Abstract
We report the case of a patient with systemic juvenile idiopathic arthritis (s-JIA) receiving tocilizumab (TCZ) who experienced relapses of s-JIA after receiving influenza vaccination. Systemic symptoms of s-JIA might be masked during TCZ therapy. Careful observation with the monitoring of serum interleukin (IL)-18 and IL-6 levels may be useful.
CASE REPORT
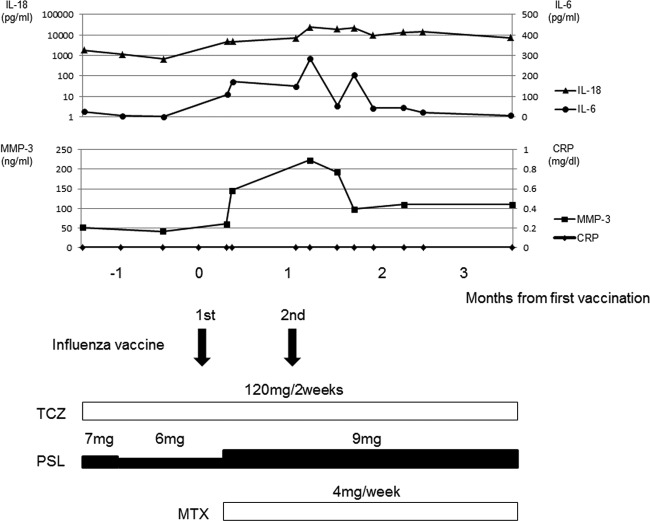
A 3-year-old girl was initially diagnosed with systemic juvenile idiopathic arthritis (s-JIA) on the basis of the presence of spiky fever, a salmon-colored erythematous rash, polyarthritis, and leukocytosis (white blood cell count [WBC], 14,700/μl) as well as elevated serum levels of C-reactive protein (CRP; 5.38 mg/dl), matrix metalloprotease-3 (MMP-3; 626.4 ng/ml), and interleukin (IL)-18 (2,230 pg/ml). Her clinical symptoms improved rapidly after the administration of prednisolone (PSL; 1 mg/kg of body weight/day). After tapering the PSL dosage (0.6 mg/kg/day), the first relapse of s-JIA occurred, manifesting as high fever and polyarthralgia. The patient again responded to steroid therapy, but frequent relapses occurred when the PSL dosage was decreased. Treatment with tocilizumab (TCZ), a humanized anti-IL-6 receptor monoclonal antibody, was initiated, and long-term remission (7 months) was achieved along with a decrease in PSL dosage (0.4 mg/kg/day). Laboratory findings showed normal serum levels of MMP-3 (41.8 ng/ml), IL-18 (675 pg/ml), and IL-6 (<3 pg/ml). The girl subsequently received a 0.2-ml subcutaneous injection of commercially available inactivated influenza vaccine on her left upper arm; the vaccine was approved for use in the 2010 to 2011 season in Japan (Kaketsuken, Kumamoto, Japan) (Fig. 1 and Table 1). Seven days later, she abruptly developed pain and limitation of the motion of her left arm, without accompanying fever or rash. Laboratory findings revealed leukocytosis (WBC, 10,880/μl) and elevated serum levels of MMP-3 (146.1 ng/ml), IL-6 (110 pg/ml), and IL-18 (4,800 IU/ml). However, her serum CRP level was normal (0.0 mg/dl). Computed tomography imaging of her left shoulder showed synovitis with effusion. After administration of a methylprednisolone pulse, treatment with PSL (0.6 mg/kg/day) and methotrexate (4 mg/week) was initiated. The girl responded to the therapy, and her clinical symptoms improved rapidly. Four weeks after the first vaccination, she received another subcutaneous injection of inactivated influenza vaccine according to the schedule previously followed in Japan (patients aged 1 to <6 years of age were inoculated twice with a dose of 0.2 ml at intervals of 1 to 4 weeks). Seven days later, pain and swelling of her left ankle joint developed, but fever and rash were absent. Laboratory findings revealed leukocytosis (WBC, 12,810/μl) and elevated serum levels of MMP-3 (223.3 ng/ml), IL-6 (284 pg/ml), and IL-18 (24,000 IU/ml). However, the serum CRP level remained normal (0.0 mg/dl). Magnetic resonance imaging of her left ankle showed synovitis with effusion. After administration of a methylprednisolone pulse, her clinical symptoms improved rapidly. She has now been in long-term remission for 17 months under combination therapy with TCZ and PSL (0.25 mg/kg/day).
Fig 1.
Patient's clinical course. IL-18, interleukin-18; IL-6, interleukin-6; MMP-3, matrix metalloprotease-3; CRP, C-reactive protein; TCZ, tocilizumab; PSL, prednisolone; MTX, methotrexate.
Table 1.
Alterations of clinical symptoms and laboratory findings during the patient's clinical coursea
| Parameter | Value or result |
||||
|---|---|---|---|---|---|
| 2 wk before first vaccine | At first vaccine | 9 days after first vaccine | At second vaccine | 7 days after second vaccine | |
| Clinical symptoms | |||||
| Fever | − | − | − | − | − |
| Rash | − | − | − | − | − |
| Arthralgia | − | − | + | − | + |
| Laboratory data | |||||
| WBC (per μl) | 8,630 | 8,590 | 10,880 | 15,160 | 12,810 |
| CRP (mg/dl) | 0 | 0 | 0 | 0 | 0 |
| AST (IU/liter) | 28 | 25 | 27 | 34 | 40 |
| MMP-3 (ng/ml) | 41.8 | ND | 146.1 | ND | 223.3 |
| IL-6 (pg/ml) | <3 | ND | 110 | 150 | 284 |
| IL-18 (pg/ml) | 675 | ND | 4,800 | 7,100 | 24,000 |
| Treatments | |||||
| Tocilizumab | 120 mg (8 mg/kg)/2 wk | ||||
| Prednisolone | 6 mg (0.4 mg/kg/day) | 9 mg (0.6 mg/kg/day) | |||
| Methotrexate | 4 mg/wk | ||||
AST, aspartate aminotransferase; −, absent; +, present; ND, not done.
Infection is an important exacerbating factor in s-JIA. In particular, influenza is a major concern because the influenza virus is highly infectious and can cause severe complications. Influenza vaccination is encouraged in s-JIA patients to prevent primary influenza infection as well as secondary bacterial infections.
With regard to influenza vaccination in patients with juvenile autoimmune diseases (including JIA), a previous report showed that after vaccination, children with JIA are able to produce an antibody response similar to that seen in healthy children, even when they are taking steroids or disease-modifying drugs (4). Furthermore, reports have shown that there is no increase in disease activity after vaccination, and researchers concluded that patients with these diseases can therefore undergo influenza vaccination (1–3, 5).
TCZ is an effective cytokine inhibitor used for the treatment of s-JIA. Shinoki et al. investigated the safety and efficacy of the influenza vaccine in 27 patients with s-JIA who were treated with TCZ and PSL (6). They found that the efficacies of vaccination did not differ significantly between the s-JIA patients and healthy controls. None of the s-JIA patients experienced severe adverse reactions or disease relapse after vaccination. Therefore, the researchers concluded that s-JIA patients treated with TCZ can be safely and effectively immunized with the influenza vaccine.
However, in our case, s-JIA relapses occurred after both injections of influenza vaccine. In some cases, s-JIA may not be completely controlled and could be exacerbated by a trigger such as influenza vaccination. Therefore, careful observation of patients with s-JIA is necessary after each dose of the influenza vaccine.
At the time of the relapses, our patient had no systemic symptoms, including fever, but she had definite features of arthritis. Furthermore, her serum CRP levels never increased. These findings are quite different from those seen during relapses in patients not receiving TCZ. Systemic symptoms of s-JIA can thus be masked by TCZ therapy. Furthermore, serum CRP levels never increase in patients receiving TCZ because of the complete blockage of IL-6 by the drug. Therefore, monitoring of serum levels of IL-18 and IL-6 may be useful for evaluating s-JIA activity during TCZ therapy. Further studies are needed to confirm that these markers are suitable for the monitoring of disease activity in s-JIA during TCZ therapy.
ACKNOWLEDGMENT
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 8 August 2012
REFERENCES
- 1. Aikawa NE, et al. 2012. Glucocorticoid: major factor for reduced immunogenicity of 2009 influenza A (H1N1) vaccine in patients with juvenile autoimmune rheumatic disease. J. Rheumatol. 39:167–173 [DOI] [PubMed] [Google Scholar]
- 2. Dell'Era L, et al. 2012. Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. Vaccine 30:936–940 [DOI] [PubMed] [Google Scholar]
- 3. Malleson PN, Tekano JL, Scheifele DW, Weber JM. 1993. Influenza immunization in children with chronic arthritis: a prospective study. J. Rheumatol. 20:1769–1773 [PubMed] [Google Scholar]
- 4. McCann LJ. 2007. Should children under treatment for juvenile idiopathic arthritis receive flu vaccination? Arch. Dis. Child. 92:366–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogimi C, Tanaka R, Saitoh A, Oh-Ishi T. 2011. Immunogenicity of influenza vaccine in children with pediatric rheumatic diseases receiving immunosuppressive agents. Pediatr. Infect. Dis. J. 30:208–211 [DOI] [PubMed] [Google Scholar]
- 6. Shinoki T, et al. 11 February 2012, posting date. Safety and response to influenza vaccine in patients with systemic-onset juvenile idiopathic arthritis receiving tocilizumab. Mod. Rheumatol. doi:10.1007/s10165-012-0595-z [DOI] [PubMed] [Google Scholar]