Abstract
Little is known about the effect of timing of antibiotic treatment on development of IgG antibodies following acute Q fever. We studied IgG antibody responses in symptomatic patients diagnosed either before or during development of the serologic response to Coxiella burnetii. Between 15 and 31 May 2009, 186 patients presented with acute Q fever, of which 181 were included in this retrospective study: 91 early-diagnosed (ED) acute Q fever patients, defined as negative IgM phase II enzyme-linked immunosorbent assay (ELISA) and positive PCR, and 90 late-diagnosed (LD) acute Q fever patients, defined as positive/dubious IgM phase II ELISA and positive immunofluorescence assay (IFA). Follow-up serology at 3, 6, and 12 months was performed using IFA (IgG phase I and II). High IgG antibody titers were defined as IgG phase II titers of ≥1:1,024 together with IgG phase I titers of ≥1:256. At 12 months, 28.6% of ED patients and 19.5% of LD patients had high IgG antibody titers (P = 0.17). No statistically significant differences were found in frequencies of IgG phase I and IgG phase II antibody titers at all follow-up appointments for adequately and inadequately treated patients overall, as well as for ED and LD patients analyzed separately. Additionally, no significant difference was found in frequencies of high antibody titers and between early (treatment started within 7 days after seeking medical attention) and late timing of treatment. This study indicates that early diagnosis and antibiotic treatment of acute Q fever do not prohibit development of the IgG antibody response.
INTRODUCTION
Q fever is a zoonosis, caused by the intracellular bacterium Coxiella burnetii (18). The presentation of the disease is extremely variable; most individuals (60%) remain asymptomatic after infection (18, 19). In symptomatic acute Q fever patients, the most common presentations range from a (self-limiting) flu-like illness to pneumonia or hepatitis. Chronic Q fever, which presents mainly as endocarditis or vascular infection, develops in approximately 2% of infected patients (5, 10). Until 2007, roughly 5 to 20 cases of acute Q fever were notified in the Netherlands each year, and the seroprevalence was low (2.4% in 2006 and 2007) (20). However, between 2007 and 2010, large Q fever outbreaks occurred in an area in the south of the Netherlands where Q fever was previously not endemic, with over 4,000 notified symptomatic cases (25).
Laboratory diagnosis of Q fever is based mainly on serologic testing for antibodies against phase I and phase II antigens (18). C. burnetii has two antigenic states: during acute Q fever infection, antibodies against phase II antigens predominate, whereas high phase I antibody titers are more prevalent in cases of chronic Q fever (4, 6, 19). The most commonly used serologic test is the immunofluorescence assay (IFA). Seroconversion usually takes place 10 to 15 days after the onset of acute disease (18), with the appearance of IgM antibodies against phase II antigens (IgM phase II), followed by IgG antibodies against phase II antigens (IgG phase II), IgM antibodies against phase I antigens (IgM phase I), and finally IgG antibodies against phase I antigens (IgG phase I) (4). Since 2009, PCR for detection of C. burnetii DNA has become an important tool in the diagnosis of acute Q fever in our laboratory (21). PCR enables diagnosis of acute Q fever early after onset of disease (the first 3 weeks after onset of symptoms), often before seroconversion has taken place (21). As the serological response develops, PCR becomes negative in patients who do not develop chronic Q fever (21). Current international recommendations advise routine follow-ups to detect patients who develop a chronic Q fever infection, consisting of at least three consecutive serologic tests in the first year after diagnosis of acute Q fever (13, 26). Most infected individuals are asymptomatic, but in the case of symptomatic individuals, symptoms of acute Q fever can last from 10 to 90 days and usually resolve spontaneously. Antibiotic treatment with doxycycline or fluoroquinolones is warranted only in symptomatic patients to shorten duration of fever and to hasten recovery of pneumonia if present (24).
For infections caused by Borrelia spp., e.g., borreliosis, which is also treated with doxycycline (3, 11), it has been reported that early antibiotic treatment may prohibit development of the IgG antibody response in patients with erythema migrans (1, 7, 16, 17) or neuroborreliosis (7). As Q fever and borreliosis can both develop into a chronic disease which can be difficult to diagnose, there may be more similarities between the two than previously thought. Little is known about the development of IgG antibodies following antibiotic treatment of acute Q fever. The purpose of this study is to investigate the IgG antibody response in symptomatic patients diagnosed and treated either before or during development of the serologic response to C. burnetii.
MATERIALS AND METHODS
Patients.
Those eligible for this retrospective study were all symptomatic patients diagnosed with acute Q fever between 15 and 31 May 2009 at the Department of Medical Microbiology and Infection Control of the Jeroen Bosch Hospital, 's-Hertogenbosch, the Netherlands. Patients excluded were those who were younger than 18 years of age, who were pregnant, who had submitted an earlier sample for Q fever testing, or who had positive serology at diagnosis that was later found to be negative when paired with a subsequent negative tested sample.
Diagnostic workups in these acute Q fever patients were performed according to a diagnostic algorithm for acute Q fever introduced on 1 May 2009 (10). In short, serum samples were screened with an enzyme-linked immunosorbent assay (ELISA) for IgM phase II (Institut Virion/Serion GmbH, Würzburg, Germany). Depending on the outcome of ELISA IgM phase II, date of onset of disease, and inpatient or outpatient setting, either an in-house PCR for C. burnetii DNA (21) or IFA for IgM and IgG antibodies against phase I and phase II antigens (Focus Diagnostics, Inc., Cypress, CA) was performed (for more details, see Jager et al. [10]). IgG antibody responses during follow-ups were evaluated by comparing two groups of patients, the early-diagnosed (ED) group (negative ELISA IgM phase II and positive PCR at the time medical attention was sought) and the late-diagnosed (LD) group (positive or dubious ELISA IgM phase II confirmed by positive IFA [IgG phase II and/or IgM phase II titers of ≥1:32] at the time medical attention was sought).
Serological follow-up.
In line with the internationally recommended routine follow-up of acute Q fever, patients in both groups were asked to provide a serum sample at 3, 6, and 12 months after diagnosis (13, 26). Follow-up serology was performed using IFA for IgG phase II and phase I antibodies. Samples were titrated up to 1:4,096 and, if still positive, were categorized as >1:4,096. Samples of patients with an IgG phase I titer of ≥1:1,024 at month 12 were analyzed using PCR, to check whether C. burnetii DNA was still present. High IgG antibody titers in follow-up samples were arbitrarily defined as IgG phase II titers of ≥1:1,024 in combination with IgG phase I titers of ≥1:256. This IgG phase I titer is two dilutions lower than the cutoff titer for chronic Q fever of ≥1:1,024 set by the Dutch Q Fever Consensus Group (27).
Data collection.
Outcome of serologic tests, the date of the onset of disease, and the date medical attention was sought were collected from the laboratory information system (LIS). If unavailable in the LIS, the date of the onset of disease was obtained by contacting the general practitioners (GP). The dates of the onset of disease extracted from the LIS were considered more pertinent than those obtained retrospectively from the GP, as the former were written on the laboratory form at the moment the patient visited the GP. In addition, data on treatment (type, dosage, start date, and duration of antibiotic treatment prescribed) were obtained from the GP. For patients diagnosed during hospitalization, data were obtained from medical records. Included patients were assigned with a unique code, and identifying information was deleted from the database. Adequate treatment was defined as treatment with a C. burnetii-covering antibiotic at the appropriate dosage for at least 10 days (e.g., doxycycline, 200 mg/day; moxifloxacin, 400 mg/day; ciprofloxacin, 1,000 mg/day; or cotrimoxazole, 1,920 mg/day). Other dosage schemes or antibiotics were considered inadequate for treating symptomatic acute Q fever patients. Early antibiotic treatment was defined as starting treatment before diagnosis or within 7 days after seeking medical attention.
Data analysis.
Descriptive characteristics of the ED and LD groups were investigated by calculating the mean and standard deviation, median and interquartile range (IQR), and relative frequencies. Depending on the variable, Student's t test (age), Mann-Whitney U tests (nonparametric data; e.g., total number of days between onset of disease and seeking medical attention for diagnosis, seeking medical attention for diagnosis and sample at 12-month follow-up, seeking medical attention for diagnosis and start of adequate antibiotic treatment; comparing the frequencies of IgG antibody titers), and Chi-square tests (gender, frequencies of high antibody titers, and adequate, inadequate, and early treatment) were performed to assess significant differences. Patients without at least one follow-up sample were included in the descriptive characteristics but were excluded from further analysis. Data were analyzed using Microsoft Excel (Microsoft Corp.) and IBM SPSS Statistics version 19.0.0 (SPSS Inc.). Additionally, a separate analysis was performed excluding LD patients without IgG phase I antibodies at 3, 6, and 12 months. This was done because it cannot be ignored that patients in the LD group, for whom no PCR was performed at the time medical attention was sought and who did not develop IgG phase I antibodies during follow-up, are in fact patients with a resolved Q fever infection and persisting IgG phase II antibodies.
RESULTS
Between 15 and 31 May 2009, 92 ED patients and 94 LD patients were diagnosed. One ED patient (<18 years of age) and four LD patients (<18 years of age, n = 1; pregnant, n = 1; submitted an earlier sample for Q fever testing, n = 1; positive serology at diagnosis that was tested negative in a paired retest with a consecutive negative tested sample, n = 1) did not meet the inclusion criteria. Therefore, 91 acute Q fever patients were included in the ED group and 90 patients in the LD group. Characteristics of both groups are presented in Table 1. The date of the onset of disease was available for 92.3% (84/91) of patients in the ED group and for 85.6% (77/90) in the LD group. Both groups showed comparable results, except for time between onset of disease to seeking medical attention (median number of days [interquartile range]: ED, 4.5 [3 to 5]; LD, 12 [7.5 to 23.5]; P = 0.00), which is in line with the definition of both groups. In 79.0% of the patients, the GP applied for the laboratory test, while in 21.0%, a hospital physician requested the test.
Table 1.
Characteristics of acute Q fever patients in the early- and late-diagnosed groups
| Characteristic | Value |
|
|---|---|---|
| Early diagnoseda (n = 91) | Late diagnosedb (n = 90) | |
| Age (mean ± SD) | 48 ± 15 | 51 ± 16 |
| No. (%) of males | 57 (62.6) | 56 (62.2) |
| Median no. of days (IQR) from onset of disease to seeking medical attention for diagnosisc | 4.5 (3 to 5)d | 12 (7.5 to 23.5)d |
| Median no. of days (IQR) between seeking medical attention for diagnosis and sample at 12-month follow-up | 364 (360 to 371) | 364 (359 to 369) |
Early-diagnosed group: negative ELISA IgM phase II and positive PCR.
Late-diagnosed group: positive or dubious ELISA IgM phase II confirmed by positive IFA (IgG phase II and/or IgM phase II titers of ≥1:32).
Based on 84 patients in the early-diagnosed group and 77 in the late-diagnosed group.
Statistical significant difference (P = 0.00): in line with the definition of the groups.
In the diagnostic sera of the LD group, IgM phase II ELISA was positive in 95.6% (86/90); the remaining four samples showed a dubious result. However, these four samples showed positive results for IFA IgM phase II and IgG phase II. IFA IgM phase I and phase II was performed for 70 LD samples: all samples showed positive results for IgM phase II, and 65.7% (46/70), 1.4% (1/70), and 32.9% (23/70) were IgM phase I positive, dubious, and negative, respectively. IgG phase II was positive for 98.9% (89/90) of samples, and one showed a dubious result, while IgG phase I was positive in 35.6% (32/90), dubious in 1.1% (1/90), and negative in 63.3% (57/90) of the LD patients. Six patients (three ED patients and three in the LD group) did not provide any follow-up samples and thus were excluded from further analysis.
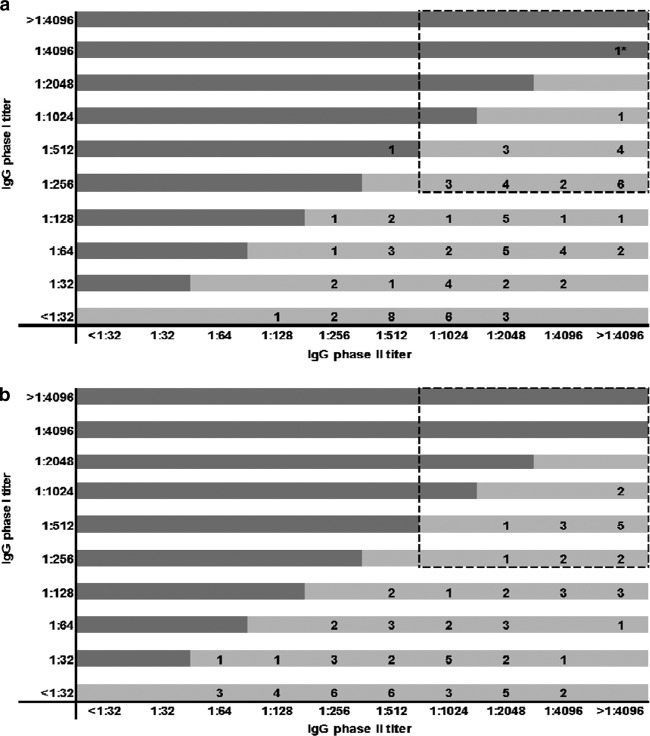
In the ED group, 26.4% (23/87) had high IgG antibody titers (IgG phase II titers of ≥1:1,024 in combination with IgG phase I titers of ≥1:256) at month 3, 24.4% (21/86) at month 6, and 28.6% (24/84) at month 12. In the LD group, 16.7% (14/84) had high IgG antibody titers at month 3, 18.5% (15/81) at month 6, and 19.5% (16/82) at month 12. Differences between both groups were not statistically significant. There were 7 patients (5 ED and 2 LD patients) that had high titers at all three follow-up appointments. In total, 71 (40 ED and 31 LD) of the 181 patients (39.2%) had a high titer in at least one of their follow-up appointments. Figure 1 presents the distribution of IgG phase I and phase II titers in both groups at 12-month follow-up. PCR was negative for all 12-month samples with an IgG phase I titer of ≥1:1,024, except for the one patient in this study that developed chronic Q fever (this will be discussed later).
Fig 1.
Distribution of IgG phase I and phase II antibody titers as determined by immunofluorescence assay at 12-month follow-up in acute Q fever patients. (a) Early-diagnosed group (negative ELISA IgM phase II and positive PCR), n = 84 samples; (b) late-diagnosed group (positive or dubious ELISA IgM phase II confirmed by positive IFA (IgG phase II and/or IgM phase II titers of ≥1:32), n = 82 samples. The dashed box indicates high antibody titers. *, Patient with proven chronic Q fever infection.
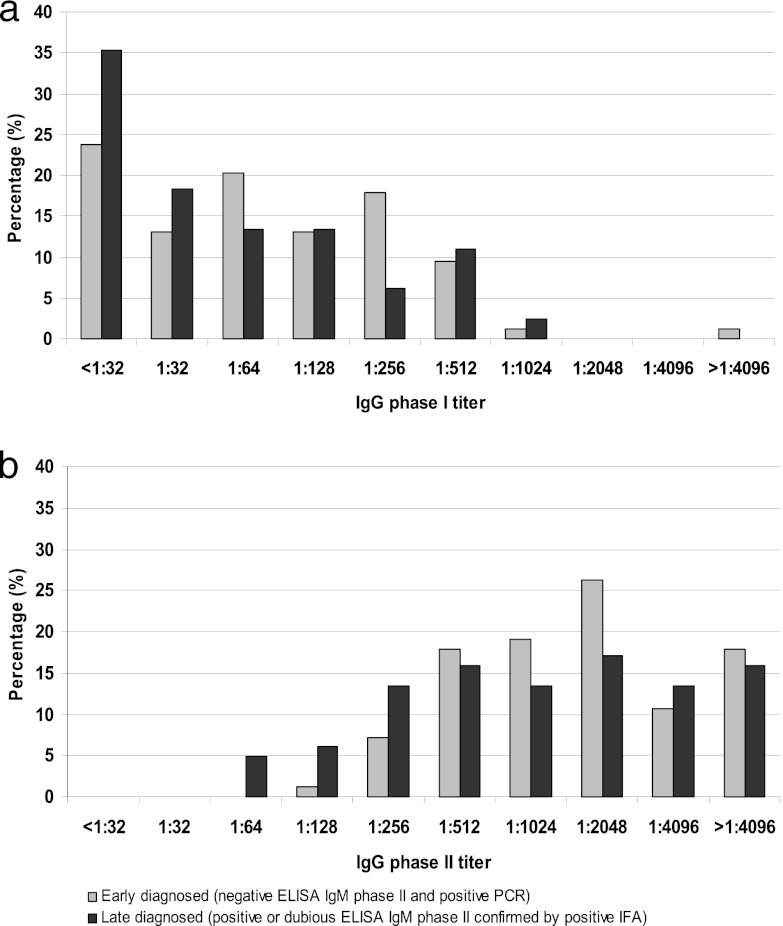
Figure 2 shows the percentage of patients with IgG phase I and phase II antibody titers at 12 months after initial diagnosis in the ED and LD groups. Although higher frequencies of the lower antibody titers were observed in the LD group, the differences between both groups were not statistically significant (IgG phase I: P = 0.07; IgG phase II: P = 0.11). There was a statistically significant difference between the IgG phase II antibody titers of the groups, with the LD group having lower titers than the ED group at 3 months after initial infection (P = 0.02). IgG phase I titers, however, showed a borderline significant difference (P = 0.05). At 6 months, a borderline significant difference was observed for IgG phase II titers (P = 0.05), while IgG phase I titers showed no statistically significant difference (P = 0.08) (data not shown).
Fig 2.
Percentage of acute Q fever patients with IgG phase I (a) and IgG phase II (b) antibody titers as determined by immunofluorescence assay at 12-month follow-up in the early-diagnosed and late-diagnosed group.
Data on antibiotic treatment for patients with at least one follow-up sample (n = 175) could be obtained for 165 patients: 85 ED and 80 LD patients (two GPs did not participate in the study, n = 3 LD patients; treatment unknown, n = 4 patients [1 ED, 3 LD]; dosage or duration of antibiotic unknown, n = 3 patients [2 ED, 1 LD]). Adequate treatment was prescribed in 83.0% (137/165) of the patients, 14.5% (24/165) received inadequate treatment, and four patients (2.4%) did not receive any treatment (Table 2). The median number of days between seeking medical attention for microbiological diagnosis and the start of any treatment as well as adequate treatment was zero for both groups, though the range between both groups differed (ED = −4 to 13, LD = −19 to 109 days; the minus sign means treatment started before the diagnostic sample was submitted). A statistically significant difference was found for early (treatment started within 7 days after seeking medical attention) or late timing of treatment between the ED and LD groups; ED patients received treatment earlier, regardless of being treated adequately or not (P = 0.04) (Table 2). Among adequately treated patients only, we also found a statistically significant difference for early treatment timing (91.5% [ED] and 75.8% [LD] received treatment within 7 days after seeking medical attention; P = 0.01). No statistically significant differences were observed in frequencies of IgG phase I and IgG phase II antibody titers at all follow-up appointments for adequately and inadequately (including no treatment) treated patients overall, as well as for ED and LD patients analyzed separately. One borderline significant result (P = 0.06) was observed for IgG phase I at the 12-month follow-up for patients treated adequately, where LD patients showed lower antibody titers than ED patients (median IgG phase I titer: ED, 1:64; LD, 1:32). Additionally, no significant difference was found in frequencies of high antibody titers and in early or late timing of treatment (data not shown).
Table 2.
Treatment of acute Q fever patients with at least one follow-up sample in the early- and late-diagnosed groups
| Characteristic | Value |
|
|---|---|---|
| Early diagnoseda (n = 85) | Late diagnosedb (n = 80) | |
| No. (%) with adequate treatmentc | 71 (83.5) | 66 (82.5) |
| No. (%) with inadequate treatment | 12 (14.1) | 12 (15.0) |
| No. (%) with no treatment | 2 (2.4) | 2 (2.5) |
| No. (%) with early treatmentd | 75 (88.2)f | 61 (76.3)f |
| Median no. of days (IQR) from seeking medical attention for diagnosis to adequate treatmente | 0 (0 to 1) | 0 (−3 to 7) |
Early-diagnosed group: negative ELISA IgM phase II and positive PCR.
Late-diagnosed group: positive or dubious ELISA IgM phase II confirmed by positive IFA (IgG phase II and/or IgM phase II).
Defined by treatment with a Coxiella burnetii-covering antibiotic at the appropriate dosage for at least 10 days (e.g., doxycycline, 200 mg/day; moxifloxacin, 400 mg/day; ciprofloxacin, 1,000 mg/day; cotrimoxazole, 1,920 mg/day).
Defined as starting treatment before diagnosis or within 7 days after seeking medical attention, regardless of treatment being adequate or not.
Based on n = 70 patients in the early-diagnosed group and n = 65 in the late-diagnosed group; the start date of treatment was unknown for 1 early- and 1 late-diagnosed patient.
Statistical significant difference: P = 0.04.
Among those who provided a sample at all follow-up appointments, absence of IgG phase I antibody titers in all three follow-up samples was observed in 2.4% (2/82) of the ED patients and 13.0% (10/77) of the LD patients (P = 0.01). Treatment data were available for nine of these 12 patients; all nine received adequate treatment. When the 10 LD patients without detectable IgG phase I antibodies at any time point were excluded from analysis, 22.2% (16/72) of the remaining cases in the LD group had high IgG antibody titers at month 12 (P = 0.37). IgG phase II antibodies were present in all ED and LD patients.
One patient with an aorta-femoral bypass (in the ED group and treated adequately) showed a chronic Q fever serologic profile at month 6 (IgG phase II titer of >1:4,096 and IgG phase I titer of >1:4,096). A positive serum PCR (threshold cycle [CT] value of 33.6) confirmed a chronic Q fever infection, and titers remained high at month 12 (both IgG phase II and phase I titers of >1:4,096) (Fig. 1a).
DISCUSSION
In this study, the development of IgG antibodies in early- and late-diagnosed and treated groups of patients with symptomatic acute Q fever infection were examined. The results show that IgG phase I and phase II responses are not inhibited by early diagnosis and subsequent treatment of the acute Q fever infection. No significant differences in high antibody titers were observed between the ED and LD groups at 3-, 6-, and 12-month follow-ups. Additionally, timing and adequateness of treatment did not result in differences in the IgG phase I and phase II antibody responses. We did find a statistically significant difference for earlier timing of treatment in ED patients compared to in LD patients.
We observed statistically significant lower IgG phase II antibody titers in the LD group than in the ED group at the 3-month follow-up and borderline or nonsignificant differences in IgG phase I and phase II titers at the other follow-ups. This observation contradicts the hypothesis that, as observed in borreliosis, early diagnosis and treatment would prohibit the IgG antibody response.
Moreover, we found a statistically significant difference between both groups in the percentage of patients without a detectable IgG phase I antibody response: 2.4% in the ED group and 13.0% in the LD group. A possible explanation for this increased proportion in the LD group is that these patients may in fact have a resolved Q fever infection with persisting IgG phase II antibodies. Transient reappearance or persistence of IgM antibodies in such patients might result from nonspecific polyclonal activation due to another (undiagnosed) infection, as has been observed in at least one case of legionellosis (unpublished). Omitting the LD patients without detectable IgG phase I antibody response from the analysis did not influence the outcome of our study, as the percentage of remaining patients with high IgG antibody titers at month 12 (22.2%) in the LD group remained lower than the percentage of ED patients (28.6%). Another possible explanation might be that in these patients, IgG phase I antibodies were produced only for a short time and therefore were not detected at the 3-, 6-, and 12-month follow-ups. There were indeed two patients in the ED group with PCR-proven acute Q fever in whom IgG phase I antibodies were not detected during follow-ups.
The hypothesis that early antibiotic treatment could hamper the development of the IgG antibody response was started by the observation of this phenomenon in several studies of patients with erythema migrans in the course of a Borrelia burgdorferi infection (1, 7, 16, 17). As Q fever and borreliosis can both develop into a chronic disease which can be difficult to diagnose and both are treated with doxycycline, there may be more similarities. Hammers-Berggren et al. found similar results for neuroborreliosis (7). It was observed that antibodies against B. burgdorferi develop gradually and slowly during the course of infection before treatment (7–9, 12, 23). Early on in infection, patients show high IgM and low IgG antibody levels, while those in the later stages have low IgM and high IgG antibody levels. Seeing that patients that had early treatment had low or no IgG antibody levels, it was suggested that antibiotic treatment prevents the development of further IgG antibody response (7, 16), but it is less likely that the IgM response is also inhibited (1). A similar observation that early antibiotic treatment affected the antibody response to cytoplasmic proteins was also observed in mice infected with Brucella melitensis (2).
As it was the third year of the 2007 to 2010 Dutch Q fever epidemic, we expected that physicians in the high-risk area were already aware of the symptoms of the acute infection. Lassche et al. performed a questionnaire study among GPs in the high-risk area with at least five acute Q fever patients in the first 6 months of 2009. In this study, 95% of the GPs (n = 20) indicated that they already started treatment when a Q fever infection was suspected or when a patient had signs of pneumonia, without awaiting the confirmation by microbiological tests (14). However, in our study, we found a lower rate of GPs prescribing antibiotic treatment before laboratory results were available (60.0% of patients received treatment on the day of sampling or before, regardless of treatment being adequate or inadequate). As we included samples submitted to the laboratory between 15 and 31 May 2009, without selecting GPs with a minimum number of diagnosed patients, the rate of starting treatment at the time of seeking medical attention of 60.0% found in this study is probably a more accurate estimate than the 95% from the questionnaire study.
Although our analysis did not show that early diagnosis and treatment of acute Q fever diminish the development of the IgG antibody response, it is of interest to evaluate if these findings can be extrapolated to clinical outcome. The development of chronic Q fever and Q fever fatigue syndrome after an acute Q fever infection are of particular interest. Up until 1 May 2012, no additional chronic Q fever patients were diagnosed among the 181 patients included, except the one adequately treated ED patient with a known vascular risk factor who was diagnosed with chronic disease at the 6-month follow-up appointment. Another interesting possibility for future research is to assess the effect of early antibiotic treatment on the time interval in which IFA IgG titers reach maximum levels (in general, 4 to 8 weeks after the onset of disease [15, 22]), but this would require more frequent acquisition of follow-up samples.
In conclusion, our observations indicate that early diagnosis and antibiotic treatment of acute Q fever do not prohibit development of the IgG antibody response after follow-up appointments at 3, 6, and 12 months after initial diagnosis.
ACKNOWLEDGMENTS
We thank all general practitioners who participated in this study.
The authors declare no conflicts of interest.
Footnotes
Published ahead of print 22 August 2012
REFERENCES
- 1. Aguero-Rosenfeld ME, et al. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowden RA, Racaro GC, Baldi PC. 1999. Effect of early antibiotic treatment on the antibody response to cytoplasmic proteins of Brucella melitensis in mice. Clin. Diagn. Lab. Immunol. 6:440–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dijkstra F, et al. 2011. Antibiotic therapy for acute Q fever in The Netherlands in 2007 and 2008 and its relation to hospitalization. Epidemiol. Infect. 139:1332–1341 [DOI] [PubMed] [Google Scholar]
- 4. Dupuis G, Péter O, Peacock M, Burgdorfer W, Haller E. 1985. Immunoglobulin responses in acute Q. fever. J. Clin. Microbiol. 22:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Centre for Disease Prevention and Control 2010. Risk assessment on Q fever. ECDC, Stockholm, Sweden [Google Scholar]
- 6. Fournier PE, Marrie TJ, Raoult D. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammers-Berggren S, et al. 1994. Serological follow-up after treatment of patients with erythema migrans and neuroborreliosis. J. Clin. Microbiol. 32:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen K, Hindersson P, Pedersen NS. 1988. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J. Clin. Microbiol. 26:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen K, Pii K, Lebech AM. 1991. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a μ-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J. Clin. Microbiol. 29:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jager MM, et al. 2011. Evaluation of a diagnostic algorithm for acute Q fever in an outbreak setting. Clin. Vaccine Immunol. 18:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karlsson M, Hammers-Berggren S, Lindquist L, Stiernstedt G, Svenungsson B. 1994. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology 44:1203–1207 [DOI] [PubMed] [Google Scholar]
- 12. Karlsson M, Stiernstedt G, Granström M, EÅsbrink Wretlind B. 1990. Comparison of flagellum and sonicate antigens for serological diagnosis of Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:169–177 [DOI] [PubMed] [Google Scholar]
- 13. Landais C, Fenollar F, Thuny F, Raoult D. 2007. From acute Q fever to endocarditis: serological follow-up strategy. Clin. Infect. Dis. 44:1337–1340 [DOI] [PubMed] [Google Scholar]
- 14. Lassche S, Schrauwen MMPW, Rietveld A, Wijkmans CJ. 2010. General practitioners in high-risk areas alert on Q-fever. Infect. Bull. 21:45–49 [Google Scholar]
- 15. Leung-Shea C, Danaher PJ. 2006. Q fever in members of the United States armed forces returning from Iraq. Clin. Infect. Dis. 43:e77–e82 doi:10.1086/507639 [DOI] [PubMed] [Google Scholar]
- 16. Lomholt H, Lebech AM, Hansen K, Brandrup F, Halkier-Sørensen L. 2000. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Derm. Venereol. 80:362–366 [DOI] [PubMed] [Google Scholar]
- 17. Luft BJ, et al. 1996. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann. Intern. Med. 124:785–791 [DOI] [PubMed] [Google Scholar]
- 18. Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 5:219–226 [DOI] [PubMed] [Google Scholar]
- 20. Schimmer B, et al. 2012. Low seroprevalence of Q fever in The Netherlands prior to a series of large outbreaks. Epidemiol. Infect. 140:27–35 [DOI] [PubMed] [Google Scholar]
- 21. Schneeberger PM, et al. 2010. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin. Vaccine Immunol. 17:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slabá K, Škultéty L, Toman R. 2005. Efficiency of various serological techniques for diagnosing Coxiella burnetii infection. Acta Virol. 49:123–127 [PubMed] [Google Scholar]
- 23. Stiernstedt G, Gustafsson R, Karlsson M, Svenungsson B, Sköldenberg B. 1988. Clinical manifestations and diagnosis of neuroborreliosis. Ann. N. Y. Acad. Sci. 539:46–55 [DOI] [PubMed] [Google Scholar]
- 24. Tissot-Dupont H, Raoult D. 2008. Q fever. Infect. Dis. Clin. North Am. 22:505–514, ix [DOI] [PubMed] [Google Scholar]
- 25. van der Hoek W, et al. 2012. Shifting priorities in the aftermath of a Q fever epidemic in 2007 to 2009 in the Netherlands: from acute to chronic infection. Euro Surveill. 17:20059. [PubMed] [Google Scholar]
- 26. Wagner-Wiening C, Brockmann S, Kimmig P. 2006. Serological diagnosis and follow-up of asymptomatic and acute Q fever infections. Int. J. Med. Microbiol. 296(Suppl 40):294–296 [DOI] [PubMed] [Google Scholar]
- 27. Wegdam-Blans MC, et al. 2012. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J. Infect. 64:247–259 [DOI] [PubMed] [Google Scholar]