Abstract
Summary: Helminth parasites infect almost one-third of the world's population, primarily in tropical regions. However, regions where helminth parasites are endemic record much lower prevalences of allergies and autoimmune diseases, suggesting that parasites may protect against immunopathological syndromes. Most helminth diseases are spectral in nature, with a large proportion of relatively asymptomatic cases and a subset of patients who develop severe pathologies. The maintenance of the asymptomatic state is now recognized as reflecting an immunoregulatory environment, which may be promoted by parasites, and involves multiple levels of host regulatory cells and cytokines; a breakdown of this regulation is observed in pathological disease. Currently, there is much interest in whether helminth-associated immune regulation may ameliorate allergy and autoimmunity, with investigations in both laboratory models and human trials. Understanding and exploiting the interactions between these parasites and the host regulatory network are therefore likely to highlight new strategies to control both infectious and immunological diseases.
INTRODUCTION
Nearly one-third of the global population is infected with helminth parasites, rendering them among the most prevalent infectious agents in the world today, and they are responsible for many debilitating diseases and syndromes (124). Moreover, helminths are widespread in livestock and cause major agricultural losses. In both human and animal hosts, helminths establish long-term chronic infections associated with significant degrees of downregulation of the host immune response (78, 121, 181). The eradication of helminth infections remains a distant goal, due to a lack of effective vaccines, limited pharmacological efficacy, emerging drug resistance, and rapid reinfection in environments where transmission cannot be interrupted. New intervention strategies based on an understanding of the immunological basis of helminth persistence are therefore urgently needed.
Helminths are multicellular worms of three taxonomic groups: cestode tapeworms, nematode roundworms, and trematode flukes. They present a striking variety of life histories, from direct fecal-oral transmission (such as the common roundworm Ascaris) to development through free-living stages (such as hookworm larvae in the environment) or dependence on invertebrate vectors (such as the schistosome snail vector). Correspondingly, helminths also have various invasion routes, including through the skin (schistosomes and hookworms), by mosquito bite (filarial worms), and, most frequently, in the gastrointestinal tract.
The extraordinary prevalence of helminth infections undoubtedly reflects their ability to manipulate the host immune system, suppressing responses that could result in their ejection. Host immunity has also developed—over eons of coevolution with parasites—mechanisms to limit pathology and to best balance susceptibility, resistance, and immune pathogenesis. Hence, immune responses often permit ongoing infection in preference to complete parasite elimination and the collateral damage that would result (3). In other cases, this balance is upset, and immune dysregulation and pathology ensue, as in the instances of hepatic fibrosis in schistosomiasis or elephantiasis in lymphatic filarial infections (Table 1).
Table 1.
Human helminth infections and their rodent models
| Human disease (pathogen[s]) | Human pathology | Global prevalencea and DALYsb | Rodent model | Reference |
|---|---|---|---|---|
| Filariasis | ||||
| Lymphatic (Brugia malayi, Wucheria bancrofti) | Lymphatic incompetence, elephantiasis | LF prevalence, 120 million; LF DALYs, 5.64 million | Mouse/gerbil for Brugia malayi/B. pahangic and Litomosoides sigmodontisd | 158 |
| Subcutaneous (Loa loa, Onchocerca volvulus) | “River blindness,” dermatitis | Ov + Ll prevalence, 50 million; Ov DALYs, 0.99 million | ||
| Schistosomiasis (Schistosoma haematobium, Schistosoma japonicum, Schistosoma mansoni) | Liver fibrosis, hepatosplenomegaly, anemia, malnutrition, bladder cancer (S. haematobium) | Prevalence, 207 million; DALYs, 1.76 million | Mouse for Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobiume | 220 |
| Ascariasis (Ascaris lumbricoides) | Cognitive impairment, intestinal obstruction | Prevalence, 807 million; DALYs, 1.18 million | Mouse for Ascaris suumf | 164 |
| Hookworm infection (Ancylostoma duodenale and Necator americanus) | Anemia, cognitive impairment | Prevalence, 576 million; DALYs, 1.83 million | Rat/mouse for Nippostronglus brasiliensisg and Heligmosomoides polygyrush | 40 |
| Trichuriasis (Trichuris trichiura) | Diarrhea, cognitive impairment | Prevalence, 604 million; DALYs, 1.65 million | Mouse for Trichuris muris | 45 |
See reference 124.
DALYs, disability-adjusted life years (309).
The full life cycle does not develop in mice; however, each life cycle stage can survive temporarily.
Natural parasite of the cotton rat Sigmodon hispidus.
Low-level infection in mice.
Only larval stages survive in mice. Ascaris suum is a natural parasite of pigs.
Truncated infection, especially in mice.
Taxonomically related to hookworms but lives entirely in the gastrointestinal tract and does not ingest blood.
Protective immunity to helminths develops only slowly, and the effector mechanisms for eliminating parasites in humans are not well delineated; however, animal models have defined a set of Th2-dependent pathways that mediate protection (3, 6). Not surprisingly, the Th2 response is itself a major target for helminth immunoregulation, as successful parasites seek to blunt the host immune attack. In this review, we set out the patterns and pathways of helminth modulation, with the perspective that this knowledge will not only provide new avenues for eradicating parasite infections from humans and animals but also offer routes to treat noncommunicable immune dysfunctions such as allergies and autoimmunity.
IMMUNE DOWNREGULATION IN HUMAN HELMINTH INFECTION
In human populations, the helminths with the largest burden of disease are the vascular-dwelling schistosomes, the filarial nematodes (Brugia malayi and Wuchereria bancrofti in the lymphatics and the subcutaneous Onchocerca volvulus), and the predominant gastrointestinal nematodes (Table 1).
The downregulation of the immune system in these infections takes effect at multiple levels. In infected populations, a large proportion of individuals may be asymptomatic carriers with depressed immune reactivity to the infecting parasite, as measured by the response of peripheral T cells to parasite antigens; as responsiveness can be rescued by curative chemotherapy, active suppression by parasites is implicated (181). Furthermore, in infected populations, responsiveness to “bystander” antigens such as vaccines, allergens, or autoantigens is attenuated in a more general manner (182). As detailed below, analyses of the cellular basis for these effects have revealed profound dysregulation across the range of innate and adaptive cell populations and pathways.
Immune Regulation and Spectrum of Disease
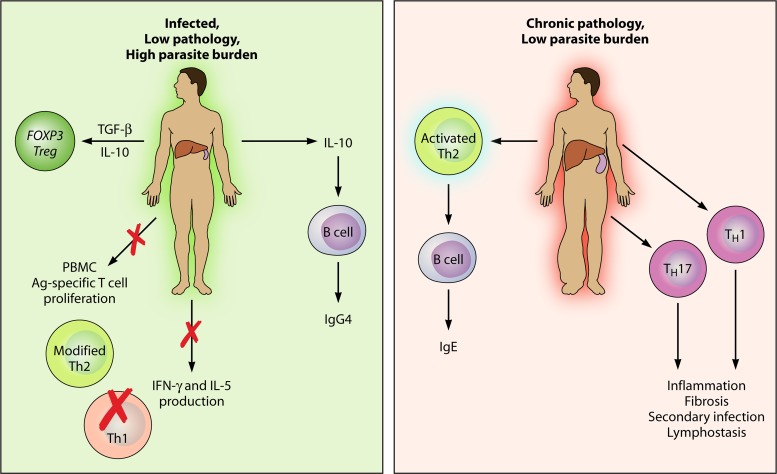
Immune regulation by parasites compromises immunity but also protects the host from damaging immunopathological reactions to the presence of parasites; this relationship is most evident in tissue settings, such as lymphatic filariasis, onchocerciasis, and schistosomiasis, in which the intensity of the infection does not necessarily positively correlate with pathology. Individuals harboring high parasite numbers may be asymptomatic, with stronger regulatory responses, while chronic pathology can occur in immunologically reactive patients with lower-level infections. The immunological mechanisms responsible for this are discussed below and are depicted in Fig. 1.
Fig 1.
Spectrum of pathology in chronic tissue helminth infections. In areas where schistosome and filarial diseases are endemic, a spectrum of pathology is seen, with some individuals developing chronic debilitating pathologies and others showing a tolerant phenotype. The tolerant phenotype is characterized by the production of transforming growth factor β and IL-10 and the expansion of Forkhead box P3+ Tregs. These cytokines lead to IgG4 production by B cells, suppressed parasite-specific T cell proliferation in PBMCs (peripheral blood mononuclear cells), reduced levels of Th2 cytokines, and ablated Th1 cytokines. Thus, the parasite survives productively in the host (schistosome eggs are deposited in the feces, or microfilariae circulate in the blood), with minimal collateral damage. In individuals with chronic pathology, the parasite may be killed or kept at low levels, at the cost of damaging immunopathology. High levels of Th1 and Th2 cytokines are seen, with the emergence of a Th17 response. B cells produce high levels of IgE against parasite antigens (Ag). Th1 and Th17 responses lead to inflammation and fibrosis around deposited schistosome eggs and lymph stasis, leading to secondary infections in lymphatic filariasis.
The spectrum of disease is best illustrated in long-term filaria-exposed populations, for whom infection outcomes range from those who are apparently immune and uninfected (endemic normal), through a large proportion of asymptomatic cases with patent infections (microfilaremic, Mf+, with bloodstream microfilariae), to a minority who experience chronic pathology in the form of lymphatic inflammation and elephantiasis (181, 216, 223). The asymptomatic phenotype shows elevated levels of the immunoregulatory cytokine interleukin-10 (IL-10) (173), a suppression of Th1 inflammatory cytokines (such as gamma interferon [IFN-γ]) as well as key Th2 components such as IL-5 (243), and increased expression levels of circulating T cells expressing the inhibitory marker CTLA-4 (cytotoxic T lymphocyte antigen 4) (265). Conversely, those cases succumbing to lymphatic pathology have deficient numbers of a key cell type, the regulatory T cell (Treg), while expressing stronger Th1 and Th17 effector components (14); the latter may be responsible for lymphatic inflammation against resident adult worms. Th17 cells are also more numerous in patients who have cleared bloodstream Mf (10), raising an interesting question of whether Mf directly downregulate Th17 responses and extend their survival by doing so.
In the related filarial infection onchocerciasis, antigen-specific responses also decline with increasing numbers of skin Mf (35), while cases of chronic pathology (dermatitis and lymphadenitis) carry low parasite numbers. In these patients, strong immune responses against subcutaneous parasites are associated with diminished levels of another immunosuppressive mediator, transforming growth factor β (TGF-β), in nodules around parasites (149). As infection intensity correlates negatively, rather than positively, with immune responsiveness, it is likely that active immunosuppression by parasites and/or their secreted products is taking place.
In schistosomiasis, the distinction between asymptomatic and pathological infections is less clear, as chronic liver fibrosis is common, with sustained granuloma formation around deposited eggs. A spectrum is evident, however, in the degree of the inflammatory response to infection. When previously unexposed travelers are infected with schistosomes, they mount an acute inflammatory immune response against the parasite, with large granulomatous reactions around deposited eggs (39). However, during chronic infection, inflammatory cytokine responses to Schistosoma soluble egg antigens (SEAs) are much reduced (202), while Th2 cytokines (including IL-10) are upregulated (39, 63), and only a small proportion of patients progress to severe hepatosplenic disease. Both acute schistosomiasis patients and severe pathology cases mount much stronger T cell proliferative responses to SEA than low-pathology but chronically infected individuals (98); the low-pathology group, however, has higher frequencies of CD4+ CD25high Tregs (282). The production of Th2 cytokines (especially IL-13), although required for resistance, can also have detrimental effects, as they are important for the generation of fibrosis and pathology in the liver and spleen during chronic schistosomiasis (62, 64).
In gastrointestinal nematode infections, particularly infections by the highly prevalent Ascaris, hookworm, and Trichuris species (Table 1), the pathology is less intense, and long-term infestation is common. Infection is associated with a regulatory set of cells and cytokines, as IL-10 and TGF-β are significantly linked with hyporesponsiveness and susceptibility (86, 285), and patients show a higher frequency of circulating CD4+ CTLA-4+ T cells (95). Thus, an immunoregulated state is a common outcome across a diverse range of helminth infections.
The profile of antibody isotypes can be a telling indicator of the regulated status of the host; in helminth infections, the relative levels of IgG4 and IgE appear to be the most important corollary of susceptibility to and protection from infection, respectively (106, 132, 179, 235). The IgG4 isotype, unlike IgE, cannot cross-link receptors on basophils, mast cells, or eosinophils and, unlike other IgG molecules, does not activate complement or act as an opsonin. It thus appears to be a canonical marker of the modified (noninflammatory) Th2 state, potentially blocking cytophilic isotypes such as IgE and preempting potentially damaging inflammation (181).
In filariasis, the production of IgG4 rather than IgE against filarial antigens is maximal in the asymptomatic Mf+ state, reaching extraordinarily high levels (132, 152). IgG4 titers fall rapidly following curative drug treatment (11). Interestingly, IgE production by B cells is promoted by IL-4 and IL-13, but in the presence of the regulatory cytokines IL-10 and TGF-β, a switch to IgG4 is favored (246). Hence, it was suggested that the extremely high IgG4 levels in many helminth-infected patients reflect a dominant regulatory environment (1, 176).
Antigen-Specific Hyporesponsiveness
A general finding is that chronic infections with filarial and schistosome parasites result in antigen-specific unresponsiveness among peripheral blood T cell populations, which fail to respond to in vitro challenge with specific parasite antigen (225, 313). A dynamic link between T cell anergy and the immunoregulatory environment in helminth infections is suggested by the strong correlation between IgG4 levels and unresponsiveness (29, 313). The fact that, as detailed below, hyporesponsiveness can be reversed by the chemotherapeutic removal of the parasite burden (101, 103, 242) argues that it reflects a direct effect of live parasites rather than an inherent incapacity of the host to respond to infection.
In filariasis, the hyporesponsive condition strongly correlates with the asymptomatic Mf carrier state (225, 313), while pathology cases are immunologically more reactive. Notably, unresponsiveness is associated with elevated levels of IL-10 and TGF-β production, and reactivity to parasite antigens can be restored, in vitro, with antibodies to these suppressive cytokines (144). During onchocerciasis, the filarial worm Onchocerca volvulus dwells in subcutaneous granulomata; T cells derived from these sites express IL-10 and TGF-β (68), with CD4+ T cell clones from this tissue possessing a regulatory phenotype and suppressive functions (245). CTLA-4 may also be an important inhibitor of effector T cell signaling, as in lymphatic filarial patients, peripheral T cells challenged with parasite antigen in the presence of anti-CTLA-4 antibodies showed improved responsiveness (265).
Intriguingly, children born to filaria-infected mothers have higher CTLA-4 levels than children of uninfected mothers, implying a maternal-fetal imprinting of regulation (265). Long-term studies of filariasis-exposed communities have revealed that the maternal infection status is highly influential in determining the Mf+ state of the offspring: children of microfilaremic mothers are more than twice as likely to become microfilaremic (154), while infants whose cord blood cells were hyporesponsive to parasite antigens and were born to Mf+ mothers are nearly 12-fold more likely to themselves become infected than infants born to uninfected mothers (183). Conversely, humans exposed to filarial parasites later in life, either as migrants relocating to an area where filariasis is endemic (219) or as military personnel in the Pacific theater of the Second World War (47), show higher incidences of pathology and lower rates of the asymptomatic “tolerant” state. Thus, early-life exposure to filarial antigens promotes tolerance, possibly by prenatal exposure to antigen presented without inflammation or through the effects of soluble parasite immunomodulators on the fetus (183, 184).
A similar pattern of immune suppression is seen for schistosome infections of humans. In early infection, T cell proliferative responses are intact but become downmodulated during chronic infection (48). Cytokine responsiveness has also been reported to be suppressed in Schistosoma haematobium-infected individuals in regard to IL-4, IL-5, and IFN-γ production (102), while parasite-specific IL-10 positively correlates with egg positivity and infection intensity (209). In mouse models, IL-10 is pivotal in the suppression of potentially protective antischistosome responses (302). Evidence that helminth parasitic infection actively suppresses immune responses also comes from studies in which responses to parasite antigens increase after the drug-induced clearance of parasites. After drug treatment of filaria-infected (227) or schistosome-infected (48, 103) patients, immune responsiveness to the respective antigens is restored. Increased Th2 responsiveness after drug cure also positively correlates with resistance to reinfection (140).
These data illustrate the ability of helminth parasites to potently induce a regulatory environment, which is exquisitely tuned to maintain effector T cell populations in a repressed, unresponsive state. Importantly, if parasites are cleared or the immune response is potentiated, immune competence can be restored to the host (281). Thus, the suppression induced by parasitic infection is active, and many of the mechanisms may be initiated by the release of helminth-derived mediators (119).
The hypoproliferative state in tissue helminth infections could reflect interference with antigen-presenting cell (APC) populations. Filarial infection subverts monocyte and APC function: in Mf+ patients, there is a preponderance of circulating CD11clow CD123low monocytic dendritic cells (DCs), which have downregulated CCR1 and may consequently be impaired in their migratory capacity (253), and lipopolysaccharide (LPS)-induced inflammatory cytokine release from Mf+ donor monocytes is significantly depressed compared to that in uninfected or chronic-pathology patients (244), with responses being partly restored following anthelmintic treatment (252). Overall, APCs show a reduced ability to upregulate TLR (Toll-like receptor) (a family of innate pattern recognition receptors) expression following stimulation, a deficiency also observed for B cells from Mf+ patients (15). Moreover, the T cell expression level of TLRs is also reduced in filarial infections (16), which is part of the general pattern of compromised TLR function during helminth infection (293). An interesting question, explored in Cellular Basis of Immunomodulation below, is whether certain APC populations are simply compromised in their function or converted into active regulatory and suppressive cell types.
Deficient Acquired Immunity
Human immunity to helminths is frequently ineffective in two key respects: the immune system is unable to clear chronic infections, and immune memory fails to protect against reinfection even after drug-mediated clearance. Indeed, classic immunity reminiscent of protection against viral and bacterial pathogens is rarely observed, with protection against helminth infection taking years to develop and seldom achieving sterile cure (5). However, effective immunity that significantly reduces the parasite burden is possible, as demonstrated by irradiated larval or cercarial vaccines in animal models of schistosomiasis, hookworm infection, and filariasis (178) as well as the more recent recombinant antigens (27). Hence, the generation of immunity is deficient in humans exposed to natural helminth infection.
Deficient immunity largely reflects inadequate Th2 responses, based on the more vigorous Th2 compartment in individuals exhibiting some degree of protection from reinfection. For example, the resistance of schistosome patients to reinfection following drug cure correlates strongly with the release of IL-4 and IL-5 (140), while in a separate study, those succumbing to reinfection showed lower-level IL-5 responses (102). Likewise, in human populations in areas where hookworm is endemic, IL-5 production correlates with resistance to reinfection after drug cure (228), and in Ascaris infections, levels of IL-4, IL-9, IL-10, and IL-13 were most positively associated with protection (284). Moreover, clear genetic links between Th2-promoting gene loci (such as IL-13 and STAT6) and resistance to infection have been reported (150, 175, 201). Clearly, during chronic helminth infection, the Th2 capacity is downregulated, and protective immunity is compromised.
However, a recent study on schistosomiasis showed that protection from infection was associated with both Th1 (IFN-γ) and Th2 (IL-4 and IL-13) gene polymorphisms, raising the possibility of Th1-derived components contributing to immunity to schistosomes (96). Consistent with this, resistant individuals mount stronger IFN-γ responses (as well as stronger overall Th2 responses) to parasite antigens, indicating that both the Th1 and Th2 arms may be modulated in susceptible individuals (29, 232). Similar observations have been reported for human filariasis and hookworm infections and are mirrored in mouse studies of filariasis and schistosomiasis, as discussed below in Mouse Models of Infection.
Regulatory Cell Populations in Human Infection
The cellular basis of immunoregulation due to helminth infection involves the modulation of both the innate and adaptive immune responses through a suite of regulatory cell types (181). Tregs, the subset of T cells that maintain self-tolerance in humans and mice (139), are emerging as the most important regulatory phenotype in helminth infections. Several distinct Treg populations can be discerned, in particular “natural” Tregs, which express the transcription factor Foxp3 following their development in the thymus; “induced” Tregs, which switch to Foxp3 expression in the periphery; and Foxp3− type 1 regulatory (Tr1) cells. All these Treg populations can produce IL-10 and TGF-β in different settings (241). As well as restraining potentially damaging autoimmune responses, however, Tregs are also activated during many infections, dampening protective immunity and suppressing immune pathology (180). Moreover, the response to infection stimulates the parallel expansion of effector and regulatory subsets (276), and the outcome may depend on changing proportions of each type (280). A central question, therefore, is whether Tregs arise as a homeostatic response to inflammation or are driven in a selective manner by pathogens, including helminth parasites, in order to sustain infection.
The importance of suppressive cell subsets in human helminth disease was evident even before regulatory T cells were properly defined, in assays of T cells from hyporesponsive Mf+ B. malayi-infected individuals (226) and in the demonstration that antibodies to IL-10 and TGF-β restored responsiveness to cultures of T cells from both filariasis (144) and schistosomiasis (145) patients. Asymptomatic Mf+ individuals show elevated levels of IL-10 (173) and a suppression of Th1 inflammatory cytokines (such as IFN-γ) as well as key Th2 components such as IL-5 (243). Conversely, those individuals succumbing to lymphatic pathology have significant Th1 and Th17 components (14); the latter may be responsible for lymphatic inflammation. By flow cytometry, recent studies have established higher frequencies of CD4+ CD25+ CD127− Foxp3+ Tregs in filariasis Mf+ cases than in controls (17, 195) as well as higher overall expression levels of CTLA-4 among the CD4+ peripheral T cell population of Mf+ patients (265). It was suggested that CTLA-4 (and PD-1 [programmed cell death 1]) may be involved in blocking inflammatory bystander responses in infected patients (13). Recently, regulatory T cells from microfilaremic individuals, but not those from uninfected individuals, were shown to suppress both Th1 and Th2 peripheral blood mononuclear cell (PBMC) cytokine production, providing further evidence of a link between Tregs and the hyporesponsive state (297). In contrast, PBMCs from filaria-infected individuals with chronic pathology fail to upregulate Foxp3 in response to filarial antigen, potentially indicating that Tregs are deficient in these patients (14), although this is subject to the caveat that Foxp3 can also be activation induced in humans (298).
In the related infection onchocerciasis, IL-10-producing Tr1 cells are readily detected in hyporesponsive patients (245) and along with Foxp3+ natural Tregs, induce IgG4 from B cells (246). Importantly, localized Treg induction around parasite nodules in tissues has been seen (148), and T cell clones from these granulomas or PBMCs included many that expressed IL-10 and/or TGF-β (68, 245). The role of DCs in inducing regulatory T cell responses is illustrated by the action of schistosome lysophosphatidylserine, which acts on human DCs to promote IL-10-producing Tr1 cells (290).
In human schistosome infections, CD4+ CD25high cell frequencies were significantly reduced after drug cure of Schistosoma mansoni infection (299), while in children, CD4+ CD25high CD127− Foxp3+ proportions were positively correlated with the S. haematobium parasite burden (213). In the latter study, in adults resistant to reinfection with the parasite, Treg proportions were negatively correlated with infection intensities (213). This finding may imply that in the early stages of infection, Tregs expand, but later in life, once resistance has developed, Treg proportions are reduced upon infection due to the expansion of the T effector population.
In human gastrointestinal infections, evidence of Treg involvement is less extensive, and the analysis of peripheral blood T cells may not be an appropriate reflection of immune populations in the gut. Nevertheless, it was found that hookworm-infected individuals display higher proportions of circulating CD4+ CD25+ Foxp3+ Tregs than uninfected controls (233). In addition, among patients with intestinal nematode infections, in vitro T cell responses to malaria and mycobacterial antigens were depressed but were rescued following Treg depletion (296).
IL-10-producing regulatory B cells (Bregs) are receiving particular interest in mouse models of helminth infection, as discussed below. Bregs have also been found in human patients infected with environmentally acquired helminths (56). IgG4-producing B cells associated with tolerance to filarial infections have been proposed to stem from IL-10-producing Bregs (131), producing an intriguing link between these two regulatory phenotypes. Thus, it seems likely that Bregs are also involved in the suppression of antihelminth immune responses.
DAMPENING BYSTANDER RESPONSES IN HUMANS
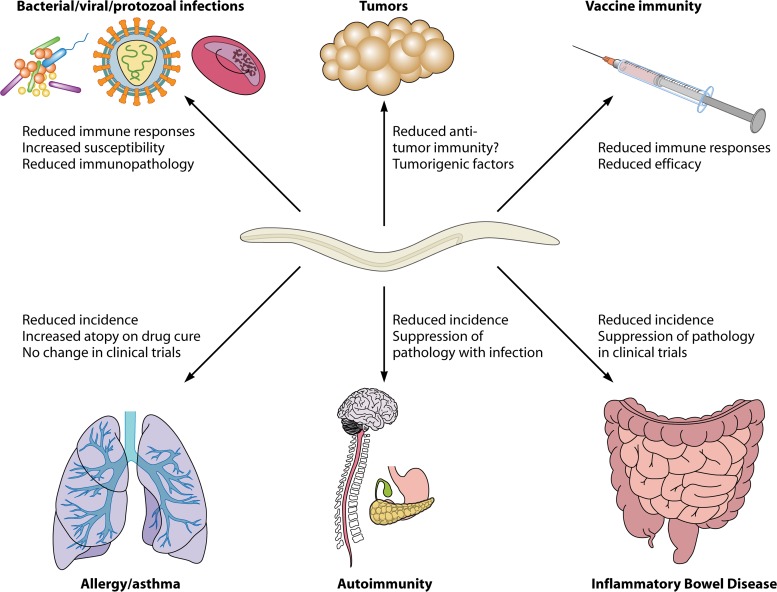
As evident from the data discussed in Immune Downregulation in Human Helminth Infection, many helminth infections can drive an antigen-specific anergic state, but suppression is not confined solely to parasite-specific responses. Indeed, there are broad effects of concurrent helminth parasite infections on vaccine, coinfection, and allergen or autoantigen responses in humans (Fig. 2), which (as discussed in Helminth Therapy in Humans) have led to human clinical trials using experimental helminth infections.
Fig 2.
Immunoregulatory effects of helminths on bystander responses. Helminths can suppress a wide range of bystander immune responses, including those of both immunopathogenic and protective natures. Coinfection with helminths suppresses antibacterial, antiviral, and antiprotozoal immunity, leading to increased susceptibility and attenuated immunopathology or, in some cases, exacerbated pathology due to higher infection burdens. Antitumor immunity may be suppressed by helminth infections, which may also release directly carcinogenic factors, potentially leading to increased numbers of malignancies in infected individuals. Vaccine efficacy is compromised by helminth infections due to suppressed immune responses. Immunopathologies such as asthma, autoimmune diseases, and inflammatory bowel diseases are all reduced in prevalence in areas where helminth disease is endemic, and direct effects of helminth infections on the suppression of disease have been shown in clinical trials for inflammatory bowel diseases.
Compromising Vaccine Efficacy
A growing concern in developing countries is the extent to which helminth infections may interfere with childhood vaccination programs (31). Patients with schistosomiasis, for example, mounted weaker IFN-γ responses to tetanus immunization (239), as did onchocerciasis and lymphatic filariasis patients, who presented higher-level IL-10 responses instead (54, 214). Moreover, vaccine responses were significantly augmented in Ascaris-infected children given anthelmintic treatment prior to immunization, compared to the responses of placebo-treated controls (53), while a similar comparison of infected and treated recipients of Mycobacterium bovis BCG showed that helminth infections depressed antimycobacterial T cell responses, with concomitant increases in levels of TGF-β release (72). Investigations using mouse models have reiterated these effects (287). For example, S. mansoni infection interferes with the protective effect of BCG vaccination (71), and Heligmosomoides polygyrus infection reduces the efficacy of vaccination against Plasmodium chabaudi (268).
A related issue is whether maternal helminth infection during pregnancy may influence infants' vaccine responsiveness; however, a large-scale clinical trial recently showed that anthelmintic treatment of pregnant mothers had no positive effect on subsequent vaccine efficacy, as judged by cytokine or antibody responses (300). As the efficacy of the anthelmintic treatment and the analysis used has been called into question (312), more studies in this area are required to confirm these results.
Coinfection with Helminths
Helminths rarely infect the human host in isolation, particularly in the settings of tropical countries, and coinfections are therefore extremely common. Interest has focused in particular on the interactions between helminths and prominent infections with high mortality rates in the tropics, such as malaria, tuberculosis, and HIV.
There is currently controversy regarding whether helminths ameliorate (211) or exacerbate (69) malarial disease, as reviewed elsewhere previously (210). It is important that the different host-parasite combinations involved as well as the intensity and duration of infection may well show opposing influences on disease (147, 260). Immunoregulatory factors are very likely to mediate this interaction, and in children coinfected with intestinal helminths and/or schistosomes, the major change in immune responsiveness was increased IL-10 levels in response to Plasmodium antigen (110). Moreover, in an area where filariasis and malaria are both endemic, PBMCs from filaria-infected subjects produced less IL-12p70, IL-17A, and IFN-γ but more IL-10 in response to malaria antigen (196, 197), consistent with diminished IL-12 responses from filariasis patients stimulated with malarial antigen (198).
Bacterial infections, such as tuberculosis, are highly prevalent in many areas where helminth infection is endemic (73). As mentioned previously, heightened CTLA-4 expression levels in filaria-coinfected patients may act with PD-1 (programmed cell death 1) to block protective Th1 and Th17 antimycobacterial responses (13). The suppression of these antimalarial/antituberculosis responses can lead to an insufficient control of the infection and increased pathology. In cholera patients, intestinal helminth infections also depress the antibacterial antibody response, particularly of the IgA isotype, which is important in protection (109). Thus, helminth coinfection can suppress immune responses to both systemic and intestinal bacterial infections.
In retroviral infections, anthelmintic treatment retards HIV disease progression (295); thus, helminths appear to encourage viral replication. This may be due to the suppression of the antiviral response or due to increased numbers of circulating antihelminth Th2 cells, in which HIV replicates preferentially (251). Indeed, in coinfection studies with rhesus monkeys, schistosome egg deposition (and the initiation of strong Th2 responses) coincided with an upsurge in circulating viral loads (12). Conversely, the suppressive effects of HIV extends to antihelminth immunity, with HIV infection inhibiting resistance to schistosome reinfection after drug cure (250) and correlating with lower levels of production of Th2 cytokines (33). HIV/AIDS predisposes individuals to other helminth parasitic infections, and pathologically intense opportunistic infections by Strongyloides species are common in areas of endemicity (33). Furthermore, human T cell lymphotropic virus type 1 (HTLV-1) infection predisposes individuals to Strongyloides stercolaris infections due to suppressed antiparasite immune responses (120), and coinfected patients show much higher Foxp3+ Treg numbers and lower-level IL-5 responses than individuals infected with either pathogen alone (203).
The Hygiene Hypothesis
Epidemiological studies have pointed to the higher frequency of allergies (such as asthma, eczema, and allergic rhinitis) in economically developed countries than in developing countries (51, 134, 174). This has been attributed to increased standards of living in the developed world leading to lower rates of childhood infections and increased immune dysregulation, known collectively as the “hygiene hypothesis” (267, 303). Much attention has since been focused on the question of whether helminth infections are one of the major factors which counteract allergies in the developing world (52, 91). A number of studies have reported that helminth-infected cohorts do indeed show a lower allergic propensity (generally, skin test reactivity to allergens such as the house dust mite), in particular in children with S. haematobium (236, 288) and S. mansoni (8, 9, 194) infections. Evidence for a causal effect of parasites on reducing allergies stems from reports that anthelmintic clearance of infection increases skin test reactivity in children (92, 289).
An important question in both helminth infection and allergy is the importance of prenatal antigen exposure to the future reactivity of the newborn. In recent studies, anthelmintic treatment of pregnant mothers in a population living in an area where hookworm and schistosome diseases are endemic was shown to significantly reduce the incidence of atopic eczema in offspring (74, 207). These data also provide the first evidence for helminths modulating clinical allergy in humans, rather than skin test atopy (91).
Good correlations have been found between the inhibition of allergic reactivity and IL-10 production from T cells challenged with parasite antigen (288). Among important cellular mediators of allergic responsiveness are basophils; notably, in helminth-infected humans, the ability of basophils to respond to either IgE- or non-IgE-mediated activation is attenuated (156). This observation parallels the situation in mice, in which unresponsiveness was further demonstrated to be dependent upon IL-10 (157). In contrast, little evidence has been found to support the concept that helminth-induced IgE blocks allergic reactivity, in part because IgE receptor expression is highly upregulated during infection (79, 200).
Gastrointestinal nematode infections may also ameliorate allergy; a meta-analysis of all published data found that both Trichuris and Ascaris protect against the development of skin test atopy (83), although an earlier meta-analysis of clinical asthma found that only hookworm infection appeared to confer any protective effect, while Ascaris increased the risk (162). Thus, intestinal helminths may be more effective at suppressing atopic sensitization than controlling later-stage pathogenic mechanisms. It was also suggested that the suppression of allergies may require a parasite burden of a certain threshold level and/or a chronicity of infection (249, 260); if so, then meta-analyses which amalgamate data from areas with both low and high infection intensities would reduce the strength of the effects observed only in the latter.
Autoimmune diseases are considerably less prevalent than allergies, and few epidemiological investigations have been conducted on the effects of helminth infections on these disorders. A recent study reported that schistosome-infected individuals had lower levels of autoantibody than infection-free residents and that levels of autoantibodies increased in infected subjects treated with the antischistosome drug praziquantel (208). In terms of overt autoimmune pathology, there is a clear negative relationship between the incidence of multiple sclerosis (MS) in different countries and the prevalence of helminth infection, although the increased incidence of MS in economically developed countries also has many noninfectious contributory factors (90).
A stronger link between helminth infection and protection from MS emerged from Argentina, where patients serendipitously acquiring a range of gastrointestinal helminths (Hymenolepis nana, Trichuris trichiura, Ascaris lumbricoides, S. stercolaris, and Enterobius vermicularis) subsequent to diagnosis remained in remission, with lower relapse rates than uninfected severity-matched patients (55). Furthermore, when a subset of the infected cohort was treated with anthelmintic drugs, these patients experienced a relapse of symptoms, in contrast to those who continued to be infected (57). Significantly, patients with a helminth-associated suppression of disease expressed elevated levels of IL-10 and TGF-β and expanded populations of both regulatory B and T cells compared to uninfected controls (55, 56).
Perhaps the most important unexplored territory is the interaction between helminths and the immune response to tumors. There is no epidemiological evidence that helminths compromise immune surveillance, although there are specific instances in which individual parasite species are linked to particular cancers. The liver flukes Opisthorchis viverrini and Clonorchis sinensis are powerful causal factors in human cholangiocarcinoma, cancer of the bile duct (112, 263). There are also causal links between S. haematobium infection and bladder cancer, possibly due to the damage caused by the egress of eggs through the bladder wall (93). Most probably, these represent cases in which parasite-derived tumor promoters (262), acting in the context of immunomodulation, contribute to a carcinogenic outcome.
MOUSE MODELS OF INFECTION
Mouse model systems have been developed for each of the highly prevalent human helminth parasites. While some human-infective schistosome species are able to infect laboratory rodents, this is not the case for most human-infective nematode parasites, such as the filariae and gastrointestinal worms. However, in most cases, closely related rodent-infective species are available, as detailed below and in Table 1.
Schistosomiasis
S. mansoni is the most widely studied schistosome model in mice, with a growing body of literature also characterizing Schistosoma japonicum infections. Both of these intestinal schistosome species are well tolerated by most mouse strains, where cercariae penetrate the skin and migrate through the lungs before developing to paired adults residing in the hepatic portal vessel. There, they release eggs, which either emerge in the feces or are swept through the vasculature to become trapped in the liver, as occurs in human infection. While the urinary schistosome S. haematobium can maintain only limited infections in mice, the damage that this parasite causes to the bladder wall can be modeled by the direct microinjection of eggs (94).
In S. mansoni-infected mice, an initial Th1-dominated immune response switches to a Th2 profile upon the deposition of eggs (220); as in humans, eggs lodging in the liver provoke the predominant pathology of liver fibrosis. IL-13 is the chief mediator of fibrosis in schistosomiasis, as in IL-13 receptor α1 (IL-13Rα1)-deficient mice (which respond to IL-4 but not IL-13), fibrosis is greatly suppressed, and the rate of survival through the chronic phase of infection is consequently increased (229).
Schistosome egg antigen (SEA) is one of the strongest known Th2-driving stimuli, and both mouse and human SEA-pulsed DCs can prompt Th2 differentiation in cocultured T cells in vitro (80, 137). The central role of the DC is reflected in the Th2 induction in vivo by SEA-pulsed DCs (172) and the loss of Th2 responses in DC-depleted mice treated with either SEA or schistosomes (224). The chief DC-stimulating molecular motif within SEA was recently defined as the RNase glycoprotein ω-1 (80, 266). The depletion of ω-1 from SEA abrogates its Th2-inducing effects in vitro; however, ω-1-depleted SEA-pulsed DCs induce identical Th2 responses in vivo, and thus, it is not the only constituent of SEA with Th2-inducing properties (80, 266).
T cell hyporesponsiveness is also observed in S. mansoni infection of mice, as the CD4+ T cell compartment becomes functionally anergic through the upregulation of the E3-ubiquitin ligase gene related to anergy (GRAIL). As this phenomenon could be replicated by the repeated stimulation of Th2 cells in vitro, it appears that this is a physiological response to chronic antigen stimulation rather than a specific effect elicited by the S. mansoni parasite (275). Thus, the host downregulates the response to schistosome infection, as it becomes more chronic, potentially damaging, and less likely to eradicate the infection. The further immunomodulatory effects of schistosomes are detailed below in Cellular Basis of Immunomodulation.
Filariasis
Laboratory models of human filarial parasites are constrained, as none can complete their life cycle in mice. For example, studies of the filarial parasite B. malayi are limited to the injection or implantation of the infective third-stage larvae (L3), adults, or microfilariae, which survive for weeks (larvae and microfilariae) to months (adults), depending on the mouse strain and sex. Nevertheless, two remarkable immunoregulatory effects on mice harboring infections with this species have been demonstrated. First, adult parasites surgically implanted into the peritoneal cavity of mice induced a novel macrophage phenotype now recognized as an alternatively activated macrophage (AAM) (2) (see “Alternatively Activated Macrophages” below); this cell type blocked the proliferation of activated T cells and hence acted in an immunosuppressive capacity. More recently, it was found that the same implantation stimulated the expansion of Foxp3+ Tregs, even among bystander (ovalbumin [OVA]-specific) T cells. Moreover, Treg induction occurred in IL-4R−/− mice, which do not develop AAMs (192). Because both AAMs (14) and Foxp3+ Tregs (14) are associated with human filariasis, these experiments suggest that the mouse model of B. malayi infection can be used to explore the generation of suppressive cell types by this human pathogen.
For many studies, it is preferable to study the full cycle of infection in mice, which is now possible with a natural filarial pathogen of cotton rats, Litomosoides sigmodontis. This parasite is transmitted by hematophagous mites and enters transcutaneously, migrating through the lymphatics to the pleural cavity, where mature adults produce circulating microfilariae (123). The L. sigmodontis model has shed light on mechanisms of immunity, specifically demonstrating that a mixed Th1/Th2 response is necessary to achieve protection. While IL-4- or IL-5-deficient mice are more susceptible to this infection (158, 264), the susceptibility of IFN-γ/IL-5 double-deficient mice is further increased (240), arguing that combinations of Th1 and Th2 elements are required for protection. As in filaria-infected humans, L. sigmodontis-infected mice develop an antigen-specific hypoproliferative defect alongside an expansion of regulatory T cells (277, 278, 280) and alternatively activated macrophages (279). Hyporesponsiveness can be reversed by manipulating GITR (glucocorticoid-induced tumor necrosis factor receptor-related gene) and CTLA-4 signaling (277, 278, 291), indicating the active and dynamic nature of suppression in vivo.
Gastrointestinal Nematodes
Ascaris, Trichuris, and hookworm species are the most prevalent human gastrointestinal helminths, the latter two of which can be appropriately studied in mouse models (Table 1). The closest model of hookworm disease in rodents is the rat parasite Nippostronglyus brasiliensis, which shares key life cycle characteristics (skin penetration and lung transit) with Necator americanus and Ancylostoma duodenale (40). In contrast to human hookworms, however, N. brasiliensis does not feed on host blood and therefore does not reproduce the chief pathological effect of anemia (125). Moreover, N. brasiliensis is ejected from the mouse or rat host within 2 weeks, while human hookworms can survive in the host for many years. To model chronic infection and immunoregulation in humans, the preferred model is Heligmosomoides polygyrus, which completes its parasitic phase entirely within the digestive tract. Most mouse strains are unable to expel a primary infection of this parasite as a result of its profoundly immunoregulatory properties (177) but become resistant to secondary infections after drug cure of a primary infection, making H. polygyrus a useful model of acquired resistance to gastrointestinal helminth infection (6).
Both mouse models have supplied invaluable information about immune responsiveness and modulation in infection. The expulsion of primary N. brasiliensis and secondary H. polygyrus infections is highly Th2 dependent, requiring CD4+ T cells and IL-4R signaling in both innate and adaptive cell populations (reviewed in references 3, 6, and 113). In addition, mast cells (113) and the goblet cell-derived defense protein resistin-like molecule β (RELM-β) are implicated in immunity to H. polygyrus (117).
The mouse model for human Trichuris trichiura infection is a closely related parasite, Trichuris muris, which can either maintain a chronic infection or be quickly ejected via a Th2-dominated CD4+ T cell response, according to the host strain and parasite dose (45). In this model, immunity is effected through IL-9-dependent mast cells, smooth muscle hypercontractility (142, 234), and increased intestinal epithelial cell turnover (46). Normal laboratory infection with high doses of parasites quickly induces a protective Th2 response in resistant mice; however, the same strain of mouse can be rendered susceptible with low-dose exposure, in particular with “trickle” infections, which model more closely the rate of naturally acquired infection (22). This is reminiscent of parasitic infections of humans, where multiple exposures to parasites are required to build up resistance (see Immune Downregulation in Human Helminth Infection).
T. muris is a particularly valuable model of susceptibility and resistance to gastrointestinal helminths because of the sparsity of data on immune regulation in the human setting. However, two recent studies have analyzed mucosal immune modulation following helminth treatment of inflammatory bowel disease (IBD) (see Helminth Therapy in Humans). First, in an ulcerative colitis patient with T. trichiura infection, parasite colonization was shown to modify IL-17 levels while inducing Th2 cytokines and IL-22 in the colonic mucosa, with associated goblet cell hyperplasia (36). Second, a clinical trial of hookworm infection in patients with celiac disease showed an upregulation of hookworm antigen-specific Th2 cytokines and basal levels of IL-22, with suppression of IL-23, IFN-γ, and IL-17A production (97, 191). Thus, immune deviation in the gastrointestinal mucosa also occurs in human parasitic infections with respect to the suppression of inflammatory Th1/17 responses. Although relevant mouse models have provided us with a wealth of data on the regulation of the response to helminth parasites, further studies are required to link this information with susceptibility or resistance to human gastrointestinal helminths.
CELLULAR BASIS OF IMMUNOMODULATION
The immune response against helminth parasites involves an impressive range of innate and adaptive pathways for the induction and amplification of highly potent effector mechanisms. However, these potentially pathogenic responses also have to be regulated by the host immune system through counterbalancing immunoregulatory mechanisms. Moreover, it is now clear that in many instances, the same mechanisms have been coopted by parasitic organisms to diminish effective antiparasite immune responses. In this section, we explore the roles of dendritic cells (DCs), alternatively activated macrophages (AAMs), regulatory T cells (Tregs), and regulatory B cells (Bregs) in the control of immune responsiveness in experimental models of helminth infections.
Dendritic Cells
Both the stimulation and modulation of the host immune system by helminths is determined by DC populations; the depletion of DCs ablates the Th2 immune response to infection, while isolated DCs pulsed with helminth antigens readily induce Th2 responsiveness (42, 81). The role of DCs in inducing regulatory responses is illustrated by the action of schistosome lysophosphatidylserine, which acts on human DCs to promote IL-10-producing Tr1 cells (290), and the egg molecule ω-1, which drives murine DCs from mice to induce Foxp3 expression in T cells in vitro (316). In the H. polygyrus model, infection expands an unusual phenotype of CD11clow CD103− DCs, which preferentially drive Foxp3 induction in naive T cells (165, 258), while there is a loss of CD8αintermediate DCs that normally traffic from the epithelium to the draining lymph node (21).
While the mechanistic pathways by which DCs induce Th1 and Th17 responsiveness via the production of IL-6, IL-12, and IL-23 and the upregulation of surface costimulatory molecules are well known, those involved in driving either Th2 or Treg outcomes are much less well understood (171). Tolerogenic DCs exhibit little evidence of maturation (upregulation of CD40, CD80, CD86, and major histocompatibility complex [MHC] class II), whereas microbial TLR ligands strongly induce these markers. However, a range of helminth molecules interfere with the ability of DCs to respond to TLR ligands and to produce IL-12 in response to stimulation (reviewed in references 81 and 119). DCs are also influenced by host “alarm” cytokines such as thymic stromal lymphopoietin (TSLP), and T. muris depends upon eliciting this product from the host to depress IL-12 responses (274), in contrast to other helminths, which are able to suppress DC IL-12 production directly without the requirement for TSLP (188).
No general pattern of receptors and signaling pathways through which helminths may inhibit DCs, or induce proregulatory DCs, has yet been established. Although some instances of TLR-mediated activation have been documented (290), it seems likely that helminth-specific pattern recognition receptors may extend well beyond the canonical TLR family, to include C-type lectin receptors (CLRs) (292), class A scavenger receptor (81), and, possibly, receptor types yet to be discovered. Furthermore, certain helminth products interfere with DC function at the intracellular level, by blocking antigen processing (187) or degrading mRNAs within the host (81). Defining the molecular interactions which result in the regulation of, and by, DCs is therefore of central importance to an understanding of host-helminth interactions.
Alternatively Activated Macrophages
Alongside the dominant Th2 response to helminth parasites, a powerful innate type 2 reaction emerges, including eosinophils, basophils, mast cells, and a distinct phenotype of macrophages, the alternatively activated macrophage (AAM) (151). AAMs are distinct from macrophages activated through IFN-γ in expressing high levels of arginine-metabolizing arginase 1, the chitinase-like molecule Chi3L3 (Ym1), and the resistin-like molecule RELM-α (170) and in exerting profoundly antiproliferative effects in vitro. Mechanistically, it is likely that arginine inhibits cell proliferation by depleting the milieu of this amino acid, while a regulatory role for RELM-α is indicated by the exaggerated Th2 responses to schistosome infection in RELM-α-deficient mice (212). In contrast to their in vitro activity, AAMs in vivo are more closely associated with wound healing (169) and metabolic homeostasis (43); indeed, whether AAMs are regulatory or effector populations in vivo remains a controversial question.
The suppression of T cell proliferation by splenic adherent cells was originally noted for rodents infected with filarial parasites (155). Subsequently, a similar immunosuppressive effect was attributed to macrophages in mice peritoneally implanted with adult B. malayi filarial parasites (2). The in vitro suppressive nature of B. malayi-induced AAMs, together with the induction in vivo of Foxp3+ Tregs by the same parasite (192), suggests that these macrophages fulfill an immunoregulatory role. In another murine filarial model, L. sigmodontis infection of the pleural cavity, AAMs are recruited to both the site of infection and the draining lymph nodes and suppress T cell responses at both sites in a TGF-β-dependent manner (279). Furthermore, in human filariasis, alternatively activated macrophage markers are upregulated in the blood of asymptomatic microfilaremics, the category displaying T cell hyporesponsiveness (18). Thus, in filarial infections at least, AAMs appear to suppress the immune response against the parasite, promoting anergy and/or tolerance.
AAMs are also induced in the gut in gastrointestinal nematode infections by pathogens such as H. polygyrus and N. brasiliensis (7, 318). Their presence can have detrimental effects on host immunity to concurrent pathogens. Coinfection with Citrobacter rodentium in H. polygyrus-infected mice results in increased C. rodentium bacterial loads and exacerbated C. rodentium-induced colitis. However, in coinfected STAT6-deficient mice, in which no AAMs are recruited to the gut, bacterial infection was not exacerbated (301). An interesting observation in these experiments was the increased levels of tumor necrosis factor (TNF) production by AAMs, a facet which may require further investigation.
The homeostatic role of AAMs also extends to protecting the host from potentially harmful inflammation. When schistosome eggs are deposited into the bloodstream and egress through the gut wall to be deposited into the feces, AAMs are required to maintain the integrity of the intestinal epithelium. In macrophage-specific IL-4Rα-deficient (LysMcre IL-4Rα−/flox) mice, commensal bacteria entered the tissues due to a lack of control of intestinal inflammation and deficient healing of the gut (116). In mice with a macrophage-specific arginase deficiency (LysMcre Arg-1−/flox), schistosome infection results in the formation of larger granulomas than those in control mice, and these granulomas do not shrink at chronic stages of infection, resulting in death due to increased fibrosis and Th2 cell-mediated inflammation (222). Importantly, macrophages from these mice can still undergo alternative activation, highlighting the role of arginase-1 in depleting arginine in the local milieu, directly suppressing T cell proliferation.
A broader question yet to be resolved is whether AAMs mediate primarily immunity rather than regulation. In both H. polygyrus and N. brasiliensis infections, the depletion of alternatively activated macrophages (using clodronate liposomes) increased susceptibility (7, 318). However, in LysMcre IL-4Rα−/flox mice, resistance to N. brasiliensis was unaltered compared to that in wild-type controls (116). Disparities in the timing of these experiments argue that in AAM-depleted mice, immunity is likely delayed rather than ablated. Moreover, clodronate depletion, or arginase inhibition, has no effect on susceptibility or immune responses to T. muris (34). Hence, AAMs can be shown to be essential for the regulation of potentially pathogenic responses while acting in different contexts to either inhibit or promote functional immune mechanisms.
Regulatory T Cells
Evidence from human studies has long suggested that Tregs are important in helminth infections (see “Regulatory Cell Populations in Human Infection”), as strongly supported by data from animal models. Tregs are induced by Brugia pahangi (99) or B. malayi filarial infections (192), with the induction of Foxp3 expression within bystander OVA-specific T cell populations being demonstrated in the latter study. In L. sigmodontis infection, natural and inducible Tregs expand after infection and are required for long-term tolerance to the parasite (280). Treg induction is accompanied by a hyporesponsive phenotype of recruited Th2 cells, which are CD4+ Foxp3− GITR+ CTLA-4+, and the induction of resistance to the parasite required both Treg depletion (using anti-CD25 antibody) and the simultaneous release of Th2 cells from suppression using either anti-GITR (277) or anti-CTLA-4 (278) antibody. Thus, Treg induction in L. sigmodontis infection results in the imprinting of a hyporesponsive phenotype onto parasite-specific Th2 cells, which finds a close parallel to the role of CTLA-4 in human filarial hyporesponsiveness (13, 265).
In mouse schistosome infections, Foxp3+ Tregs are also recruited to the draining lymph nodes and the periphery of egg-induced granulomas in the liver (24, 159), and the forced retroviral expression of Foxp3 in schistosome-infected mice results in a reduction of granuloma size (257). In this infection, Tregs control granulomatous pathology at the intestinal site; a reduction in granuloma size correlates with increased numbers of CD4+ CD25+ CD103+ Foxp3+ cells, and the depletion of these cells results in larger granulomas in the colon (286). As noted above, in the case of ω-1, schistosome eggs release factors which enhance Treg induction in the presence of DCs, TGF-β, and retinoic acid (316), potentially modulating pathology.
In mice as in humans, the Th2 response in later stages of chronic schistosome infection is progressively downregulated, and granulomas reduce in size (32). Both regulatory cells and cytokines contribute to this downmodulation: Treg depletion with anti-CD25 antibody results in decreased egg production and increased granulomatous pathology (160). Among the cytokines, IL-10 is the most critical in dampening pathology and is produced by both Tregs and CD4+ Foxp3− Th2 cells during infection (65, 118). Moreover, in IL-10/IL-12 double-deficient mice, which express exaggerated Th2 responses, excessive fibrosis results in a high mortality rate (122). However, Th1- or Th17-dominated inflammatory responses can also prove lethal in schistosomiasis, as in IL-10 (310) or IL-4 singly deficient mice, which succumb to severe liver damage and cachexia (38), or in the CBA mouse strain, which mounts an ultimately lethal Th17 response (237). Therefore, cytopathic effector responses, whether of the Th1/Th17 or Th2 type, must be regulated to ensure the survival of the host.
In mouse gastrointestinal parasitic infections, there is particularly strong evidence for the involvement of Tregs. A dramatic consequence of infection with H. polygyrus is the expansion and activation of regulatory T cells in both the lamina propria (199) and draining lymph nodes (88, 230). A less-well-defined population of CD8+ Tregs also expands in the lamina propria of H. polygyrus-infected mice (199). Excretory/secretory products of H. polygyrus (HES) can induce Treg differentiation in vitro, through the TGF-β pathway (100), and the inhibition of TGF-β receptor (TGF-βR) signaling in vivo leads to the expulsion of worms and a heightened Th2 response in chronically infected mice. The depletion of Foxp3+ Tregs during H. polygyrus infection in DEREG mice (which express the diphtheria toxin receptor under the control of the Foxp3 promoter) increases Th2 responses without increasing resistance to the parasite, at least when assessed at the early time point of day 14 postinfection (231).
An expansion of CD4+ Foxp3+ Tregs is also seen in other gastrointestinal parasite infections of mice, including Strongyloides ratti in the intestine (28), and around the muscle-stage larvae of Trichinella spiralis (25) or in the spleen of rats similarly infected (104). The depletion of Tregs in DEREG mice immediately after infection with S. ratti resulted in the expression of protective immunity (28), but the depletion of Tregs using anti-CD25 antibody during T. spiralis infection results only in increased Th2 responses without affecting parasite burden. However, the ablation or blockade of both IL-10 and TGF-β (presumably from a non-Treg source) does increase resistance to the parasite (25).
In T. muris infections, Tregs accumulate in the lamina propria. Depletion using anti-CD25 antibody does not enhance worm expulsion but provokes increased gut pathology, indicating that conventional Tregs in this infection control pathological but not effective antiparasite immune responses (60). T. muris infection was also recently shown to induce the expansion of suppressive IL-35-producing CD4+ Foxp3− “Tr35” cells in the intestine (49). Whether these are a common feature of parasitic infections or unique to T. muris remains to be established.
Regulatory B Cells
As well as their prototypic role in producing antibodies, B cells also produce inflammatory and immunoregulatory cytokines, as in the example of IL-10-producing B cells (sometimes known as B10 cells or Bregs), which can control autoimmune disorders such as experimental autoimmune encephalomyelitis (EAE) in mice (87). Suppressive B cells are activated during murine infections with S. mansoni (260) and H. polygyrus (305); in the latter case, Bregs from infected IL-10-deficient mice suppressed allergy and autoimmunity in uninfected recipients. However, in S. mansoni infections, IL-10 was found to be essential for suppression (260). Furthermore, the transfer of S. mansoni-induced Bregs induced the recruitment of Foxp3+ Tregs to the inflammatory airways in an IL-10-dependent manner (4). IL-10 in S. mansoni infection was further shown to be important both for the expansion of IgG1-producing plasma cells in the liver and for the suppression of granulomatous responses during chronic infection. These results could be replicated in mice lacking the ability to class switch antibody (82). Thus, both immunoregulatory cytokine production and antibody production by B cells are important for immunomodulation in chronic schistosome infections.
Although definitive Breg surface markers or transcription factors in mice or humans have yet to be identified, recent work indicated that TIM-1 may be restricted in expression to Bregs (66). Future studies of Bregs using this or other markers may be able to define their mode of activity, and their dialogue with other regulatory cell populations, much more closely.
THE HYGIENE HYPOTHESIS IN MICE
The inverse relationship of allergy, autoimmunity, and inflammatory bowel diseases with parasitic infections in humans has attracted much attention as the hygiene hypothesis has evolved (see “the Hygiene Hypothesis”). Mouse models of parasitic infection and immune pathology are ideal for testing the mechanistic relationship between infection, pathology, and immune status, as reflected in studies investigating this relationship (Tables 2 and 3 and Fig. 3).
Table 2.
Regulation of immunopathology by helminth infections in micea
| Parasite and model | Mechanism type | Reference(s) |
|---|---|---|
| Fasciola hepatica | ||
| EAE | TGF–β dependent, IL–10 independent | 294 |
| Heligmosomoides polygyrus | ||
| Peanut-specific food allergy | IL-10 dependent | 23 |
| House dust mite-specific airway allergy | Treg dependent, IL-10 independent | 304 |
| Breg dependent, IL-10 independent | 305 | |
| OVA-specific airway allergy | Treg dependent, IL-10 independent?g | 111, 146, 304 |
| NOD diabetes | Treg and IL-10 independent | 167 |
| NOD diabetes | 247 | |
| EAE | Breg dependent (IL-10 independent) | 305 |
| TNBS colitis | 273 | |
| IL-10 dependent | 254 | |
| IL-10-deficient T cell transfer colitis | IL-10 independent | 107 |
| IL-10-deficient piroxicam-induced colitis | CD8+ cell dependent, IL-10 independent | 76, 199 |
| OVA-specific colitis | 163 | |
| Hymenolepis diminuta | ||
| Col II-specific arthritis | IL-4Rα, CD4, and IL-10 dependent | 256 |
| DNBS colitis | Macrophage and IL-10 dependent | 129, 130 |
| Litomosoides sigmodontis | ||
| OVA-specific airway allergyb | TGF-β and CD25+ Treg independent | 67 |
| NOD diabetes | TGF-β dependent; Th2, IL-10, and CD25+ Treg independent | 126, 127 |
| Nippostrongylus brasiliensis | ||
| OVA-specific airway allergyc | IL-10 dependent | 307 |
| Schistosoma japonicum | ||
| Col II-specific arthritisd | 114 | |
| TNBS colitis | 319 | |
| Schistosoma mansoni | ||
| Penicillin-specific anaphylaxisd | IL-10-producing Breg dependent; Treg, macrophage, and TGF-β independent | 185 |
| OVA-specific airway allergyd,e,f | CD1dhi B cell-dependent, IL-10-dependent expansion of Tregse | 186 |
| T or B cells transferred protection, IL-10 dependentd | 4e | |
| CD25+ Treg dependent (IL-10 independent)f | 260d | |
| 217d,f | ||
| NOD diabetesd,f | 50d | |
| NKT cell dependentf | 317f | |
| Col II-specific arthritisd | 215 | |
| DSS colitise | Macrophage and IL-10 dependent (but not alternatively activated macrophages) | 259 |
| TNBS colitise | 204 | |
| Symphacia obvelata | ||
| CFA-induced arthritis | 221 | |
| Trichinella spiralis | ||
| NOD diabetes | 247 | |
| DNBS colitis | 141 | |
| EAE | Dose dependent, transferred with splenocytes | 104, 105 |
EAE, experimental autoimmune encephalomyelitis; OVA, chicken egg ovalbumin; NOD, nonobese diabetic; TNBS, trinitrobenzene sulfonic acid; DNBS, dinitrobenzene sulfonic acid; TGF-β, transforming growth factor β; Treg, regulatory T cell; Breg, regulatory B cell; NKT, natural killer T; Col II, collagen II.
L. sigmodontis adults implanted into peritoneal cavity prior to OVA-alum injection.
Dependent on infection time: suppresses only when infection is prior to but not after sensitization.
Mixed-sex infection.
Male-only infection.
Egg injection.
Table 3.
Regulation of immunopathology by helminth products in micea
| Parasite and preparation | Model | Mechanism(s) | Reference(s) |
|---|---|---|---|
| Acanthocheilonema viteae | |||
| Recombinant Av17 cystatin | OVA–specific airway allergy | Macrophage and IL–10 dependent, partially Treg dependent | 248 |
| Recombinant Av17 cystatin | DSS colitis | 248 | |
| ES-62 | Collagen-induced arthritis, airway allergy | Multiple interactions with B cells, dendritic cells, mast cells, and T cells | 108, 190 |
| Ancylostoma caninum | |||
| ES | TNBS colitis | 238 | |
| Ancylostoma ceylanicum | |||
| ES or adult worm antigen | DSS colitis | 41 | |
| Anisakis simplex | |||
| Macrophage migration-inhibitory factor homolog | OVA-specific airway allergy | Increased IL-10, TGF-β, and Tregs | 218 |
| Ascaris suum | |||
| Adult worm antigen | OVA-specific airway allergy | 166 | |
| Ascaris suum PAS-1 | A. suum APAS-3-specific allergy | 135 | |
| Clonorchis sinensis | |||
| Type 1 cystatin | DSS colitis | 136 | |
| Heligmosomoides polygyrus | |||
| ES | OVA-specific airway allergy | Induces Tregs in vitro, which suppress upon transfer | 100 |
| Hymenolepis diminuta | |||
| Adult worm antigen | DNBS colitis | IL-10 independent | 138 |
| Litomosoides sigmodontis | |||
| Adult worm antigen | NOD diabetes | 127 | |
| Nippostrongylus brasiliensis | |||
| ES | OVA-specific airway allergy | Dependent on protein fold; independent of TLR2/4 signaling and IL-10 | 283 |
| Schistosoma japonicum | |||
| SEA | OVA-specific airway allergy | 311 | |
| Schistosoma mansoni | |||
| SEA or SWAP | NOD diabetes | Increased NKT cells, galectin-1/3, TGF-β, and Tregs | 314, 315, 317 |
| SEA | EAE | Switch of EAE response from Th1 to Th2 | 320 |
| SWAP | TNBS colitis | 238 | |
| Toxascaris leonina | |||
| Galectin-9 homolog | DSS colitis | 143 | |
| Trichinella spiralis | |||
| Homogenized muscle stage larvae | DNBS colitis | 206 | |
| Recombinant TsP53 | TNBS colitis | 70 |
ES, excretory/secretory products; SEA, soluble egg antigen; SWAP, soluble worm adult preparation; OVA, chicken egg ovalbumin; DSS, dextran sulfate sodium; DNBS, dinitrobenzene sulfonic acid; EAE, experimental autoimmune encephalomyelitis; TNBS, trinitrobenzene sulfonic acid; APAS-3, allergenic protein of A.suum; Treg, regulatory T cell; NKT, natural killer T; TGF-β, transforming growth factor β.
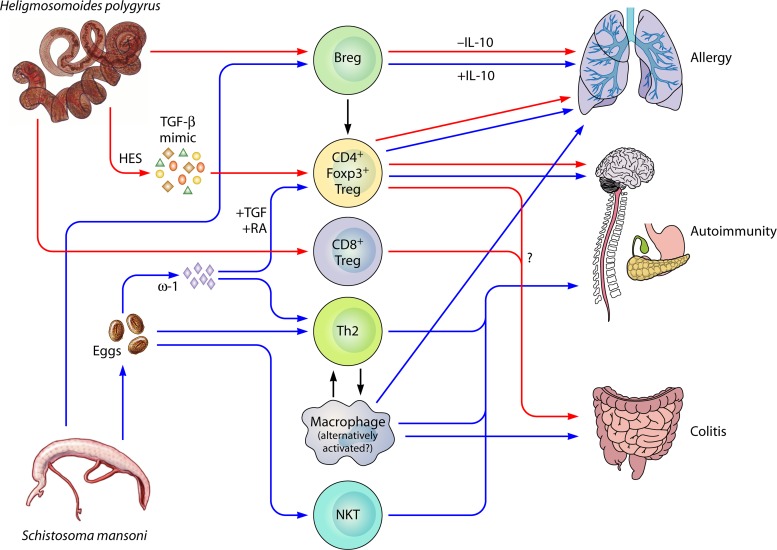
Fig 3.
Suppressive mechanisms of Heligmosomoides polygyrus and Schistosoma mansoni on immunopathologies. Of all the helminths that infect mice, the effects of H. polygyrus (red arrows) and S. mansoni (blue arrows) on immunopathology models have been best characterized. Both parasites effectively suppress immune responses and pathology in models of allergy, autoimmunity, and colitis. CD4+ Foxp3+ (Forkhead box p3) Tregs (regulatory T cells) are induced by both H. polygyrus and S. mansoni through secretory molecules affecting the TGF-β (transforming growth factor β) and/or retinoic acid pathways, and these Tregs can suppress all immunopathologies. Suppressive Bregs (regulatory B cells) are also induced by both parasites, and functional suppression has been shown in allergy and autoimmunity through B cell IL-10 production (S. mansoni) or an IL-10-independent mechanism (H. polygyrus). H. polygyrus has also been shown to induce a regulatory population of CD8+ T cells in the gut, which may, along with CD4+ Foxp3+ Tregs, be involved in the suppression of colitis in an IL-10-independent, TGF-βR-dependent manner. S. mansoni eggs and their products (especially the RNase ω-1) are powerful Th2 (T helper type 2) inducers, and skewing toward a Th2 response is protective in some models of autoimmunity. Th2 cytokines also induce the alternative activation of macrophages, which are functionally suppressive. Macrophages induced by S. mansoni infections (whether alternatively activated or not) are suppressive in all models of immunopathology studied. Finally, S. mansoni eggs induce the expansion of NKT (natural killer T) cells, which are deficient in the diabetic NOD mouse, and protect against disease.
Allergy
A wide range of helminth species have been demonstrated to modulate allergic responses (78, 303), most notably the intestinal nematode H. polygyrus, which suppresses IgE and anaphylaxis in a model of dietary peanut allergy (23) as well as airway hyperresponsiveness, lung histopathology, eosinophil recruitment, and Th2 cytokines in alum-sensitized models of airway allergy (111, 304). The suppression of allergy was dependent on CD25+ Tregs but was unaffected by anti-IL-10R treatment and could be transferred from infected, allergen-naive mice to uninfected, allergen-sensitized mice with CD4+ cells, CD4+ CD25+ Tregs (304), or B cells (305). However, a subsequent study found that the suppression of airway allergy in H. polygyrus infection was lost in IL-10-deficient mice, indicating that IL-10 may play an important role in some settings (146). Interestingly, the same parasite is unable to modulate skin allergy, although this was tested some 10 weeks following infection of mice (111). As mentioned above, H. polygyrus excretory/secretory products (HES) can induce Tregs in vitro through the TGF-βR pathway. The transfer of Tregs induced in vitro by HES, or by mammalian TGF-β, also abrogated airway allergic inflammation, confirming the ability of helminth-induced Tregs to suppress allergic pathology (100). When administered with an allergen in vivo, HES can also abolish the subsequent allergic response to airway challenge (193).
Filarial parasites similarly suppress pathology in allergy models. The implantation of L. sigmodontis adults into the peritoneal cavity of mice reduces airway allergy upon subsequent sensitization and challenge (67). Both TGF-β and CD25+ Tregs are enhanced in this system, although the depletion of these factors in vivo affected only physiological hyperreactivity and not tissue inflammation. Recombinantly expressed filarial cystatin (Av17, from the related species Acanthocheilonema viteae) can also inhibit allergic pathology when coadministered at the time of allergen sensitization or, subsequently, prior to challenge in a macrophage-, IL-10-, and partially Treg-dependent manner (248).
Infection with the gastrointestinal helminth N. brasiliensis prior to (but not after) allergen sensitization also blocked eosinophil recruitment to the lungs in an IL-10-dependent manner (307). However, in this model, N. brasiliensis stimulates an allergic response against its own components. As previous work established that hookworm-secreted proteases degrade eotaxin (59), it would be interesting to know if the related pathogen N. brasiliensis used a similar mechanism to suppress eosinophil accumulation. The excretory/secretory products of N. brasiliensis (NES), moreover, can potently suppress eosinophil infiltration, Th2 cytokines, anti-OVA IgE, and pathology (by histological scoring or plethysmography) when administered with a sensitizing allergen in a TLR2- and TLR4-independent manner (283). A number of other helminth products can similarly interfere with allergen priming, including whole-body extracts of the human roundworm Ascaris suum (166) and a purified product (PAS-1) that suppresses allergic responses induced by another component of the same parasite (135).
Schistosomes are also potent inhibitors of allergies in mice, and animals infected with S. mansoni show attenuated cellular and cytokine responses to allergen challenge (217, 260). Similarly, the administration of S. japonicum egg antigens to mice leads to the suppression of airway Th2 cytokines and responsiveness and histological inflammation, which was abrogated by the depletion of CD25+ Tregs (311). However, the strength of protection depends on multiple factors, including the parasite load, the stage of infection, and the gender of the parasite (186, 260), as allergy is intensified in some settings. For example, productive (egg-laying) infections with S. mansoni exacerbated pathology, while male-only (and egg-free) infections suppressed allergen-specific lung responses in a CD4-independent and IL-10- and B cell-dependent manner (186). Protection from allergy correlated quantitatively with the worm burden, and only the most highly infected animals were significantly suppressed (261), a finding which resonates with a recent study of S. haematobium-infected humans showing that the suppression of atopy correlated with infection intensity (236).
These subtle but crucial factors may explain why mechanistic insights into the S. mansoni-associated suppression of allergy differ between studies. Thus, Pacifico et al. showed that protection by S. mansoni was unchanged with anti-IL-10R administration, while Treg depletion using anti-CD25 antibody abrogated the suppression of eosinophil recruitment to the lungs (217). However, other authors reported that protection against allergy by S. mansoni is IL-10 dependent and could be transferred with B cells (4, 185) and, in one study, by CD4+ T cells (260).
Autoimmunity
Multiple models of autoimmunity in rodents have revealed the suppressive capability of helminths in Th1/Th17-mediated immunopathologies (78, 189). An early example of this interaction was reported for rats infected with the nematode parasite Symphacia obvelata, in which complete Freund's adjuvant (CFA)-induced arthritis was reduced (221). In type II collagen-induced arthritis (CIA), infection with S. mansoni reduced collagen II-specific antibodies and inflammatory cytokine production, while pathology negatively correlated with parasite burden (215). Similarly, S. japonicum was also shown to suppress CIA; however, in a stage-specific manner, early-stage infection (2 weeks postinfection) significantly suppressed arthritis, collagen II-specific antibody titers, and inflammatory cytokine production, while productive egg-laying infection (7 weeks postinfection) had no effect (114). Thus, the schistosome suppression of arthritis pathology may depend on a lack of egg-induced inflammation, as seen in asthma models of S. mansoni infection (186). The rat tapeworm, Hymenolepis diminuta, also downregulates CFA-induced arthritis in mice in an IL-4Rα-, CD4-, and IL-10-dependent manner, consistent with suppression being due to Th2 induction in these mice (256). Collagen-induced arthritis can also be alleviated by the filarial nematode molecule ES-62 (190). This modulator has direct effects on T cells, B cells, and innate cells, acting by altering intracellular signaling (108).
Nonobese diabetic (NOD) mice develop spontaneous diabetes over the first 6 months of life. Infection with H. polygyrus (167, 247), T. spiralis (247), L. sigmodontis (126, 127), or S. mansoni, either by live infection (50) or by nonliving schistosome (314, 315, 317) or filarial (127) antigen administration, delays onset or ameliorates disease in these mice. Proposed mechanisms of protection by live infection include a skewing of the inflammatory response toward Th2 cells (50, 247) or a TGF-β-dependent mechanism independent of Th2 cells, Tregs, or IL-10 (126). S. mansoni egg antigens may confer protection by the expansion of invariant natural killer T (iNKT) cells (which are deficient in NOD mice) (317) or by the expansion of Tregs, cell-bound TGF-β, or alternatively activated macrophages (314, 315).
The development of pathology in the experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis can be ablated by many parasites, as first shown with S. mansoni (153, 255). During H. polygyrus infection, B cells transfer protection against EAE in an IL-10-independent manner, similar to the B cell-mediated suppression shown in asthma (305). In rats, EAE pathology is controlled by T. spiralis infection in a dose-dependent manner by a mediator that could be transferred with nylon wool-depleted, T cell-enriched splenocytes (105). S. mansoni SEA administration is beneficial, as it skews the antimyelin response from Th1/Th17 to Th2 (320). Infection with the liver fluke Fasciola hepatica also results in a delayed onset and an ameliorated pathology of EAE through an IL-10-independent, TGF-β-dependent mechanism, while the control of the antiparasite response is IL-10 dependent (294). Thus, multiple mechanisms may control parasite-specific and bystander immunosuppression. Not all parasitic infections control EAE, however, as infection of rats with Strongyloides venezuelensis did not alter EAE progression by any of the parameters measured (44).
As helminth parasites can be thought of as long-term xenotransplants, the possibility that they would prolong allogeneic transplants in animals has been investigated. Notably, kidney allograft survival in rats was prolonged 3-fold by infection with N. brasiliensis, which caused a marked reduction in numbers of graft-infiltrating CD8+ T cells and an overall increase in IL-4 levels (161). A similar shift was achieved by administering whole-worm extract prior to grafting, suggesting that the induction of a potent Th2 response was able to inhibit the CD8+ T cell-mediated transplant rejection. That same group subsequently demonstrated that cardiac transplant survival in mice was similarly promoted by N. brasiliensis and provided evidence that infection shifted the CD8+ T cell population into a type 2 (“Tc2”) state (168).
Inflammatory Bowel Disease
Human inflammatory bowel diseases represent important and often severe pathologies, including ulcerative colitis and Crohn's disease (128). Several mouse models for IBD-like colitis differ in their speed of onset, severity, chronicity, location of inflammation, and mechanism of action, depending on the relative contributions of the adaptive immune system, the innate immune system, and/or commensals in the gut (77, 78, 306). In largely innate cell-mediated dextran sulfate sodium (DSS) colitis, male-only S. mansoni infections (similarly to protection in airway allergy [186]) could suppress pathology through a macrophage- and IL-10-dependent mechanism (259). In T cell-dependent trinitrobenzene sulfonic acid (TNBS)-induced colitis, schistosome infections (204), eggs (75, 319), or whole-adult-worm extract (238) suppressed pathology and Th1/Th17 cytokines and induced the Th2 cytokines IL-10 and TGF-β.
H. polygyrus can similarly counteract pathology in bacterially induced colitis (301), TNBS-induced colitis (273), T cell transfer colitis (107), and spontaneous colitis in IL-10-deficient (76) but not TGF-βR-compromised (133) mice. Thus, the suppressive effects of H. polygyrus appear to depend on TGF-βR, but not IL-10R, signaling.
The administration of Ancylostoma hookworm excretory/secretory products can also suppress DSS-induced (41) and TNBS-induced (238) colitis, and products of the tapeworm Hymenolepis diminuta are able to dampen pathology in T cell-mediated dinitrobenzene sulfonic acid (DNBS)-induced colitis (138). The downregulatory effects of H. diminuta were found to be dependent on alternatively activated macrophage induction, and in fact, the administration of alternatively activated macrophages could protect against colitis in naive mice (130). Intriguingly, remission in human Crohn's disease was also found to be associated with the accumulation of alternatively activated macrophages in the gut, further highlighting their wound-healing role (130). Filarial recombinant Av17 cystatin could also suppress DSS-induced colitis, and although the mechanism of protection against colitis was not dissected, protection against airway allergy using this protein was macrophage and IL-10 dependent and partially Treg dependent (248).
T. spiralis infection, or administration of T. spiralis antigens into the rectal submucosa, suppresses pathology in DNBS-induced colitis (141, 206). Recently, this effect was replicated in the TNBS-induced colitis model with the subcutaneous administration of rTsP53, a 53-kDa protein of T. spiralis (70).
Although the suppressive effects of administering helminth infection or helminth products have focused largely on immunoregulatory mechanisms, an important mechanism of protection may be goblet cell hyperplasia and increased mucous production seen in antiparasite responses, which could also ameliorate the barrier defect seen in inflammatory bowel diseases (308).
HELMINTH THERAPY IN HUMANS
The epidemiological and immunological evidence for the suppression of immune dysregulatory diseases such as allergy, autoimmunity, and inflammatory bowel disease has led to several clinical trials of treatment with live helminth parasites (Table 4). These trials can be seen as the ultimate test for the hygiene hypothesis: if parasitic infection prevents immune dysregulation, then infection of affected individuals with parasites should cure disease. However, a number of caveats should be borne in mind when assessing these trials.
Table 4.
Regulation of immunopathology in human trialsa
| Disease | Parasite treatment (regimen) | No. of subjects | Effect(s) | Reference(s) |
|---|---|---|---|---|
| Allergic rhinitis | Trichuris suis (2,500 ova every 3 wk for 24 wk) | 96 (49 infected, 47 placebo) | No change in clinical disease | 19 |
| Allergic rhinoconjuctivitis | Necator americanus (10 larvae) | 30 (15 infected, 15 placebo) | No clinical benefit; no suppressive immune response or reduction of allergic immune response | 30, 84 |
| Celiac disease | Necator americanus (15 larvae total) | 20 (10 infected, 10 placebo) | No clinical benefit; suppression of intestinal inflammatory cytokines | 61, 191 |
| Crohn's disease | Necator americanus | 9 (all infected) | Trend toward reduced disease; CDAI at baseline, 165; CDAI at wk 20 postinfection, 64 (P = 0.132) | 58 |
| Crohn's disease | Trichuris suis (2,500 ova every 3 wk for 24 wk) | 29 (all infected) | Significant suppression of pathology; 79.3% response, 72.4% remission | 270 |
| Crohn's disease and ulcerative colitis | Trichuris suis (2,500 ova initially, boosts of 2,500 ova every 3 wk) | CD, 4 infected, 2 given boost; UC, 3 infected, 2 given boost | CD, 3/4 patients entered remission; UC, 3/3 improved (mean, 43% improvement) | 269 |
| Ulcerative colitis | Trichuris suis (2,500 ova every 2 wk for 12 wk) | 52 (29 infected, 23 placebo) | Significant suppression of pathology; UCDAI, 8.75–7.5 for placebo (P = 0.1167), 8.77–6.1 for infected (P = 0.0004); simple index, no change for placebo (P > 0.05), decrease for infected (P < 0.0001) | 271 |
| Ulcerative colitis | Trichuris trichiura (500, 1,000, and 200 eggs) | 1 (infected) | Disease remission after infection; downregulation of mucosal inflammatory cytokines; switch to IL-22 | 36 |
| Multiple sclerosis | Various (3 Hymenolepis nana, 3 Trichuris trichiura, 3 Ascaris lumbricoides, 2 Strongyloides stercolaris, 1 Enterobius vermicularis) | 24 (12 infected, 12 matched controls) | Significantly fewer exacerbations when infected; drug cure of 4 patientsb led to increase of symptoms to uninfected control levels; MARR, 0 for infected, 1.09 for uninfected (P < 0.0001); increase after drug cure, P = 0.007; decreased inflammatory cytokine levels, increased IL-10, TGF-β, and Treg levels with parasitic infection | 55–57 |
| Multiple sclerosis | Trichuris suis ova (2,500 ova every 2 wk for 24 wk) | 5 (all infected) | Trend toward reduced disease; no change in peripheral Tregs or alternatively activated macrophages | 26, 89 |
CDAI, Crohn's disease activity index; CD, Crohn's disease; UC, ulcerative colitis; UCDAI, ulcerative colitis disease activity index; MARR, median annual relapse rate.
Two patients were infected with A. lumbricoides, 1 was infected with T. trichiura, and 1 was infected with S. stercolaris.
First, individuals who live in areas where helminth disease is endemic are exposed to parasitic infections from an early age (and potentially in the womb), prior to the development of immune dysregulatory disease. Therefore, parasitic infection may be required at an early age to encourage the appropriate development of the immune system. Due to ethical and practical considerations, most clinical trials have been carried out on individuals already affected by immune dysregulatory disease, and it may be much more difficult to suppress an ongoing reaction than prevent its development.
Second, due to ethical considerations, many trials using parasitic infection must use an asymptomatic dose (for example, in N. americanus infections), which may be much lower than that received during natural infections (205) and may not be efficacious in suppressing pathology. Finally, good quality controls are often unavailable, if they are used at all. Where blinded matched controls are used, participants may realize that they are infected due to mild symptoms associated with infection, such as skin rash, cough, and gastrointestinal symptoms (20, 205). A placebo effect is therefore difficult to discount in many trials. Nevertheless, trials have been ongoing for the last 10 years, beginning with the administration of Trichuris suis ova (TSO) in inflammatory bowel disease by Summers et al. (269). Since this success, many other trials have been carried out, using T. suis ova or T. trichiura or N. americanus infections.
The first published clinical trial tested TSO in Crohn's disease and ulcerative colitis patients (269). T. suis is the pig whipworm, which, although closely related to the human whipworm, T. trichiura, cannot maintain a chronic infection in humans and is ejected within a few weeks. Thus, TSO treatment regimens consist of repeated administration every 2 to 3 weeks. In the first trial, TSO treatment showed a trend toward the suppression of both Crohn's disease and colitis (269), which led to further larger-scale trials for Crohn's disease (270) and a placebo-controlled trial for ulcerative colitis (271), both of which showed a significant suppression of disease.
More recently, TSO therapy has been used in allergic rhinitis, with no change in clinical symptoms (19). Some researchers have argued that this may be due to the timing of the treatment, as it was initiated only just prior to the pollen season and, hence, allergen exposure (272), or to the low dose of parasites at a site distant from pathology (115). In addition, a trial for multiple sclerosis showed a trend toward reduced numbers of new lesions by magnetic resonance imaging (89). However, no changes in inflammatory or regulatory cytokines or cells were seen, and the mechanism of action of TSO treatment remains unknown. T. suis ova are now produced under good manufacturing practice (GMP) guidelines and are available commercially (Ovamed GmbH). Clinical trials are also now planned (http://clinicaltrials.gov/) for the use of TSO for allergies to peanuts and tree nuts (clinical trial identifier NCT01070498) and irritability in autism (clinical trial identifier NCT01040221).
T. suis is a swine parasite that is not adapted to humans and causes significant side effects, including gastrointestinal upset and diarrhea (20). These symptoms appear to abate after several weeks of treatment, implying a suppression of the inflammatory response in the gut during chronic stimulation. Some researchers have turned to human pathogens for an alternative to TSO, and several studies using human hookworm have been carried out. Hookworms have been linked most strongly of all parasites studied to the suppression of asthma in populations living in an area of endemicity (162), and at low levels experimental infections can be tolerated without significant pathology (205). As hookworm is a human-adapted parasite, it also maintains chronic infections for months to years, with the life span of the adult worm alone being between 1 and 18 years (37).
The first clinical trial of hookworms as a therapeutic agent was for Crohn's disease, where infection resulted in a trend toward decreased pathology in an unblinded open-label trial (58). Subsequently, trials for celiac disease (61) and allergic rhinoconjunctivitis (85) have been carried out. Although neither of these small-scale trials showed a significant suppression of pathology, the celiac disease trial did show some suppression of inflammatory cytokines in the gut (191), associated with a switch to a gluten-specific Th2 response (191). Thus, it remains to be seen whether human hookworm infection will be a viable option for the treatment of human immunopathologies.
Although clinical trials using helminth infection have yielded interesting prospects for the treatment of immune dysregulatory disease, the ultimate goal of this work is to identify the molecular mechanism by which parasites achieve this and to replicate this using a commercially produced drug. An intermediate step in this process is the use of parasite-derived products to replicate the protective effect of the infection and, thus, identify individual molecular motifs responsible for suppression. Although positive results have come from administering parasite products in mouse models of immune dysregulatory disease (Table 3), no parasite products have yet been administered as treatments for human disease.
CONCLUSIONS
Immunoregulation by helminths is now understood to be a major survival mechanism that has evolved through the close host-parasite relationship over eons of time (3). Moreover, many exciting immunological insights and therapeutic possibilities are developing from the study of helminth parasites. Epidemiological indications of a protective effect against some of the maladies of the industrialized world have been supported by in-depth field studies as well as extensive laboratory experimentation. This information now suggests future strategies which can exploit both the general principles elucidated and the specific modulatory mediators discovered for the control of both infectious and immunopathological diseases.
Biographies

Henry J. McSorley obtained his undergraduate degree in Immunology from the University of Glasgow, Glasgow, Scotland, in 2004. In 2008, he completed an Immunoparasitology Ph.D. with Professor Richard M. Maizels at the University of Edinburgh before taking up positions as a postdoctoral scientist working on the effects of hookworms on colitis in mice and humans at the Queensland Institute of Medical Research in Brisbane, Australia, and the James Cook University in Cairns, Australia, with Professor Alex Loukas. Since 2010, he has been working as a postdoctoral scientist with Professor Rick M. Maizels at the University of Edinburgh, working on the effects of Heligmosomoides polygyrus products on mouse models of allergic airway disease.

Rick M. Maizels is in the University of Edinburgh's Institute of Immunology and Infection Research. He is an immunologist interested in fundamental questions of how and why parasites manipulate the sophisticated mammalian immune system and how that system has evolved in the face of parasite immunomodulatory strategies. His laboratory (http://maizelsgroup.biology.ed.ac.uk/) focuses on the most complex parasites to invade the body, multicellular helminths, which cause widespread tropical diseases while displaying a profound immunoregulatory capability. The central questions which his group addresses are (i) how do parasites manipulate the host immune system, (ii) what is the impact of this modulation on immunological diseases, and (iii) what parasite molecules are responsible for immunomodulation? Before taking the Chair of Zoology at Edinburgh in 1995, Dr. Maizels was Professor of Parasite Immunology at Imperial College London, where he moved in 1983. Prior to this, he held positions at the National Institute for Medical Research in London (1973 to 1976 and 1979 to 1983) as well as the University of California, Los Angeles (1977 to 1979), and the California Institute of Technology (1976 to 1977).
REFERENCES
- 1. Adjobimey T, Hoerauf A. 2010. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann. Trop. Med. Parasitol. 104:455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen JE, Lawrence RA, Maizels RM. 1996. Antigen presenting cells from mice harboring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int. Immunol. 8:143–151 [DOI] [PubMed] [Google Scholar]
- 3. Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11:375–388 [DOI] [PubMed] [Google Scholar]
- 4. Amu S, et al. 2010. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 125:1114–1124 [DOI] [PubMed] [Google Scholar]
- 5. Anderson RM, May RM. 1985. Helminth infection of humans: mathematical model, population dynamics and control. Adv. Parasitol. 24:1–101 [DOI] [PubMed] [Google Scholar]
- 6. Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7:975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anthony RM, et al. 2006. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12:955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Araujo MI, de Carvalho EM. 2006. Human schistosomiasis decreases immune responses to allergens and clinical manifestations of asthma. Chem. Immunol. Allergy 90:29–44 [DOI] [PubMed] [Google Scholar]
- 9. Araujo MI, et al. 2000. Inverse association between skin response to aeroallergen and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145–148 [DOI] [PubMed] [Google Scholar]
- 10. Arndts K, et al. 2012. Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti. PLoS Negl. Trop. Dis. 6:e1611 doi:10.1371/journal.pntd.0001611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atmadja AK, et al. 1995. Differential decline in filarial-specific IgG1, IgG4 and IgE antibodies following diethylcarbamazine chemotherapy of Brugia malayi infected patients. J. Infect. Dis. 172:1567–1572 [DOI] [PubMed] [Google Scholar]
- 12. Ayash-Rashkovsky M, et al. 2007. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infect. Immun. 75:1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Babu S, et al. 2009. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 200:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babu S, et al. 2009. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl. Trop. Dis. 3:e420 doi:10.1371/journal.pntd.0000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. 2005. Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J. Immunol. 175:1170–1176 [DOI] [PubMed] [Google Scholar]
- 16. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. 2006. Diminished T cell TLR expression and function modulates the immune response in human filarial infection. J. Immunol. 176:3885–3889 [DOI] [PubMed] [Google Scholar]
- 17. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176:3248–3256 [DOI] [PubMed] [Google Scholar]
- 18. Babu S, Kumaraswami V, Nutman TB. 2009. Alternatively activated and immunoregulatory monocytes in human filarial infections. J. Infect. Dis. 199:1827–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bager P, et al. 2010. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J. Allergy Clin. Immunol. 125:123–130 [DOI] [PubMed] [Google Scholar]
- 20. Bager P, et al. 2011. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS One 6:e22346 doi:10.1371/journal.pone.0022346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balic A, Smith KA, Harcus Y, Maizels RM. 2009. Dynamics of CD11c+ dendritic cell subsets in lymph nodes draining the site of intestinal nematode infection. Immunol. Lett. 127:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bancroft AJ, Else KJ, Humphreys NE, Grencis RK. 2001. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int. J. Parasitol. 31:1627–1637 [DOI] [PubMed] [Google Scholar]
- 23. Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. 2002. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 169:3284–3292 [DOI] [PubMed] [Google Scholar]
- 24. Baumgart M, Tomkins F, Leng J, Hesse M. 2006. Naturally-occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 176:5374–5387 [DOI] [PubMed] [Google Scholar]
- 25. Beiting DP, et al. 2007. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-β. J. Immunol. 178:1039–1047 [DOI] [PubMed] [Google Scholar]
- 26. Benzel F, et al. 2012. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. J. Helminthol. 86:339–347 [DOI] [PubMed] [Google Scholar]
- 27. Bethony JM, et al. 2011. Vaccines to combat the neglected tropical diseases. Immunol. Rev. 239:237–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blankenhaus B, et al. 2011. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 186:4295–4305 [DOI] [PubMed] [Google Scholar]
- 29. Bloch P, Nielsen NO, Meyrowitsch DW, Malecela MN, Simonsen PE. 2011. A 22 year follow-up study on lymphatic filariasis in Tanzania: analysis of immunological responsiveness in relation to long-term infection pattern. Acta Trop. 120:258–267 [DOI] [PubMed] [Google Scholar]
- 30. Blount D, et al. 2009. Immunologic profiles of persons recruited for a randomized, placebo-controlled clinical trial of hookworm infection. Am. J. Trop. Med. Hyg. 81:911–916 [DOI] [PubMed] [Google Scholar]
- 31. Borkow G, Bentwich Z. 2008. Chronic parasite infections cause immune changes that could affect successful vaccination. Trends Parasitol. 24:243–245 [DOI] [PubMed] [Google Scholar]
- 32. Boros DL, Pelley RP, Warren KS. 1975. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J. Immunol. 114:1437–1441 [PubMed] [Google Scholar]
- 33. Bourke CD, Maizels RM, Mutapi F. 2011. Acquired immune heterogeneity and its sources in human helminth infection. Parasitology 138:139–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bowcutt R, et al. 2011. Arginase-1-expressing macrophages are dispensable for resistance to infection with the gastrointestinal helminth Trichuris muris. Parasite Immunol. 33:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brattig NW, et al. 2002. Onchocerca volvulus-exposed persons fail to produce interferon-gamma in response to O. volvulus antigen but mount proliferative responses with interleukin-5 and IL-13 production that decrease with increasing microfilarial density. J. Infect. Dis. 185:1148–1154 [DOI] [PubMed] [Google Scholar]
- 36. Broadhurst MJ, et al. 2010. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2:60ra88 doi:10.1126/scitranslmed.3001500 [DOI] [PubMed] [Google Scholar]
- 37. Brooker S, Bethony J, Hotez PJ. 2004. Human hookworm infection in the 21st century Adv. Parasitol. 58:197–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. 1997. IL-4 protects against TNF-α-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159:777–785 [PubMed] [Google Scholar]
- 39. Caldas IR, et al. 2008. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop. 108:109–117 [DOI] [PubMed] [Google Scholar]
- 40. Camberis M, Le Gros G, Urban J., Jr 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus, p 19.12.11–19.12.27 In Coico R. (ed), Current protocols in immunology. John Wiley & Sons, Inc, Hoboken, NJ: [DOI] [PubMed] [Google Scholar]
- 41. Cançado GG, et al. 2011. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm. Bowel Dis. 17:2275–2286 [DOI] [PubMed] [Google Scholar]
- 42. Carvalho L, et al. 2009. Mechanisms underlying helminth modulation of dendritic cell function. Immunology 126:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chawla A, Nguyen KD, Goh YP. 2011. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11:738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiuso-Minicucci F, et al. 2011. Experimental autoimmune encephalomyelitis evolution was not modified by multiple infections with Strongyloides venezuelensis. Parasite Immunol. 33:303–308 [DOI] [PubMed] [Google Scholar]
- 45. Cliffe LJ, Grencis RK. 2004. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57:255–307 [DOI] [PubMed] [Google Scholar]
- 46. Cliffe LJ, et al. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308:1463–1465 [DOI] [PubMed] [Google Scholar]
- 47. Coggeshall LT. 1946. Filariasis in the serviceman; retrospect and prospect. JAMA 131:8–12 [DOI] [PubMed] [Google Scholar]
- 48. Colley DG, et al. 1986. Immune responses during human schistosomiasis. XII. Differential responsiveness in patients with hepatosplenic disease. Am. J. Trop. Med. Hyg. 35:793–802 [PubMed] [Google Scholar]
- 49. Collison LW, et al. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 11:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cooke A, et al. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21:169–176 [DOI] [PubMed] [Google Scholar]
- 51. Cooper PJ. 2009. Interactions between helminth parasites and allergy. Curr. Opin. Allergy Clin. Immunol. 9:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cooper PJ, Barreto ML, Rodrigues LC. 2006. Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br. Med. Bull. 79–80:203–218 [DOI] [PubMed] [Google Scholar]
- 53. Cooper PJ, et al. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. 1998. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J. Infect. Dis. 178:1133–1138 [DOI] [PubMed] [Google Scholar]
- 55. Correale J, Farez M. 2007. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. 61:97–108 [DOI] [PubMed] [Google Scholar]
- 56. Correale J, Farez M, Razzitte G. 2008. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann. Neurol. 64:187–199 [DOI] [PubMed] [Google Scholar]
- 57. Correale J, Farez MF. 2011. The impact of parasite infections on the course of multiple sclerosis. J. Neuroimmunol. 233:6–11 [DOI] [PubMed] [Google Scholar]
- 58. Croese J, et al. 2006. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 55:136–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Culley FJ, et al. 2000. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J. Immunol. 165:6447–6453 [DOI] [PubMed] [Google Scholar]
- 60. D'Elia R, Behnke JM, Bradley JE, Else KJ. 2009. Regulatory T cells: a role in the control of helminth driven intestinal pathology and worm survival. J. Immunol. 182:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daveson AJ, et al. 2011. Effect of hookworm infection on wheat challenge in celiac disease—a randomised double-blinded placebo controlled trial. PLoS One 6:e17366 doi:10.1371/journal.pone.0017366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Jesus AR, et al. 2004. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect. Immun. 72:3391–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Jesus AR, et al. 2002. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J. Infect. Dis. 185:98–105 [DOI] [PubMed] [Google Scholar]
- 64. Dessein A, et al. 2004. Interleukin-13 in the skin and interferon-gamma in the liver are key players in immune protection in human schistosomiasis. Immunol. Rev. 201:180–190 [DOI] [PubMed] [Google Scholar]
- 65. Dewals B, Hoving JC, Horsnell WG, Brombacher F. 2010. Control of Schistosoma mansoni egg-induced inflammation by IL-4-responsive CD4(+)CD25(−)CD103(+)Foxp3(−) cells is IL-10-dependent. Eur. J. Immunol. 40:2837–2847 [DOI] [PubMed] [Google Scholar]
- 66. Ding Q, et al. 2011. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 121:3645–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dittrich AM, et al. 2008. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J. Immunol. 180:1792–1799 [DOI] [PubMed] [Google Scholar]
- 68. Doetze A, et al. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-b but not by a Th1 to Th2 shift. Int. Immunol. 12:623–630 [DOI] [PubMed] [Google Scholar]
- 69. Druilhe P, Tall A, Sokhna C. 2005. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 21:359–362 [DOI] [PubMed] [Google Scholar]
- 70. Du L, et al. 2011. The protective effect of the recombinant 53-kDa protein of Trichinella spiralis on experimental colitis in mice. Dig. Dis. Sci. 56:2810–2817 [DOI] [PubMed] [Google Scholar]
- 71. Elias D, et al. 2005. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 23:1326–1334 [DOI] [PubMed] [Google Scholar]
- 72. Elias D, Britton S, Aseffa A, Engers H, Akuffo H. 2008. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine 26:3897–3902 [DOI] [PubMed] [Google Scholar]
- 73. Elias D, Britton S, Kassu A, Akuffo H. 2007. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev. Anti Infect. Ther. 5:475–484 [DOI] [PubMed] [Google Scholar]
- 74. Elliott AM, et al. 2011. Treatment with anthelminthics during pregnancy: what gains and what risks for the mother and child? Parasitology 138:1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Elliott DE, et al. 2003. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284:G385–G391 doi:10.1152/ajpgi.00049.2002 [DOI] [PubMed] [Google Scholar]
- 76. Elliott DE, et al. 2004. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 34:2690–2698 [DOI] [PubMed] [Google Scholar]
- 77. Elliott DE, Summers RW, Weinstock JV. 2005. Helminths and the modulation of mucosal inflammation. Curr. Opin. Gastroenterol. 21:51–58 [PubMed] [Google Scholar]
- 78. Elliott DE, Summers RW, Weinstock JV. 2007. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 37:457–464 [DOI] [PubMed] [Google Scholar]
- 79. Erb KJ. 2007. Helminths, allergic disorders and IgE-mediated immune responses: where do we stand? Eur. J. Immunol. 37:1170–1173 [DOI] [PubMed] [Google Scholar]
- 80. Everts B, et al. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206:1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Everts B, Smits HH, Hokke CH, Yazdankbakhsh M. 2010. Sensing of helminth infections by dendritic cells via pattern recognition receptors and beyond: consequences for T helper 2 and regulatory T cell polarization. Eur. J. Immunol. 40:1525–1537 [DOI] [PubMed] [Google Scholar]
- 82. Fairfax KC, et al. 2012. IL-10R blockade during chronic schistosomiasis mansoni results in the loss of B cells from the liver and the development of severe pulmonary disease. PLoS Pathog. 8:e1002490 doi:10.1371/journal.ppat.1002490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Feary J, Britton J, Leonardi-Bee J. 2011. Atopy and current intestinal parasite infection: a systematic review and meta-analysis. Allergy 66:569–578 [DOI] [PubMed] [Google Scholar]
- 84. Feary J, et al. 2009. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin. Exp. Allergy 39:1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feary JR, et al. 2010. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin. Exp. Allergy 40:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Figueiredo CA, et al. 2010. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun. 78:3160–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944–950 [DOI] [PubMed] [Google Scholar]
- 88. Finney CAM, Taylor MD, Wilson MS, Maizels RM. 2007. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 37:1874–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fleming J, et al. 2011. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult. Scler. 17:743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fleming JO, Cook TD. 2006. Multiple sclerosis and the hygiene hypothesis. Neurology 67:2085–2086 [DOI] [PubMed] [Google Scholar]
- 91. Flohr C, Quinnell RJ, Britton J. 2009. Do helminth parasites protect against atopy and allergic disease? Clin. Exp. Allergy 39:20–32 [DOI] [PubMed] [Google Scholar]
- 92. Flohr C, et al. 2010. Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double-blind, placebo-controlled trial in Vietnam. Clin. Exp. Allergy 40:131–142 [DOI] [PubMed] [Google Scholar]
- 93. Fried B, Reddy A, Mayer D. 2011. Helminths in human carcinogenesis. Cancer Lett. 305:239–249 [DOI] [PubMed] [Google Scholar]
- 94. Fu C-L, Odegaard JI, De'Broski RH, Hsieh MH. 2012. A novel mouse model of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 8:e1002605 doi:10.1371/journal.ppat.1002605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. García-Hernández MH, et al. 2009. Regulatory T cells in children with intestinal parasite infection. Parasite Immunol. 31:597–603 [DOI] [PubMed] [Google Scholar]
- 96. Gatlin MR, et al. 2009. Association of the gene polymorphisms IFN-γ +874, IL-13-1055 and IL-4-590 with patterns of reinfection with Schistosoma mansoni. PLoS Negl. Trop. Dis. 3:e375 doi:10.1371/journal.pntd.0000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gaze S, et al. 2012. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 8:e1002520 doi:10.1371/journal.ppat.1002520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gazzinelli G, et al. 1987. Immune response in different clinical groups of schistosomiasis patients. Mem. Inst. Oswaldo Cruz 82(Suppl 4):95–100 [DOI] [PubMed] [Google Scholar]
- 99. Gillan V, Devaney E. 2005. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect. Immun. 73:4034–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grainger JR, et al. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207:2331–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Greene BM, Gbakima AA, Albiuz EJ, Taylor HR. 1985. Humoral and cellular immune responses to Onchocerca volvulus in humans. Rev. Infect. Dis. 7:789–795 [DOI] [PubMed] [Google Scholar]
- 102. Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. 1998. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 177:1433–1437 [DOI] [PubMed] [Google Scholar]
- 103. Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. 1996. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur. J. Immunol. 26:1365–1370 [DOI] [PubMed] [Google Scholar]
- 104. Gruden-Movsesijan A, et al. 2010. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 32:450–459 [DOI] [PubMed] [Google Scholar]
- 105. Gruden-Movsesijan A, et al. 2008. Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp. Parasitol. 118:641–647 [DOI] [PubMed] [Google Scholar]
- 106. Hagan P, Blumenthal UJ, Dunn D, Simpson AJG, Wilkins HA. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243–245 [DOI] [PubMed] [Google Scholar]
- 107. Hang L, et al. 2010. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J. Immunol. 185:3184–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Harnett W, Harnett MM. 2009. Immunomodulatory activity and therapeutic potential of the filarial nematode secreted product, ES-62. Adv. Exp. Med. Biol. 666:88–94 [DOI] [PubMed] [Google Scholar]
- 109. Harris JB, et al. 2009. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl. Trop. Dis. 3:e403 doi:10.1371/journal.pntd.0000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hartgers FC, et al. 2009. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 199:1528–1535 [DOI] [PubMed] [Google Scholar]
- 111. Hartmann S, et al. 2009. Gastrointestinal nematode infection interferes with experimental allergic airway inflammation but not atopic dermatitis. Clin. Exp. Allergy 39:1585–1596 [DOI] [PubMed] [Google Scholar]
- 112. Haswell-Elkins MR, et al. 1994. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int. J. Cancer 59:505–509 [DOI] [PubMed] [Google Scholar]
- 113. Hayes KS, Bancroft AJ, Grencis RK. 2004. Immune-mediated regulation of chronic intestinal nematode infection. Immunol. Rev. 201:75–88 [DOI] [PubMed] [Google Scholar]
- 114. He Y, et al. 2010. The inhibitory effect against collagen-induced arthritis by Schistosoma japonicum infection is infection stage-dependent. BMC Immunol. 11:28 doi:10.1186/1471-2172-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hepworth MR, Hamelmann E, Lucius R, Hartmann S. 2010. Looking into the future of Trichuris suis therapy. J. Allergy Clin. Immunol. 125:767–768 [DOI] [PubMed] [Google Scholar]
- 116. Herbert DR, et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20:623–635 [DOI] [PubMed] [Google Scholar]
- 117. Herbert DR, et al. 2009. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. J. Exp. Med. 206:2947–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hesse M, et al. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172:3157–3166 [DOI] [PubMed] [Google Scholar]
- 119. Hewitson JP, Grainger JR, Maizels RM. 2009. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 167:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hirata T, et al. 2006. Impairment of host immune response against Strongyloides stercoralis by human T cell lymphotropic virus type 1 infection. Am. J. Trop. Med. Hyg. 74:246–249 [PubMed] [Google Scholar]
- 121. Hoerauf A, Satoguina J, Saeftel M, Specht S. 2005. Immunomodulation by filarial nematodes. Parasite Immunol. 27:417–429 [DOI] [PubMed] [Google Scholar]
- 122. Hoffmann KF, Cheever AW, Wynn TA. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406–6416 [DOI] [PubMed] [Google Scholar]
- 123. Hörauf A, Fleischer B. 1997. Immune responses to filarial infection in laboratory mice. Med. Microbiol. Immunol. 185:207–215 [DOI] [PubMed] [Google Scholar]
- 124. Hotez PJ, et al. 2008. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 118:1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hotez PJ, et al. 2004. Hookworm infection. N. Engl. J. Med. 351:799–807 [DOI] [PubMed] [Google Scholar]
- 126. Hübner MP, et al. 2012. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J. Immunol. 188:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hübner MP, Stocker JT, Mitre E. 2009. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology 127:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Huibregtse IL, van Lent AU, van Deventer SJ. 2007. Immunopathogenesis of IBD: insufficient suppressor function in the gut? Gut 56:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hunter MM, Wang A, Hirota CL, McKay DM. 2005. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J. Immunol. 174:7368–7375 [DOI] [PubMed] [Google Scholar]
- 130. Hunter MM, et al. 2010. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138:1395–1405 [DOI] [PubMed] [Google Scholar]
- 131. Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. 2011. Regulatory B-cell induction by helminths: implications for allergic disease. J. Allergy Clin. Immunol. 128:733–739 [DOI] [PubMed] [Google Scholar]
- 132. Hussain R, Grögl M, Ottesen EA. 1987. IgG antibody subclasses in human filariasis. Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J. Immunol. 139:2794–2798 [PubMed] [Google Scholar]
- 133. Ince MN, et al. 2009. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur. J. Immunol. 39:1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. ISAAC 1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 351:1225–1232 [PubMed] [Google Scholar]
- 135. Itami DM, et al. 2005. Modulation of murine experimental asthma by Ascaris suum components. Clin. Exp. Allergy 35:873–879 [DOI] [PubMed] [Google Scholar]
- 136. Jang SW, et al. 2011. Parasitic helminth cystatin inhibits DSS-induced intestinal inflammation via IL-10+F4/80+ macrophage recruitment. Korean J. Parasitol. 49:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jankovic D, Kullberg MC, Caspar P, Sher A. 2004. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J. Immunol. 173:2419–2427 [DOI] [PubMed] [Google Scholar]
- 138. Johnston MJG, et al. 2010. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect. Immun. 78:1364–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Josefowicz SZ, Lu LF, Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Joseph S, et al. 2004. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. J. Infect. Dis. 190:835–842 [DOI] [PubMed] [Google Scholar]
- 141. Khan WI, et al. 2002. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 70:5931–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Khan WI, et al. 2003. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect. Immun. 71:2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kim JY, et al. 2010. Inhibition of dextran sulfate sodium (DSS)-induced intestinal inflammation via enhanced IL-10 and TGF-β production by galectin-9 homologues isolated from intestinal parasites. Mol. Biochem. Parasitol. 174:53–61 [DOI] [PubMed] [Google Scholar]
- 144. King CL, et al. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Invest. 92:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. King CL, et al. 1996. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J. Immunol. 156:4715–4721 [PubMed] [Google Scholar]
- 146. Kitagaki K, et al. 2006. Intestinal helminths protect in a murine model of asthma. J. Immunol. 177:1628–1635 [DOI] [PubMed] [Google Scholar]
- 147. Knowles SCL. 2011. The effect of helminth co-infection on malaria in mice: a meta-analysis. Int. J. Parasitol. 41:1041–1051 [DOI] [PubMed] [Google Scholar]
- 148. Korten S, et al. 2008. Natural death of adult Onchocerca volvulus and filaricidal effects of doxycycline induce local FOXP3+/CD4+ regulatory T cells and granzyme expression. Microbes Infect. 10:313–324 [DOI] [PubMed] [Google Scholar]
- 149. Korten S, Hoerauf A, Kaifi J, Büttner DW. 2011. Low levels of transforming growth factor-β (TGF-β) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis. Parasitology 138:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kouriba B, et al. 2005. Analysis of the 5q31-q33 locus shows an association between IL13-1055C/T IL-13-591A/G polymorphisms and Schistosoma haematobium infections. J. Immunol. 174:6274–6281 [DOI] [PubMed] [Google Scholar]
- 151. Kreider T, Anthony RM, Urban JF, Jr, Gause WC. 2007. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kurniawan A, et al. 1993. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 150:3941–3950 [PubMed] [Google Scholar]
- 153. La Flamme AC, Ruddenklau K, Backstrom BT. 2003. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect. Immun. 71:4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lammie PJ, Hitch WL, Walker Allen EM, Hightower W, Eberhard ML. 1991. Maternal filarial infection as risk factor for infection in children. Lancet 337:1005–1006 [DOI] [PubMed] [Google Scholar]
- 155. Lammie PJ, Katz SP. 1983. Immunoregulation in experimental filariasis. I. In vitro suppression of mitogen-induced blastogenesis by adherent cells from jirds chronically infected with Brugia pahangi. J. Immunol. 130:1381–1385 [PubMed] [Google Scholar]
- 156. Larson D, et al. 2012. Helminth infection is associated with decreased basophil responsiveness in humans. J. Allergy Clin. Immunol. 130:270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Larson D, et al. 2012. Chronic helminth infection reduces basophil responsiveness in an IL-10-dependent manner. J. Immunol. 188:4188–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lawrence RA, Devaney E. 2001. Lymphatic filariasis: parallels between the immunology of infection in humans and mice. Parasite Immunol. 23:353–361 [DOI] [PubMed] [Google Scholar]
- 159. Layland LE, et al. 2010. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J. Immunol. 184:713–724 [DOI] [PubMed] [Google Scholar]
- 160. Layland LE, Rad R, Wagner H, da Costa CU. 2007. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 37:2174–2184 [DOI] [PubMed] [Google Scholar]
- 161. Ledingham DL, et al. 1996. Prolongation of rat kidney allograft survival by nematodes. Transplantation 61:184–188 [DOI] [PubMed] [Google Scholar]
- 162. Leonardi-Bee J, Pritchard D, Britton J. 2006. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 174:514–523 [DOI] [PubMed] [Google Scholar]
- 163. Leung J, et al. 2012. Heligmosomoides polygyrus abrogates antigen-specific gut injury in a murine model of inflammatory bowel disease. Inflamm. Bowel Dis. 18:1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lewis R, Behnke JM, Stafford P, Holland CV. 2006. The development of a mouse model to explore resistance and susceptibility to early Ascaris suum infection. Parasitology 132:289–300 [DOI] [PubMed] [Google Scholar]
- 165. Li Z, et al. 2011. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int. J. Parasitol. 41:1129–1137 [DOI] [PubMed] [Google Scholar]
- 166. Lima C, et al. 2002. Eosinophilic inflammation and airway hyperresponsiveness are profoundly inhibited by a helminth (Ascaris suum) extract in a murine model of asthma. Clin. Exp. Allergy 32:1659–1666 [DOI] [PubMed] [Google Scholar]
- 167. Liu Q, et al. 2009. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect. Immun. 77:5347–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liwski R, Zhou J, McAlister V, Lee TD. 2000. Prolongation of allograft survival by Nippostrongylus brasiliensis is associated with decreased allospecific cytotoxic T lymphocyte activity and development of T cytotoxic cell type 2 cells. Transplantation 69:1912–1922 [DOI] [PubMed] [Google Scholar]
- 169. Loke P, et al. 2007. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179:3926–3936 [DOI] [PubMed] [Google Scholar]
- 170. Loke P, et al. 2002. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3:7 doi:10.1186/1471-2172-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. MacDonald AS, Maizels RM. 2008. Alarming dendritic cells for Th2 induction. J. Exp. Med. 205:13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. MacDonald AS, Straw AD, Bauman B, Pearce EJ. 2001. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982–1988 [DOI] [PubMed] [Google Scholar]
- 173. Mahanty S, et al. 1996. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 173:769–773 [DOI] [PubMed] [Google Scholar]
- 174. Maizels RM. 2005. Infections and allergy—helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 17:656–661 [DOI] [PubMed] [Google Scholar]
- 175. Maizels RM. 2009. Parasite immunomodulation and polymorphisms of the immune system. J. Biol. 8:62 doi:10.1186/jbiol166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Maizels RM, Bundy DAP, Selkirk ME, Smith DF, Anderson RM. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797–805 [DOI] [PubMed] [Google Scholar]
- 177. Maizels RM, et al. 2012. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp. Parasitol. 132:76–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Maizels RM, Holland M, Falcone FH, Zang XX, Yazdanbakhsh M. 1999. Vaccination against helminth parasites: the ultimate challenge for immunologists? Immunol. Rev. 171:125–148 [DOI] [PubMed] [Google Scholar]
- 179. Maizels RM, et al. 1995. T cell activation and the balance of antibody isotypes in human filariasis. Parasitol. Today 11:50–56 [DOI] [PubMed] [Google Scholar]
- 180. Maizels RM, Smith KA. 2011. Regulatory T cells in infection. Adv. Immunol. 112:73–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Maizels RM, Yazdanbakhsh M. 2003. Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733–743 [DOI] [PubMed] [Google Scholar]
- 182. Maizels RM, Yazdanbakhsh M. 2008. T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem. Immunol. Allergy 94:112–123 [DOI] [PubMed] [Google Scholar]
- 183. Malhotra I, et al. 2006. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J. Infect. Dis. 193:1005–1013 [DOI] [PubMed] [Google Scholar]
- 184. Malhotra I, et al. 2003. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect. Immun. 71:5231–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mangan NE, et al. 2004. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173:6346–6356 [DOI] [PubMed] [Google Scholar]
- 186. Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. 2006. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J. Immunol. 176:138–147 [DOI] [PubMed] [Google Scholar]
- 187. Manoury B, Gregory WF, Maizels RM, Watts C. 2001. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 11:447–451 [DOI] [PubMed] [Google Scholar]
- 188. Massacand JC, et al. 2009. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl. Acad. Sci. U. S. A. 106:13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Matisz CE, McDougall JJ, Sharkey KA, McKay DM. 2011. Helminth parasites and the modulation of joint inflammation. J. Parasitol. Res. 2011:942616 doi:10.1155/2011/942616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. McInnes IB, et al. 2003. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J. Immunol. 171:2127–2133 [DOI] [PubMed] [Google Scholar]
- 191. McSorley HJ, et al. 2011. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One 6:e24092 doi:10.1371/journal.pone.0024092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. 2008. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite, Brugia malayi. J. Immunol. 181:6456–6466 [DOI] [PubMed] [Google Scholar]
- 193. McSorley HJ, et al. 18 June 2012. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur. J. Immunol. [Epub ahead of print.] doi:10.1002/eji.201142161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Medeiros M, Jr, et al. 2003. Schistosoma mansoni infection is associated with a reduced course of asthma. J. Allergy Clin. Immunol. 111:947–951 [DOI] [PubMed] [Google Scholar]
- 195. Metenou S, et al. 2010. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 184:5375–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Metenou S, et al. 2009. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J. Immunol. 183:916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Metenou S, et al. 2011. Filarial infection suppresses malaria-specific multifunctional Th1 and th17 responses in malaria and filarial coinfections. J. Immunol. 186:4725–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Metenou S, et al. 2012. Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection. Eur. J. Immunol. 42:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Metwali A, et al. 2006. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G253–259 [DOI] [PubMed] [Google Scholar]
- 200. Mitre E, Norwood S, Nutman TB. 2005. Saturation of immunoglobulin E (IgE) binding sites by polyclonal IgE does not explain the protective effect of helminth infections against atopy. Infect. Immun. 73:4106–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Moller M, et al. 2007. Genetic haplotypes of Th-2 immune signalling link allergy to enhanced protection to parasitic worms. Hum. Mol. Genet. 16:1828–1836 [DOI] [PubMed] [Google Scholar]
- 202. Montenegro SML, et al. 1999. Cytokine production in acute versus chronic human Schistosomiasis mansoni: the cross-regulatory role of interferon-γ and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J. Infect. Dis. 179:1502–1514 [DOI] [PubMed] [Google Scholar]
- 203. Montes M, et al. 2009. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl. Trop. Dis. 3:e456 doi:10.1371/journal.pntd.0000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Moreels TG, et al. 2004. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 53:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mortimer K, et al. 2006. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am. J. Trop. Med. Hyg. 75:914–920 [PubMed] [Google Scholar]
- 206. Motomura Y, et al. 2009. Helminth antigen-based strategy to ameliorate inflammation in an experimental model of colitis. Clin. Exp. Immunol. 155:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mpairwe H, et al. 2011. Anthelminthic treatment during pregnancy is associated with increased risk of infantile eczema: randomised-controlled trial results. Pediatr. Allergy Immunol. 22:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mutapi F, et al. 2011. Schistosome infection intensity is inversely related to auto-reactive antibody levels. PLoS One 6:e19149 doi:10.1371/journal.pone.0019149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mutapi F, et al. 2007. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect. Dis. 7:139 doi:10.1186/1471-2334-7-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Nacher M. 2008. Worms and malaria: blind men feeling the elephant? Parasitology 135:861–868 [DOI] [PubMed] [Google Scholar]
- 211. Nacher M, et al. 2002. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am. J. Trop. Med. Hyg. 66:304–309 [DOI] [PubMed] [Google Scholar]
- 212. Nair MG, et al. 2009. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206:937–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nausch N, Midzi N, Mduluza T, Maizels RM, Mutapi F. 2011. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS One 6:e16860 doi:10.1371/journal.pone.0016860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. 2004. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect. Immun. 72:2598–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. 2009. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int. J. Parasitol. 39:457–464 [DOI] [PubMed] [Google Scholar]
- 216. Ottesen EA. 1984. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans. R. Soc. Trop. Med. Hyg. 78(Suppl):9–18 [DOI] [PubMed] [Google Scholar]
- 217. Pacifico LG, et al. 2009. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infect. Immun. 77:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Park SK, et al. 2009. Macrophage migration inhibitory factor homologs of anisakis simplex suppress Th2 response in allergic airway inflammation model via CD4+CD25+Foxp3+ T cell recruitment. J. Immunol. 182:6907–6914 [DOI] [PubMed] [Google Scholar]
- 219. Partono F. 1984. Elephantiasis and its relation to filarial immunity. Southeast Asian J. Trop. Med. Public Health 13:275–279 [PubMed] [Google Scholar]
- 220. Pearce EJ, MacDonald AS. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499–511 [DOI] [PubMed] [Google Scholar]
- 221. Pearson DJ, Taylor G. 1975. The influence of the nematode Syphacia oblevata on adjuvant arthritis in the rat. Immunology 29:391–396 [PMC free article] [PubMed] [Google Scholar]
- 222. Pesce JT, et al. 2009. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5:e1000371 doi:10.1371/journal.ppat.1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Pfarr KM, Debrah AY, Specht S, Hoerauf A. 2009. Filariasis and lymphoedema. Parasite Immunol. 31:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Phythian-Adams AT, et al. 2010. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 207:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Piessens WF, et al. 1980. Immune responses in human infections with Brugia malayi. Specific cellular unresponsiveness to filarial antigens. J. Clin. Invest. 65:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Piessens WF, et al. 1982. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N. Engl. J. Med. 307:144–148 [DOI] [PubMed] [Google Scholar]
- 227. Piessens WF, et al. 1981. Effect of treatment with diethylcarbamazine on immune responses to filarial antigens in patients infected with Brugia malayi. Acta Trop. 38:227–234 [PubMed] [Google Scholar]
- 228. Quinnell RJ, Pritchard DI, Raiko A, Brown AP, Shaw M- A. 2004. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 190:430–438 [DOI] [PubMed] [Google Scholar]
- 229. Ramalingam TR, et al. 2008. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 9:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Rausch S, et al. 2008. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 76:1908–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Rausch S, et al. 2009. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur. J. Immunol. 39:3066–3077 [DOI] [PubMed] [Google Scholar]
- 232. Ribeiro de Jesus A, et al. 2000. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect. Immun. 68:2797–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Ricci ND, et al. 2011. Induction of CD4+CD25+FOXP3+ regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl. Trop. Dis. 5:e1383 doi:10.1371/journal.pntd.0001383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. 2000. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc. Natl. Acad. Sci. U. S. A. 97:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. 1991. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 27:2679–2686 [DOI] [PubMed] [Google Scholar]
- 236. Rujeni N, et al. 2012. Atopy is inversely related to schistosome infection intensity: a comparative study in Zimbabwean villages with distinct levels of Schistosoma haematobium infection. Int. Arch. Allergy Immunol. 158:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rutitzky LI, Stadecker MJ. 2011. Exacerbated egg-induced immunopathology in murine Schistosoma mansoni infection is primarily mediated by IL-17 and restrained by IFN-γ. Eur. J. Immunol. 41:2677–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Ruyssers NE, et al. 2008. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin. Dev. Immunol. 2008:567314 doi:10.1155/2008/567314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269–272 [DOI] [PubMed] [Google Scholar]
- 240. Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. 2003. Synergism of gamma interferon and interleukin-5 in the control of murine filariasis. Infect. Immun. 71:6978–6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775–787 [DOI] [PubMed] [Google Scholar]
- 242. Sartono E, et al. 1995. Elevated cellular responses and interferon-γ release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J. Infect. Dis. 171:1683–1687 [DOI] [PubMed] [Google Scholar]
- 243. Sartono E, Kruize YCM, Kurniawan-Atmadja A, Maizels RM, Yazdanbakhsh M. 1997. Depression of antigen-specific interleukin-5 and interferon-γ responses in human lymphatic filariasis as a function of clinical status and age. J. Infect. Dis. 175:1276–1280 [DOI] [PubMed] [Google Scholar]
- 244. Sasisekhar B, et al. 2005. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect. Immun. 73:3385–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Satoguina J, et al. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291–1300 [DOI] [PubMed] [Google Scholar]
- 246. Satoguina JS, et al. 2008. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-β. Eur. J. Immunol. 38:3101–3113 [DOI] [PubMed] [Google Scholar]
- 247. Saunders KA, Raine T, Cooke A, Lawrence CE. 2007. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Schnoeller C, et al. 2008. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J. Immunol. 180:4265–4272 [DOI] [PubMed] [Google Scholar]
- 249. Schopf L, et al. 2005. Differential modulation of allergic eye disease by chronic and acute Ascaris infection. Invest. Ophthalmol. Vis. Sci. 46:2772–2780 [DOI] [PubMed] [Google Scholar]
- 250. Secor WE. 2005. Immunology of human schistosomiasis: off the beaten path. Parasite Immunol. 27:309–316 [DOI] [PubMed] [Google Scholar]
- 251. Secor WE, Sundstrom JB. 2007. Below the belt: new insights into potential complications of HIV-1/schistosome coinfections. Curr. Opin. Infect. Dis. 20:519–523 [DOI] [PubMed] [Google Scholar]
- 252. Semnani RT, et al. 2006. Filaria-induced monocyte dysfunction and its reversal following treatment. Infect. Immun. 74:4409–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Semnani RT, et al. 2010. Expanded numbers of circulating myeloid dendritic cells in patent human filarial infection reflect lower CCR1 expression. J. Immunol. 185:6364–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Setiawan T, et al. 2007. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 75:4655–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sewell D, et al. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 15:59–69 [DOI] [PubMed] [Google Scholar]
- 256. Shi M, et al. 2011. Infection with an intestinal helminth parasite reduces Freund's complete adjuvant-induced monoarthritis in mice. Arthritis Rheum. 63:434–444 [DOI] [PubMed] [Google Scholar]
- 257. Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. 2005. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology 114:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Smith KA, et al. 2011. Chronic helminth infection mediates tolerance in vivo through dominance of CD11clo CD103− DC population. J. Immunol. 186:7098–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Smith P, et al. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 178:4557–4566 [DOI] [PubMed] [Google Scholar]
- 260. Smits HH, et al. 2007. Protective effect of Schistosoma mansoni infection on allergic asthma depends on intensity and chronicity of infection. J. Allergy Clin. Immunol. 120:932–940 [DOI] [PubMed] [Google Scholar]
- 261. Smits HH, Yazdanbakhsh M. 2007. Chronic helminth infections modulate allergen-specific immune responses: protection against development of allergic disorders? Ann. Med. 39:428–439 [DOI] [PubMed] [Google Scholar]
- 262. Smout MJ, et al. 2009. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 5:e1000611 doi:10.1371/journal.ppat.1000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Smout MJ, et al. 2011. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol. Biosyst. 7:1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Specht S, Volkmann L, Wynn T, Hoerauf A. 2004. Interleukin-10 (IL-10) counterregulates IL-4-dependent effector mechanisms in murine filariasis. Infect. Immun. 72:6287–6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Steel C, Nutman TB. 2003. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J. Immunol. 170:1930–1938 [DOI] [PubMed] [Google Scholar]
- 266. Steinfelder S, et al. 2009. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 206:1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Strachan DP. 1989. Hay fever, hygiene, and household size. BMJ 299:1259–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Su Z, Segura M, Stevenson MM. 2006. Reduced protective efficacy of a blood-stage malaria vaccine by concurrent nematode infection. Infect. Immun. 74:2138–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Summers RW, et al. 2003. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 98:2034–2041 [DOI] [PubMed] [Google Scholar]
- 270. Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. 2005. Trichuris suis therapy in Crohn's disease. Gut 54:87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. 2005. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128:825–832 [DOI] [PubMed] [Google Scholar]
- 272. Summers RW, Elliott DE, Weinstock JV. 2010. Trichuris suis might be effective in treating allergic rhinitis. J. Allergy Clin. Immunol. 125:766–767 [DOI] [PubMed] [Google Scholar]
- 273. Sutton TL, et al. 2008. Anti-inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect. Immun. 76:4772–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Taylor BC, et al. 2009. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. 2009. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J. Clin. Invest. 119:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Taylor JJ, Mohrs M, Pearce EJ. 2006. Regulatory T cell responses develop in parallel to Th responses, and control the magnitude and phenotype of the Th effector population. J. Immunol. 176:5839–5847 [DOI] [PubMed] [Google Scholar]
- 277. Taylor M, et al. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174:4924–4933 [DOI] [PubMed] [Google Scholar]
- 278. Taylor MD, et al. 2007. CTLA-4+ and CD4+CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 179:4626–4634 [DOI] [PubMed] [Google Scholar]
- 279. Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. 2006. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J. Immunol. 176:6918–6927 [DOI] [PubMed] [Google Scholar]
- 280. Taylor MD, et al. 2009. Early recruitment of natural CD4+Foxp3+ regulatory T cells by infective larvae determines the outcome of filarial infection. Eur. J. Immunol. 39:192–206 [DOI] [PubMed] [Google Scholar]
- 281. Taylor MD, van der Werf N, Maizels RM. 2012. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 33:181–189 [DOI] [PubMed] [Google Scholar]
- 282. Teixeira-Carvalho A, et al. 2008. Cytokines, chemokine receptors, CD4+CD25HIGH+ T-cells and clinical forms of human schistosomiasis. Acta Trop. 108:139–149 [DOI] [PubMed] [Google Scholar]
- 283. Trujillo-Vargas CM, et al. 2007. Helminth derived products inhibit the development of allergic responses in mice. Am. J. Respir. Crit. Care Med. 175:336–344 [DOI] [PubMed] [Google Scholar]
- 284. Turner J, et al. 2003. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 188:1768–1775 [DOI] [PubMed] [Google Scholar]
- 285. Turner JD, et al. 2008. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 197:1204–1212 [DOI] [PubMed] [Google Scholar]
- 286. Turner JD, et al. 2011. CD4+CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl. Trop. Dis. 5:e1269 doi:10.1371/journal.pntd.0001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Urban JF, Jr, et al. 2007. Infection with parasitic nematodes confounds vaccination efficacy. Vet. Parasitol. 148:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. van den Biggelaar A, et al. 2000. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356:1723–1727 [DOI] [PubMed] [Google Scholar]
- 289. van den Biggelaar AH, et al. 2004. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J. Infect. Dis. 189:892–900 [DOI] [PubMed] [Google Scholar]
- 290. van der Kleij D, et al. 2002. A novel host-parasite lipid cross talk: schistosomal lysophosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277:48122–48129 [DOI] [PubMed] [Google Scholar]
- 291. van der Werf N, et al. 2011. Th2 responses to helminth parasites can be therapeutically enhanced by, but are not dependent upon, GITR-GITR ligand costimulation In vivo. J. Immunol. 187:1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. van Liempt E, et al. 2007. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 44:2605–2615 [DOI] [PubMed] [Google Scholar]
- 293. Venugopal PG, Nutman TB, Semnani RT. 2009. Activation and regulation of Toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 43:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Walsh KP, Brady MT, Finlay CM, Boon L, Mills KHG. 2009. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J. Immunol. 183:1577–1586 [DOI] [PubMed] [Google Scholar]
- 295. Walson JL, Herrin BR, John-Stewart G. 2009. Deworming helminth co-infected individuals for delaying HIV disease progression. Cochrane Database Syst. Rev. 2009:CD006419 doi:10.1002/14651858.CD006419.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Wammes LJ, et al. 2010. Regulatory T cell in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 40:437–442 [DOI] [PubMed] [Google Scholar]
- 297. Wammes LJ, et al. 2012. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl. Trop. Dis. 6:e1655 doi:10.1371/journal.pntd.0001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. 2007. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 37:129–138 [DOI] [PubMed] [Google Scholar]
- 299. Watanabe K, et al. 2007. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am. J. Trop. Med. Hyg. 77:676–682 [PMC free article] [PubMed] [Google Scholar]
- 300. Webb EL, et al. 2011. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet 377:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Weng M, et al. 2007. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 179:4721–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA. 2011. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 7:e1002171 doi:10.1371/journal.ppat.1002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Wilson MS, Maizels RM. 2004. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 26:35–49 [DOI] [PubMed] [Google Scholar]
- 304. Wilson MS, et al. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Wilson MS, et al. 2010. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur. J. Immunol. 40:1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Wirtz S, Neufert C, Weigmann B, Neurath MF. 2007. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2:541–546 [DOI] [PubMed] [Google Scholar]
- 307. Wohlleben G, et al. 2004. Helminth infection modulates the development of allergen-induced airway inflammation. Int. Immunol. 16:585–596 [DOI] [PubMed] [Google Scholar]
- 308. Wolff MJ, Broadhurst MJ, Loke P. 2012. Helminthic therapy: improving mucosal barrier function. Trends Parasitol. 28:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. World Health Organization 2002. Reducing risks, promoting healthy life. The World Health Report 2002. Annex Table 3. Burden of disease in DALYs by cause, sex and mortality stratus in WHO regions, estimates for 2001. World Health Organization, Geneva, Switzerland [Google Scholar]
- 310. Wynn TA, et al. 1998. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 159:4473–4480 [PubMed] [Google Scholar]
- 311. Yang J, et al. 2007. Schistosoma japonicum egg antigens modulate airway inflammation by producing CD4+CD25+ T cells in a murine model of asthma. Immunology 120:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Yazdanbakhsh M, Luty AJ. 2011. Wormy mothers, healthy babies: case closed or conundrum? Lancet 377:6–8 [DOI] [PubMed] [Google Scholar]
- 313. Yazdanbakhsh M, et al. 1993. T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J. Infect. Dis. 167:925–931 [DOI] [PubMed] [Google Scholar]
- 314. Zaccone P, et al. 2009. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 39:1098–1107 [DOI] [PubMed] [Google Scholar]
- 315. Zaccone P, et al. 2010. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J. Biomed. Biotechnol. 2010:795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Zaccone P, et al. 2011. The S. mansoni glycoprotein ω-1 induces Foxp3 expression in NOD mouse CD4+ T cells. Eur. J. Immunol. 41:2709–2718 [DOI] [PubMed] [Google Scholar]
- 317. Zaccone P, et al. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33:1439–1449 [DOI] [PubMed] [Google Scholar]
- 318. Zhao A, et al. 2008. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135:217–225.e211 doi:10.1053/j.gastro.2008.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Zhao Y, Zhang S, Jiang L, Jiang J, Liu H. 2009. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid-induced colitis and bacterial translocation in mice. J. Gastroenterol. Hepatol. 24:1775–1780 [DOI] [PubMed] [Google Scholar]
- 320. Zheng X, et al. 2008. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J. Neuroimmunol. 194:107–114 [DOI] [PubMed] [Google Scholar]