Abstract
Lyme arthritis, caused by Borrelia burgdorferi, has similarities to rheumatoid arthritis and its experimental murine model, collagen-induced arthritis (CIA). Currently, no common strain exists for examination of arthritis models of Lyme arthritis and CIA, which are typically studied in C3H/HeJ and DBA/1 mice, respectively. The aim of this study was to define the characteristics of Borrelia burgdorferi infection and arthritis in the DBA/1 murine strain. Murine Lyme arthritis was induced in C3H/HeJ and DBA/1 mice by subcutaneous infection with B. burgdorferi. Tibiotarsal joints were measured during infection, and mice were sacrificed for histologic, microbiologic, and serologic analysis on days 14 and 42 postinfection. All bladder cultures obtained from C3H/HeJ and DBA/1 mice at 14 days postinfection grew Borrelia. There was no significant difference in spirochetal burdens in hearts and tibiotarsal joints at days 14 and 42 postinfection. Tibiotarsal joint swelling and histologic scoring were not significantly different between the two strains. Serologic analysis revealed increased IgG2a production in C3H/HeJ mice compared to DBA/1 mice. Analysis of 2-dimensional immunoblots revealed several specific antigens (LA7, BBA03, BBA64, BBA73, OspA, and VlsE) which were not recognized by DBA/1 sera. We conclude that the DBA/1 murine strain is a suitable model for the study of Lyme arthritis and experimental B. burgdorferi infection, allowing direct comparison between Lyme arthritis and collagen-induced arthritis. The specificity of the humoral immune response differs between the two strains, further study of which may reveal important findings about how individual strains respond to B. burgdorferi infection.
INTRODUCTION
Lyme disease is the most common reported arthropod-borne infection in the United States, with over 30,000 new cases diagnosed annually (2, 22). Arthritis occurred in 1/3 of cases reported to the Centers for Disease Control and Prevention (2) and is the most common cause of morbidity and persistent symptoms. Lyme arthritis is typically a monoarticular process resulting in chronic joint swelling and mild clinical complaints, distinguishing it from pyogenic arthritis. The original description of Lyme arthritis by Steere et al. in 1977 noted similarities with autoimmune arthritis, including the duration of symptoms, appearance of the affected joints, and concentration in pediatric patients (22). The discovery of the spirochete Borrelia burgdorferi confirmed the infectious etiology of Lyme disease and has led to investigations into the pathophysiology of joint disease occurring during infection (4, 14).
Previous work has demonstrated similar phenotypes for Lyme arthritis and autoimmune arthritis (19, 21, 24). Histologic evaluation of the joint in Lyme arthritis reveals significant lymphocytic and neutrophilic infiltration with synovitis and pannus formation, which is distinct from what is seen for other pyogenic arthritis typically caused by Staphylococcus aureus. Critical immunologic mediators of Lyme arthritis include tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β), T and B cell involvement, and antibody responses (7, 13). Collagen-induced arthritis (CIA), an experimental murine model of autoimmune arthritis, has been well characterized and also has phenotypic and immunologic similarities to Lyme disease (6, 12). The most common strain used for the study of CIA is the DBA/1 mouse. Conversely, the primary strain for study of Lyme arthritis is the C3H congenic mouse, typically C3H/HeJ or HeN strains. Both strains demonstrate phenotypic and histologic evidence of arthritis after infection, but they are not susceptible to CIA. While most other murine strains are susceptible to B. burgdorferi infection, arthritis develops in a limited number (3). Subcutaneous or intradermal infection of C57BL/6 and DBA/2 mice leads to minimal or absent joint disease (3). However, there are no published data regarding the study of Lyme arthritis in the DBA/1 strain. A common murine strain for the study of both CIA and Lyme arthritis would allow new opportunities for comparative investigation of these two arthritides.
In the current study, we examined the phenotypes, histopathologies, infectivities, and serologic responses of C3H/HeJ and DBA/1 mice infected with B. burgdorferi. We demonstrate that the DBA/1 mouse is a novel strain for the study of experimental Lyme disease, including arthritis, allowing direct comparison of murine models of CIA and B. burgdorferi infection.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C3H/HeJ mice (Jackson Laboratory, Bar Harbor, ME) or male DBA/1 mice (Harlan Laboratories, Indianapolis, IN) were housed in accordance with the University of Pittsburgh School of Medicine Institutional Animal Care and Use Committee protocols and fed pathogen-free food and water ad libitum. Tibiotarsal joints were measured in duplicate by a blinded observer prior to infection and at least twice weekly in the anterio-posterior diameter with digital calipers. The absolute change in anterio-posterior width was used as a measure of arthritis. The absolute joint diameter was not different between strains at day 0 (data not shown), so changes in joint diameter were compared directly. Mice were sacrificed at 14 days postinfection (for examination of early disease, such as carditis) or at 42 days postinfection (for examination of arthritis) by carbon dioxide inhalation.
B. burgdorferi culture and infection.
Low-passage nonclonal B31 strain B. burgdorferi was cultured in BSK-H medium (Sigma) at 35°C and 5% CO2. The bacteria were shifted to pH 7.0 BSK-H and grown to mid-log phase (∼5 × 107 spirochetes/ml) as enumerated by dark-field microscopy. Groups of 10 mice were infected with 1 × 106 spirochetes subcutaneously in the mid back, with sham-infected mice being injected with medium alone. Prior to infection, plasmid profiles were verified by PCR for lp25, lp28-1, and lp28-4 to ensure virulence. All infected mice were inoculated with spirochetes derived from the same culture to ensure exposure to similar bacterial populations. Bladders were collected upon sacrifice, immediately placed in 5-ml Falcon tubes filled with BSK-H plus rifampin, phosphomycin, and amphotericin, and incubated at 35°C and 5% CO2 for 28 days. These cultures were evaluated weekly by dark-field microscopy for detection of viable spirochetes. Any observation of viable spirochetes was considered a positive culture.
Histologic analysis of tibiotarsal joints and hearts.
Upon sacrifice, one ankle from each mouse and one half of each bisected heart were placed in 10% neutral buffered formalin (Fischer Scientific, Pittsburgh, PA) until processing. Joints were decalcified, and joints/hearts were paraffin embedded, sectioned, and stained with hematoxylin-eosin (H&E). Joints and hearts were blindly scored as follows on a scale of 0 to 3 by an independent pathologist: 0, normal, with no inflammation or synovial proliferation; 1, focal mild synovial proliferation and/or inflammation; 2, marked inflammation and/or synovial proliferation affecting a portion of the specimen; and 3, marked inflammation and synovial proliferation involving most or all of the specimen.
DNA extraction from infected tissues.
Control and infected mice were sacrificed at 14 and 42 days postinfection, and one rear ankle joint and one half of the heart were stored immediately in dry ice and transferred to −80°C until the time of DNA extraction. Each tissue was pulverized with liquid nitrogen in a prechilled mortar and pestle and transferred to 2.5 ml of a 1-mg/ml collagenase A (Boehringer Mannheim) solution in phosphate-buffered saline (pH 7.4). Digestions were carried out for 4 h at 37°C. An equal volume of proteinase K solution (0.2 mg of proteinase K per ml, 200 mM NaCl, 20 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1% sodium dodecyl sulfate) was added to collagenase-digested tissues, and the mixture was incubated overnight at 55°C. DNA was recovered by extraction of the digested sample with phenol-chloroform and subsequent ethanol precipitation. Resuspended samples were digested with 0.1 mg of DNase-free RNase per ml for 1 h at 37°C. Extractions and precipitations were repeated, and DNA was resuspended in 0.5 ml of Tris-EDTA (TE). The DNA yield was determined, and samples were used for quantitative PCR (qPCR).
Measurement of spirochetal density by real-time qPCR.
One hundred nanograms of extracted tissue DNA was used in 25-μl reaction mixtures containing SYBR Green JumpStart Taq ReadyMix (Sigma) using the iCycler iQ detection system (Bio-Rad, Hercules, CA). Each reaction mixture contained either OspC primers (forward, TACGGATTCTAATGCGGTTTTAC; reverse, GTGATTATTTTCGGTATCCAAACCA) or mouse β-actin primers (forward, AGAGGGAAATCGTGCGTGAC; reverse, CAATAGTGATGACCTGGCCGT) at a 1 μM concentration. Cycle parameters were as follows: 1 cycle at 95°C for 3 min and then 50 cycles of 95°C for 15 s followed by 55°C for 30 s. Melting curves were generated by 80 cycles of 50°C for 10 s with 0.5°C increments. qPCRs were performed in triplicate at least two times with comparable results. The results were calculated using the ΔΔCT method, where relative amounts of B. burgdorferi DNA were compared to amounts of the murine β-actin gene as an internal standard.
One-dimensional electrophoresis and immunoblotting.
We used membrane-associated protein fractions for all electrophoresis studies, prepared as previously described from fractionation of B. burgdorferi strain B31 (16). Briefly, B31 strain spirochetes from the same clonal isolate used in murine infections were grown to a BSK-H culture density of 5 × 107 bacteria per ml and pelleted at low speed (6,000 × g for 15 min at 25°C). Pellets were washed with HN buffer (10 mM HEPES with 50 mM NaCl) twice and then resuspended in HN buffer with protease inhibitor cocktail (Amersham). Cells were fractionated by lysis with a French press, using 2 passes at 18,000 lb/in2. Subcellular fractions (soluble and membrane proteins) were separated using ultracentrifugation (340,00 × g for 60 min at 25°C). For one-dimensional electrophoresis, 15 μg of membrane-associated proteins was separated on sodium dodecyl sulfate-polyacrylamide gels with an SE600 gel apparatus (Hoefer Scientific, San Francisco, CA). Gels were transferred to nitrocellulose (Bio-Rad Laboratories) as described by Towbin et al. (23) with a Bio-Rad Trans Blot Cell (60 V for 2.5 h at 4°C). After transfer, proteins were visualized with amido blue (0.1% amido blue dye in 1.0% acetic acid), and standards were marked. Membranes were blocked with blocking buffer (overnight at 4°C) and probed with 1:5,000 infected mouse serum in blocking buffer for 1 h at 25°C. After washing, membranes were probed with 1:5,000 horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG-IgM for 1 h at 25°C. Membrane bands were visualized with the ECL Western blotting detection reagents (Amersham Biosciences) in accordance with the manufacturer's specifications.
Two-dimensional electrophoresis.
Prior to two-dimensional electrophoresis, B. burgdorferi membrane-associated proteins were precipitated using trichloroacetic acid (TCA)-acetone (1 volume of saturated TCA to 8 volumes cold acetone) as described previously (16). Precipitated protein samples were solubilized for isoelectric focusing using immobilized pH gradient (IPG) buffer {7 M urea, 2 M thiourea, 4.0% [wt/vol] 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 1.0% [vol/vol] Triton X-100, 100 mM dithiothreitol [DTT], and 0.5% IPGphor buffer, pH 3 to 10 [GE Health Sciences]}. Samples were clarified by ultracentrifugation (435,700 × g for 30 min at 23°C). Fifty micrograms was loaded onto 13-cm pH 3 to 10 IPG strips (GE Health Sciences) and focused for 82,000 V · h using the IPGphor III system (GE Health Sciences) (running conditions of 500 V for 1 h, 1,500 V for 1 h, and 8,000 V for 80,000 V · h). IPG strips were stored at −80°C until separated by mass using SDS-PAGE as described above. Prior to SDS-PAGE, IPG strips were equilibrated twice in 10 ml of SDS equilibration buffer (3 M urea, 2.0% SDS, 1% DTT, and 10% [vol/vol] glycerol in 125 mM Tris [pH 8.8]) for 10 min at 23°C. Standard SDS-PAGE was performed with broad-range protein standards (Promega). SDS-polyacrylamide gels were transferred to nitrocellulose membranes and Western blotting performed as described for one-dimensional electrophoresis.
B. burgdorferi enzyme-linked immunosorbent assay (ELISA).
Nunc Immunumodule MaxiSorp F8 flat-bottom 96-well plates (Fisher Scientific) were coated with 200 ng of B. burgdorferi B31 membrane proteins prepared as described above, covered, and incubated overnight at 4°C. Wells were washed twice with H2O and blocked with 5% milk in TBS-T20 (Tris-buffered saline [TBS] with 0.02% Tween 20) (blocking buffer) for 1 h at 37°C. The blocking buffer was removed, and wells were incubated for 1 h at 37°C with infected mouse serum in blocking buffer. Murine sera were used at dilutions of 1:5,000 (IgG-IgM and IgG), 1:1,000 (IgM and IgG2a), or 1:200 (IgG1), depending on the secondary antibody indicated. After washing with TBS-T20 (wash buffer), wells were incubated for 1 h at 37°C with 5 μg/ml biotinylated anti-murine IgG-IgM, IgM (KPL), IgG (Vector), IgG1, or IgG2a (BD Pharmingen), respectively. Wells were washed and incubated with streptavidin-conjugated horseradish peroxidase (HRP) for 20 min at room temperature and then washed with wash buffer. After incubation with tetramethylbenzidine (TMB) substrate developer (BD Pharmingen), the reaction was stopped with 1 M H2SO4 and absorbance at 450 nm with a 570-nm reference was determined. Wells were read in triplicate. Individual mouse serum was added in triplicate to each well at dilutions as described above. For each secondary antibody, a standard curve was generated using a serial dilution of pooled C3H/HeJ serum, and the absorbance at the dilution for each secondary antibody was assigned a value of 1 relative unit (RU).
Statistical analysis.
All statistics were performed using Graph Pad Prism 5 (GraphPad, La Jolla, CA). Parametric data were analyzed using an unpaired Student t test with or without Welch's correction. Graphical data were depicted with mean values with error bars demonstrating standard errors of the means where appropriate.
RESULTS
DBA/1 and C3H/HeJ mice show similar infectivities with B. burgdorferi.
We examined infectivity of the DBA/1 strain by comparing outgrowth from murine bladder cultures after sacrifice of mice at 14 days postinfection. C3H/HeJ and DBA/1 murine bladders were removed, cultured in BSK-H medium, and observed for 14 days. The proportions of cultures with B. burgdorferi were identical (100% versus 100%) in C3H/HeJ and DBA/1 mice. These data showed successful infection and dissemination of spirochetes in DBA/1 mice which were comparable to those in C3H/HeJ controls.
DBA/1 and C3H/HeJ mice develop comparable arthritis after infection with B. burgdorferi.
Having confirmed infectivity in DBA/1 mice, we examined whether B. burgdorferi infection would result in an arthritic phenotype. DBA/1 mice developed arthritis with timing and severity that were similar to those for C3H/HeJ mice following infection with equivalent doses of B31 B. burgdorferi (Fig. 1). Both strains were compared with uninfected controls and showed significant increases in the anterio-posterior diameter (ΔAP) of tibiotarsal joints as a measure of arthritis severity. Joints were not noted to be different in absolute diameter at the time of infection between mice infected with different strains (data not shown). Onset of joint swelling was observed slightly earlier in DBA/1 mice, i.e., at 24 days, versus 31 days in the C3H/HeJ mice. The mean ΔAPs on day 42 postinfection were 0.61 ± 0.05 mm and 0.45 ± 0.07 mm in DBA/1 and C3H/HeJ mice, respectively. Gross examination of the joints revealed predominantly monoarticular tibiotarsal swelling in both strains, and the appearance of joints with respect to redness and swelling was not noted to be different. As with other models of B. burgdorferi arthritis, mice retained mobility and feeding despite the presence of the inflamed joint. As a negative control, a group of C57BL/6 mice was also infected with the B31 strain, and absence of arthritis was noted (data not shown).
Fig 1.
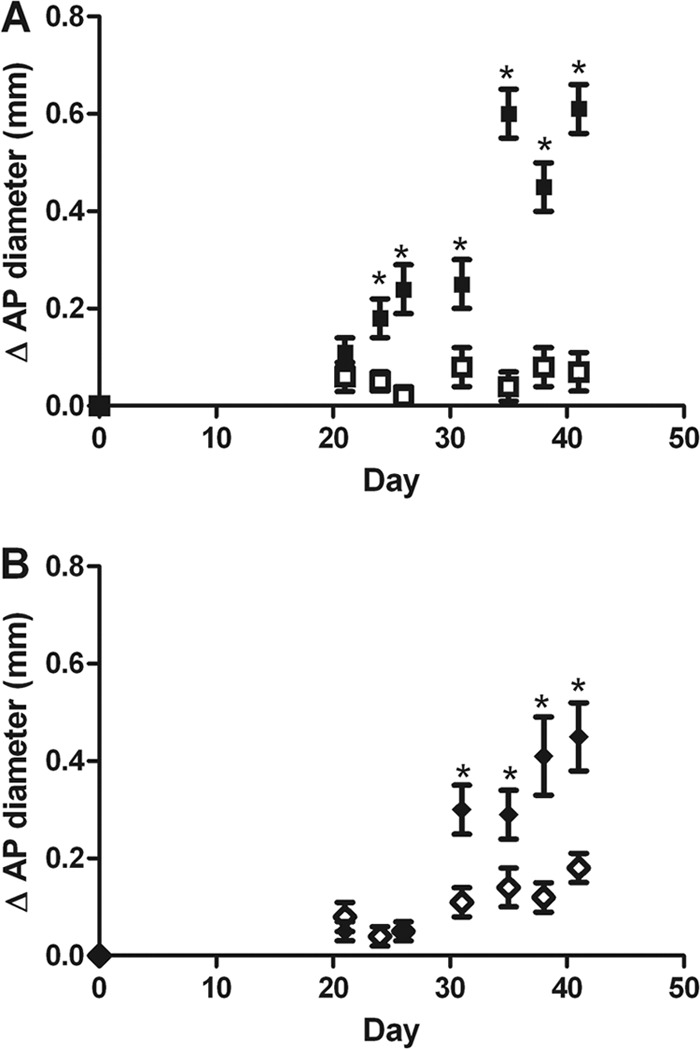
DBA/1 and C3H/HeJ mice develop similar tibiotarsal joint swelling following B. burgdorferi infection. DBA/1 (A) and C3H/HeJ (B) mice were infected with B. burgdorferi or control medium (n = 10/group). The anterio-posterior (AP) tibiotarsal joint diameter was measured prior to infection and periodically throughout infection. The average change in AP diameter was calculated for each group and is shown with standard error for each time point. *, P < 0.05.
Histologic evidence of arthritis is comparable for DBA/1 and C3H/HeJ mice.
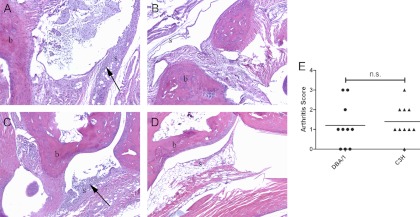
Tibiotarsal joint histology sections from infected and uninfected DBA/1 mice (Fig. 2A and B) and C3H/HeJ mice (Fig. 2C and D) at 42 days postinfection were compared to assess the severity of arthritis in each strain. Sections from infected mice of both strains showed infiltrates of lymphocytes and neutrophils in joint tissue, with involvement of bone and soft tissue structures surrounding the tibiotarsal joints noted in some animals. Control mice showed no evidence of inflammation or significant lymphocytic infiltration. Figure 2E shows a comparison of the histologic scores of tibiotarsal joints from infected DBA/1 and C3H/HeJ mice. Scoring of histologic severity of arthritis was not significantly different for the two strains, and the histologic appearances of individual joints were similar. Consistent with previous reports and the pauciarticular nature of experimental arthritis induced by B. burgdorferi, some joints had minimal inflammation present on histology. The mean scores of joints from the two strains were not different (1.2 ± 0.4 for DBA/1 versus 1.4 ± 0.3 for C3H/HeJ; P = 0.66). Though not the focus of this study, cardiac inflammation was also scored and was not different between groups (data not shown).
Fig 2.
DBA/1 and C3H/HeJ mice develop similar histologic evidence of arthritis in tibiotarsal joints. DBA/1 and C3H/HeJ (n = 10 each) tibiotarsal joints were collected at 42 days after B. burgdorferi infection or sham infection and then decalcified and H&E stained. (A to D) Representative images at a magnification of ×10 are shown for B. burgdorferi-infected (A) or sham-infected (B) DBA/1 mice and for B. burgdorferi-infected (C) or sham-infected (D) C3H/HeJ mice. b, bone; s, synovium. Proliferative synovitis with leukocyte infiltrates is indicated by arrows. (E) Histologic scoring of arthritis severity was not different for the two strains. n.s., not significant (P = 0.66).
Tissue-specific infection with B. burgdorferi is comparable in DBA/1 and C3H/HeJ mice.
While joint measurements and histologic scoring were similar for the two murine strains studied, we also sought to examine if bacterial dissemination to joints and other target organs differed. Using quantitative PCR, we examined bacterial density in tibiotarsal joints and hearts at 14 and 42 days postinfection, time points which correlate with maximal predicted carditis and arthritis, respectively. Spirochete density was measured by qPCR of ospC genome copies normalized to murine actin. At 14 days postinfection, both joint and heart tissues showed detectable spirochetal genomes in all tissues analyzed (Fig. 3A). No significant difference was found in mean spirochetal density (OspC copies/106 actin copies) between DBA/1 and C3H/HeJ tissues (joint, 1,564 ± 635 versus 1,927 ± 755, respectively; heart, 41 ± 5.7 versus 82 ± 20.4, respectively). Analysis at 42 days postinfection (Fig. 3B) again revealed similar spirochetal burdens in the C3H/HeJ and DBA/1 strains (joint, 2,328 ± 1,128 versus 2,429 ± 714, respectively; heart, 62 ± 16.5 versus 183 ± 127.8, respectively).
Fig 3.
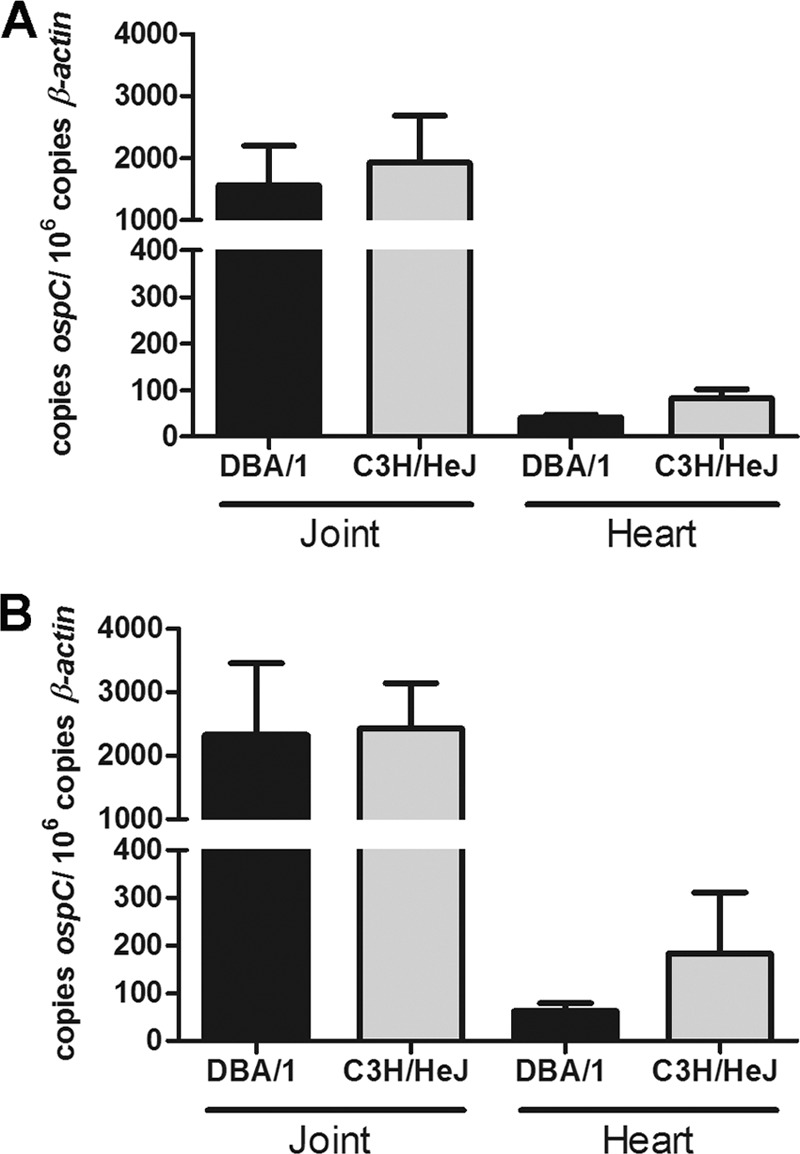
DBA/1 and C3H/HeJ mice have similar tissue densities of B. burgdorferi. B. burgdorferi-infected C3H/HeJ and DBA/1 tibiotarsal joints and hearts were collected at 14 (A) and 42 (B) days and DNA extracted. Tissue infectious density was determined using qPCR of ospC copies per 106 β-actin copies at each time point. No difference was found between murine strains at each time point in each organ. Mean values are depicted with standard errors.
The humoral response to B. burgdorferi infection differs in DBA/1 and C3H/HeJ mice.
Given the different genetic backgrounds of the murine strains, we sought to characterize their humoral responses to B. burgdorferi infection. Figure 4 shows ELISA data obtained using class- and isotype-specific secondary antibodies to detect averages of individual murine serum reactivity with B. burgdorferi cell lysates. Sera were used from animals sacrificed at 42 days postinfection. Figure 4 shows a general trend toward decreased antibody production against B. burgdorferi antigens in the DBA/1 strain. Figure 4A indicates that total IgG/IgM levels against B. burgdorferi antigens were not significantly different between strains, with lower mean antibody titers in DBA/1 mice (0.682 ± 0.10 RU) than in C3H/HeJ mice (0.857 ± 0.07 RU) (P = 0.17). Analysis of total IgM (Fig. 4C) again showed a nonsignificant trend toward higher titers in C3H/HeJ mice (DBA/1, 0.714 ± 0.07 RU; C3H/HeJ, 0.831 ± 0.05 RU; P = 0.18). Total IgG levels (Fig. 4B) also did not differ between DBA/1 and C3H/HeJ mice (DBA/1, 0.654 ± 0.12 RU; C3H/HeJ, 0.868 ± 0.08 RU; P = 0.15). We further analyzed IgG production by subclass and found a significant difference in IgG2a (Fig. 4E) but not IgG1 production (Fig. 4D). DBA/1 mice showed lower mean levels of IgG1 (0.925 ± 0.17 versus 1.463 ± 0.28 RU; P = 0.14) and significantly decreased IgG2a levels (0.489 ± 0.13 RU versus 0.958 ± 0.15 RU; P = 0.031) compared to those in C3H/HeJ mice at 42 days postinfection.
Fig 4.
The humoral response to B. burgdorferi proteins varies between DBA/1 and C3H/HeJ mice. ELISA plates coated with B. burgdorferi cell lysate were probed with DBA/1 and C3H/HeJ infected mouse sera (n = 10 per strain) and secondary antibodies specific for murine class- and isotype-specific antibodies. Antibody densities were compared for each class and isotype as indicated. No differences were found between total IgG-IgM (A), IgG (B), IgM (C), or IgG1 (D) levels. IgG2a density (E) was significantly decreased in DBA/1 versus C3H/HeJ mice (P < 0.05). Mean values are depicted with standard errors.
Specific antigens recognized by the humoral response to B. burgdorferi differ in DBA/1 and C3H/HeJ mice.
Having determined differences in antibody isotype responses in DBA/1 and C3H/HeJ mice infected by B. burgdorferi, we sought to identify what specific differences in B. burgdorferi antigen recognition might account for the varying antibody production. Figure 5A shows Western blots of pooled DBA/1 and C3H/HeJ mouse sera against B. burgdorferi membrane proteins at 42 days postinfection. Individual arrows highlight obvious differences in reactivity between the two strains. Because one-dimensional SDS-PAGE separation did not allow specific identification of antigenic targets, we performed 2-dimensional electrophoresis using an immobilized pH gradient (IPG) for first-dimension separation by isoelectric point and traditional SDS-PAGE for second-dimension separation by mass. Using Western blotting of 2-dimensional gels, Fig. 5B and C show the immunoreactivities of pooled DBA/1 and C3H/HeJ murine sera with B. burgdorferi membrane proteins separated by pI and mass. Using data from our previous mapping of the B. burgdorferi immunome, we identified individual protein antigens recognized by each pool and compared these responses. The overall number of recognized antigens was greater in the C3H/HeJ pool. This included a number of antigens not recognized by DBA/1 immune sera, including LA7, BBA03, BBA64, BBA73, OspA, and VlsE. All of these except OspA are lipoproteins expressed during mammalian infection. There were no antigens recognized by DBA/1 sera that were not also detected on Western blotting by C3H/HeJ immune sera.
Fig 5.
B. burgdorferi membrane-associated protein antigen recognition differs between DBA/1 and C3H/HeJ mice at 42 days postinfection. (A) B. burgdorferi membrane-associated proteins (MAP) (15 μg) separated by one-dimensional SDS-PAGE were probed with pooled sera (n = 10 per group) from C3H/HeJ and DBA/1 mice at 42 days postinfection, with detection of polyclonal IgG-IgM reactivity. (B and C) Fifty micrograms of B. burgdorferi MAP was separated using an IPG followed by SDS-PAGE and transferred to nitrocellulose membrane. Pooled sera from murine strains C3H/HeJ (B) and DBA/1 (C) (n = 10) were used to probe blots, followed by detection of polyclonal IgG-IgM reactivity. The resulting two-dimensional serologic maps were correlated with protein identifications. Boxes indicate MAP recognized by C3H/HeJ but not DBA/1 serum.
DISCUSSION
Similarities between Lyme disease and autoimmune arthritis have been noted since Steere and colleagues first described Lyme arthritis in a cohort of predominantly pediatric patients (20, 22). Since that time, experimental models for the study of both diseases have been developed in genetically distinct murine strains, preventing direct comparisons (1, 3, 6, 24). The DBA/1 strain is commonly employed for the collagen-induced arthritis model (1), while C3H congenic strains represent the most common experimental model of Lyme arthritis. Other murine strains used for B. burgdorferi infection have a limited (C57BL/6) or absent (DBA/2) arthritis phenotype (3, 18). Studies examining allelic markers for arthritogenic responses to infection and CIA have shown a distinct separation along H-2 haplotypes, which govern major histocompatibility complex (MHC)-restricted responses. While B. burgdorferi arthritis-susceptible strains (C3H/HeJ) segregate to the H-2k haplotype, arthritis-resistant strains (DBA/2) are most commonly H-2d, with mild arthritis seen with strains representing the H-2b, H-2j, and H-2r haplotypes (18). The DBA/1 strain, despite sharing a common ancestry with H-2d DBA/2 mice, has an H-2q haplotype and has not been previously studied in experimental models of Lyme arthritis.
Although it is an infectious arthritis, numerous immunologic aspects of murine Lyme arthritis suggest a phenotype with similarities to that of CIA. Both processes involve a diverse array of proinflammatory cytokines, including TNF-α and IL-1β (12, 13). A similar balance of Th1 and Th2 immunity has been noted, and more recent work highlights a role for Th17 cells in the pathogenesis of both diseases (5, 11, 15). The histology of arthritis in the two models is similar. As opposed to the destructive neutrophilic infiltrates of more typical pyogenic bacterial arthritis, B. burgdorferi infection produces a proliferative synovial response which is more reminiscent of the antigen-driven joint disease seen in CIA. A lack of published data on the infectivity of DBA/1 mice with B. burgdorferi led us to examine its utility as a novel model strain for the study of Lyme arthritis.
Our data establish that the DBA/1 strain is comparable to the C3H/HeJ model for the study of murine Lyme arthritis. DBA/1 and C3H/HeJ murine bladders exhibited identical outgrowth of Borrelia after subcutaneous infection, indicating that the infectivities of the two murine stains were comparable. When the specific tissue burden of infection was quantitated using qPCR to measure B. burgdorferi genome copies, there was no significant difference in spirochetal numbers at early (14 days) and later (42 days) time points in cardiac and tibiotarsal joint tissues. Taken together, these data suggest similar capacities for infection and degrees of dissemination to target tissues. B. burgdorferi infection alone, however, may not lead to an arthritic phenotype (3). Examination of the DBA/1 strain for phenotypic and histologic signs of joint disease was therefore critical to this study. Figures 1 and 2 confirm the similarity in timing, duration, and severity of tibiotarsal joint involvement in both the DBA/1 and C3H/HeJ strains, with joint swelling noted to be slightly greater in DBA/1 mice. Importantly, there was also no difference in the joint size at the time of infection, which may have influenced the degree of arthritis. Analysis of tibiotarsal joint histology revealed synovial proliferation and the presence of lymphocytic and neutrophilic infiltrates in both joint tissues as well as occasional inflammation of local soft tissue. There was no significant difference in the grading of arthritis severity, and sections from the two animals were not distinct in their arthritic phenotypes. These data confirm the utility of the DBA/1 strain in further exploration of CIA and Lyme arthritis, allowing for direct comparisons between the two disease models.
In natural infection, the humoral response (both T cell dependent and independent) is a critical component of the development and control of Lyme arthritis but is inadequate to completely clear spirochetes (8, 10). Multiple models have shown that B cell responses develop in the absence of T cell or innate immune components (Toll-like receptor 2 [TLR2] and MyD88), and passive antibody transfer or immunization of these mice is partially protective. While we detected similar infectivity and arthritis in DBA/1 and C3H/HeJ mice, we also sought to compare their humoral immune responses to B. burgdorferi antigens. Production of both IgM and IgG was similar for the two strains, though with a trend toward decreased total antibody production in the DBA/1 strain. Specific analysis of IgG isotypes, specifically IgG1 (Th2-type) and IgG2a (Th1-type) responses, showed significantly decreased IgG2a production in DBA/1 versus C3H/HeJ sera. The implication of this result is mixed, and it seems to suggest a relative decrease in the total intensity of humoral responses in the DBA/1 mouse rather than a specific loss of one isotype. When we qualitatively confirmed antibody differences with Western blotting with pooled sera, we observed variation in the recognition of B. burgdorferi antigens. Our previous work mapping the proteomic correlates of antibody responses in infected mice allowed us to dissect specific antigen reactivity in both murine strains. Two-dimensional immunoblot analysis indicated that the pooled sera from DBA/1 and C3H/HeJ mice recognized a common set of antigens, including OspC and BmpA. However, a number of lipoproteins were detected only by C3H/HeJ, including OspA and several other proteins from the virulence-associated Borrelia plasmid lp54. Interestingly, the more limited humoral responses in the DBA/1 strain did not diminish arthritis after B. burgdorferi infection, suggesting that the differential humoral response to these antigens did not influence the development or resolution of arthritis. The absence of the antibody response to these specific lipoproteins in DBA/1 mice also did not alter the course of infection as determined by culture positivity or tissue-specific spirochetal burden. These data are naturally limited by the poor expression in vitro of some proteins required for pathogenesis in vivo, such as VlsE. However, they do identify a wide range of membrane-associated antigens which are critical for mammalian infection, including OspC, BBA64, and others. The specific impact of variation in the humoral response in DBA/1 mice thus represents an area for future examination. One could hypothesize that antigens recognized in only the C3H-congenic mice are not critical to the development of Lyme disease and arthritis and may be suboptimal targets for vaccine development or serologic diagnostic tests in the clinical setting.
Some limitations of our study derive primarily from the infectious dose employed. We used 106 organisms, a dose above the threshold for infectivity. While this dose was used to ensure the infection and development of arthritis in all hosts, we cannot conclude that lower-dose infection will produce similar phenotypes of infection. While higher infectious doses can have a partial immunization effect due to spirochete burden (9, 17), we did not observe significant OspA reactivity, which would be the most common finding in immunized subjects as we have previously shown (16). Previous studies of DBA/2 mice have also shown that they are resistant to the development of arthritis at even higher doses, suggesting that our observations in DBA/1 mice at this dose are valid (17). Future studies should focus on varied infectious doses for evaluation of the immune response to Borrelia infection in DBA/1 mice, further characterizing this novel strain for the study of murine Lyme arthritis. We also evaluated humoral responses using pooled sera, which may mask the individual variability of serologic response to specific antigens. We did, however, observe that on individual one-dimensional immunoblots, the frequency of individual reactivity correlated well with antigen recognition of pooled sera (data not shown). The nonclonal isolate of Borrelia employed in this study also raises the possibility that subpopulations of spirochetes might have been responsible for the variation in antigen recognition. However, all mice were simultaneously infected with a single culture of Borrelia, which should limit differential antigen exposure in vivo.
The value of a novel murine model strain for the study of Lyme arthritis that is common to CIA is significant. The results of our study suggest that the wealth of previous knowledge of CIA in DBA/1 mice may be directly compared and contrasted with data for Lyme arthritis in the same strain. The opportunity to evaluate critical mediators of the immunopathogenesis of CIA and disease-modifying agents in Lyme arthritis is an exciting potential benefit. Further, study of experimental Lyme disease and arthritis in the DBA/1 mouse may provide insight into determinants of susceptibility to B. burgdorferi infection and the clinical manifestations of Lyme disease.
ACKNOWLEDGMENTS
The authors have no conflicts of interest.
Grant support for this work was from the National Institutes of Health grants R01 AR056959 and T32 AR052282 and the Children's Hospital of Pittsburgh Research Advisory Committee.
Footnotes
Published ahead of print 1 August 2012
REFERENCES
- 1. Asquith DL, Miller AM, McInnes IB, Liew FY. 2009. Animal models of rheumatoid arthritis. Eur. J. Immunol. 39:2040–2044 [DOI] [PubMed] [Google Scholar]
- 2. Bacon RM, Kugeler KJ, Mead PS. 2008. Surveillance for Lyme disease—United States, 1992-2006. MMWR Surveill. Summ. 57:1–9 [PubMed] [Google Scholar]
- 3. Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133–138 [DOI] [PubMed] [Google Scholar]
- 4. Burgdorfer W, et al. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319 [DOI] [PubMed] [Google Scholar]
- 5. Codolo G, et al. 2008. Borrelia burgdorferi NapA-driven Th17 cell inflammation in lyme arthritis. Arthritis Rheum. 58:3609–3617 [DOI] [PubMed] [Google Scholar]
- 6. Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. 1980. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 283:666–668 [DOI] [PubMed] [Google Scholar]
- 7. Crandall H, et al. 2006. Gene expression profiling reveals unique pathways associated with differential severity of lyme arthritis. J. Immunol. 177:7930–7942 [DOI] [PubMed] [Google Scholar]
- 8. Dickinson GS, Alugupalli KR. 2012. Deciphering the role of Toll-like receptors in humoral responses to Borreliae. Front. Biosci. 4:699–712 [DOI] [PubMed] [Google Scholar]
- 9. Gern L, Schaible UE, Simon MM. 1993. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J. Infect. Dis. 167:971–975 [DOI] [PubMed] [Google Scholar]
- 10. Kraiczy P, Skerka C, Kirschfink M, Zipfel PF, Brade V. 2002. Immune evasion of Borrelia burgdorferi: insufficient killing of the pathogens by complement and antibody. Int. J. Med. Microbiol. 291(Suppl. 33):141–146 [DOI] [PubMed] [Google Scholar]
- 11. Lubberts E. 2010. Th17 cytokines and arthritis. Semin. Immunopathol. 32:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McInnes IB, Schett G. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7:429–442 [DOI] [PubMed] [Google Scholar]
- 13. Miller JC, Ma Y, Crandall H, Wang X, Weis JJ. 2008. Gene expression profiling provides insights into the pathways involved in inflammatory arthritis development: murine model of Lyme disease. Exp. Mol. Pathol. 85:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nardelli DT, Callister SM, Schell RF. 2008. Lyme arthritis: current concepts and a change in paradigm. Clin. Vaccine Immunol. 15:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nardelli DT, et al. 2010. Significant differences between the Borrelia-infection and Borrelia-vaccination and -infection models of Lyme arthritis in C3H/HeN mice. FEMS Immunol. Med. Microbiol. 60:78–89 [DOI] [PubMed] [Google Scholar]
- 16. Nowalk AJ, Gilmore RD, Jr., Carroll JA. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 74:3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaible UE, et al. 1993. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol. Lett. 36:219–226 [DOI] [PubMed] [Google Scholar]
- 18. Schaible UE, Kramer MD, Wallich R, Tran T, Simon MM. 1991. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur. J. Immunol. 21:2397–2405 [DOI] [PubMed] [Google Scholar]
- 19. Steere AC. 1991. Clinical definitions and differential diagnosis of Lyme arthritis. Scand. J. Infect. Dis. Suppl. 77:51–54 [PubMed] [Google Scholar]
- 20. Steere AC, et al. 1979. Chronic Lyme arthritis. Clinical and immunogenetic differentiation from rheumatoid arthritis. Ann. Intern. Med. 90:896–901 [DOI] [PubMed] [Google Scholar]
- 21. Steere AC, Glickstein L. 2004. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 4:143–152 [DOI] [PubMed] [Google Scholar]
- 22. Steere AC, et al. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 20:7–17 [DOI] [PubMed] [Google Scholar]
- 23. Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wooten RM, Weis JJ. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4:274–279 [DOI] [PubMed] [Google Scholar]