Abstract
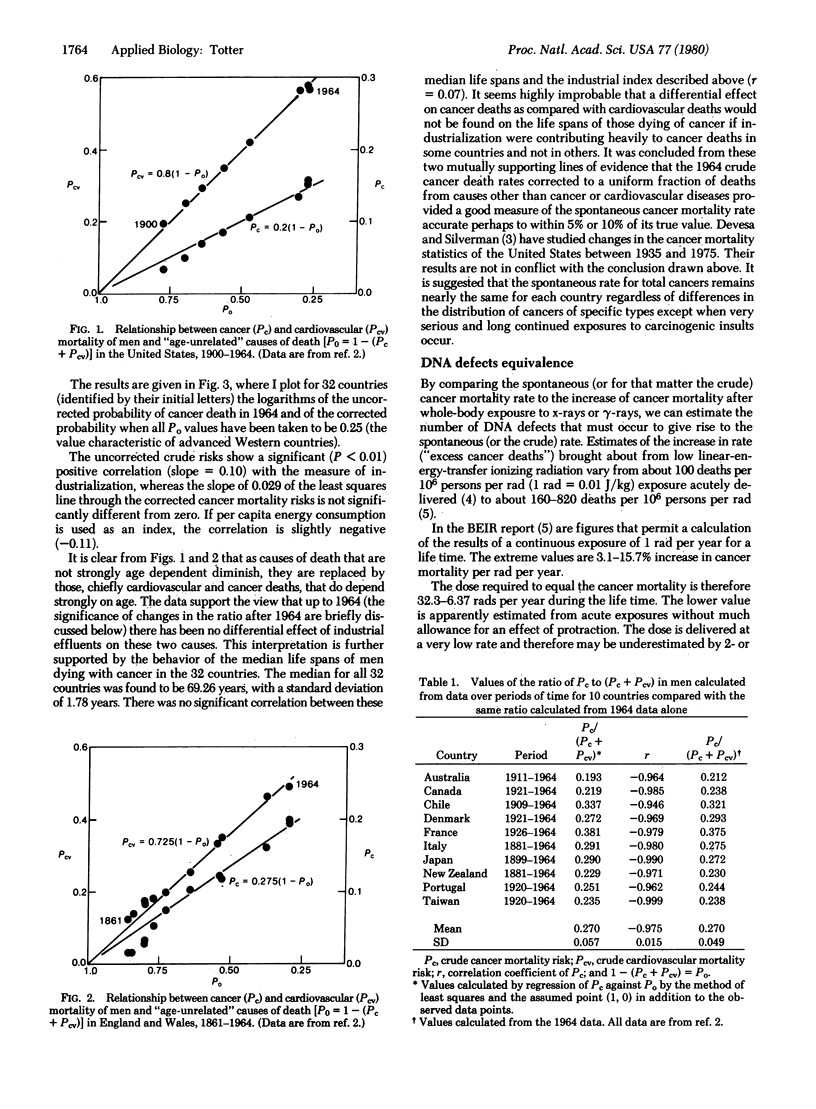
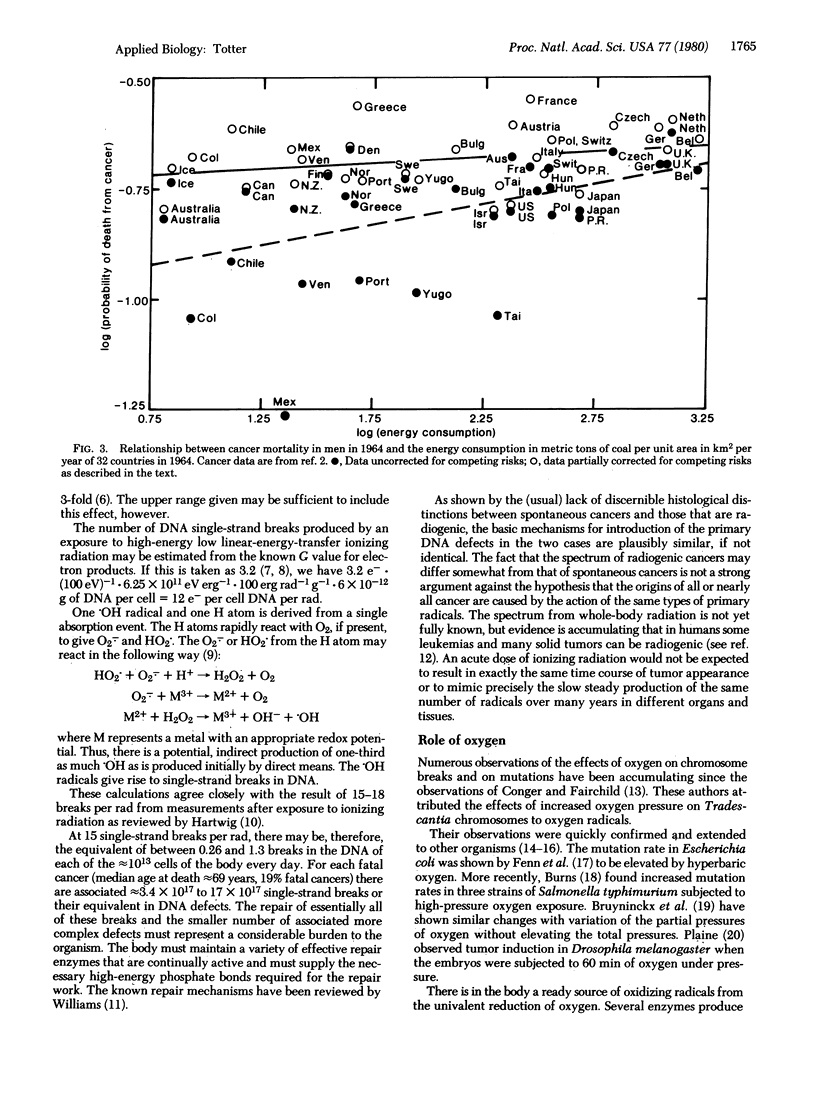
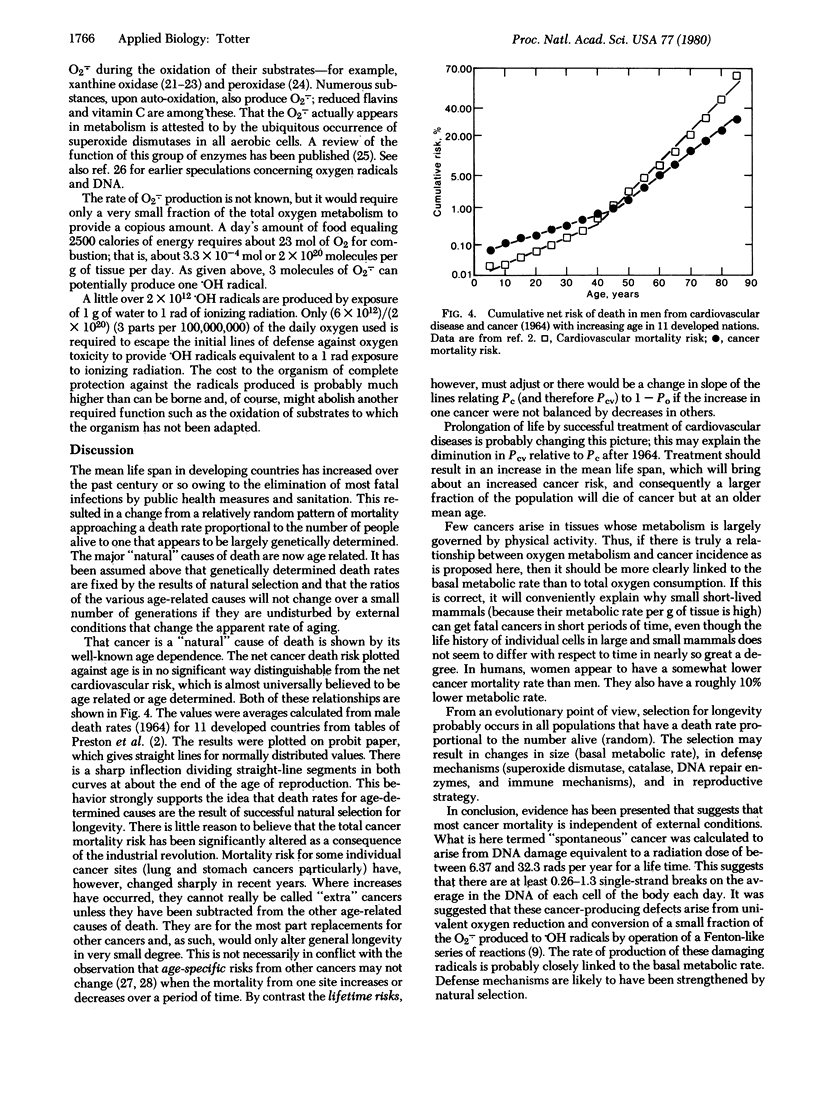
Mortality statistics for cancer in various countries and periods of time indicate that there has been little effect of industrialization on the inherent or spontaneous rate of occurrence of cancer. From U.S. cancer statistics and the BEIR values [Report of the Advisory Committee on the Biological Effects of Ionizing Radiation (1979)] for radiation dose response, the ionizing radiation exposure required to produce a number of cancers equal to this spontaneous incidence was estimated to lie between 450 and 2100 rads (1 rad = 0.01 J/kg). From these "cancer equivalent" doses the number of single-strand DNA breaks required to produce the spontaneous cancers is estimated to be 0.26-1.3 per cell DNA per day. It is suggested that the univalent reduction of oxygen in normal metabolism to O2- and subsequent production of more harmful radicals is the source of the DNA defects that, in cases where the defense mechanisms fail, lead to spontaneous cancer in the individual.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruyninckx W. J., Mason H. S., Morse S. A. Are physiological oxygen concentrations mutagenic? Nature. 1978 Aug 10;274(5671):606–607. doi: 10.1038/274606a0. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W., Leuthauser S. W. The effect of iron on the distribution of superoxide and hydroxyl radicals as seen by spin trapping and on the superoxide dismutase assay. Photochem Photobiol. 1978 Oct-Nov;28(4-5):693–695. doi: 10.1111/j.1751-1097.1978.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Conger A. D., Fairchild L. M. Breakage of Chromosomes by Oxygen. Proc Natl Acad Sci U S A. 1952 Apr;38(4):289–299. doi: 10.1073/pnas.38.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devesa S. S., Silverman D. T. Cancer incidence and mortality trends in the United States: 1935-74. J Natl Cancer Inst. 1978 Mar;60(3):545–571. doi: 10.1093/jnci/60.3.545. [DOI] [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. III. Sulfite oxidation as an ultra sensitive assay. J Biol Chem. 1958 Dec;233(6):1578–1580. [PubMed] [Google Scholar]
- Fenn W. O., Gerschman R., Gilbert D. L., Terwilliger D. E., Cothran F. V. MUTAGENIC EFFECTS OF HIGH OXYGEN TENSIONS ON ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1957 Dec 15;43(12):1027–1032. doi: 10.1073/pnas.43.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Freese E. B. Mutagenic and inactivating DNA alterations. Radiat Res. 1966;(Suppl):97+–97+. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- KRONSTAD W. E., NILAN R. A., KONZAK C. F. Mutagenic effect of oxygen on barley seeds. Science. 1959 Jun 12;129(3363):1618–1618. doi: 10.1126/science.129.3363.1618. [DOI] [PubMed] [Google Scholar]
- Plaine H. L. The Effect of Oxygen and of Hydrogen Peroxide on the Action of a Specific Gene and on Tumor Induction in Drosophila Melanogaster. Genetics. 1955 Mar;40(2):268–280. doi: 10.1093/genetics/40.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOTTER J. R., DE DUGROS E. C., RIVEIRO C. The use of chemiluminescent compounds as possible indicators of radical production during xanthine oxidase action. J Biol Chem. 1960 Jun;235:1839–1842. [PubMed] [Google Scholar]
- Wynder E. L., Gori G. B. Contribution of the environment to cancer incidence: an epidemiologic exercise. J Natl Cancer Inst. 1977 Apr;58(4):825–832. doi: 10.1093/jnci/58.4.825. [DOI] [PubMed] [Google Scholar]



