, lymphocytes, monocytes/macrophages, and endothelial and tumor cells. After cleavage from the cell surface, suPAR can be found in the blood and other organic fluids in all individuals. suPAR takes part in various immunological functions, including cell adhesion, migration, chemotaxis, proteolysis, immune activation, tissue remodeling, invasion, and signal transduction (83). The clinical utility of suPAR in patients with sepsis is poorly addressed; from existing observational studies, it may be concluded that suPAR has a poor accuracy in the diagnosis of sepsis compared to those of CRP and PCT. suPAR is not a specific marker for bacteremia or sepsis but rather a marker of the severity of disease (76). The measurement of suPAR levels had little value as a single marker to detect community-acquired infection in patients with SIRS (129) (Table 1). A marked association between high suPAR levels and high mortality rates has been observed for patients with HIV, tuberculosis, malaria, and Crimean-Congo hemorrhagic fever (83) and also for ill patients with no obvious infectious disease diagnosis (215). Overall, the diagnostic value of suPAR is low for sepsis; it may have some value for outcome predictions and for monitoring the response to treatment (13).
Midregional proadrenomedullin.
Serum adrenomedullin (ADM), a 52-amino-acid peptide, is modulated by the complement system and has potent vasodilating as well as bactericidal effects. ADM levels were found to be elevated in patients with sepsis (107). Because circulating ADM is rapidly broken down and masked by a binding protein (complement factor H), the midregional fragment of proadrenomedullin (Pro-ADM), consisting of amino acids 45 to 92, is measured instead (262). In several studies that enrolled nearly 2,000 patients with different severities of community-acquired pneumonia, Pro-ADM levels measured at admission emerged as good predictors of the severity and outcome of CAP with a prognostic accuracy similar to that of clinical pneumonia severity scores and a better prognostic accuracy than those of commonly measured clinical and laboratory parameters, including procalcitonin (55, 56, 114).
Polymorphonuclear CD64 index.
During the systemic inflammatory response, circulating polymorphonuclear (PMN) cells bind to endothelial cells monolayers and express CD64, the high-affinity Fc receptor for IgG. The percentage of PMNs expressing CD64 was higher in SIRS patients than in non-SIRS patients (214). The upregulation of CD64 expression on the cell surface of PMN cells and monocytes is considered to be a very early step of the immune host response to bacterial infection. The CD64 index has been found to be useful for the early detection of sepsis in critically ill neonates (118), was suggested to be an adjunctive marker in combination with more sensitive markers in medical ICU patients (98), and showed high sensitivity and specificity (84.4% and 95.2%, respectively) for the detection of infection in unselected ICU patients (91) (Table 1). Levels of a number of other leukocyte cell surface markers, such as CD10, CD11b, and CD11c, were found to be decreased whereas levels of CD48 and CD64 were increased in patients with sepsis compared to levels in healthy controls; other surface markers, such as CD14, CD18, CD25, and CD28, distinguished between survivors and nonsurvivors at 28 days (207).
Panels of Biomarkers
Combinations of biomarkers reflecting various aspects of the host response have been proposed to overcome limitations of single biomolecules. A panel consisting of serum concentrations of soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) and procalcitonin (PCT) and the expression of the polymorphonuclear CD64 index in flow cytometry, called the “bioscore,” was higher in patients with sepsis than in all others (P < 0.001 for the three markers) (91). These biomarkers were all independent predictors of infection, with the best receiver-operator characteristic curve being obtained for the PMN CD64 index, while the performance of the combination was better than that of each individual biomarker and was externally confirmed in a validation cohort (91) (Table 1).
In critically ill patients, the combined measurement of PCT concentrations and the presence of a biphasic transmittance waveform (BPW), an atypical BPW due to decreased light transmission from the coagulation marker activated partial thromboplastin time (aPTT), increased the specificity of either marker alone for the detection of sepsis (308) (Table 1).
In the emergency department, the usefulness of a combination of a multiplex immunoassay measuring suPAR, sTREM-1, and MIF in parallel with standard measurements of CRP, PCT, and neutrophils was evaluated in patients with SIRS to detect community-acquired bacterial infections. The area under the concentration-time curve (AUC) of the six-marker test was significantly greater than that of any single marker (129) (Table 1).
Thus, the combination of several biomarkers holds some promise to increase sensitivity and specificity; however, the clinical utility and cost-effectiveness regarding the management of septic patients need further prospective testing (250) by the use of appropriate statistical methods that can be applied to high-dimensional data (163).
Molecular Strategies To Improve Pathogen Detection
Blood culture (BC) reflects the current gold standard for the detection of bloodstream infection, since viable microorganisms isolated from the blood can be analyzed to identify species and susceptibility to antimicrobial therapy. The practical value of BC in the diagnosis of sepsis, however, is impaired by the delay in the time to results and the fact that positive blood cultures can be found for only approximately 30% of these patients (48). Furthermore, it is known that the sensitivity for many slow-growing and fastidious organisms is low (256).
A number of molecular approaches to improve conventional culture-based identification, including PCR, have been suggested (205), such as matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, which may decrease the time to result to 4 h once the BC has become positive. However, a broader clinical evaluation of this approach is still missing. Another alternative strategy is the extraction and amplification of microbial nucleic acids from a positive BC and subsequent hybridization on a microarray platform to detect the gyrB, parE, and mecA genes of 50 bacterial species, which has recently been evaluated in an observational multicenter design with conventional BC as the comparator. Eighty-six percent of positive blood culture samples included a pathogen covered by the molecular assay. The assay had a sensitivity of 94.7% and a specificity of 98.8%, and the sensitivity and specificity were both 100% for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. On average, the assay was 18 h faster than conventional BC (271). Thus, this study provides a proof of concept that molecular assays can assist in shortening the time to results, while shortcomings include an incomplete coverage of pathogens, the inability of the test to be applied directly to a biological sample, and restricted information regarding antimicrobial susceptibility (primarily regarding multiresistant Gram-negative bacteria) despite an excellent performance in the detection of MRSA. While the PCR-based detection of MRSA (and also vancomycin-resistant enterococci) is feasible due to a limited number of resistance genes, the need to identify the large and continuously evolving set of genotypes encoding extended-spectrum beta-lactamases renders a conventional PCR-based approach unreliable.
Similar shortcomings, obviously with the exception of a need for prior culture, also hold true PCR-based approaches, which amplify microbial nucleic acids directly from the bloodstream. This has led to the recommendation that currently available PCR tests can supplement but not replace BC. In a variety of clinical studies, such a combined diagnostic approach has been documented to lead to higher rates of detection of an alleged pathogen than with each method alone and decreased the time to results in well-defined risk populations, such as patients with episodes of fungal infections (104). The culture-independent, nucleic acid amplification-based detection of pathogen-associated molecular patterns carries the potential to improve the detection of infection with typical easy-to-culture sepsis-associated bacteria, such as streptococci and staphylococci (40), and also polymicrobial infections, including fastidious, multiresistant, and difficult-to-culture species such as fungi (75, 89, 175, 205, 296). Thus, despite the above-described inherent limitations, PCR may reflect a promising approach to decrease the time to results in a way that may favorably influence decision making for antibiotic therapy in the septic host.
Comparable data are currently available only from studies which used SeptiFast multiplex PCR (161, 300). Data obtained from patients with sepsis, endocarditis, or bloodstream infection show that the concordance of PCR and BC results with respect to the recovery of blood culture-positive results by PCR was moderate to good in most but not all studies (Fig. 3; study data are shown in Table 3). It is noteworthy that a significant share of blood culture findings was typically not reproduced by PCR. Cumulative data document the consistent inability to identify approximately 20 to 30% of culture-positive results by multiplex PCR, even if the pathogen should be covered by a primer pair (Fig. 3). Given the additional limitation in comprehensively assessing resistance patterns, the clinical utility of PCR remains to be defined and currently may be used as a supplement to rather than a replacement of BC.
Fig 3.
Positivity rates and concordance of multiplex PCR and blood culture (BC) results from 27 published studies. The left two box plots indicate positive results (in percentages of blood samples); the right two box plots indicate the concordance of negative or positive paired samples (in percentages of all samples) or, with regard to isolates, the concordance of negative results and of identified isolates in paired samples (in percentages of the sum of isolates and negative results). Study data are given in Table 1. Box plots indicate the median, interquartile range, and 10th and 90th percentiles, and dots denote outlying single studies. A total of 7,814 samples/episodes were evaluated for positivity rates, 7,347 samples/episodes were evaluated for the concordance of BC and PCR results, and 6,586 samples/episodes were evaluated for the concordance of isolates (sum of isolates and BC-negative/PCR-negative sample pairs equals 6,847).
Table 3.
Patient data from 23 studies applying multiplex PCR to identify the presence of pathogen DNA in the bloodstreama
| Reference, yr | Population(s) | Design | No. of patients | No. of samples |
|---|---|---|---|---|
| 12, 2010 | Adults, emergency department, suspected bloodstream infection, at least 2 SIRS criteria | Single center | 144 | 144 |
| 23, 2010 | Adults, surgical intensive care unit, severe sepsis | Multicenter, prospective | 142 | 236 |
| 23, 2010 | Adults, intensive care unit, elective surgery | Multicenter, prospective | 63 | 111 |
| 73, 2009 | Adults, presumed sepsis, medical and surgical patients | Single center, retrospective | 77 | 101 |
| 84, 2010 | Adults, endocarditis, BC and PCR results not corresponding | Single center, prospective | 15 | 15 |
| 97, 2012 | Presumed sepsis, intensive care unit, general ward | Single center, prospective | 61 | 71 |
| 125, 2011 | Adults, infectious diseases department, patients subjected to BC | Single center, prospective | 1,093 | 1,093 |
| 138, 2010 | Adults, isolation ward, hematological patients undergoing chemotherapy or hematopoietic stem cell transplantation with febrile neutropenia | Single center, prospective | 86 | 141 |
| 142, 2009 | Adults, emergency department, intensive care unit, general ward, presumed sepsis | Multicenter, retrospective | 436 | 467 |
| 143, 2010 | Adults, surgical intensive care unit, presumed severe sepsis | 2 center, prospective | 108 | 453 |
| 290, 2009 | Adults, hematology ward, febrile neutropenia | Single center, prospective | 70 | 119 |
| 158, 2009 | Adults, surgical intensive care unit, screening 2×/wk | Single center, prospective | 52 | 258 |
| 157, 2011 | Adults, surgical intensive care unit, SIRS | Single center | 104 | 148 |
| 161, 2008 | Adults, emergency department, intensive care unit, general ward, suspected bloodstream infection, at least 2 SIRS criteria | Single center, prospective | 200 | 200 |
| 162, 2011 | Neonates, pediatrics, emergency department, intensive care unit, general wards, neonatology, presumed sepsis | Single center, retrospective | 803 | 1,673 |
| 167, 2008 | Adults, febrile neutropenia | Single center | 34 | 103 |
| 174, 2012 | Adults, children, febrile immunocompromised patients (neutropenia, immunosuppressive therapy, hematological malignancies, solid tumors) | Single center | 79 | 79 |
| 195, 2011 | Adults, emergency department, intensive care unit, general ward, presumed sepsis | Single center | 54 | 78 |
| 202, 2009 | Neonates, presumed sepsis | Single center, retrospective | 34 | 34 |
| 204, 2012 | Presumed sepsis, internal medicine | Single center | 391 | 391 |
| 219, 2012 | Presumed sepsis after liver transplantation or major abdominal surgery, intensive care unit | Single center, retrospective | 170 | 225 |
| 221, 2010 | Adults, intensive care unit, presumed sepsis | Single center, prospective | 72 | 106 |
| 275, 2011 | Adults, trauma and emergency surgery, burn patients | Single center, prospective | 50 | 50 |
| 277, 2010 | Adults, emergency department, presumed sepsis | 2 center, prospective | 306 | 306 |
| 285, 2009 | Adults, children, hematology/oncology ward, febrile neutropenia | Single center | 100 | 130 |
| 286, 2008 | Intensive care unit, hematological/general ward, presumed sepsis | Dual centric | 36 | 39 |
| 293, 2010 | Adults, intensive care unit, patients with fever or hypothermia | Single center, prospective | 72 | 100 |
| 300, 2009 | Presumed sepsis | Multicenter | 359 | 558 |
| 307, 2010 | Emergency department, intensive car unit, general ward, presumed sepsis | Multicenter, prospective | 212 | 400 |
Studies were searched for in PubMed using the terms “pathogen” and “multiplex PCR,” where blood samples for culture and PCR were drawn at the same time for comparison. Comparable data for larger cohorts of patients are currently available only for the SeptiFast multiplex PCR test. Further important proof-of-concept studies which applied different platforms are discussed in the text.
Several avenues have been pursued to address the opposite problem, i.e., a PCR-positive but blood-culture negative result: a study by Louie et al. assessed PCR in addition to blood culture and other microbiological specimens from the alleged focus along with a chart review (which can be considered a constructed gold standard). PCR from DNA prepared from whole blood led to an increase in presumably true-positive results in septic patients, although a share of the PCR results still remained unconfirmed (161). In this study, PCR detected potentially significant bacteria and fungi in 45 cases, compared to 37 cases detected by BC, in 200 patients from the emergency department, intensive care unit, and general wards who presented with SIRS. Over 68% of the PCR results were confirmed in that study by blood, urine, and catheter cultures. It is also noteworthy that all MRSA bacteremic episodes were detected by PCR (161).
The assessment of the association of the PCR status with measures of morbidity and mortality reflects a complementary strategy to validate the significance of an amplicon in the absence of a concomitant blood culture result. In our study (23), which was restricted to cases with severe sepsis, 34.7% of PCRs were positive, compared to 16.5% of BCs. Consistently, 78% of positive BCs had a corresponding PCR result, while only 23% of PCR results were confirmed by BC. Compared to patients with negative PCR results at enrollment, those testing positive had higher organ dysfunction scores and a trend toward higher mortality rates (PCR negative, 25.3%; PCR positive, 39.1%; P = 0.115). In predefined cohorts of patients with a fungal amplicon, these were observed twice as frequently as cultures positive for fungi, and both results were associated with approximately 90% mortality. These data lend support to the concept that the presence of a pathogen-associated DNA amplicon reflects a meaningful event in severe sepsis. PCR warrants further investigation regarding its suitability to guide anti-infective therapy, particularly in regard to fastidious and difficult-to-culture pathogens, such as Candida species, or specific resistance problems, such as MRSA. The strategy using PCR in order to close the gap regarding those pathogens not covered by guideline-recommended initial treatment has been advocated lately to address emerging clinical needs (208). This could be achieved by technically less-demanding tests with higher accuracy and more cost-effectiveness.
Although recovery rates of PCR are generally higher than the ones obtained by conventional culture, the identification of an allegedly causative microorganism fails in the majority of sepsis episodes even in the most severely sick septic patients. This has prompted efforts to improve the preanalytical handling of blood samples to increase sensitivity. Whole blood as a template for PCR faces limitations due to its very high human DNA background level. An increase of the ratio of pathogen DNA to host DNA may improve the diagnostic performance of PCR to amplify pathogen DNA against the eukaryotic background. The discrimination of “self” as opposed to bacterial DNA is achieved by immunocompetent cells via species-specific cytidylate-phosphate-deoxyguanylate (CpG) motif recognition via TLR9. This process is not restricted to TLR9 but is also observed via a human CpG-binding protein (hCGBP) (or CXXC finger protein 1) (128, 291), a mammalian transcriptional activator which avidly binds unmethylated CpG dinucleotide motifs by the recognition of the sequence (A/C)CG(A/C) with an even larger number of potential binding sites than TLR9 (18, 140). A cloned truncated protein derived from CXXC finger protein 1 can be used for affinity chromatography to achieve a relative enrichment of bacterial DNA out of blood samples from septic patients. In comparison to BC, an approximate 3-fold increase in sensitivity was achieved in a limited cohort of patients with episodes of bacteremia or “DNAemia,” while the time to result was reduced, as in other PCR-based approaches, to less than 8 h (234) despite the additional preanalytic step.
However, it needs to be emphasized that culture-independent nucleic acid amplification-based approaches are not fast enough to postpone empirical anti-infective therapy and are hampered by a limited capability to assess resistance, in particular for Gram-negative infections. They have potential as a supplement to BC to reduce the time to results to readjust antimicrobial therapy, specifically for infections such as those by Candida or MRSA, which are not covered by guideline-recommended treatment. Such strategies which modify empirical antibiotic therapy to restrict the duration and narrow the spectrum of therapy are currently considered valuable and may minimize the development of resistant pathogens and help to contain costs.
The Promise of Systems Biology and “Omics” Technologies
There is great hope that systems biology approaches using high-throughput technologies, such as transcriptomics, proteomics, and metabolomics, will contribute to a better understanding of the pathophysiology of sepsis and systemic inflammation and will allow the development of better sepsis biomarkers (103, 248, 302). There are recent data that suggest that transcriptomic profiling by multiplexed quantitative PCR (qPCR) and metabolite detection by liquid chromatography-tandem mass spectrometry (LC-MS/MS) may have potential in the clinical development of diagnostic tests that are capable of overcoming the limitations of single molecules to differentiate between infectious and noninfectious causes of systemic inflammation and help to determine the status of the patient's immune system (240, 263). Mass spectrometry-based proteomic profiling technologies, such as ProteinChip surface-enhanced laser desorption ionization (SELDI), have been used to identify host response signature proteins for the diagnosis of pathological conditions such as early-onset neonatal sepsis, intra-amniotic inflammation, and severe acute respiratory syndrome (46, 201, 210, 233). The great potential of an unbiased approach to the identification of novel host response biomarkers for the early and accurate diagnosis of septicemia by quantitative proteome profiling with MALDI-TOF mass spectrometry was recently demonstrated in an excellent case-control study of preterm infants with neonatal septicemia and necrotizing enterocolitis (NEC) (191). Proapolipoprotein CII (Pro-ApoC2) and a desarginine variant of serum amyloid A (SAA) were identified as the most promising biomarkers and were validated in a prospective cohort study with 104 consecutively suspected sepsis/NEC episodes by using an ApoSAA score computed from plasma ApoC2 and SAA concentrations. The stratification of infants into different risk categories by the ApoSAA score enabled the withholding of treatment of 45% of nonsepsis infants and the enactment of an early stoppage of antibiotics in 16% of nonsepsis infants. The negative predictive value of this antibiotic policy was 100% (Table 1) (191).
Expression profiling is an approach that involves the use of microarray technology to simultaneously measure mRNAs of thousands of transcripts. This provides a rapid method for the unbiased screening of thousands of molecular species in a single assay (58, 62). Insights from such genome-wide transcriptomic profiling studies that involved trauma and burn patients (306) as well as data from endotoxin challenges in human volunteers (51) suggest that all these stressors induce a “genomic storm” in which up to 80% of the leukocyte transcriptome is altered, involving the systemic inflammatory, innate immune, and compensatory anti-inflammatory responses as well as the suppression of genes that regulate adaptive immunity (306). The authors of that study concluded that there is no other clinical condition that is associated with “such diversity and magnitude of genomic changes”; moreover, they could not align their findings at the level of the transcriptome with the current SIRS/CARS paradigm (51, 306). Interestingly, all genes moved in the same direction regardless of the patient's clinical course, and delayed clinical recovery with organ injury was not associated with dramatic qualitative differences in the leukocyte transcriptome. In these trauma and burn patients, there was no evidence of a single gene or cluster of genes where expression changed in association with the clinical course and outcome (306). These conclusions, however, are limited to the fact that they were based on transcriptomic changes, and measurements of the proteomic and metabolomic levels are missing. In this respect, transcriptomic profiling using a whole-genome approach is obviously less suitable to reflect the clinical course of systemic inflammation than single biomarkers such as interleukin-6, procalcitonin, and pro-ADM (102, 106, 114, 187, 200).
However, genome-wide analysis across the spectrum of pediatric SIRS, sepsis, and septic shock demonstrated that there were some common patterns of gene expression and repression across the entire spectrum, with upregulated genes relating broadly to innate immunity and inflammation. Furthermore, there also existed patterns of gene expression that were relatively specific to each of these three clinical syndromes, in particular for septic shock (303).
The authors of a recent systematic review that comprised 12 studies with genome-wide expression data for early and late sepsis in children and adults did not find strong genomic evidence to support the classical two-phase model of sepsis (268). The most consistent finding in those studies was an upregulation of pathogen recognition and signal transduction pathway genes during the early and late phases of sepsis. In contrast, changes in the inflammatory pathways were much less consistent, and the early, transient increase in levels of some proinflammatory mediators was evident only in a minority of studies. In some studies, the expression of anti-inflammatory genes dominated over the expression of proinflammatory genes (268). McDunn et al. investigated the hypothesis that transcriptional profiles of circulating leukocytes can be used to monitor the host response in such a way that the onset of an infection-specific transcriptional program may precede the clinical diagnosis of pneumonia in patients (176).
Potential of gene expression profiling to distinguish SIRS and sepsis.
Some studies suggested that gene expression profiling may help to differentiate between sterile SIRS and early sepsis (123) and enable the discrimination of patients with acute infections (217). A set of 85 leukocyte genes (circulating leukocyte RNA profiles or “riboleukograms”) detected ventilator-associated pneumonia (VAP) in 158 intubated patients after blunt trauma (57 [36%] of whom developed VAP) with a sensitivity of 57% and a specificity of 69% (61, 176). In ventilated children, expression patterns of 48 genes appeared to discriminate children with and children without VAP (299). Another research group identified a molecular signature of 138 genes expressed in peripheral blood mononuclear cells that could differentiate between sepsis and SIRS among 70 critically ill patients with 80% accuracy (sensitivity of 0.81 and specificity of 0.79) (269).
A recent prospective multicenter study provided a proof of concept that a set of 42 molecular markers could differentiate between postsurgical SIRS and bacteremia with an accuracy of between 86 and 92% for the detection of sepsis (263). In that study, 145 human white blood cell (WBC) genes associated with acute infection and inflammation were identified as being abnormally expressed. From these, a panel of 42 genes linked with innate and early adaptive immune function/activation pathways were identified a priori by using preclinical research outcomes, allowing a platform transfer to a multiplex qPCR format, retaining sensitivity and specificity, with a time to result within hours (263). The clinical utility of both molecular tests to identify pathogens and the ensuing host response still has to be evaluated with sufficiently powered prospective clinical utility trials with a design to allow assessments of their potential to distinguish SIRS from sepsis and to guide therapy.
There is hope that improved molecular diagnostics and prognostics based on transcriptomic, proteomic, or metabolic profiling will not only result in a better understanding of the complexity of systemic inflammation but also lead to new diagnostic tools which help to recognize infection and sepsis earlier and differentiate between infectious and noninfectious inflammation. Such tools also hold promise for the identification of new therapeutic targets and the identification of patient populations that may benefit from specific interventions that are aimed at these targets.
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of Andreas Kortgen, who preformed the review of studies involving multiplex PCR for the detection of microbial DNA.
K.R. cofounded SIRS-Lab, a university spinoff biotechnology company, which produces a multiplex PCR test for culture-independent pathogen detection. In the past, all authors served as scientific advisors for SIRS-Lab. K.R. served as an advisor to Roche Diagnostics until 2007, received speaker fees and honoraria and served as an advisor for BRAHMS Diagnostics until 2008, and served as an advisor to Eli Lilly and EISAI for the development of adjunctive sepsis therapies. N.C.R. is the cofounder and CEO of InflaRx GmbH, Germany, a company developing new therapeutic antibodies indicated for acute inflammatory diseases.
Biographies

Konrad Reinhart is a Board-Certified Anesthesiologist and a Professor of Anesthesiology and Intensive Care at the Jena University Hospital of the Friedrich Schiller University of Jena, Germany. He received his M.D. from the Free University of Berlin. Since 1993, he has been Chairman of the Department of Anesthesiology and Intensive Care Medicine. He was the founding president of the German Sepsis Society in 2001 and became the founding chairman of the Global Sepsis Alliance in 2010. He is a member of the Council of the World Federation of Societies of Intensive & Critical Care Medicine (WFSICCM) and the International Sepsis Forum (ISF). As a speaker for the publicly founded nationwide German research network SepNet, he initiated a number of large, multicenter, randomized clinical trials on the efficacy and safety of new diagnostic and therapeutic approaches to sepsis. His research activities in the field of sepsis and intensive care medicine led to more than 250 peer-reviewed publications. He received the Research Award of the Federal State of Thuringia in 2008 and became a member of the German National Academy of Sciences Leopoldina in 2011.

Michael Bauer is Professor of Anesthesiology and Intensive Care and Chief Executive Director of the Center for Sepsis Control Care (CSCC) at the Jena University Hospital of the Friedrich Schiller University of Jena, Germany. He received his M.D. from Saarland University Medical School and postgraduate training in molecular biology at Johns Hopkins University (1993 to 1995). After Board Certification in Anesthesiology and Intensive Care, he was visiting professor at the Department of Biology at the University of North Carolina in Charlotte (1998 to 1999). Dr. Bauer has published more than 50 peer-reviewed papers in the fields of molecular mechanisms of sepsis and shock-related organ dysfunction.

Niels C. Riedemann holds a position as Vice Director of Intensive Care at the University Hospital of the Friedrich Schiller University, Jena, Germany, and he is a Board-Certified General Surgeon and Intensive Care Physician. He has spent several years working as a researcher in the laboratory of Peter Ward at the University of Michigan, Ann Arbor, MI, and was appointed Professor of Experimental Surgery at the Hannover Medical School, Germany, in 2007. He has published extensively about the pathophysiological role of the complement system in sepsis and acute inflammation and received various national and international awards for his research. He is a founding member of the Center for Innovation Competence Septomics at the Friedrich Schiller University of Jena, which was funded with a multi-million-dollar federal grant in 2010. He is also cofounder and CEO of InflaRx GmbH, Germany, a young biotechnology company which develops highly innovative new therapeutic antibodies in the complement field.

Christiane S. Hartog is a lecturer at the Department of Anesthesiology and Intensive Care and Principal Investigator at the Center for Sepsis Control and Care at the Jena University Hospital. She received her M.D. from the University of Erlangen-Nuremberg, Board Certification for General Medicine from the University of Jena, and training in molecular biology at the Free University Berlin (1994 to 1997). In 1998, she joined Professor Reinhart and has published articles in peer-reviewed journals on the epidemiology and treatment of sepsis.
REFERENCES
- 1. Adib-Conquy M, Cavaillon JM. 2009. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 101:36–47 [PubMed] [Google Scholar]
- 2. Agnelli G, Becattini C. 2010. Acute pulmonary embolism. N. Engl. J. Med. 363:266–274 [DOI] [PubMed] [Google Scholar]
- 3. Ahn S, et al. 2011. Role of procalcitonin and C-reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respi. Viruses 5:398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Subaie N, et al. 2010. C-reactive protein as a predictor of outcome after discharge from the intensive care: a prospective observational study. Br. J. Anaesth. 105:318–325 [DOI] [PubMed] [Google Scholar]
- 5. Andaluz-Ojeda D, et al. 2012. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine 57:332–336 [DOI] [PubMed] [Google Scholar]
- 6. Angus DC. 2012. Drotrecogin alfa (activated)…a sad final fizzle to a roller-coaster party. Crit. Care 16:107 doi:10.1186/cc11152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angus DC. 2010. The lingering consequences of sepsis: a hidden public health disaster? JAMA 304:1833–1834 [DOI] [PubMed] [Google Scholar]
- 8. Angus DC, et al. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 9. Annane D, et al. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871 [DOI] [PubMed] [Google Scholar]
- 10. Assicot M, et al. 1993. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkinson AJ, et al. 2001. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69:89–95 [DOI] [PubMed] [Google Scholar]
- 12. Avolio M, et al. 2010. Molecular identification of bloodstream pathogens in patients presenting to the emergency department with suspected sepsis. Shock 34:27–30 [DOI] [PubMed] [Google Scholar]
- 13. Backes Y, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barati M, et al. 2010. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J. Crit. Care 25:362.e1–362.e6 doi:10.1016/j.jcrc.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 15. Barochia AV, et al. 2010. Bundled care for septic shock: an analysis of clinical trials. Crit. Care Med. 38:668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bateman BT, Schmidt U, Berman MF, Bittner EA. 2010. Temporal trends in the epidemiology of severe postoperative sepsis after elective surgery: a large, nationwide sample. Anesthesiology 112:917–925 [DOI] [PubMed] [Google Scholar]
- 17. Baue AE. 1975. Multiple, progressive, or sequential systems failure, a syndrome of the 1970s. Arch. Surg. 110:779–781 [DOI] [PubMed] [Google Scholar]
- 18. Bauer S, et al. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 98:9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becker K, et al. 2001. Calcitonin gene family of peptides, p 520–534 In Becker K. (ed), Principles and practice of endocrinology and metabolism, 3rd ed JB Lippincott Co, Philadelphia, PA [Google Scholar]
- 20. Becker KL, Nylen ES, White JC, Muller B, Snider RH., Jr 2004. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis. A journey from calcitonin back to its precursors. J. Clin. Endocrinol. Metab. 89:1512–1525 [DOI] [PubMed] [Google Scholar]
- 21. Bernard GR, et al. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699–709 [DOI] [PubMed] [Google Scholar]
- 22. Berner R, et al. 1998. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr. Res. 44:469–477 [DOI] [PubMed] [Google Scholar]
- 23. Bloos F, et al. 2010. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 36:241–247 [DOI] [PubMed] [Google Scholar]
- 24. Bo L, Wang F, Zhu J, Li J, Deng X. 2011. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit. Care 15:R58 doi:10.1186/cc10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bogar L, Molnar Z, Kenyeres P, Tarsoly P. 2006. Sedimentation characteristics of leucocytes can predict bacteraemia in critical care patients. J. Clin. Pathol. 59:523–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bohoun C, Petitjean S, Assicot M. 1994. Blood procalcitonin is a new biological marker of the human septic response. New data on the specificity. Clin. Intensive Care 5(Suppl 2): 89–98 [Google Scholar]
- 27. Bone RC. 1994. Gram-positive organisms and sepsis. Arch. Intern. Med. 154:26–34 [PubMed] [Google Scholar]
- 28. Bone RC. 1996. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 24:1125–1128 [DOI] [PubMed] [Google Scholar]
- 29. Bone RC, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Chest 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 30. Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA. 1987. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 92:1032–1036 [DOI] [PubMed] [Google Scholar]
- 31. Bone RC, et al. 1989. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group Crit. Care Med. 17:389–393 [PubMed] [Google Scholar]
- 32. Boomer JS, et al. 2011. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306:2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bottazzi B, et al. 2009. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol. Rev. 227:9–18 [DOI] [PubMed] [Google Scholar]
- 34. Bouadma L, et al. 2010. Procalcitonin in intensive care units: the PRORATA trial—authors' reply. Lancet 375:1606–1607 [DOI] [PubMed] [Google Scholar]
- 35. Bouadma L, et al. 2010. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375:463–474 [DOI] [PubMed] [Google Scholar]
- 36. Bouchon A, Dietrich J, Colonna M. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995 [DOI] [PubMed] [Google Scholar]
- 37. Bouchon A, Facchetti F, Weigand MA, Colonna M. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103–1107 [DOI] [PubMed] [Google Scholar]
- 38. Boyle JJ. 2005. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr. Vasc. Pharmacol. 3:63–68 [DOI] [PubMed] [Google Scholar]
- 39. Bozza FA, et al. 2004. Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock 22:309–313 [DOI] [PubMed] [Google Scholar]
- 40. Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. 2005. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation 111:1415–1421 [DOI] [PubMed] [Google Scholar]
- 41. Briel M, et al. 2008. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 168:2000–2007; discussion 2007-2008 [DOI] [PubMed] [Google Scholar]
- 42. Brun-Buisson C, Meshaka P, Pinton P, Vallet B. 2004. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 30:580–588 [DOI] [PubMed] [Google Scholar]
- 43. Brunkhorst F, Sakr Y, Hagel S, Reinhart K. 2007. Protein C concentrations correlate with organ dysfunction and predict outcome independent of the presence of sepsis. Anesthesiology 107:15–23 [DOI] [PubMed] [Google Scholar]
- 44. Brunkhorst FM, Eberhard OK, Brunkhorst R. 1999. Discrimination of infectious and noninfectious causes of early acute respiratory distress syndrome by procalcitonin. Crit. Care Med. 27:2172–2176 [DOI] [PubMed] [Google Scholar]
- 45. Brunkhorst FM, Heinz U, Forycki ZF. 1998. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 24:888–889 [DOI] [PubMed] [Google Scholar]
- 46. Buhimschi CS, et al. 2007. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 4:e18 doi:10.1371/journal.pmed.0040018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burkhardt O, et al. 2010. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur. Respir. J. 36:601–607 [DOI] [PubMed] [Google Scholar]
- 48. Calandra T, Cohen J. 2005. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 33:1538–1548 [DOI] [PubMed] [Google Scholar]
- 49. Calandra T, Roger T. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Calfee CS, et al. 2010. Plasma interleukin-8 is not an effective risk stratification tool for adults with vasopressor-dependent septic shock. Crit. Care Med. 38:1436–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calvano SE, et al. 2005. A network-based analysis of systemic inflammation in humans. Nature 437:1032–1037 [DOI] [PubMed] [Google Scholar]
- 52. Chalupa P, Beran O, Herwald H, Kasprikova N, Holub M. 2011. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection 39:411–417 [DOI] [PubMed] [Google Scholar]
- 53. Charles PE, et al. 2006. Serum procalcitonin measurement contribution to the early diagnosis of candidemia in critically ill patients. Intensive Care Med. 32:1577–1583 [DOI] [PubMed] [Google Scholar]
- 54. Christ-Crain M, et al. 2004. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 363:600–607 [DOI] [PubMed] [Google Scholar]
- 55. Christ-Crain M, et al. 2006. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit. Care 10:R96 doi:10.1186/cc4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christ-Crain M, et al. 2005. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit. Care 9:R816–R824 doi:10.1186/cc3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Christaki E, Anyfanti P, Opal SM. 2011. Immunomodulatory therapy for sepsis: an update. Expert Rev. Anti Infect. Ther. 9:1013–1033 [DOI] [PubMed] [Google Scholar]
- 58. Christie JD. 2005. Microarrays. Crit. Care Med. 33:S449–S452 doi:10.1097/01.CCM.0000186078.26361.96 [DOI] [PubMed] [Google Scholar]
- 59. Christofilopoulou S, Charvalos E, Petrikkos G. 2002. Could procalcitonin be a predictive biological marker in systemic fungal infections? Study of 14 cases. Eur. J. Intern. Med. 13:493–495 [DOI] [PubMed] [Google Scholar]
- 60. Clec'h C, et al. 2006. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit. Care Med. 34:102–107 [DOI] [PubMed] [Google Scholar]
- 61. Cobb JP, et al. 2009. Validation of the riboleukogram to detect ventilator-associated pneumonia after severe injury. Ann. Surg. 250:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cobb JP, O'Keefe GE. 2004. Injury research in the genomic era. Lancet 363:2076–2083 [DOI] [PubMed] [Google Scholar]
- 63. Cohen J. 2001. TREM-1 in sepsis. Lancet 358:776–778 [DOI] [PubMed] [Google Scholar]
- 64. Cuquemelle E, et al. 2011. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 37:796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dalton WS, Friend SH. 2006. Cancer biomarkers—an invitation to the table. Science 312:1165–1168 [DOI] [PubMed] [Google Scholar]
- 66. Davis BH, Bigelow NC. 2005. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts, and automated hematology analyzer flags as indicators of infection or sepsis. Lab. Hematol. 11:137–147 [PubMed] [Google Scholar]
- 67. de Kruif MD, et al. 2008. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 34:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Kruif MD, et al. 2010. PTX3 predicts severe disease in febrile patients at the emergency department. J. Infect. 60:122–127 [DOI] [PubMed] [Google Scholar]
- 69. Delannoy B, Guye ML, Slaiman DH, Lehot JJ, Cannesson M. 2009. Effect of cardiopulmonary bypass on activated partial thromboplastin time waveform analysis, serum procalcitonin and C-reactive protein concentrations. Crit. Care 13:R180 doi:10.1186/cc8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dellinger RP, et al. 2008. Surviving Sepsis Campaign. International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296–327 [DOI] [PubMed] [Google Scholar]
- 70a. Dellinger RP, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med., in press [DOI] [PubMed] [Google Scholar]
- 71. Dev D, et al. 1998. Value of C-reactive protein measurements in exacerbations of chronic obstructive pulmonary disease. Respir. Med. 92:664–667 [DOI] [PubMed] [Google Scholar]
- 72. Dhainaut JF, et al. 2005. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit. Care Med. 33:341–348 [DOI] [PubMed] [Google Scholar]
- 73. Dierkes C, et al. 2009. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect. Dis. 9:126 doi:10.1186/1471-2334-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Docke WD, et al. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678–681 [DOI] [PubMed] [Google Scholar]
- 75. Domann E, et al. 2003. Culture-independent identification of pathogenic bacteria and polymicrobial infections in the genitourinary tract of renal transplant recipients. J. Clin. Microbiol. 41:5500–5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Donadello K, Scolletta S, Covajes C, Vincent JL. 2012. suPAR as a prognostic biomarker in sepsis. BMC Med. 10:2 doi:10.1186/1741-7015-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dornbusch HJ, et al. 2005. Procalcitonin—a marker of invasive fungal infection? Support Care Cancer 13:343–346 [DOI] [PubMed] [Google Scholar]
- 78. Drancourt M, Aboudharam G, Signoli M, Dutour O, Raoult D. 1998. Detection of 400-year-old Yersinia pestis DNA in human dental pulp: an approach to the diagnosis of ancient septicemia. Proc. Natl. Acad. Sci. U. S. A. 95:12637–12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eberhard OK, et al. 1997. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum. 40:1250–1256 [DOI] [PubMed] [Google Scholar]
- 80. Engel A, Mack E, Kern P, Kern WV. 1998. An analysis of interleukin-8, interleukin-6 and C-reactive protein serum concentrations to predict fever, gram-negative bacteremia and complicated infection in neutropenic cancer patients. Infection 26:213–221 [DOI] [PubMed] [Google Scholar]
- 81. Engel C, et al. 2007. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 33:606–618 [DOI] [PubMed] [Google Scholar]
- 82. Eriksson S, Granstrom L, Olander B, Wretlind B. 1995. Sensitivity of interleukin-6 and C-reactive protein concentrations in the diagnosis of acute appendicitis. Eur. J. Surg. 161:41–45 [PubMed] [Google Scholar]
- 83. Eugen-Olsen J. 2011. suPAR—a future risk marker in bacteremia. J. Intern. Med. 270:29–31 [DOI] [PubMed] [Google Scholar]
- 84. Fernandez AL, et al. 2010. Evaluation of a multiplex real-time PCR assay for detecting pathogens in cardiac valve tissue in patients with endocarditis. Rev. Esp. Cardiol. 63:1205–1208 [DOI] [PubMed] [Google Scholar]
- 85. Ferrer R, et al. 2008. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 299:2294–2303 [DOI] [PubMed] [Google Scholar]
- 86. Fry DE, Pearlstein L, Fulton RL, Polk HC., Jr 1980. Multiple system organ failure. The role of uncontrolled infection. Arch. Surg. 115:136–140 [DOI] [PubMed] [Google Scholar]
- 87. Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448–454 [DOI] [PubMed] [Google Scholar]
- 88. Gaini S, Koldkjaer OG, Pedersen C, Pedersen SS. 2006. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit. Care 10:R53 doi:10.1186/cc4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gasanov U, Hughes D, Hansbro PM. 2005. Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: a review. FEMS Microbiol. Rev. 29:851–875 [DOI] [PubMed] [Google Scholar]
- 90. Gendrel D, et al. 1998. Procalcitonin, C-reactive protein and interleukin 6 in bacterial and viral meningitis in children. Presse Med. 27:1135–1139 (In French.) [PubMed] [Google Scholar]
- 91. Gibot S, et al. 2012. Combination biomarkers to diagnose sepsis in the critically ill patient. Am. J. Respir. Crit. Care Med. 186:65–71 [DOI] [PubMed] [Google Scholar]
- 92. Gibot S, et al. 2005. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit. Care Med. 33:792–796 [DOI] [PubMed] [Google Scholar]
- 93. Gibot S, et al. 2004. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N. Engl. J. Med. 350:451–458 [DOI] [PubMed] [Google Scholar]
- 94. Gibot S, et al. 2004. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann. Intern. Med. 141:9–15 [DOI] [PubMed] [Google Scholar]
- 95. Gibot S, et al. 2005. Surface and soluble triggering receptor expressed on myeloid cells-1: expression patterns in murine sepsis. Crit. Care Med. 33:1787–1793 [DOI] [PubMed] [Google Scholar]
- 96. Gogos CA, Drosou E, Bassaris HP, Skoutelis A. 2000. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J. Infect. Dis. 181:176–180 [DOI] [PubMed] [Google Scholar]
- 97. Grif K, et al. 2012. Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis. Wien. Klin. Wochenschr. 124:266–270 [DOI] [PubMed] [Google Scholar]
- 98. Gros A, et al. 2012. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 38:445–452 [DOI] [PubMed] [Google Scholar]
- 99. Gruithuisen VPF. (ed). 1814. Hippocrates des Zweyten ächte medizinische Schriften, ins Deutsche übersetzt. Ignaz Josef Lentner, Munich, Germany [Google Scholar]
- 100. Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. 2011. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS data brief, vol 62 National Center for Health Statistics, Hyattsville, MD: [PubMed] [Google Scholar]
- 101. Hamano K, et al. 1998. Increased serum interleukin-8: correlation with poor prognosis in patients with postoperative multiple organ failure. World J. Surg. 22:1077–1081 [DOI] [PubMed] [Google Scholar]
- 102. Harbarth S, et al. 2001. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 164:396–402 [DOI] [PubMed] [Google Scholar]
- 103. Hartlova A, Krocova Z, Cerveny L, Stulik J. 2011. A proteomic view of the host-pathogen interaction: the host perspective. Proteomics 11:3212–3220 [DOI] [PubMed] [Google Scholar]
- 104. Hebart H, et al. 2009. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 43:553–561 [DOI] [PubMed] [Google Scholar]
- 105. Hebra F. 1947. Höchst wichtige Erfahrungen über die Ätiologie der in Gebäranstalten epidemischen Puerperalfieber. Zeitschr. Ges. Arzte Wien 4:242–244 [Google Scholar]
- 106. Heper Y, et al. 2006. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur. J. Clin. Microbiol. Infect. Dis. 25:481–491 [DOI] [PubMed] [Google Scholar]
- 107. Hirata Y, et al. 1996. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J. Clin. Endocrinol. Metab. 81:1449–1453 [DOI] [PubMed] [Google Scholar]
- 108. Ho KM, Lee KY, Dobb GJ, Webb SA. 2008. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a prospective cohort study. Intensive Care Med. 34:481–487 [DOI] [PubMed] [Google Scholar]
- 109. Hochreiter M, et al. 2009. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit. Care 13:R83 doi:10.1186/cc7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hoffmann G, et al. 2001. In vitro modulation of inducible nitric oxide synthase gene expression and nitric oxide synthesis by procalcitonin. Crit. Care Med. 29:112–116 [DOI] [PubMed] [Google Scholar]
- 111. Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. 2009. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 15:496–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hotchkiss RS, Karl IE. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150 [DOI] [PubMed] [Google Scholar]
- 113. Hotchkiss RS, Opal S. 2010. Immunotherapy for sepsis—a new approach against an ancient foe. N. Engl. J. Med. 363:87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Huang DT, et al. 2009. Midregional proadrenomedullin as a prognostic tool in community-acquired pneumonia. Chest 136:823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huber W, Schweigart U, Bottermann P. 1997. Failure of PCT to indicate severe fungal infection in two immunodeficient patients. Infection 25:377–378 [DOI] [PubMed] [Google Scholar]
- 116. Hurley JC. 1995. Endotoxemia: methods of detection and clinical correlates. Clin. Microbiol. Rev. 8:268–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huttunen R, et al. 2011. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS One 6:e17653 doi:10.1371/journal.pone.0017653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Icardi M, et al. 2009. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J. Clin. Microbiol. 47:3914–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Iwashyna TJ, Ely EW, Smith DM, Langa KM. 2010. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jacob L. 1909. Ueber Allgemeininfektion durch Bacterium coli commune. Dtsch. Arch. Klin. Med. 97:303–347 [Google Scholar]
- 121. Jensen JU, et al. 2011. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit. Care Med. 39:2048–2058 [DOI] [PubMed] [Google Scholar]
- 122. Jiyong J, Tiancha H, Wei C, Huahao S. 2009. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med. 35:587–595 [DOI] [PubMed] [Google Scholar]
- 123. Johnson SB, et al. 2007. Gene expression profiles differentiate between sterile SIRS and early sepsis. Ann. Surg. 245:611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jones AE, Fiechtl JF, Brown MD, Ballew JJ, Kline JA. 2007. Procalcitonin test in the diagnosis of bacteremia: a meta-analysis. Ann. Emerg. Med. 50:34–41 [DOI] [PubMed] [Google Scholar]
- 125. Josefson P, et al. 2011. Evaluation of a commercial multiplex PCR test (SeptiFast) in the etiological diagnosis of community-onset bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 30:1127–1134 [DOI] [PubMed] [Google Scholar]
- 126. Karlsson S, et al. 2008. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 34:1046–1053 [DOI] [PubMed] [Google Scholar]
- 127. Karzai W, Meisner M, Reinhart K. 1999. New approaches to the diagnosis of sepsis. Curr. Opin. Crit. Care 5:357–362 [Google Scholar]
- 128. Klose RJ, Bird AP. 2006. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 31:89–97 [DOI] [PubMed] [Google Scholar]
- 129. Kofoed K, et al. 2007. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit. Care 11:R38 doi:10.1186/cc5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kollef KE, et al. 2008. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 134:281–287 [DOI] [PubMed] [Google Scholar]
- 131. Komiya K, et al. 14 December 2011. Plasma C-reactive protein levels are associated with mortality in elderly with acute lung injury. J. Crit. Care [Epub ahead of print.] doi:10.1016/j.jcrc.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 132. Kopterides P, Siempos, Tsangaris I, Tsantes A, Armaganidis A. 2010. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 38:2229–2241 [DOI] [PubMed] [Google Scholar]
- 133. Kristoffersen KB, et al. 2009. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin. Microbiol. Infect. 15:481–487 [DOI] [PubMed] [Google Scholar]
- 134. Kruger S, et al. 2008. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur. Respir. J. 31:349–355 [DOI] [PubMed] [Google Scholar]
- 135. Kumar A, et al. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 136. Kumar V, Cotran RS, Robbins SL. (ed). 2003. Robbins basic pathology, 7th ed Saunders, Philadelphia, PA [Google Scholar]
- 137. Kutukculer N, Caglayan S, Aydogdu F. 1998. Study of pro-inflammatory (TNF-alpha, IL-1alpha, IL-6) and T-cell-derived (IL-2, IL-4) cytokines in plasma and synovial fluid of patients with juvenile chronic arthritis: correlations with clinical and laboratory parameters. Clin. Rheumatol. 17:288–292 [DOI] [PubMed] [Google Scholar]
- 138. Lamoth F, et al. 2010. Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J. Clin. Microbiol. 48:3510–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Latour-Perez J, et al. 2010. Diagnostic accuracy of sTREM-1 to identify infection in critically ill patients with systemic inflammatory response syndrome. Clin. Biochem. 43:720–724 [DOI] [PubMed] [Google Scholar]
- 140. Lee JH, Voo KS, Skalnik DG. 2001. Identification and characterization of the DNA binding domain of CpG-binding protein. J. Biol. Chem. 276:44669–44676 [DOI] [PubMed] [Google Scholar]
- 141. Lee TH, Goldman L. 2000. Evaluation of the patient with acute chest pain. N. Engl. J. Med. 342:1187–1195 [DOI] [PubMed] [Google Scholar]
- 142. Lehmann LE, et al. 2009. Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit. Care Med. 37:3085–3090 [DOI] [PubMed] [Google Scholar]
- 143. Lehmann LE, et al. 2010. Improved detection of blood stream pathogens by real-time PCR in severe sepsis. Intensive Care Med. 36:49–56 [DOI] [PubMed] [Google Scholar]
- 144. Lehmann LE, et al. 2001. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Med. 27:1412–1415 [DOI] [PubMed] [Google Scholar]
- 145. Levi M, Ten Cate H. 1999. Disseminated intravascular coagulation. N. Engl. J. Med. 341:586–592 [DOI] [PubMed] [Google Scholar]
- 146. Levin J, Bang FB. 1968. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb. Diath. Haemorrh. 19:186–197 [PubMed] [Google Scholar]
- 147. Levy MM, et al. 2010. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit. Care Med. 38:367–374 [DOI] [PubMed] [Google Scholar]
- 148. Levy MM, et al. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 29:530–538 [DOI] [PubMed] [Google Scholar]
- 149. Li H, Luo YF, Blackwell TS, Xie CM. 2011. Meta-analysis and systematic review of procalcitonin-guided therapy in respiratory tract infections. Antimicrob. Agents Chemother. 55:5900–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Li L, et al. 26 June 2012. Diagnostic value of soluble triggering receptor expressed on myeloid cells-1 in critically ill, postoperative patients with suspected sepsis. Am. J. Med. Sci. [Epub ahead of print.] doi:10.1097/MAJ.0b013e318253a1a6 [DOI] [PubMed] [Google Scholar]
- 151. Limaye AP, et al. 2008. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. 2010. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J. Infect. 60:409–416 [DOI] [PubMed] [Google Scholar]
- 153. Linscheid P, et al. 2004. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 32:1715–1721 [DOI] [PubMed] [Google Scholar]
- 154. Lister J. 1867. On a new method of treating compound fracture, abscess, etc.: with observations on the conditions of suppuration. Lancet 89:387–389 [Google Scholar]
- 155. Litton E, Ho KM, Chamberlain J, Dobb GJ, Webb SA. 2007. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a nested case-control study. Crit. Care Resusc. 9:19–25 [PubMed] [Google Scholar]
- 156. Lobo SM, et al. 2003. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest 123:2043–2049 [DOI] [PubMed] [Google Scholar]
- 157. Lodes U, Bohmeier B, Lippert H, Konig B, Meyer F. 2011. PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS. Langenbecks Arch. Surg. 397:447–455 [DOI] [PubMed] [Google Scholar]
- 158. Lodes U, Meyer F, Konig B, Lippert H. 2009. Microbiological sepsis screening in surgical ICU patients with the “lightCycler” Septifast test—a pilot study. Zentralbl. Chir. 134:249–253 (In German.) [DOI] [PubMed] [Google Scholar]
- 159. Long W, et al. 2011. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology 16:819–824 [DOI] [PubMed] [Google Scholar]
- 160. Long W, et al. 2009. The value of serum procalcitonin in treatment of community acquired pneumonia in outpatient. Zhonghua Nei Ke Za Zhi 48:216–219 (In Chinese.) [PubMed] [Google Scholar]
- 161. Louie RF, et al. 2008. Multiplex polymerase chain reaction detection enhancement of bacteremia and fungemia. Crit. Care Med. 36:1487–1492 [DOI] [PubMed] [Google Scholar]
- 162. Lucignano B, et al. 2011. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J. Clin. Microbiol. 49:2252–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lvovschi V, et al. 2011. Cytokine profiles in sepsis have limited relevance for stratifying patients in the emergency department: a prospective observational study. PLoS One 6:e28870 doi:10.1371/journal.pone.0028870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Machiavelli N. 2002. The Prince, translated by WK Marriott. eBooks@Adelaide, The University of Adelaide Library, University of Adelaide, Adelaide, South Australia, Australia [Google Scholar]
- 165. Majno G, Joris I. 2004. Cells, tissues, and disease: principles of general pathology, 2nd ed Oxford University Press, New York, NY [Google Scholar]
- 166. Malaguarnera M, et al. 1997. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J. Gastroenterol. 32:211–215 [DOI] [PubMed] [Google Scholar]
- 167. Mancini N, et al. 2008. Molecular diagnosis of sepsis in neutropenic patients with haematological malignancies. J. Med. Microbiol. 57:601–604 [DOI] [PubMed] [Google Scholar]
- 168. Marshall JC. 2000. SIRS and MODS: what is their relevance to the science and practice of intensive care? Shock 14:586–589 [PubMed] [Google Scholar]
- 169. Marshall JC, et al. 2004. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J. Infect. Dis. 190:527–534 [DOI] [PubMed] [Google Scholar]
- 170. Marshall JC, Reinhart K. 2009. Biomarkers of sepsis. Crit. Care Med. 37:2290–2298 [DOI] [PubMed] [Google Scholar]
- 171. Marshall JC, et al. 2003. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit. Care Med. 31:1560–1567 [DOI] [PubMed] [Google Scholar]
- 172. Matzinger P. 2002. The danger model: a renewed sense of self. Science 296:301–305 [DOI] [PubMed] [Google Scholar]
- 173. Mauri T, et al. 2010. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 36:621–629 [DOI] [PubMed] [Google Scholar]
- 174. Mauro MV, et al. 2012. Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients. Diagn. Microbiol. Infect. Dis. 73:308–311 [DOI] [PubMed] [Google Scholar]
- 175. McDonald RR, et al. 2005. Development of a triplex real-time PCR assay for detection of Panton-Valentine leukocidin toxin genes in clinical isolates of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:6147–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. McDunn JE, et al. 2008. Plasticity of the systemic inflammatory response to acute infection during critical illness: development of the riboleukogram. PLoS One 3:e1564 doi:10.1371/journal.pone.0001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454:428–435 [DOI] [PubMed] [Google Scholar]
- 178. Meisel C, et al. 2009. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 180:640–648 [DOI] [PubMed] [Google Scholar]
- 179. Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schuttler J. 1998. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 24:680–684 [DOI] [PubMed] [Google Scholar]
- 180. Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. 1999. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit. Care 3:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mesters RM, et al. 2000. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit. Care Med. 28:2209–2216 [DOI] [PubMed] [Google Scholar]
- 182. Miyazaki T, et al. 1995. (1→3)-Beta-D-glucan in culture fluid of fungi activates factor G, a Limulus coagulation factor. J. Clin. Lab. Anal. 9:334–339 [DOI] [PubMed] [Google Scholar]
- 183. Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. 2009. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 146:72–81 [DOI] [PubMed] [Google Scholar]
- 184. Monneret G, et al. 1997. Procalcitonin and C-reactive protein levels in neonatal infections. Acta Paediatr. 86:209–212 [DOI] [PubMed] [Google Scholar]
- 185. Monneret G, Venet F, Pachot A, Lepape A. 2008. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol. Med. 14:64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Muller B, et al. 2000. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 28:977–983 [DOI] [PubMed] [Google Scholar]
- 187. Muller B, et al. 2007. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect. Dis. 7:10 doi:10.1186/1471-2334-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Muller F, et al. 2010. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 138:121–129 [DOI] [PubMed] [Google Scholar]
- 189. Nast-Kolb D, et al. 1997. Indicators of the posttraumatic inflammatory response correlate with organ failure in patients with multiple injuries. J. Trauma 42:446–454; discussion 454-455 [DOI] [PubMed] [Google Scholar]
- 190. Nathan C. 2002. Points of control in inflammation. Nature 420:846–852 [DOI] [PubMed] [Google Scholar]
- 191. Ng PC, et al. 2010. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J. Clin. Invest. 120:2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ng PC, Lam HS. 2006. Diagnostic markers for neonatal sepsis. Curr. Opin. Pediatr. 18:125–131 [DOI] [PubMed] [Google Scholar]
- 193. Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. 2008. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am. J. Respir. Crit. Care Med. 177:498–505 [DOI] [PubMed] [Google Scholar]
- 194. O'Grady NP, et al. 2008. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care Med. 36:1330–1349 [DOI] [PubMed] [Google Scholar]
- 195. Obara H, et al. 2011. The role of a real-time PCR technology for rapid detection and identification of bacterial and fungal pathogens in whole-blood samples. J. Infect. Chemother. 17:327–333 [DOI] [PubMed] [Google Scholar]
- 196. Opal SM, et al. 1999. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180:1584–1589 [DOI] [PubMed] [Google Scholar]
- 197. Otto GP, et al. 2011. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 15:R183 doi:10.1186/cc10332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Reference deleted.
- 199. Ozsu S, et al. 2011. C-reactive protein alone or combined with cardiac troponin T for risk stratification of respiratory intensive care unit patients. Respir. Care 56:1002–1008 [DOI] [PubMed] [Google Scholar]
- 200. Panacek EA, et al. 2004. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab′)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 32:2173–2182 [DOI] [PubMed] [Google Scholar]
- 201. Pang RT, et al. 2006. Serum proteomic fingerprints of adult patients with severe acute respiratory syndrome. Clin. Chem. 52:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Paolucci M, et al. 2009. Laboratory diagnosis of late-onset sepsis in newborns by multiplex real-time PCR. J. Med. Microbiol. 58:533–534 [DOI] [PubMed] [Google Scholar]
- 203. Papadopoulos N, Kinzler KW, Vogelstein B. 2006. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat. Biotechnol. 24:985–995 [DOI] [PubMed] [Google Scholar]
- 204. Pasqualini L, et al. 2012. Diagnostic performance of a multiple real-time PCR assay in patients with suspected sepsis hospitalized in an internal medicine ward. J. Clin. Microbiol. 50:1285–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4:751–760 [DOI] [PubMed] [Google Scholar]
- 206. Pettila V, Pentti J, Pettila M, Takkunen O, Jousela I. 2002. Predictive value of antithrombin III and serum C-reactive protein concentration in critically ill patients with suspected sepsis. Crit. Care Med. 30:271–275 [DOI] [PubMed] [Google Scholar]
- 207. Pierrakos C, Vincent JL. 2010. Sepsis biomarkers: a review. Crit. Care 14:R15 doi:10.1186/cc8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Pletz MW, Wellinghausen N, Welte T. 2011. Will polymerase chain reaction (PCR)-based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med. 37:1069–1076 [DOI] [PubMed] [Google Scholar]
- 209. Ploug M, et al. 1991. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J. Biol. Chem. 266:1926–1933 [PubMed] [Google Scholar]
- 210. Poon TC. 2007. Opportunities and limitations of SELDI-TOF-MS in biomedical research: practical advices. Expert Rev. Proteomics 4:51–65 [DOI] [PubMed] [Google Scholar]
- 211. Povoa P, Souza-Dantas VC, Soares M, Salluh JF. 2011. C-reactive protein in critically ill cancer patients with sepsis: influence of neutropenia. Crit. Care 15:R129 doi:10.1186/cc10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Povoa P, Teixeira-Pinto AM, Carneiro AH. 2011. C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Crit. Care 15:R169 doi:10.1186/cc10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Quartin AA, Schein RM, Kett DH, Peduzzi PN. 1997. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA 277:1058–1063 [PubMed] [Google Scholar]
- 214. Qureshi SS, et al. 2001. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS). Clin. Exp. Immunol. 125:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Rabna P, et al. 2009. High mortality risk among individuals assumed to be TB-negative can be predicted using a simple test. Trop. Med. Int. Health 14:986–994 [DOI] [PubMed] [Google Scholar]
- 216. Radimerski TM, et al. 2010. Role of calcium in lipopolysaccharide-induced calcitonin gene expression in human adipocytes. Innate Immun. 17:403–413 [DOI] [PubMed] [Google Scholar]
- 217. Ramilo O, et al. 2007. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 109:2066–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ranzani OT, et al. 6 January 2012. Failure to reduce C-reactive protein levels more than 25% in the last 24 hours before intensive care unit discharge predicts higher in-hospital mortality: a cohort study. J. Crit. Care [Epub ahead of print.] doi:10.1016/j.jcrc.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 219. Rath PM, et al. 2012. Multiplex PCR for rapid and improved diagnosis of bloodstream infections in liver transplant recipients. J. Clin. Microbiol. 50:2069–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Rau B, et al. 1997. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 41:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Regueiro BJ, et al. 2010. Automated extraction improves multiplex molecular detection of infection in septic patients. PLoS One 5:e13387 doi:10.1371/journal.pone.0013387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Reinhart K, Meisner M. 2011. Biomarkers in the critically ill patient: procalcitonin. Crit. Care Clin. 27:253–263 [DOI] [PubMed] [Google Scholar]
- 223. Reinhart K, et al. 2001. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study. Crit. Care Med. 29:765–769 [DOI] [PubMed] [Google Scholar]
- 224. Reinhart K, et al. 1996. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit. Care Med. 24:733–742 [DOI] [PubMed] [Google Scholar]
- 225. Riedemann NC, Guo RF, Ward PA. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 9:517–524 [DOI] [PubMed] [Google Scholar]
- 226. Rivers E, et al. 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 227. Rivers EP, et al. 2007. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit. Care Med. 35:2016–2024 [DOI] [PubMed] [Google Scholar]
- 228. Robak T, Gladalska A, Stepien H, Robak E. 1998. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm. 7:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Roberts RR, et al. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin. Infect. Dis. 49:1175–1184 [DOI] [PubMed] [Google Scholar]
- 230. Roldan AL, et al. 1990. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 9:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Roth RI, Levin FC, Levin J. 1990. Optimization of detection of bacterial endotoxin in plasma with the Limulus test. J. Lab. Clin. Med. 116:153–161 [PubMed] [Google Scholar]
- 232. Rubulotta FM, et al. 2009. An international survey: public awareness and perception of sepsis. Crit. Care Med. 37:167–170 [DOI] [PubMed] [Google Scholar]
- 233. Ruetschi U, et al. 2005. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J. Proteome Res. 4:2236–2242 [DOI] [PubMed] [Google Scholar]
- 234. Sachse S, et al. 2009. Truncated human cytidylate-phosphate-deoxyguanylate-binding protein for improved nucleic acid amplification technique-based detection of bacterial species in human samples. J. Clin. Microbiol. 47:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Said EA, et al. 2010. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16:452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. 2008. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit. Care Med. 36:2014–2022 [DOI] [PubMed] [Google Scholar]
- 237. Sakr Y, Reinhart K, Hagel S, Kientopf M, Brunkhorst F. 2007. Antithrombin levels, morbidity, and mortality in a surgical intensive care unit. Anesth. Analg. 105:715–723 [DOI] [PubMed] [Google Scholar]
- 238. Sakr Y, Sponholz C, Tuche F, Brunkhorst F, Reinhart K. 2008. The role of procalcitonin in febrile neutropenic patients: review of the literature. Infection 36:396–407 [DOI] [PubMed] [Google Scholar]
- 239. Sands KE, et al. 1997. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group JAMA 278:234–240 [PubMed] [Google Scholar]
- 240. Schmerler D, et al. 2012. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J. Lipid Res. 53:1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Schottmueller H. 1914. Wesen und Behandlung der Sepsis. Inn. Med. 31:257–280 [Google Scholar]
- 242. Schroeder S, et al. 2009. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch. Surg. 394:221–226 [DOI] [PubMed] [Google Scholar]
- 243. Schuetz P, et al. 2010. Serum procalcitonin, C-reactive protein and white blood cell levels following hypothermia after cardiac arrest: a retrospective cohort study. Eur. J. Clin. Invest. 40:376–381 [DOI] [PubMed] [Google Scholar]
- 244. Schuetz P, Albrich W, Mueller B. 2011. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 9:107 doi:10.1186/1741-7015-9-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Schuetz P, Chiappa V, Briel M, Greenwald JL. 2011. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch. Intern. Med. 171:1322–1331 [DOI] [PubMed] [Google Scholar]
- 246. Schuetz P, et al. 2009. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 302:1059–1066 [DOI] [PubMed] [Google Scholar]
- 247. Schuetz P, Mueller B, Trampuz A. 2007. Serum procalcitonin for discrimination of blood contamination from bloodstream infection due to coagulase-negative staphylococci. Infection 35:352–355 [DOI] [PubMed] [Google Scholar]
- 248. Serkova NJ, Standiford TJ, Stringer KA. 2011. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am. J. Respir. Crit. Care Med. 184:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Sexton PM, et al. 2008. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit. Care Med. 36:1637–1640 [DOI] [PubMed] [Google Scholar]
- 250. Shapiro NI, et al. 2009. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit. Care Med. 37:96–104 [DOI] [PubMed] [Google Scholar]
- 251. Shaw AC. 1991. Serum C-reactive protein and neopterin concentrations in patients with viral or bacterial infection. J. Clin. Pathol. 44:596–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Shenkin A, et al. 1989. The serum interleukin 6 response to elective surgery. Lymphokine Res. 8:123–127 [PubMed] [Google Scholar]
- 253. Sherwin C, et al. 2008. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am. J. Perinatol. 25:629–636 [DOI] [PubMed] [Google Scholar]
- 254. Silvestre J, Coelho L, Povoa P. 2010. Should C-reactive protein concentration at ICU discharge be used as a prognostic marker? BMC Anesthesiol. 10:17 doi:10.1186/1471-2253-10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. 2004. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 39:206–217 [DOI] [PubMed] [Google Scholar]
- 256. Socan M, Marinic-Fiser N, Kese D. 1999. Comparison of serologic tests with urinary antigen detection for diagnosis of Legionnaires' disease in patients with community-acquired pneumonia. Clin. Microbiol. Infect. 5:201–204 [DOI] [PubMed] [Google Scholar]
- 257. Spink WW. 1978. Infectious diseases. Prevention and treatment in the nineteenth and twentieth centuries. University of Minnesota Press, Minneapolis, MN [Google Scholar]
- 258. Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. 2006. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit. Care 10:R145 doi:10.1186/cc5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Sprung CL, et al. 2008. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 358:111–124 [DOI] [PubMed] [Google Scholar]
- 260. Stolz D, et al. 2007. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 131:9–19 [DOI] [PubMed] [Google Scholar]
- 261. Stolz D, et al. 2009. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur. Respir. J. 34:1364–1375 [DOI] [PubMed] [Google Scholar]
- 262. Struck J, Tao C, Morgenthaler NG, Bergmann A. 2004. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 25:1369–1372 [DOI] [PubMed] [Google Scholar]
- 263. Sutherland A, et al. 2011. Development and validation of a novel molecular biomarker diagnostic test for the early detection of sepsis. Crit. Care 15:R149 doi:10.1186/cc10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Svaldi M, et al. 2001. Procalcitonin-reduced sensitivity and specificity in heavily leucopenic and immunosuppressed patients. Br. J. Haematol. 115:53–57 [DOI] [PubMed] [Google Scholar]
- 265. Svoboda P, Kantorova I, Scheer P, Radvanova J, Radvan M. 2007. Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology 54:359–363 [PubMed] [Google Scholar]
- 266. Tamayo E, et al. 2011. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 22:82–87 [DOI] [PubMed] [Google Scholar]
- 267. Tang BM, Eslick GD, Craig JC, McLean AS. 2007. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect. Dis. 7:210–217 [DOI] [PubMed] [Google Scholar]
- 268. Tang BM, Huang SJ, McLean AS. 2010. Genome-wide transcription profiling of human sepsis: a systematic review. Crit. Care 14:R237 doi:10.1186/cc9392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. 2009. Gene-expression profiling of peripheral blood mononuclear cells in sepsis. Crit. Care Med. 37:882–888 [DOI] [PubMed] [Google Scholar]
- 270. Tarnow-Mordi W, Gebski V. 2010. Procalcitonin in intensive care units: the PRORATA trial. Lancet 375:1605 doi:10.1016/S0140-6736(10)60695-2 [DOI] [PubMed] [Google Scholar]
- 271. Tissari P, et al. 2011. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet 375:224–230 [DOI] [PubMed] [Google Scholar]
- 272. Tobias PS, et al. 1992. Participation of lipopolysaccharide-binding protein in lipopolysaccharide-dependent macrophage activation. Am. J. Respir. Cell Mol. Biol. 7:239–245 [DOI] [PubMed] [Google Scholar]
- 273. Tobias PS, Soldau K, Ulevitch RJ. 1986. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J. Exp. Med. 164:777–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Tracey KJ, et al. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662–664 [DOI] [PubMed] [Google Scholar]
- 275. Tran NK, et al. 2011. Multiplex polymerase chain reaction pathogen detection in patients with suspected septicemia after trauma, emergency, and burn surgery. Surgery 151:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Tsalik EL, et al. 2012. Discriminative value of inflammatory biomarkers for suspected sepsis. J. Emerg. Med. 43:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Tsalik EL, et al. 2010. Multiplex PCR to diagnose bloodstream infections in patients admitted from the emergency department with sepsis. J. Clin. Microbiol. 48:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. 2011. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J. Crit. Care 26:54–64 [DOI] [PubMed] [Google Scholar]
- 279. Tschaikowsky K, Hedwig-Geissing M, Schmidt J, Braun GG. 2011. Lipopolysaccharide-binding protein for monitoring of postoperative sepsis: complemental to C-reactive protein or redundant? PLoS One 6:e23615 doi:10.1371/journal.pone.0023615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. 2006. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit. Care Med. 34:1996–2003 [DOI] [PubMed] [Google Scholar]
- 281. van der Poll T, Opal SM. 2008. Host-pathogen interactions in sepsis. Lancet Infect. Dis. 8:32–43 [DOI] [PubMed] [Google Scholar]
- 282. van Genderen ME, et al. 2011. Serum C-reactive protein as a predictor of morbidity and mortality in intensive care unit patients after esophagectomy. Ann. Thorac. Surg. 91:1775–1779 [DOI] [PubMed] [Google Scholar]
- 283. van Nieuwkoop C, et al. 2010. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit. Care 14:R206 doi:10.1186/cc9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Vanska M, et al. 2011. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica 96:1385–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Varani S, et al. 2009. Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR. J. Infect. 58:346–351 [DOI] [PubMed] [Google Scholar]
- 286. Vince A, et al. 2008. LightCycler SeptiFast assay as a tool for the rapid diagnosis of sepsis in patients during antimicrobial therapy. J. Med. Microbiol. 57:1306–1307 [DOI] [PubMed] [Google Scholar]
- 287. Vincent JL. 1997. Dear SIRS, I'm sorry to say that I don't like you. Crit. Care Med. 25:372–374 [DOI] [PubMed] [Google Scholar]
- 288. Vincent JL, et al. 2006. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34:344–353 [DOI] [PubMed] [Google Scholar]
- 289. Volk HD, Reinke P, Docke WD. 2000. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem. Immunol. 74:162–177 [DOI] [PubMed] [Google Scholar]
- 290. von Lilienfeld-Toal M, et al. 2009. Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia. J. Clin. Microbiol. 47:2405–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Voo KS, Carlone DL, Jacobsen BM, Flodin A, Skalnik DG. 2000. Cloning of a mammalian transcriptional activator that binds unmethylated CpG motifs and shares a CXXC domain with DNA methyltransferase, human trithorax, and methyl-CpG binding domain protein 1. Mol. Cell. Biol. 20:2108–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME. 2011. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med. 37:747–762 [DOI] [PubMed] [Google Scholar]
- 293. Wallet F, et al. 2010. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin. Microbiol. Infect. 16:774–779 [DOI] [PubMed] [Google Scholar]
- 294. Wang CH, Gee MJ, Yang C, Su YC. 2006. A new model for outcome prediction in intra-abdominal sepsis by the linear discriminant function analysis of IL-6 and IL-10 at different heart rates. J. Surg. Res. 132:46–51 [DOI] [PubMed] [Google Scholar]
- 295. Wang H, Yang H, Tracey KJ. 2004. Extracellular role of HMGB1 in inflammation and sepsis. J. Intern. Med. 255:320–331 [DOI] [PubMed] [Google Scholar]
- 296. Wellinghausen N, Kothe J, Wirths B, Sigge A, Poppert S. 2005. Superiority of molecular techniques for identification of gram-negative, oxidase-positive rods, including morphologically nontypical Pseudomonas aeruginosa, from patients with cystic fibrosis. J. Clin. Microbiol. 43:4070–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Welsch T, et al. 2008. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery 143:20–28 [DOI] [PubMed] [Google Scholar]
- 298. Wenzel RP, Edmond MB. 2000. Managing antibiotic resistance. N. Engl. J. Med. 343:1961–1963 [DOI] [PubMed] [Google Scholar]
- 299. Werner JA, et al. 31 May 2011. Preliminary evidence for leukocyte transcriptional signatures for pediatric ventilator-associated pneumonia. J. Intensive Care Med. [Epub ahead of print.] doi:10.1177/0885066611406835 [DOI] [PubMed] [Google Scholar]
- 300. Westh H, et al. 2009. Multiplex real-time PCR and blood culture for identification of bloodstream pathogens in patients with suspected sepsis. Clin. Microbiol. Infect. 15:544–551 [DOI] [PubMed] [Google Scholar]
- 301. Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. 2000. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood 96:218–223 [PubMed] [Google Scholar]
- 302. Wong HR. 2012. Genetics and genomics in pediatric septic shock. Crit. Care Med. 40:1618–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Wong HR, et al. 2009. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit. Care Med. 37:1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Wong HR, et al. 2008. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am. J. Respir. Crit. Care Med. 178:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. 1994. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 180:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Xiao W, et al. 2011. A genomic storm in critically injured humans. J. Exp. Med. 208:2581–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Yanagihara K, et al. 2010. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit. Care 14:R159 doi:10.1186/cc9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Zakariah AN, et al. 2008. Combination of biphasic transmittance waveform with blood procalcitonin levels for diagnosis of sepsis in acutely ill patients. Crit. Care Med. 36:1507–1512 [DOI] [PubMed] [Google Scholar]
- 309. Zhang Q, et al. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]