Abstract
New malaria vaccines are urgently needed to improve vaccine protective efficacy. PfCelTOS is a recombinant malaria vaccine antigen that has shown protective efficacy in a small-animal challenge model when combined with a water-in-oil emulsion adjuvant (Montanide ISA 720). In this report, we show that PfCelTOS vaccines containing GLA-SE (a stable oil-in-water emulsion combined with a Toll-like receptor 4 [TLR4] agonist) elicit strong Th1-type immune responses in BALB/c mice. These responses include higher antigen-specific IgG2a antibody titers and more gamma interferon (IFN-γ) production than those seen with a PfCelTOS vaccine containing Montanide ISA 720. Furthermore, reducing the emulsion dose from 2% to 1% or 0.5% (vol/vol) squalene in GLA-SE did not compromise immunogenicity. Emulsion dose titration in the absence of formulated GLA caused some reduction in humoral and cellular immune responses compared to those with the 2% squalene emulsion dose.
INTRODUCTION
Oil-in-water (o/w) emulsions have been used safely and successfully as adjuvants in modern vaccines. The most notable o/w emulsions are MF59 and AS03, which are produced by Novartis and GSK Biologicals, respectively. Both of these adjuvants contain squalene at ∼2.5% (vol/vol) in the final vaccine formulation (13). However, until recently, only a few reports regarding the motivation for selecting this squalene concentration were published (9, 21, 25). Moreover, a recent study showed that dilution of MF59 did not compromise the immune response in a pandemic influenza vaccine clinical trial (20). If adjuvant activity can be maintained with a reduction of the squalene dose, then the local reactogenicity of o/w emulsions can potentially be reduced. Furthermore, the vaccine cost could decrease while the available adjuvant supply would increase, thus making vaccine and adjuvant production in resource-poor countries more achievable.
The recombinant malaria antigen PfCelTOS (Plasmodium falciparum cell traversal protein for ookinetes and sporozoites) combined with an emulsion adjuvant (Montanide ISA 720) protected 60% of mice in a heterologous challenge model (8). PfCelTOS inhibits sporozoite motility and hepatocyte infectivity and could be an important component of new malaria vaccines. Both cellular and humoral immune responses are important for protective efficacy directed against this antigen (7, 8).
In this work, we evaluated squalene-based stable emulsion (SE) adjuvant dose effects on humoral and cellular immune responses to PfCelTOS. Moreover, we investigated the effect of including a formulated synthetic Toll-like receptor 4 (TLR4) agonist, glucopyranosyl lipid adjuvant (GLA), in the vaccine formulation. We show that squalene concentrations of <2% (vol/vol) in GLA-SE may induce adjuvant responses equivalent to those seen with a 2% (vol/vol) squalene concentration. This finding has important implications for vaccine adjuvant production and dosing as well as for novel routes of administration (such as intradermal routes), which may be more sensitive to oil concentrations. Moreover, we show that the presence of GLA-based adjuvant formulations shapes immune activity toward a Th1-type response, eliciting higher levels of IgG2a antibody titers, more splenocytes producing gamma interferon (IFN-γ), and more long-lived antibody-secreting plasma cells (ASPC), all of which may be important for vaccine efficacy.
MATERIALS AND METHODS
Vaccine formulations.
Shark liver squalene (≥98% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Glycerol and α-tocopherol were purchased from Spectrum Chemical (Gardena, CA). Poloxamer 188 (Pluronic F68) was obtained from BASF (Ludwigshafen, Germany) or Spectrum Chemical. Egg phosphatidylcholine (PC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and glucopyranosyl lipid adjuvant (phosphorylated hexaacyl disaccharide [PHAD]) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). Ammonium phosphate buffer components were obtained from J. T. Baker. The emulsion formulation was prepared by making separate aqueous and oil phases. Poloxamer 188, glycerol, and buffer components were dissolved in the aqueous phase by stirring, whereas egg phosphatidylcholine was dissolved in the oil phase by sonication and heating. The aqueous and oil phases (10% [vol/vol] oil) were then mixed at high speed and microfluidized under high pressure as described earlier (14). A GLA-aqueous nanoparticle suspension formulation (GLA-AF) was formed by premixing GLA with DPPC at a 4.2:1 molar ratio in organic solvent, followed by solvent evaporation and hydration with ultrapure water. Water bath sonication at ∼70°C formed the nanoparticle aqueous suspension. On the day of immunization, the emulsion was diluted with phosphate-buffered saline (PBS) to the specified oil concentration and then mixed with antigen and GLA-AF (where applicable). Formulations were monitored by size and GLA concentration at 5°C throughout the duration of the immunization schedule. Particle size was measured by dynamic light scattering as described previously (14). The GLA concentration was monitored by high-performance liquid chromatography–charged aerosol detection (HPLC-CAD) (1). Montanide ISA 720 was purchased from Seppic Inc. (Fairfield, NJ) and was prepared following the manufacturer's instructions, by mixing 70% Montanide with 30% (vol/vol) aqueous phase prior to immunization. Throughout this report, the emulsion dose is referred to as the % (vol/vol) oil in the final vaccine formulation; the ratio of oil to emulsifier is kept constant at all doses. The codon-harmonized recombinant Plasmodium falciparum malarial protein PfCelTOS was developed and produced at the Walter Reed Army Institute of Research and provided to the Infectious Disease Research Institute (IDRI) as a purified bulk in phosphate buffer.
Mice.
Female BALB/c mice (5 to 7 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed under specific-pathogen-free conditions at the IDRI animal facility. All procedures were performed in accordance with the regulations and guidelines of the IDRI Animal Care and Use Committee.
Immunizations.
Two separate experiments are described in the text. The first experiment employed 5 mice/group for all assays. The second experiment consisted of 5 mice/group for antibody titer and enzyme-linked immunosorbent spot (ELISPOT) assays and 4 mice/group for the multiplex bead assay. Mice were immunized by subcutaneous (s.c.) injection. Formulations were mixed with antigen immediately prior to injection to provide a final formulation consisting of 0.5, 1, or 2% (vol/vol) oil, either with or without GLA (GLA-SE), in a 100-μl total injection volume. Montanide ISA 720 was used based on the manufacturer's instructions (using a 70:30 ratio of Montanide to PBS, i.e., 70 μl of Montanide and 30 μl of PBS per mouse per injection). The PfCelTOS antigen was used at 10 μg per dose. Mice were immunized three times, with doses given 3 weeks apart. Serum was collected by retro-orbital bleeding into Microtainer serum collection tubes (VWR International) before each injection or 3 weeks after the final injection.
Antibody responses.
Sera were analyzed for antigen-specific IgG, IgG1, and IgG2a antibodies by antibody capture enzyme-linked immunosorbent assays (ELISAs). Wells of Polysorp ELISA plates (Nunc) were coated with PfCelTOS (0.2 μg/100 μl of 0.1 M bicarbonate coating buffer) and incubated overnight at 4°C. Plates were blocked with PBS-0.5% Tween and 1% bovine serum albumin (BSA) (Sigma) and then washed, and sera were diluted serially (1:5 dilutions) across the plate. Plates were incubated (2 h, room temperature [RT]) and washed, and 100 μl anti-mouse IgG–horseradish peroxidase (HRP) (1:4,000), IgG1-HRP (1:2,000), or IgG2a-HRP (1:2,000) (Southern Biotech) was added per well. Plates were incubated at RT for 1 h, washed, and developed using SureBlue tetramethylbenzidine (TMB) substrate solution (Kirkegaard and Perry Laboratories). The enzymatic reaction was stopped with 50 μl/well of 1 N H2SO4, and plates were read at 450 nm (ELX808; Bio-Tek Instruments Inc.). The endpoint titer was determined using Prism software (GraphPad Software), following a sigmoidal fit (variable slope) of the values determined at dilution, and corresponded to a cutoff value (C) determined as described previously (16), according to the following equation: C = X + SDf, where X is the average optical density (OD), SD is the standard deviation for the negative-control serum, and f is the multiplier at the 99.9% confidence level. A cutoff OD of 0.1 was assigned if the cutoff value determined as described above was <0.1. On the rare occasion that an individual well registered an abnormally high reading for one of the higher dilutions, Grubb's test was used to evaluate the abnormal value for exclusion from the sigmoidal fit by comparing it to the 4 dilutions surrounding the dilution in question at the 99% confidence level (18).
Enumeration of long-lived ASPC.
A bone marrow ELISPOT assay was used to determine the induction of vaccine-specific long-lived ASPC 3 weeks following the last immunization, as previously described (5), with minor modifications. Briefly, femurs were collected, bone marrow was extracted, single-cell suspensions were prepared and seeded at 1.0 × 106 cells/well, and 1:3 dilutions were added to the next three wells in MultiScreen HA 96-well plates (Millipore) coated with 10 μg of CelTOS protein. Anti-mouse IgG–HRP was added and incubated at 4°C overnight. Plates were developed with 3-amino-9-ethylcarbazole (AEC) substrate for 30 min, and the reaction was stopped with a distilled water rinse. Spots were counted by an ELISPOT plate reader (CTL Serie3A analyzer; Cellular Technology Ltd., Cleveland, OH), and the data were analyzed using Immunospot software (CTL Analyzer LLC).
Cytokine ELISPOT assays.
IFN-γ and interleukin-5 (IL-5) ELISPOT kits (eBioscience) were used to measure cytokine responses to PfCelTOS. These assays were performed as previously described (3, 15), with minor modifications. Plates were coated with 100 μl/well of capture antibody solution (for IFN-γ or IL-5) and incubated at 4°C overnight. After incubation, the plates were washed and blocked, and 100 μl of medium alone or medium with PfCelTOS (10 μg/ml) or concanavalin A (ConA) (0.75 μg/ml) was added to each well. Splenocytes were added (2 × 105 cells/well), and plates were incubated at 37°C for 48 h and then washed. Biotinylated detection antibody was added to each well. The plates were incubated for 2 h at RT, washed, incubated with avidin-horseradish peroxidase reagent (100 μl/well) for 45 min at RT, and washed again. The peroxidase substrate AEC was added to the wells and allowed to react for 30 min at RT. The reaction was stopped with a distilled water rinse. The plates were counted by an ELISPOT plate reader, and data were analyzed using Immunospot software.
Multiplex cytokine bead assay.
Cytokine levels in 72-h antigen-stimulated splenocyte cultures were determined by the Luminex multiplex cytokine bead assay. Spleens were harvested from 4 mice/group 3 weeks after the third immunization, and single-cell suspensions were prepared as previously described (5). Splenocytes were stimulated with medium, ConA (0.75 μg/ml), or PfCelTOS protein (10 μg/ml). Mouse multiplex assays containing antibodies to IL-2, IL-5, IL-10, IL-13, IL-17, IFN-γ, and tumor necrosis factor alpha (TNF-α) were purchased from Affymetrix (Fremont, CA) and performed following the manufacturer's instructions. Briefly, 50 μl of sample supernatant was incubated with antibody-coated polystyrene beads. After washing, the beads were incubated with detection antibodies, washed, and incubated with streptavidin-phycoerythrin (streptavidin-PE). After the final wash, bead-bound PE was measured on a Luminex 200 instrument (Millipore, Billerica, MA), and cytokine concentrations were determined using MasterPlex QT software (MiraiBio Group, South San Francisco, CA). Each analyte was detected across a range of 2.4 to 40,000 pg/ml.
RESULTS
GLA-AF and GLA-SE are stable nanoparticle formulations (11). GLA-AF is an aqueous nanosuspension of GLA and phospholipid particles. SE is a squalene-based nanoemulsion typically used at 2% (vol/vol) squalene for immunization. In order to facilitate emulsion dose titration while keeping the GLA concentration constant, GLA-AF was added to the specified concentration of SE immediately prior to immunization for the experimental groups labeled GLA-SE. Mean particle sizes based on dynamic light scattering intensity distributions of the formulation batches used in the present study were between 88 and 98 nm, although the size polydispersity of GLA-AF was higher than that of GLA-SE (∼0.24 and ∼0.05, respectively). The GLA concentration for these batches of GLA-AF remained constant for at least 6 months, including the entire time encompassed by the studies described in this report. Montanide ISA 720 is a water-in-oil adjuvant that is typically used at 70% (vol/vol) squalene for immunization and previously showed significant sterile protection with PfCelTOS in a malaria challenge model (8). Montanide ISA 720 was therefore employed as a positive control for the immunogenicity studies described below.
To understand the impact of squalene emulsion concentration on PfCelTOS vaccine immunogenicity, BALB/c mice were immunized 3 times at 3-week intervals with a range of SE concentrations (0.02 to 2%), with or without 5 μg GLA. IgG1 and IgG2a antibody endpoint titers were analyzed following the 2nd and 3rd immunizations, as a measure of Th2- and Th1-type responses, respectively, and total IgG and the frequencies of long-lived plasma cells were also measured. There was a clear dose-dependent effect on IgG, IgG1, and IgG2a levels with the emulsions that did not contain GLA (Fig. 1; see Fig. S1 in the supplemental material). After the 3rd immunization (Fig. 1), the impact of SE dose titration was evident by reduced IgG1 and IgG2a responses of the 0.02% SE group compared to the 2% SE group. The 0.1% and 0.5% SE groups also showed lower IgG2a responses than that of the 2% SE group. However, the addition of formulated GLA appeared to negate this SE dose titration effect: all GLA-SE groups, regardless of emulsion dose, elicited equivalent IgG, IgG1, and IgG2a responses. In fact, the GLA-AF group (containing no emulsion) elicited antibody responses equivalent to those of the GLA-SE groups. Clearly, the presence of formulated GLA (whether SE or AF) elicited a stronger IgG2a response than that with the same vaccine composition without GLA. The Montanide group showed the highest IgG1 responses, although they were not significantly different from those of the 2% SE or 0.5% GLA-SE group. Antibody responses after the 2nd immunization showed similar patterns to those described above, although overall titers were somewhat lower. Moreover, the 0.02%, 0.1%, and 2% GLA-SE groups showed significantly higher IgG2a responses than the GLA-AF group (P < 0.05). Finally, larger numbers of antibody-secreting plasma cells in the bone marrow were elicited by vaccines containing formulated GLA (SE or AF), Montanide, or 2% SE than those in the antigen-only group (Fig. 2). Interestingly, 0.02% SE showed reduced levels of antibody-secreting plasma cells compared to the full 2% SE dose, mirroring the antibody endpoint titers.
Fig 1.
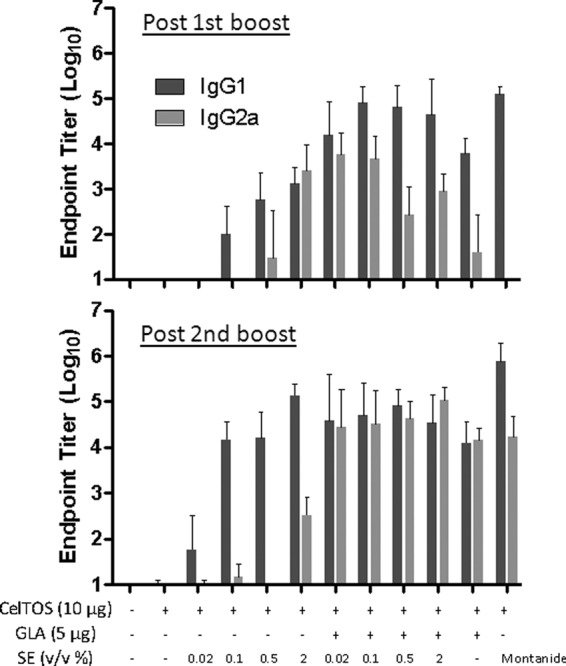
Antibody endpoint titers induced by PfCelTOS vaccines containing various adjuvant formulations with emulsion doses of 0.02% to 2% (vol/vol) oil, as measured 3 weeks after the 2nd and 3rd immunizations. Each group consisted of 5 mice, and error bars represent the standard deviations of the means. Statistically significant differences (P < 0.05) were seen for the following groups: for postboost IgG1, all adjuvant groups (except the 0.02% and 0.1% SE groups) versus PfCelTOS alone, all GLA-SE groups versus SE groups at the same emulsion concentrations, 2% SE versus 0.02% or 0.1% SE, Montanide versus 0.02% to 2% SE, 0.02% or 0.1% GLA-SE versus GLA-AF, and GLA-AF versus 0.02% to 0.5% SE; for postboost IgG2a, all GLA-SE groups and 2% SE versus PfCelTOS alone, 0.02% or 0.1% GLA-SE versus SE at the same emulsion concentration, 2% SE versus 0.02% to 0.5% SE, all GLA-SE groups and 2% SE versus Montanide, and all GLA-SE groups (except the 0.5% group) and 2% SE versus GLA-AF; for post-2nd boost IgG1, all adjuvant groups (except the 0.02% SE group) versus PfCelTOS alone, 0.02% GLA-SE versus 0.02% SE, 2% SE versus 0.02% SE, Montanide versus all adjuvant groups except the 2% SE and 0.5% GLA-SE groups, and GLA-AF versus 0.02% SE; and for post-2nd boost IgG2a, all adjuvant groups (except the 0.02% to 0.5% SE groups) versus PfCelTOS alone, all GLA-SE groups versus SE groups at the same emulsion concentrations, 2% SE versus 0.02% to 0.5% SE, Montanide versus 0.02% to 2% SE, and GLA-AF versus 0.02% to 2% SE.
Fig 2.
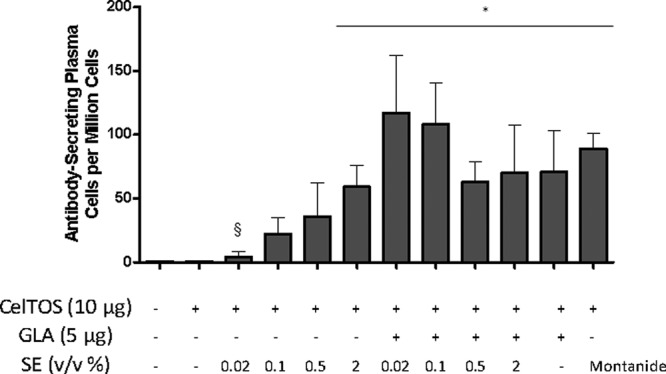
Long-lived antibody-secreting cells in the bone marrow, induced by PfCelTOS vaccines containing various adjuvant formulations with emulsion doses of 0.02% to 2% (vol/vol) oil and detected 3 weeks after the 3rd immunization. Each group consisted of 5 mice, and error bars represent the standard deviations of the means. Additional statistically significant differences (P < 0.05) that were not plotted include the following: 0.02% or 0.1% GLA-SE versus SE group at the same emulsion concentration, Montanide versus 0.02% to 0.5% SE, and GLA-AF versus 0.02% SE. *, P < 0.05 versus CelTOS alone; §, P < 0.05 versus 2% SE.
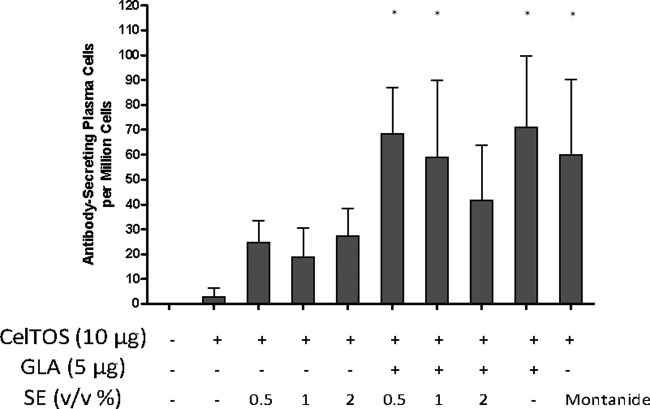
In another experiment, a narrower range of SE dose titration was employed (0.5% to 2%), and cellular as well as humoral responses were investigated. In Fig. 3, the total IgG1 and IgG2a antibody endpoint titers measured after the 2nd and 3rd immunizations are presented, and total IgG endpoint titers are shown in Fig. S2 in the supplemental material. After the 3rd immunization, all adjuvanted groups showed higher total IgG responses than those seen with antigen alone. However, the 0.5% dose of SE failed to elicit significantly higher IgG1 antibodies than those seen with antigen alone, and all SE doses failed to induce higher IgG2a responses than those with antigen alone. In contrast, all groups receiving formulated GLA showed higher IgG, IgG1, and IgG2a responses than those seen with antigen alone. GLA-based formulations dramatically increased IgG2a responses in all groups compared to those seen with the same composition without GLA. While the Montanide group showed significantly higher IgG1 titers than those of all other groups (P < 0.05), the GLA-SE groups at the 0.5% and 1% SE doses showed significantly higher IgG2a responses than those seen in the Montanide group (P < 0.05). Similar patterns were evident after the 2nd immunization, although the 0.5% SE dose elicited high IgG1 responses, in contrast to the results observed post-3rd immunization. Larger numbers of antibody-secreting plasma cells were elicited with formulated GLA- or Montanide-containing vaccines (Fig. 4), although no dose titration effect was evident with the various SE concentrations.
Fig 3.
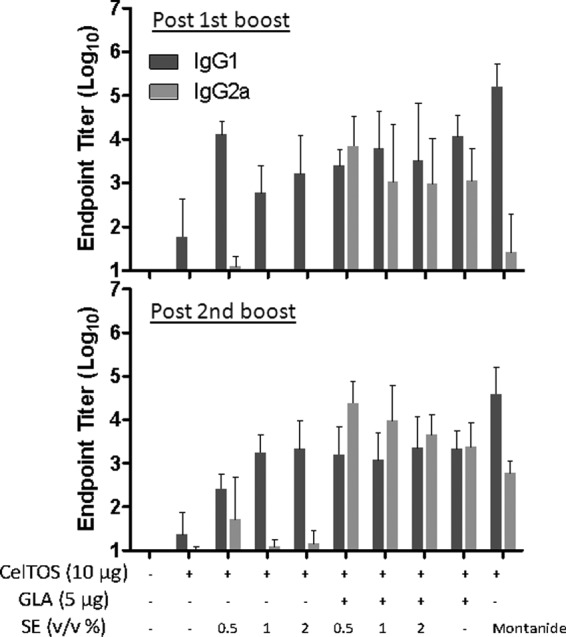
Antibody endpoint titers induced by PfCelTOS vaccines containing various adjuvant formulations with emulsion doses of 0.5% to 2% (vol/vol) oil, as measured 3 weeks after the 2nd and 3rd immunizations. Each group consisted of 5 mice, and error bars represent the standard deviations of the means. Statistically significant differences (P < 0.05) were seen for the following groups: for postboost IgG1, all adjuvant groups (except the 1% and 2% SE groups) versus PfCelTOS alone and Montanide versus 1% or 2% SE and GLA-SE at 0.05% or 2%; for postboost IgG2a, all GLA-SE groups and GLA-AF versus PfCelTOS alone, all GLA-SE groups versus SE groups at the same emulsion concentrations, all GLA-SE groups and GLA-AF versus Montanide, and GLA-AF versus all SE groups; for post-2nd boost IgG1, all adjuvant groups except the 0.5% SE group versus PfCelTOS alone and Montanide versus all groups; and for post-2nd boost IgG2a, all GLA-SE groups, GLA-AF, and Montanide versus PfCelTOS alone, all GLA-SE groups versus SE groups at same emulsion concentrations, 0.5% or 1% GLA-SE versus Montanide, Montanide versus 1% or 2% SE, and GLA-AF versus all SE groups.
Fig 4.
Long-lived antibody-secreting cells in the bone marrow, induced by PfCelTOS vaccines containing various adjuvant formulations with emulsion doses of 0.5% to 2% (vol/vol) oil and detected 3 weeks after the 3rd immunization. Each group consisted of 5 mice, and error bars represent the standard deviations of the means. Additional statistically significant differences (P < 0.05) that were not plotted include the following: 0.5% GLA-SE versus 0.5% SE and GLA-AF versus all SE groups. *, P < 0.05 versus CelTOS alone.
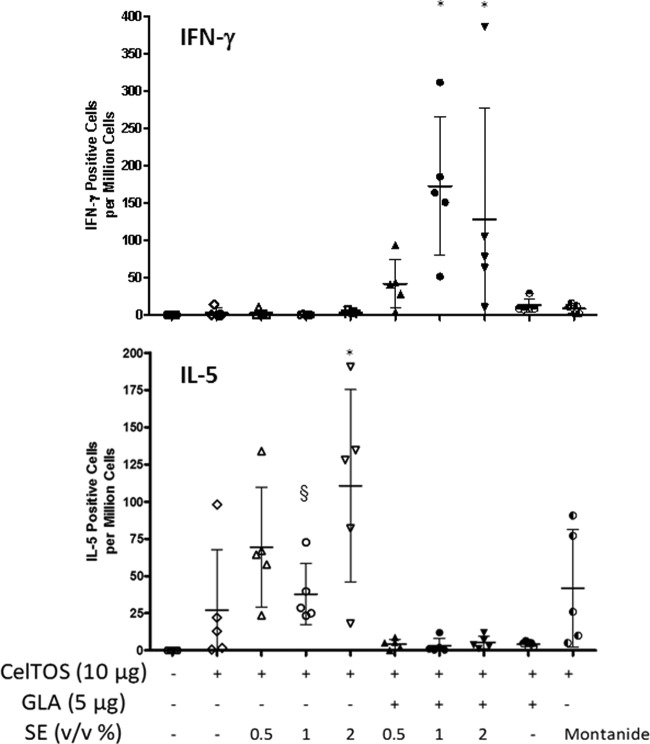
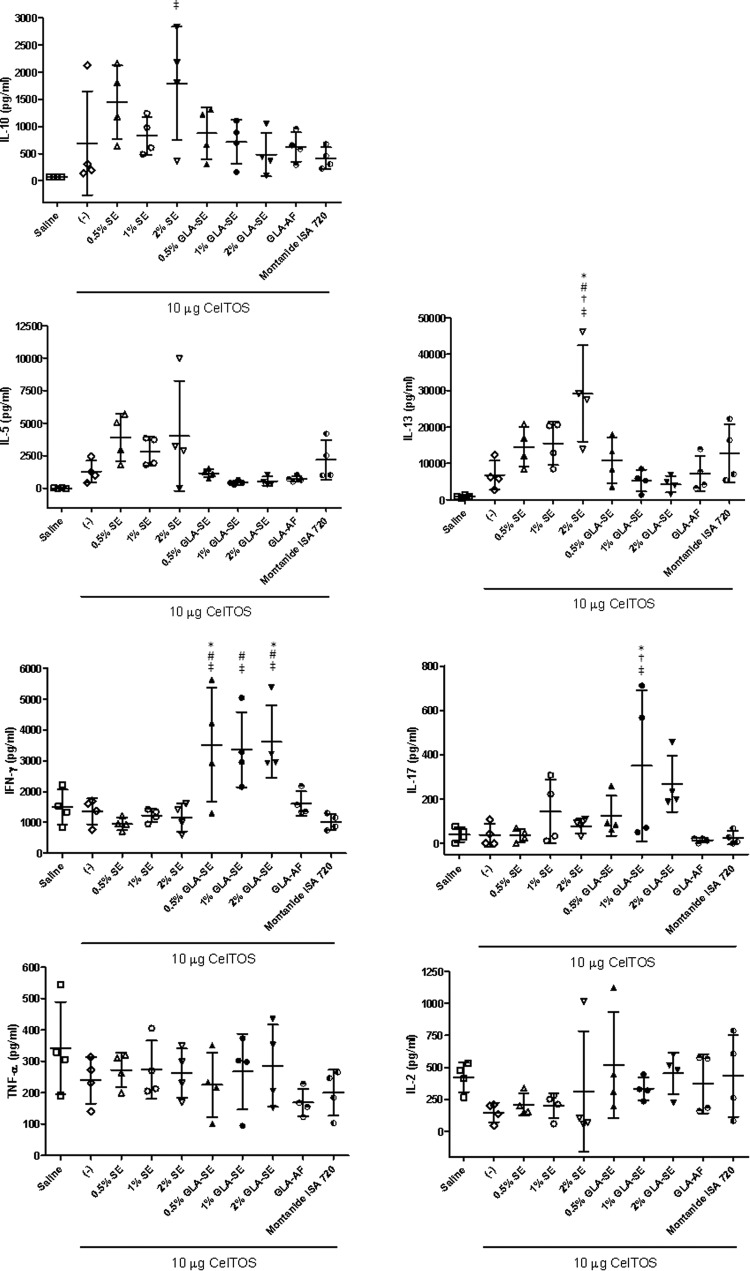
The number of cells responding with IFN-γ production was investigated by a cytokine ELISPOT assay. Significantly higher levels of IFN-γ-producing cells were elicited by the GLA-SE groups at the 1% or 2% SE dose than by the antigen-only, GLA-AF, SE without GLA, and Montanide groups (Fig. 5) (P < 0.05). In contrast, the GLA-based formulation groups showed negligible levels of IL-5-secreting cells, whereas the 2% SE group elicited significantly higher levels of IL-5-secreting cells than the antigen-only group or the Montanide group (P < 0.05). A similar trend is evident in the multiplex cytokine bead assay results (Fig. 6). In these analyses, the Th1 cytokine IFN-γ was induced to similar levels by GLA-SE immunization, regardless of emulsion concentration, whereas increased Th2 cytokines, such as IL-5, IL-10, and IL-13, were associated with the emulsion groups not containing GLA formulations. The 2% SE dose elicited the largest Th2 cytokine cellular responses (e.g., IL-5 and IL-13).
Fig 5.
Antigen-specific cytokine-producing cells (IFN-γ and IL-5) detected by ELISPOT assay 3 weeks after the 3rd immunization. Each group consisted of 5 mice, and error bars represent the standard deviations of the means. Additional statistically significant differences (P < 0.05) that were not plotted include the following: for IFN-γ, 1% or 2% GLA-SE group versus SE group at same emulsion concentration, 1% or 2% GLA-SE versus Montanide, and 1% GLA-SE versus GLA-AF; and for IL-5, 2% SE versus 2% GLA-SE, GLA-AF, or Montanide. *, P < 0.05 versus CelTOS alone; §, P < 0.05 versus 2% SE.
Fig 6.
Antigen-specific cytokine production detected by multiplex bead assay (Luminex) 3 weeks after the 3rd immunization. Each group consisted of 4 mice, and error bars represent the standard deviations of the means. *, P < 0.05 versus CelTOS alone; #, P < 0.05 versus same % oil, with or without GLA; †, P < 0.05 versus GLA-AF; ‡, P < 0.05 versus Montanide.
DISCUSSION
Inclusion of formulated GLA in the PfCelTOS vaccine composition shapes the immune response toward a Th1 bias, as demonstrated by the production of significantly higher IgG2a antibody titers and IFN-γ levels. Total antibody production did not correlate with protective efficacy in earlier PfCelTOS studies (7, 8); however, antibody isotype analyses were not performed in those studies, and therefore it is unclear whether more IgG2a antibody production could correlate with increased protective potential. In contrast, IFN-γ production does relate to vaccine efficacy (7, 8). Thus, GLA-SE adjuvants may be good candidates for generating protective efficacy with the PfCelTOS vaccine or other subunit malaria vaccines. While the immunogenicity of GLA-SE described in this work compared favorably with that of the positive control (Montanide), the ability of GLA-SE to induce similar levels of protective immunity was not evaluated here but rather is part of a related ongoing study that will be published separately. We note here that the emulsion (i.e., GLA-SE groups) appeared to be necessary for IFN-γ production, while antibody titers of the GLA-AF groups were similar to those of the GLA-SE groups after the 3rd immunization. The mechanism by which GLA-AF induces antibody titers equivalent to those of GLA-SE without appreciable IFN-γ production is unknown; perhaps IFN-γ levels are present but remain at low levels, or IFN-γ-independent factors may be responsible (17, 23). An analogous situation is reported by Przetak et al. (27), who showed that another synthetic TLR4 agonist increased IgG2a antibody responses and suppressed IL-5 production but did not produce appreciable IFN-γ. Interestingly, reduction of the emulsion dose in GLA-SE from 2% to 1% or 0.5% did not reduce antibody production or IFN-γ production as measured by multiplex cytokine bead assay, although in the IFN-γ ELISPOT assay the 0.5% concentration of GLA-SE did not achieve statistical significance versus PfCelTOS alone. Finally, inclusion of GLA-based formulations also induced significantly more long-lived antibody-secreting cells than those induced by antigen alone, which may indicate a more durable immune response.
Overall, GLA formulations shape the immune response toward a Th1 bias and induce larger numbers of antibody-secreting plasma cells in the bone marrow, although it appears that emulsion concentrations of <2% may be sufficient for these effects. These findings confirm the Th1-enhancing adjuvant effects described for GLA formulations in other vaccine models (4, 10). Similarly, the TLR9 agonist CpG was reported to induce a balanced Th1/Th2 response while reducing the amount of oil necessary in emulsion adjuvants (19). It is interesting that the IFN-γ and IL-5 results clearly indicate a Th1 bias of the GLA-containing emulsions and a Th2 bias of the emulsions without GLA, whereas the antibody data show a more balanced profile of IgG1 and IgG2a responses induced by the GLA-based adjuvants. This phenomenon has been noted in earlier studies and may be explained in part because IgG2a switching is downstream of IgG1 and may be affected by multiple cytokines (29). Another study reported IgG2a induction independent of IFN-γ (23), which may explain why the Montanide adjuvant in the present work induced some IgG2a after three immunizations, without any detectable effect on IFN-γ production. Adjuvant formulations containing GLA have caused dendritic cell-mediated upregulation of IL-12p40, leading to enhanced IFN-γ production from adaptive Th1-type immune responses and to downregulation of IL-5 (Th2-type immune response) for many of the vaccines tested previously (5, 11). This behavior is not specific to formulations of GLA but also has been reported for other TLR agonists formulated with an oil-in-water emulsion, including TLR4 and TLR9 ligands, which not only boost IFN-γ levels but also actively suppress IL-5 (6, 27). Besides dendritic cells, other cell types, such as macrophages, monocytes, granulocytes, and myocytes, are also activated by vaccine adjuvants such as MF59 and aluminum salts and may play significant roles in biological activity (24, 28), although this remains to be explored further for GLA-containing adjuvants.
The antigen-specific multiplex bead assay results serve to confirm and strengthen the antibody isotype and cytokine ELISPOT results described above regarding immune response quality. As expected, the level of the classic Th1-associated cytokine IFN-γ was highest in the GLA-SE groups. IL-10, which downregulates Th1-type immune activation, was more apparent in the SE groups, although only 2% SE showed statistical significance (compared to the Montanide control). Likewise, 2% SE produced high levels of the Th2 cytokine IL-13, and there was a trend for more IL-5 (another Th2 cytokine) in the SE groups than in the GLA-containing groups, but no statistical significance was achieved in this case. No differences between vaccine groups and the background responses (saline controls) were apparent in the IL-2 and TNF-α data. Interestingly, increased IL-17 was apparent in the 1% GLA-SE group; intranasal administration of GLA formulations in an HIV vaccine context also induced a Th17-type immune response characterized by high IL-17 production (2). In another disease model, IL-17 was shown to lead to protective Th1-type immune responses (22). While IFN-γ, IL-17, and TNF-α can also be produced by innate cellular responses (i.e., from NK cells, γδ cells, and dendritic cells, respectively), we emphasize that these cytokine responses are vaccine antigen specific and are probably desirable for generating a prophylactic immune response (e.g., IFN-γ in the present vaccine model is correlated with protection), whereas nonspecific, systemic, and unchecked proinflammatory cytokine production would be cause for concern due to autoimmunity implications.
In emulsion groups not containing GLA, the effects of reduced adjuvant concentration were apparent. Thus, levels of IL-5-producing cells were lower in the 1% SE group than in the 2% SE group, and only the 2% SE group showed larger amounts of IL-13 than those seen with antigen alone (Fig. 6). The effect of emulsion dose titration was demonstrated in the antibody endpoint titers, especially for the group with the lowest emulsion concentration (0.02% SE), which elicited lower IgG1 and IgG2a levels as well as lower levels of antibody-secreting plasma cells than those of the 2% SE group. However, SE concentrations of 0.5% and 1% appeared to elicit similar levels of IgG and IgG1 antibody titers as well as similar levels of antibody-secreting plasma cells compared to the full 2% SE dose. In assessing the overall immune response to the PfCelTOS vaccines using lower concentrations of SE, the responses appeared to be somewhat reduced, especially at the highest dilution (0.02% SE).
Reduction of the emulsion concentration in vaccines can have multiple applications, such as dose sparing, cost saving, reduced local reactogenicity, alternative routes of delivery, and facilitating immunization of young children. We have shown that adjuvants containing smaller amounts of emulsion in the presence of a TLR4 agonist may produce immune responses equivalent to those obtained with the full 2% oil emulsion dose. Recent clinical studies have evaluated adjuvant dose effects by the leading oil-in-water emulsion products, MF59 and AS03, both approved for use in Europe (neither formulation contains a TLR agonist). For example, a pandemic influenza vaccine clinical trial found that the typical (∼2.3%) oil dose of the MF59 adjuvant could be reduced by half (but not 4-fold) without compromising immunogenicity, while still meeting the European criteria for pandemic vaccine licensure (20). Furthermore, a dose-dependent decrease in injection site pain was noted in trial participants. A seasonal influenza vaccine trial in an elderly population established optimal immunogenicity but also an increased frequency of mild to moderate local reactions with the full dose of MF59 (12). In contrast, a study employing seasonal influenza virus antigen and MF59 at doses of 0.125, 0.25, 0.5, and the full dose in 6- to 36-month-old children found no difference in reactogenicity between the different adjuvant doses (9). Antibody responses followed a dose-response relationship according to adjuvant concentration, although all formulations met the European Committee for Medicinal Products for Human Use (CHMP) criteria after the 2nd immunization.
Two different doses of AS03 (AS03A, containing the full dose of 2.5% squalene and 2.5% α-tocopherol, versus AS03B, containing a half dose) were evaluated in another influenza vaccine clinical trial, where it was concluded that while initial immune responses elicited by the two different adjuvant doses were equivalent, the durability of the response measured at 182 days postvaccination was somewhat better with the higher adjuvant dose (21). Furthermore, the 41- to 64-year-old age group showed significantly reduced antibody responses at the lower adjuvant dose. In addition, modest pain reduction was noted at the lower adjuvant dose compared to the full dose. Finally, clinical trial results showed reduced reactogenicity in children receiving a malaria vaccine containing AS01 (a liposome adjuvant system containing MPL and QS21) compared to the same vaccine containing AS02 (an emulsion adjuvant system containing MPL and QS21) (26). Taken together, the MF59 and AS series clinical trials demonstrate that reduction or removal of oil content has the potential to reduce local reactogenicity, although this effect may depend on each vaccine's composition and its intended recipients.
Conclusions.
PfCelTOS vaccines containing GLA-SE adjuvant elicited strong Th1-type immune responses (including more IFN-γ production than that with a PfCelTOS vaccine containing Montanide ISA 720) and long-lived antibody-secreting cells in BALB/c mice. The emulsion dose could be reduced from 2% to 0.5% without compromising immunogenicity in the GLA-SE adjuvant groups. In general, a reduction of the emulsion dose in SE groups (not containing the TLR4 agonist GLA) showed somewhat reduced immunogenicity, especially at the lowest adjuvant dose (0.02%). Further evaluation of PfCelTOS with GLA-SE is ongoing, including a phase I clinical trial initiated in 2012; furthermore, determining the impact of antibody isotype as well as adjuvant oil concentration on protective efficacy in the mouse model is planned for future evaluation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Lin, Sandra Sivananthan, Traci Mikasa, Tim Dutill, Tara Evers, Farah Mompoint, and Alison Bernard for providing excellent technical assistance, Martin Friede for helpful discussions, and Randy Howard for his valuable comments and careful review of the manuscript. We gratefully acknowledge Elke Bergmann-Leitner and Chris Ockenhouse at WRAIR for providing PfCelTOS and for helpful discussions.
This research was supported in part by grant 42387 from the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print 15 August 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1. Anderson RC, et al. 2010. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf. B Biointerfaces 75:123–132 [DOI] [PubMed] [Google Scholar]
- 2. Arias MA, et al. 2012. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS One 7:e41144 doi:10.1371/journal.pone.0041144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldwin SL, et al. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baldwin SL, et al. 2012. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J. Immunol. 188:2189–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldwin SL, et al. 2009. Enhanced humoral and type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine 27:5956–5963 [DOI] [PubMed] [Google Scholar]
- 6. Baudner BC, et al. 2009. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm. Res. 26:1477–1485 [DOI] [PubMed] [Google Scholar]
- 7. Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse C, Angov E. 2011. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine 29:5940–5949 [DOI] [PubMed] [Google Scholar]
- 8. Bergmann-Leitner ES, et al. 2010. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS One 5:e12294 doi:10.1371/journal.pone.0012294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cioppa GD, Vesikari T, Sokal E, Lindert K, Nicolay U. 2011. Trivalent and quadrivalent MF59-adjuvanted influenza vaccine in young children: a dose- and schedule-finding study. Vaccine 29:8696–8704 [DOI] [PubMed] [Google Scholar]
- 10. Coler RN, et al. 2010. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One 5:e13677 doi:10.1371/journal.pone.0013677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coler RN, et al. 2011. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One 6:e16333 doi:10.1371/journal.pone.0016333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Della Cioppa G, et al. 2012. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59 adjuvant: results from a dose-finding clinical trial in older adults. Hum. Vaccine Immunother. 8:216–227 [DOI] [PubMed] [Google Scholar]
- 13. Fox CB. 2009. Squalene emulsions for parenteral vaccine and drug delivery. Molecules 14:3286–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. 2011. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine 29:9563–9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. 2012. Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. AAPS PharmSciTech 13:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frey A, Di Canzio J, Zurakowski D. 1998. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221:35–41 [DOI] [PubMed] [Google Scholar]
- 17. Gao N, Dang T, Yuan D. 2001. IFN-γ-dependent and -independent initiation of switch recombination by NK cells. J. Immunol. 167:2011–2018 [DOI] [PubMed] [Google Scholar]
- 18. Hibbert DB, Gooding JJ. 2006. Data analysis for chemistry. Oxford University Press, New York, NY [Google Scholar]
- 19. Ioannou XP, et al. 2002. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine 21:127–137 [DOI] [PubMed] [Google Scholar]
- 20. Keitel W, et al. 2010. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 28:840–848 [DOI] [PubMed] [Google Scholar]
- 21. Langley JM, et al. 2010. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J. Infect. Dis. 201:1644–1653 [DOI] [PubMed] [Google Scholar]
- 22. Lin Y, et al. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markine-Goriaynoff D, et al. 2000. IFN-gamma-independent IgG2a production in mice infected with viruses and parasites. Int. Immunol. 12:223–230 [DOI] [PubMed] [Google Scholar]
- 24. Mosca F, et al. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. U. S. A. 105:10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ott G, et al. 1995. MF59: design and evaluation of a safe and potent adjuvant for human vaccines, p 277–296 In Powell MF, Newman MJ. (ed), Vaccine design: the subunit and adjuvant approach. Plenum Press, New York, NY: [DOI] [PubMed] [Google Scholar]
- 26. Owusu-Agyei S, et al. 2009. Randomized controlled trial of RTS,S/AS02D and RTS,S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS One 4:e7302 doi:10.1371/journal.pone.0007302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Przetak M, et al. 2003. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine 21:961–970 [DOI] [PubMed] [Google Scholar]
- 28. Seubert A, Monaci E, Pizza M, O'Hagan DT, Wack A. 2008. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J. Immunol. 180:5402–5412 [DOI] [PubMed] [Google Scholar]
- 29. Yip HC, et al. 1999. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J. Immunol. 162:3942–3949 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.