Abstract
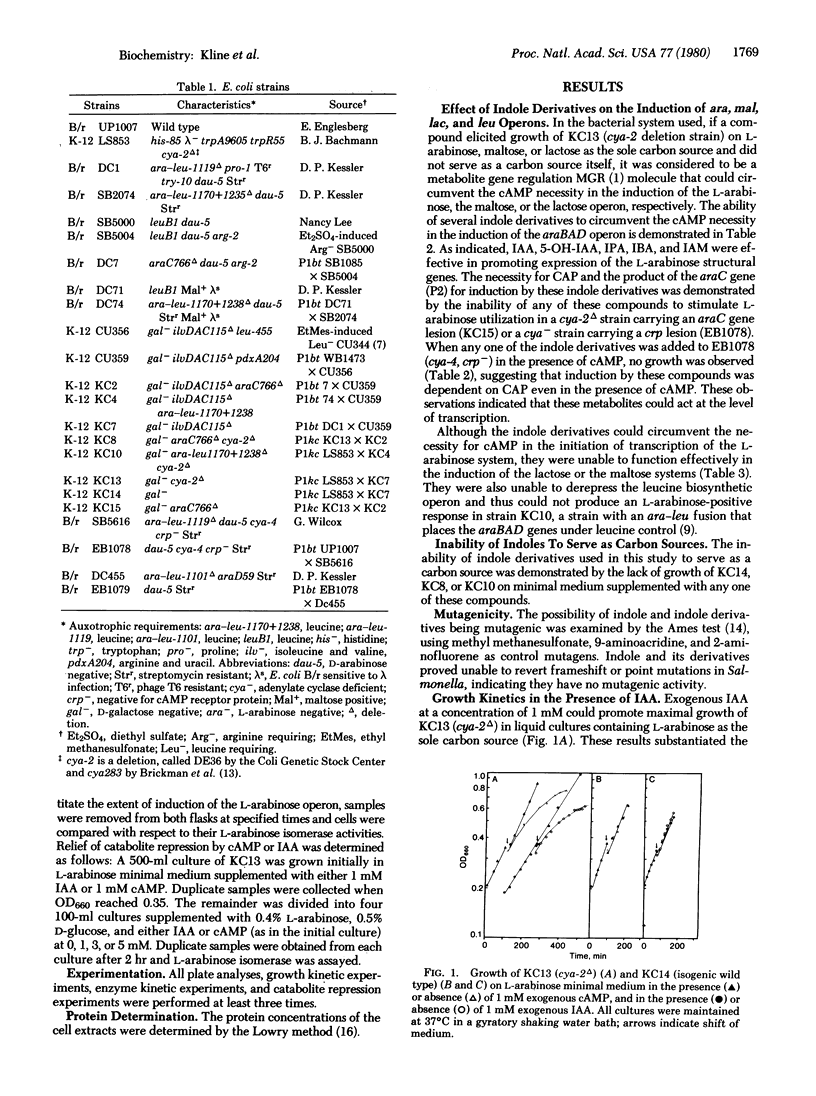
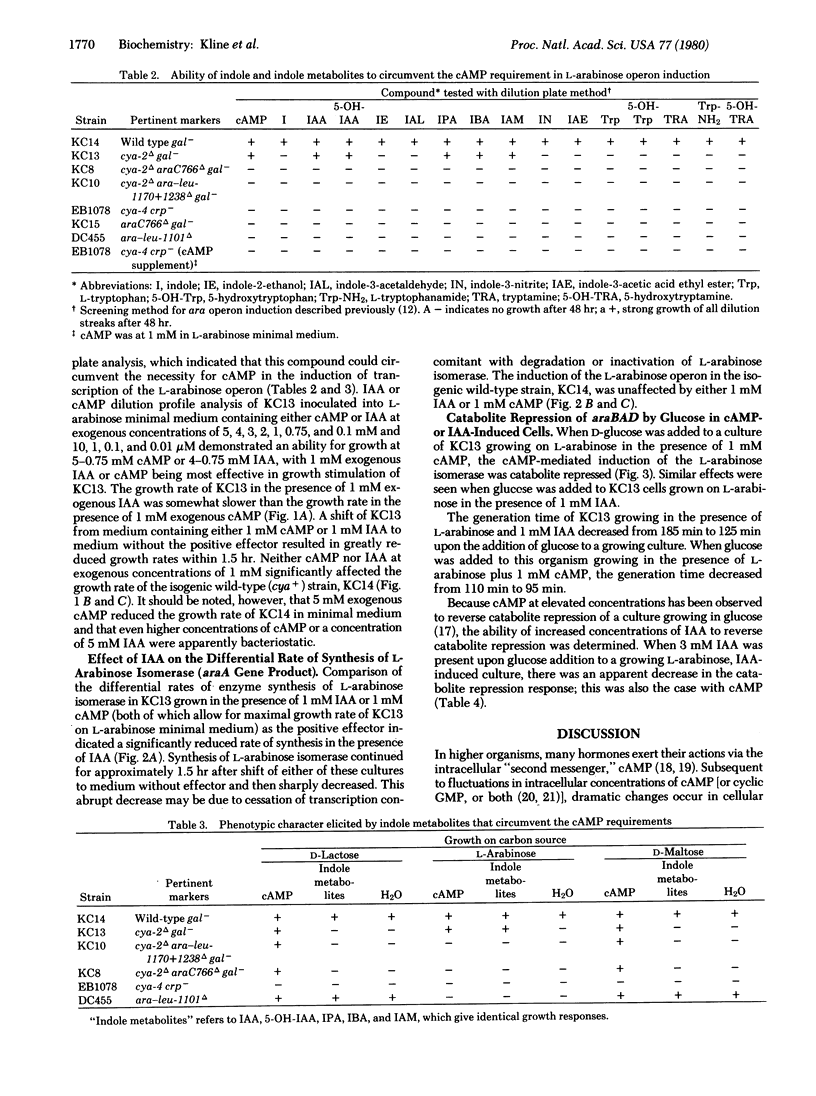
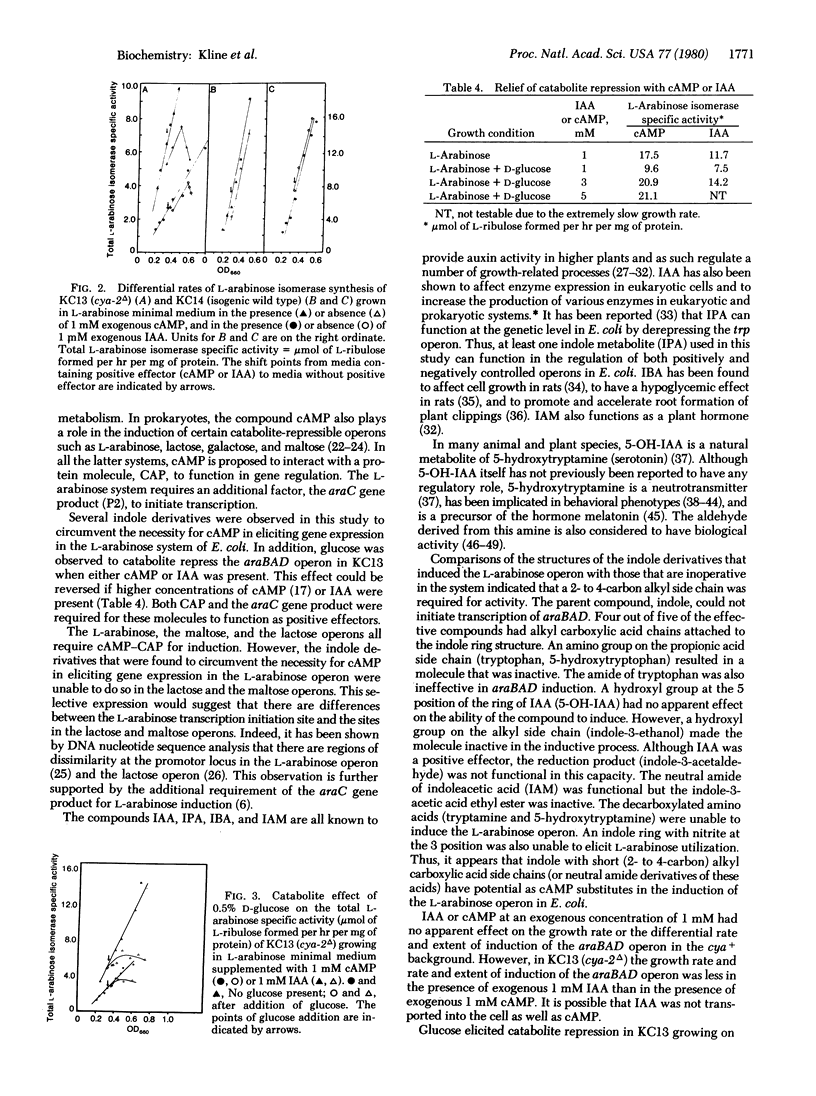
The ability of indole derivatives to facilitate RNA polymerase transcription of the L-arabinose operon in Escherichia coli was shown to require the catabolite activator protein (CAP) as well as the araC gene product. Adenosine 3',5'-monophosphate (cAMP) was not obligatory for araBAD transcription when the cells were grown in the presence of 1 mM indole-3-acetic acid or in the presence of indole-3-acetamide, indole-3-propionic acid, indole-3-butyric acid, or 5-hydroxyindole-3-acetic acid. However, these indole derivatives were unable to circumvent the cAMP requirement for the induction of the lactose and the maltose operons. Catabolic repression occurred when glucose was added to cells grown in the presence of L-arabinose and 1 mM indoleacetic acid or 1 mM cAMP. This effect was reversed at higher concentrations of indoleacetic acid or cAMP. The induction and the catabolite repression phenomena were quantitated by measuring the differential rate of synthesis of L-arabinose isomerase (the araA gene product). These results indicated that indole metabolites from various living systems may regulate gene expression and may be involved in "metabolite gene regulation."
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WEISSBACH H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science. 1960 Apr 29;131(3409):1312–1312. doi: 10.1126/science.131.3409.1312. [DOI] [PubMed] [Google Scholar]
- Alivisatos S. G., Ungar F. Incorporation of radioactivity from labeled serotonin and tryptamine into acid-insoluble material from subcellular fractions of brain. I. The nature of the substrate. Biochemistry. 1968 Jan;7(1):285–292. doi: 10.1021/bi00841a035. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- BARONDES S. H. The influence of neuroamines on the oxidation of glucose by the anterior pituitary. I. The role of monoamine oxidase. J Biol Chem. 1962 Jan;237:204–207. [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. S., West R., Hilderman R. H., Bayliss F. T., Klines E. L. A new locus (leuK) affecting the regulation of branched-chain amino acid, histidine, and tryptophan biosynthetic enzymes. J Bacteriol. 1978 Aug;135(2):542–550. doi: 10.1128/jb.135.2.542-550.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENGOTT D., MIRSKY I. A., PERISUTTI G. The hypoglycemic and insulinase-inhibitory action of some plant growth regulators. Endocrinology. 1956 Dec;59(6):715–718. doi: 10.1210/endo-59-6-715. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. E., Peterkofsky A. Diverse directional changes of cGMP relative to cAMP in E. coli. Biochem Biophys Res Commun. 1975 Nov 3;67(1):190–197. doi: 10.1016/0006-291x(75)90301-0. [DOI] [PubMed] [Google Scholar]
- Good N. E., Andreae W. A., Ysselstein M. W. Studies on 3-Indoleacetic Acid Metabolism. II. Some Products of the Metabolism of Exogenous Indoleacetic Acid in Plant Tissues. Plant Physiol. 1956 May;31(3):231–235. doi: 10.1104/pp.31.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Boone T., Wilcox G. DNA sequence of the araBAD promoter in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4724–4728. doi: 10.1073/pnas.75.10.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan L., Bass R., Englesberg E. Mutations affecting catabolite repression of the L-arabinose regulon in Escherichia coli B/r. J Bacteriol. 1976 Jun;126(3):1119–1131. doi: 10.1128/jb.126.3.1119-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry L. B., Witham F. H., Chapman O. L. Gene regulation: the involvement of stereochemical recognition in DNA-small molecule interactions. Perspect Biol Med. 1977 Autumn;21(1):120–130. doi: 10.1353/pbm.1977.0018. [DOI] [PubMed] [Google Scholar]
- Hendry L. B., Witham F. H. Stereochemical recognition in nucleic acid-amino acid interactions and its implications in biological coding: a model approach. Perspect Biol Med. 1979 Spring;22(3):333–345. doi: 10.1353/pbm.1979.0002. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Englesberg E. L-arabinose binding protein from Escherichia coli B-r. J Bacteriol. 1969 Oct;100(1):423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L., Bankaitis V., Brown C. S., Montefiori D. Imidazole acetic acid as a substitute for cAMP. Biochem Biophys Res Commun. 1979 Mar 30;87(2):566–574. doi: 10.1016/0006-291x(79)91832-1. [DOI] [PubMed] [Google Scholar]
- Kline E. L. New amino acid regulatory locus having unusual properties in heterozygous merodiploids. J Bacteriol. 1972 Jun;110(3):1127–1134. doi: 10.1128/jb.110.3.1127-1134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAAS J. W. Neurochemical differences between two strains of mice. Science. 1962 Aug 24;137(3530):621–622. doi: 10.1126/science.137.3530.621. [DOI] [PubMed] [Google Scholar]
- MAAS J. W. Neurochemical differences between two strains of mice. Nature. 1963 Jan 19;197:255–257. doi: 10.1038/197255a0. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- NISHIDA S. Potent diphtheria toxin within the cells of C. diphtheriae. Nature. 1954 Nov 20;174(4438):970–970. doi: 10.1038/174970a0. [DOI] [PubMed] [Google Scholar]
- PESONEN S. Tumours resulting from parthenogenesis induced by plant hormones in albino rats. Acta Endocrinol (Copenh) 1950;5(6):409–412. doi: 10.1530/acta.0.0050409. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pauley R. J., Fredricks W. W., Smith O. H. Effect of tryptophan analogs on derepression of the Escherichia coli tryptophan operon by indole-3-propionic acid. J Bacteriol. 1978 Oct;136(1):219–226. doi: 10.1128/jb.136.1.219-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- SUDAK H. S., MAAS J. W. CENTRAL NERVOUS SYSTEM SEROTONIN AND NOREPINEPHRINE LOCALIZATION IN EMOTIONAL AND NON-EMOTIONAL STRAINS IN MICE. Nature. 1964 Sep 19;203:1254–1256. doi: 10.1038/2031254a0. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Formation of adenosine-3,5-phosphate (cyclic adenylate) and its relation to the action of several neurohormones or hormones. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 50):171–174. doi: 10.1530/acta.0.xxxivs171. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Tabakoff B., Groskopf W., Anderson R., Alivisatos S. G. "Biogenic" aldehyde metabolism relation to pentose shunt activity in brain. Biochem Pharmacol. 1974 Jun 15;23(12):1707–1719. doi: 10.1016/0006-2952(74)90398-0. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., WEISSBACH H., BOGDANSKI D. F. Biochemical findings relating to the action of serotonin. Ann N Y Acad Sci. 1957 Mar 14;66(3):602–608. doi: 10.1111/j.1749-6632.1957.tb40750.x. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., WEISSBACH H., BOGDANSKI D. F. Effect of iproniazid on serotonin metabolism in vivo. J Pharmacol Exp Ther. 1957 Jun;120(2):255–260. [PubMed] [Google Scholar]
- UDENFRIEND S., WEISSBACH H., BOGDANSKI D. F. Increase in tissue serotonin following administration of its precursor 5-hydroxytryptophan. J Biol Chem. 1957 Feb;224(2):803–810. [PubMed] [Google Scholar]
- Wasmuth J., Umbarger H. E., Dempsey W. B. A role for a pyridoxne derivative in the multivalent repression of the isoleucine and valine biosynthetic enzymes. Biochem Biophys Res Commun. 1973 Mar 5;51(1):158–164. doi: 10.1016/0006-291x(73)90522-6. [DOI] [PubMed] [Google Scholar]
- Witham F. H., Hendry L. B., Chapman O. L. Chirality and stereochemical recognition in DNA-phytohormone interactions: a model approach. Orig Life. 1978 Sep;9(1):7–15. doi: 10.1007/BF00929709. [DOI] [PubMed] [Google Scholar]


