Abstract
Durum wheat (Triticum turgidum L. var durum) cultivars exhibit lower Zn efficiency than comparable bread wheat (Triticum aestivum L.) cultivars. To understand the physiological mechanism(s) that confers Zn efficiency, this study used 65Zn to investigate ionic Zn2+ root uptake, binding, and translocation to shoots in seedlings of bread and durum wheat cultivars. Time-dependent Zn2+ accumulation during 90 min was greater in roots of the bread wheat cultivar. Zn2+ cell wall binding was not different in the two cultivars. In each cultivar, concentration-dependent Zn2+ influx was characterized by a smooth, saturating curve, suggesting a carrier-mediated uptake system. At very low solution Zn2+ activities, Zn2+ uptake rates were higher in the bread wheat cultivar. As a result, the Michaelis constant for Zn2+ uptake was lower in the bread wheat cultivar (2.3 μm) than in the durum wheat cultivar (3.9 μm). Low temperature decreased the rate of Zn2+ influx, suggesting that metabolism plays a role in Zn2+ uptake. Ca inhibited Zn2+ uptake equally in both cultivars. Translocation of Zn to shoots was greater in the bread wheat cultivar, reflecting the higher root uptake rates. The study suggests that lower root Zn2+ uptake rates may contribute to reduced Zn efficiency in durum wheat varieties under Zn-limiting conditions.
Soils that contain insufficient levels of the essential plant micronutrient Zn are common throughout the world. As a result, Zn deficiency is a widespread problem in crop plants, especially cereals (Graham et al., 1992). The importance of plant foods as sources of Zn, particularly in the marginal diets of developing countries, is well established (Welch, 1993). The development of crop plants that are efficient Zn accumulators is therefore a potentially important endeavor. In addition to its effects on nutrition, Zn deficiency in crops is relevant to other areas of human health. Another consequence of Zn-deficient soils is the tendency for plants grown in such soils to accumulate heavy metals. For example, in the Great Plains region of North America, where soil Zn levels are low and naturally occurring Cd is present, durum wheat (Triticum turgidum L. var durum) grains accumulate Cd to relatively high concentrations (Wolnik et al., 1983). The presence of Cd in food represents a potential human health hazard and, in response, international trade standards have been proposed to limit the levels of Cd in exported grain (Codex Alimentarius Commission, 1993). Thus, there is a need to understand the physiological processes that control acquisition of Zn from soil solution by roots and mobilization of Zn within plants.
It has been demonstrated in recent years that crop plants vary in their ability to take up Zn, particularly when its availability to roots is limited. Zn efficiency, defined as the ability of a plant to grow and yield well in Zn-deficient soils, varies among wheat cultivars (Graham and Rengel, 1993). In field trials, durum wheat cultivars have been shown to be consistently less Zn efficient than bread wheat (Triticum aestivum L.) cultivars (Graham et al., 1992). Similarly, durum wheat varieties were reported to be less Zn efficient than bread wheat varieties when grown in chelate-buffered hydroponic nutrient culture (Rengel and Graham, 1995a).
The physiological mechanism(s) that confers Zn efficiency has not been identified. Processes that could influence the ability of a plant to tolerate limited amounts of available Zn include higher root uptake, more efficient utilization of Zn, and enhanced Zn translocation within the plant. Cakmak et al. (1994) showed that a Zn-inefficient durum wheat cultivar exhibited Zn-deficiency symptoms earlier and more intensely than a Zn-efficient bread wheat cultivar even though the Zn tissue concentrations were similar in both lines, suggesting differential utilization of Zn in the two cultivars. Rates of Zn translocation to shoots were shown to vary among sorghum cultivars, although correlations with Zn efficiency were not established (Ramani and Kannan, 1985). Root uptake kinetics have been reported to vary between rice cultivars having different Zn requirements, with high-Zn-requiring cultivars exhibiting consistently higher root uptake rates (Bowen, 1986). In contrast, a correlation between Zn efficiency and rates of root Zn uptake in bread and durum wheat cultivars could not be demonstrated (Rengel and Graham, 1995b).
In grasses Zn influx into the root symplasm has been hypothesized to occur as the free Zn2+ ion (Halvorson and Lindsay, 1977), as well as in the form of Zn complexes with nonprotein amino acids known as phytosiderophores (Tagaki et al., 1984) or phytometallophores (Welch, 1993). Concentration-dependent uptake of free Zn2+ ions has been shown to be saturable in several species, including maize (Mullins and Sommers, 1986), barley (Veltrup, 1978), and wheat (Chaudhry and Loneragan, 1972), suggesting that ionic uptake in grasses occurs via a carrier-mediated system. However, several of these studies have been criticized on the basis that excessively high (and physiologically unrealistic) Zn2+ concentrations were used (Kochian, 1993).
This study was undertaken to examine unidirectional Zn2+ influx and translocation to shoots in Zn-efficient bread wheat lines and Zn-inefficient durum wheat lines. Experiments were performed in the absence of added phytometallophores and results are presumed to represent influx of ionic Zn2+. Zn activities in the nanomolar range were used to more closely mimic free Zn2+ levels occurring naturally in soil solution. The results presented here indicate that a Zn-efficient bread wheat cultivar maintained higher rates of Zn uptake than a Zn-inefficient durum wheat cultivar, particularly at low (and physiologically relevant) solution Zn2+ activities.
MATERIALS AND METHODS
Seedling Growth
Seeds of durum wheat (Triticum turgidum L. var durum cv Renville) and bread wheat (Triticum aestivum L. cv Grandin) were germinated and planted in hydroponic medium as described elsewhere (Hart et al., 1998). Seeds were germinated on moistened filter paper after surface sterilization and then were transferred to a hydroponic system consisting of mesh-bottomed black polyethylene film cups positioned above a solution in light-sealed, black 5-L polyethylene pots fitted with aeration tubes. Growth solutions consisted of a complete nutrient solution, including a chelate buffer to control the activities of metal micronutrients at levels adequate for normal growth (Norvell and Welch, 1993). Seedlings in pots were placed in a growth chamber with a photon flux density of 400 to 500 μmol m−2 s−1 and day/night temperature of 20°C/15°C (16/8 h).
Uptake Experiments
Roots of intact 8-d-old bread wheat or 10-d-old durum wheat seedlings were removed from nutrient solution, immersed for 2 min in deionized water, and then placed for 30 min in modified uptake solution (2 mm Mes-Tris [pH 6.0], 0.2 mm CaSO4, 12.5 μm H3BO3 [to help maintain membrane integrity], and 0.15 nm ZnSO4 [to continue the approximate level of free Zn2+ to which roots had been exposed in nutrient solution]). Roots were then transferred to wells (two roots per well) of a custom-built uptake apparatus described previously (Hart et al., 1993). Wells were filled with 60 mL of aerated uptake solution containing 5 mm Mes-Tris (pH 6.0), 0.2 mm CaSO4, and 12.5 μm HBO3. After 45 min, wells were emptied and refilled with fresh uptake solution. Experiments were then initiated by addition of 0.012 to 1.8 μCi of 65ZnCl2 (NEN) plus nonradiolabeled ZnSO4 as needed to achieve the desired Zn2+ concentration.
In experiments measuring uptake from solutions containing free Zn2+ activities of less than 300 nm, EDTA was included in the uptake solution and free Zn2+ activities were calculated using the speciation program GEOCHEM-PC (Parker et al., 1994). In experiments measuring uptake at 2°C, uptake wells were packed in ice. To measure Zn2+ binding to root cell walls, roots were treated to disrupt and remove cellular contents. This was achieved by immersing roots in methanol:chloroform (2:1, v/v) for 3 d, followed by a rinse for 2 d in deionized water. Roots subjected to this treatment have been reported to yield a morphologically intact, lipid-free root cell wall preparation (DiTomaso et al., 1992). Unless noted otherwise, all experiments used a 20-min uptake period.
For pulse-labeled translocation experiments, seedlings were removed from wells following the 20-min uptake period in 65Zn2+ and transferred to 1-L flasks containing nonradiolabeled ZnSO4 in uptake solution. For continuously radiolabeled translocation experiments, seedlings were placed in 1-L flasks containing 4 μm 65Zn2+. The uptake solution was replaced with fresh uptake solution containing 4 μm 65Zn2+ after each harvest of a subset of the seedlings at specific time points. In all experiments, desorption was initiated at the end of the uptake period by replacing uptake solution with a 2°C desorption solution that contained 5 mm Mes-Tris (pH 6.0), 5 mm CaSO4, 12.5 μm H3BO3, and 100 μm ZnSO4. In translocation experiments, seedlings were transferred from flasks to uptake wells for desorption. After 15 min of desorption (with a change of desorption solution midway through the desorption period), seedlings were removed from uptake wells and placed on damp paper towels to remove excess solution from roots. Roots were excised, weighed, and analyzed for 65Zn in an Auto-Gamma 5530 gamma counter (Packard, Meriden, CT).
RESULTS
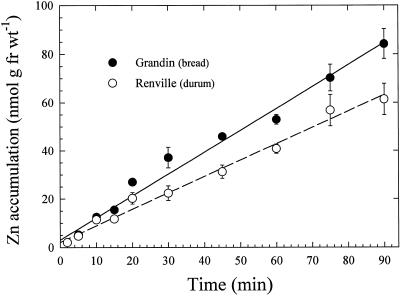
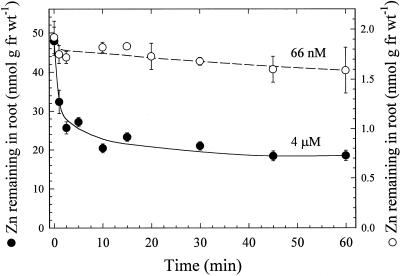
Time-dependent Zn2+ accumulation in desorbed roots of bread and durum wheat varieties was linear for at least 90 min (Fig. 1). During this period, roots were immersed in a solution containing 4 μm Zn2+, and roots of the durum wheat cultivar accumulated less Zn2+ than roots of the bread wheat cultivar. Regression lines through the data points had r2 values for bread and durum varieties of 0.984 and 0.987, respectively, and intercepted the y axis slightly above the origin. The amount of Zn2+ desorbed from roots of both wheat lines was dependent on the activity of Zn2+ in the uptake solution (Fig. 2). In the durum cultivar, after 60 min, approximately 60% of Zn2+ was desorbed from roots that had accumulated 65Zn from a solution containing 4 μm Zn2+, whereas about 15% was removed from roots that had absorbed 65Zn from a 66 nm Zn2+ solution. Desorption was rapid in both cases, with 76% and 60% of the total Zn2+, which was removed after 60 min of desorption, dissociating from roots within the first 2.5 min after incubation in 4 μm and 66 nm Zn2+, respectively. Results were similar for the bread wheat variety (not shown). In both wheat varieties the percentage of Zn2+ desorbed from roots increased as the activity of Zn2+ to which roots were exposed increased (Table I).
Figure 1.
Time course of 65Zn2+ accumulation in bread and durum wheat seedlings. Roots were incubated in a solution containing 4 μm 65ZnSO4 and desorbed in a solution containing 100 μm nonradiolabeled Zn. Data points and bars represent means and se values of four replicates. Error bars do not extend outside some data points. fr wt, Fresh weight.
Figure 2.
Desorption of 65Zn2+ from intact durum wheat roots after a 20-min uptake period in solutions containing 66 nm or 4 μm ZnSO4. Data points and bars represent means and se values of four replicates. fr wt, Fresh weight.
Table I.
65Zn accumulation in intact wheat roots with and without desorption
| Cultivar | Accumulation
|
||||
|---|---|---|---|---|---|
| Zn2+ activity | w/o Desorption | Zn2+ activity | w/Desorption | Desorbed | |
| μm | nmol g−1 h−1 | μm | nmol g−1 h−1 | % | |
| Bread | 0.0056 | 0.275 (0.022) | 0.0062 | 0.248 (0.033) | 10 |
| 0.055 | 1.68 (0.08) | 0.059 | 1.67 (0.04) | 1 | |
| 0.79 | 13.0 (0.7) | 0.77 | 11.1 (0.4) | 15 | |
| 8.0 | 76.1 (5.0) | 8.0 | 37.7 (3.9) | 50 | |
| Durum | 0.0060 | 0.300 (0.021) | 0.0059 | 0.283 (0.014) | 6 |
| 0.059 | 1.89 (0.11) | 0.061 | 1.79 (0.06) | 6 | |
| 0.79 | 14.6 (0.6) | 0.78 | 12.2 (0.4) | 16 | |
| 8.0 | 74.8 (4.2) | 8.0 | 37.7 (3.8) | 50 | |
Roots were immersed for 20 min in solutions containing varying activities of 65ZnSO4. Desorption solution contained 5 mm Mes-Tris (pH 6.0), 5 mm CaSO4, 12.5 μm H3BO3, and 100 μm ZnSO4. Desorption time was 15 min (two consecutive 7.5-min periods). Undesorbed roots were quickly rinsed twice with deionized water before harvesting. Accumulation data represent means and se values of four replications.
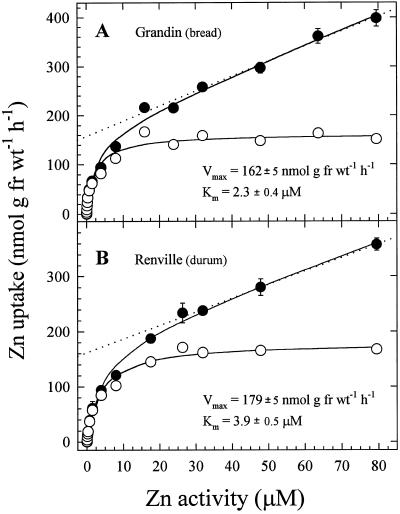
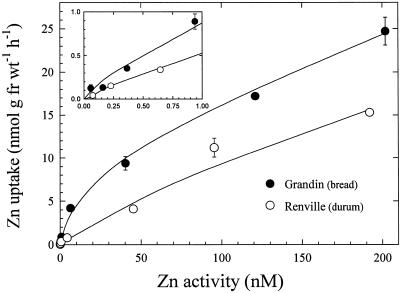
Concentration-dependent uptake kinetics for both wheat varieties were characterized by nonsaturating curves that became linear at Zn2+ activities greater than 20 μm (Fig. 3). These curves could be graphically dissected into saturable and linear components. Kinetic constants for the saturable components were derived by fitting a hyperbolic curve to the calculated saturable data points. Both Km and Vmax values were higher in the durum than in the bread wheat cultivar. At low Zn2+ activities, Zn2+ uptake rates were higher in the bread wheat variety (Fig. 4).
Figure 3.
Concentration-dependent uptake of 65Zn2+ in intact bread and durum wheat roots. The data in each panel are from two separate experiments (Zn activities: 0.1–300 nm and 0.5–80 μm). Filled symbols depict total uptake. Dotted lines represent linear components derived from regression lines through the five highest concentration data points. Open symbols represent saturable components derived by subtracting the linear component from the total-uptake points. Data points and bars represent means and se values of four replicates. Error bars do not extend outside some data points. fr wt, Fresh weight.
Figure 4.
Concentration-dependent uptake of 65Zn2+ in intact bread and durum wheat roots. Uptake solutions contained 250 nm EDTA and varying concentrations of 65ZnSO4 (50–800 nm). Zn2+ activities shown on the x axis were calculated using the speciation program GEOCHEM-PC. Data points and bars represent means and se values of four replicates. Error bars do not extend outside some data points. Inset, Low Zn2+ activity data points plotted on expanded axes. fr wt, Fresh weight.
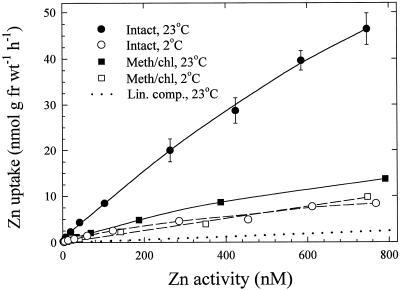
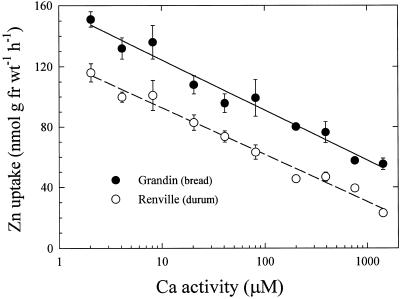
Zn2+ uptake in both varieties was dramatically inhibited when roots were subjected to cold temperature or treated to remove cellular contents (Fig. 5). Zn2+ uptake in intact roots at 2°C was inhibited 70% to 85% in the bread wheat cultivar (Fig. 5) and 80% to 85% in the durum wheat cultivar (not shown) compared with uptake at 23°C. Methanol:chloroform-treated roots showed 70% to 80% (bread) and 60% to 80% (durum) reduction in uptake at 23°C compared with intact roots. At 2°C, Zn2+ uptake in methanol:chloroform-treated roots was reduced further in both varieties to about 85% inhibition. Zn2+ influx in both wheat varieties was also inhibited by Ca (Fig. 6). Increasing Ca activity caused greater inhibition of Zn2+ uptake, with a similar response in both cultivars.
Figure 5.
Concentration-dependent uptake of 65Zn2+ at 23°C and 2°C in intact bread wheat roots and in bread wheat roots treated to remove cellular contents. The dotted line represents the linear component (Lin. comp.) derived from Figure 3A. Data points and bars represent means and se values of four replicates. Error bars do not extend outside some data points. Meth/chl, Methanol:chloroform treated; fr wt, Fresh weight.
Figure 6.
Uptake of 65Zn2+ in roots of bread and durum wheat seedlings. Uptake solutions contained 2 μm 65ZnSO4 and varying concentrations of Ca. Data points and bars represent means and se values of four replicates. Error bars do not extend outside some data points. fr wt, Fresh weight.
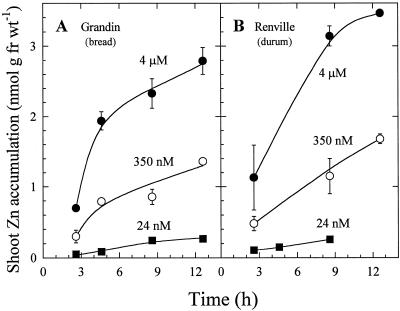
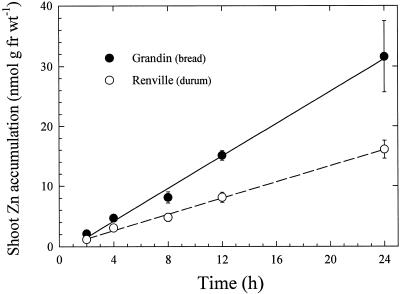
In seedlings with roots exposed to 65Zn2+ at three concentrations for 20 min and then transferred to solutions containing similar concentrations of unlabeled Zn2+, translocation of Zn2+ to shoots was time and concentration dependent in both wheat varieties (Fig. 7). Shoot Zn2+ concentration increased in both varieties during a 12-h period, with the greatest shoot concentrations measured in seedlings exposed to an uptake solution containing 4 μm Zn2+. When seedling roots were exposed continuously to 4 μm 65Zn2+, shoot Zn2+ levels increased linearly for at least 24 h (Fig. 8). The bread wheat cultivar accumulated approximately twice as much Zn2+ as the durum wheat cultivar (Fig. 8). The partitioning of absorbed Zn within the plant (measured as shoot:root 65Zn ratio) was similar in both varieties (Table II).
Figure 7.
Time-dependent translocation of 65Zn from roots to shoots in intact seedlings of bread (A) and durum (B) wheat cultivars. Roots were immersed for 20 min in solutions containing different activities of 65Zn2+, and then transferred to solutions containing similar concentrations of nonradiolabeled ZnSO4. Data points and bars represent means and se values of three replicates. Error bars do not extend outside some data points. fr wt, Fresh weight.
Figure 8.
Time-dependent shoot translocation of 65Zn in intact seedlings of bread and durum wheat cultivars. Roots were immersed continuously in solutions containing 4 μm 65ZnSO4. Data points represent means and se values of four replicates. Error bars do not extend outside some data points. fr wt, Fresh weight.
Table II.
65Zn partitioning in intact wheat seedlings
| Time | Bread | Durum |
|---|---|---|
| h | Shoot [Zn]:root [Zn]a | |
| 2 | 8.2 (1.3) | 8.1 (0.8) |
| 4 | 14.2 (1.3) | 14.7 (1.2) |
| 8 | 17.1 (2.1) | 16.0 (3.1) |
| 12 | 24.6 (5.0) | 19.7 (1.8) |
| 24 | 45.7 (6.8) | 42.1 (1.9) |
Roots were immersed in a solution containing 4 μm 65ZnSO4, 2 mm Mes-Tris (pH 6.0), 0.2 mm CaSO4, and 12.5 μm H3BO3. After the given uptake period, roots were desorbed for 15 min in a 2°C solution containing 5 mm Mes-Tris (pH 6.0), 5 mm CaSO4, 12.5 μm H3BO3, and 100 μm ZnSO4. Shoot:root concentration data represent means and se values (in parentheses) of four replications.
Shoot [Zn] is in nmol g fresh wt−1 × 103; root [Zn] is in nmol g fresh wt−1.
DISCUSSION
Zn2+ Binding
Several lines of evidence indicate that a limited amount of nonexchangeable Zn2+ binding to wheat root cell walls occurred in these experiments. The regression lines drawn through the data points in Figure 1 intersect the y axis slightly above the origin, indicating a relatively small quantity of rapidly bound Zn2+ that was not removed from roots in our 15-min desorption regimen. The data in Figure 2 show that most of the freely dissociable Zn2+ was removed from roots during the first 5 min of desorption and is likely to have come from the cell wall free space. This interpretation is consistent with the efflux data of Santa Maria and Cogliatti (1988), who measured a half-life of about 4 min for Zn2+ release from the wheat root cell wall free space. In addition, the larger amounts of Zn that were desorbed from roots exposed to higher Zn2+ activities (Fig. 2; Table I) suggest a proportional release of Zn2+ from the cell wall free space and a limited degree of strong binding to cell wall or membrane components.
The linear kinetic component for Zn2+ influx (Fig. 3), which predominates at high Zn2+ activity in the uptake solution, can be interpreted as representing nonspecific Zn2+ binding to cell wall components that remains after desorption. Evidence for divalent cation binding to root cell walls that resists desorption when exposed to high cation concentration has been observed previously (DiTomaso et al., 1992; Hart et al., 1992; Lasat et al., 1996). As a means of estimating Zn2+ binding to wheat root cell walls, uptake was measured in intact roots exposed to low temperatures during the uptake period. Low temperature has been shown to inhibit uptake of Zn2+ in barley (Schmid et al., 1965) and sugarcane (Bowen, 1969).
The greatly reduced Zn2+ uptake in bread wheat roots under low-temperature conditions (Fig. 5) agrees with those earlier results. Furthermore, the plot of Zn2+ uptake under cold conditions displays a predominantly linear quality that is consistent with the linear components in Figure 3. For purposes of comparison, the linear component from Figure 3A has been replotted in Figure 5. The larger amount of root-associated Zn2+ in the low-temperature uptake experiment compared with the replotted linear component probably represents a low level of Zn2+ uptake under cold conditions in addition to nonspecific Zn2+ binding. The slightly saturable nature of the low-temperature uptake plot supports this interpretation.
Association of Zn2+ with roots treated to remove cellular contents must consist of nonspecific Zn2+ binding, and the plots in Figure 5 show much lower amounts of Zn2+ associated with roots subjected to this treatment. The higher amounts of Zn2+ binding in methanol:chloroform-treated roots compared with the replotted linear component (Fig. 5) may reflect greater accessibility to interior cell wall-binding sites exposed by the removal of the root symplasm. In addition, analysis of methanol:chloroform-treated roots (data not shown) revealed the presence of residual protein, which also could have contributed to higher levels of Zn2+ binding. Furthermore, the fresh weights of methanol:chloroform-treated roots were about 25% lower than those of intact roots because of the absence of intact cells, and this would lead to an overestimation of Zn2+ binding calculated on a per weight basis.
Taken together, the evidence from experiments with intact roots subjected to low temperature, as well as that from methanol:chloroform-treated roots, supports the interpretation that the linear components in Figure 3 represent nonspecific binding of Zn2+ to apoplasmic binding sites. As a consequence, the saturable components likely represent metabolically coupled transport of Zn2+ across the plasma membrane via a Zn2+ (or divalent cation) transporter.
The low level of cell wall binding seen in wheat roots in this work contrasts with the pronounced binding in barley roots reported previously (Schmid et al., 1965; Veltrup, 1978). The cause of this difference may be related to differences in root exposure to Zn2+ before the start of uptake experiments. In this work roots were grown in full nutrient solution containing Zn2+ (albeit at a low activity), whereas in the cited work, roots were grown without added Zn2+, either in low-salt medium (Schmid et al., 1965) or in nutrient solution (Veltrup, 1978). It is possible that in our experiments, growth in the presence of Zn2+ saturated Zn2+-binding sites in root cell walls and resulted in little additional binding of 65Zn2+ in uptake experiments. Conversely, in the cited papers, Zn-deficient roots may have had binding sites with high affinity for Zn2+ occupied by Ca or other cations, which quickly exchanged with added Zn2+.
Zn2+ Uptake
The difference in Zn2+ levels measured in intact roots and Zn2+ associated with methanol:chloroform-treated roots or intact roots incubated at low temperature (Fig. 5) must represent Zn2+ taken up across the root plasma membrane. This interpretation is supported by the results shown in Figure 7, which demonstrate that in seedlings pulse loaded for the same 20 min period used in root uptake experiments, 65Zn2+ appeared in shoots within 3 h of root exposure. Because the path of Zn2+ movement from root surface to shoot includes a symplasmic component (because of apoplastic blockage by the endodermis), translocation to shoots is indicative of Zn2+ movement across root cell plasma membranes. Moreover, the linear time course of accumulation in both cultivars (Fig. 1) shows that symplasmic Zn2+ uptake is unidirectional for at least 90 min. Similar patterns of time-dependent root accumulation of Zn2+ have been reported for barley (Schmid et al., 1965; Veltrup, 1978; Bowen, 1981), rice (Bowen, 1986), and wheat (Santa Maria and Cogliatti, 1988), and were interpreted as resulting from cellular uptake of Zn2+.
The saturating curves for Zn2+ concentration-dependent uptake kinetics in both varieties (Fig. 3) is consistent with a carrier-mediated Zn2+ uptake system. Evidence for carrier-mediated Zn2+ transport has been reported in a variety of biological systems, including mammalian (Tacnet et al., 1990; Bobilya et al., 1992), fungal (Gadd et al., 1987; White and Gadd, 1987), and plant systems (Veltrup, 1978; Mullins and Sommers, 1986; Lasat et al., 1996). In the bread and durum wheat lines measured here, Km values ranged between 2.3 and 3.9 μm (Fig. 3). Similar Km values were reported for Zn uptake in roots of other gramineous crop plants, including barley (Veltrup, 1978), maize (Mullins and Sommers, 1986), and wheat (Chaudhry and Loneragan, 1972), as well as for fungal (White and Gadd, 1987; Budd, 1988; Sabie and Gadd, 1990) and animal (Bobilya et al., 1992) cells. The similar kinetic parameters for Zn2+ uptake among a wide variety of life-forms suggests conserved transport systems for Zn2+ or a common adaptation to similar ambient levels of this essential micronutrient.
The very low Zn2+ activities shown in Figure 4 were achieved by the use of the metal chelate buffer, EDTA. The chemical speciation program GEOCHEM PC was used to predict the free Zn2+ activities in the presence of varying total Zn2+ concentrations (50–800 nm) and a single EDTA concentration (250 nm). Experimental evidence from our laboratory clearly shows that in short-term experiments, Zn is taken up by wheat roots predominantly in the form of the free Zn2+ ion, and not as the Zn-EDTA complex (data not presented). Therefore, the free Zn2+ activities in the uptake solution in Figure 4 represent good estimates of the true free Zn2+ activities in solution in these experiments.
The significantly lower rates of Zn2+ uptake at low-solution Zn2+ activity in the durum wheat variety (Fig. 4) suggest that the Zn2+ uptake system is different from that in the bread wheat line. At higher Zn2+ concentrations, kinetic differences between the two wheat types were not as clearly resolved (Fig. 3). However, higher Zn accumulation (Fig. 1) and translocation to shoots (Fig. 8) over longer periods in the bread wheat cultivar were consistent with a higher capacity for net Zn uptake in bread wheat. Furthermore, the substantial difference in Zn2+ uptake rates between bread and durum wheats at low Zn2+ activities (Fig. 4) suggests that Zn efficiency may be related to the capacity for Zn2+ uptake from Zn-deficient soils.
Correlation between root Zn2+ uptake and Zn efficiency has been reported previously in studies of whole-plant net Zn2+ uptake rates in bread and durum wheat cultivars grown in Zn-deficient soils (Graham et al., 1992; Dong et al., 1995). Those studies showed lower Zn2+ uptake in Zn-inefficient durum wheat grown in long-term field and greenhouse pot experiments. However, a solution culture study that used chelate buffer to control Zn2+ activities at very low levels failed to establish a correlation between Zn efficiency and long-term (22 d) whole-plant net uptake rates (Rengel and Graham, 1995b).
It is important to note that the Km values for both cultivars (2.3–3.9 μm) are higher than the soil-solution Zn2+ concentrations found in normal soils (1 nm–1 μm; Welch, 1995). In Zn-deficient soils, where the Zn-efficiency trait is expressed most clearly, Zn2+ activity in soil solution can be much lower, reaching a low to subnanomolar concentration range (Lindsay, 1991), far below the Km values of the bread and durum wheat cultivars studied here. This suggests that short-term root uptake rates measured at Zn2+ concentrations much higher than those found in Zn-deficient soils may not be good predictors of Zn efficiency. The large difference in Zn2+ uptake rates of bread and durum cultivars measured at very low solution Zn2+ activities (Fig. 4) supports this view.
Responses of the Zn2+ transport system to certain external factors appear to be similar in both bread and durum wheat lines. Zn2+ uptake in both cultivars is inhibited dramatically by low temperature (Fig. 5) and by Ca (Fig. 6). The large inhibition of Zn2+ uptake at low temperature suggests a metabolic requirement for Zn2+ transport. As discussed by Kochian (1993), uptake of Zn2+ is likely to be a thermodynamically passive process, driven by the inwardly directed negative membrane potential across the plasmalemma, and low-temperature uptake inhibition is likely to result indirectly from a reduction in the membrane potential. Low-temperature-induced reduction in Zn2+ uptake was reported previously for sugarcane leaves (Bowen, 1969), barley roots (Schmid et al., 1965), and wheat roots (Chaudhry and Loneragan, 1972). The dramatic inhibition of Zn2+ uptake by Ca (Fig. 6) is also similar to findings from previous reports with rice (Giordano et al., 1974) and wheat seedlings (Chaudhry and Loneragan, 1972). In the latter study, transformation of uptake data into double-reciprocal plots revealed a noncompetitive interaction between Zn2+ and Ca2+, which suggested that Zn2+ and Ca2+ do not share a common transport mechanism. The parallel inhibitory response to low temperature and Ca in bread and durum wheat cultivars in this study implies that there are similarities in the Zn2+-transport systems of these two cultivars.
Zn2+ Translocation
The appearance of 65Zn2+ in shoots within 3 h of root exposure (Figs. 7 and 8) indicates that Zn2+ taken up by roots enters the vascular tissue and is rapidly translocated to the shoot. Seedlings exposed to a 20-min pulse of varying activities of radiolabeled Zn2+ and then placed in nonradiolabeled solutions containing the same Zn2+ activity showed Zn2+ movement to shoots at rates dependent on the root solution activity (Fig. 7). This result confirms that Zn2+ was taken up symplasmically during the 20-min uptake period and was not simply bound to the apoplasm.
In Figure 7, the decline in 65Zn2+ accumulation in shoots with time should not be interpreted as saturation of shoots, but rather as the result of decreasing 65Zn2+ specific activity caused by the replacement of radiolabeled solution by a solution containing nonradiolabeled Zn2+. When roots were exposed continuously to solutions containing 65Zn2+ at constant specific activity, translocation exhibited a linear time dependence for at least 24 h (Fig. 8). The larger amounts of Zn2+ translocated to shoots of bread wheat compared with durum wheat at a 4 μm Zn2+ root solution reflects the greater root uptake rate in the bread wheat variety. However, the similar shoot:root 65Zn ratios (Table II) indicate that Zn partitioning was not different in the two varieties.
In summary, this work has provided evidence for carrier-mediated Zn2+ influx into the root symplasm in both durum and bread wheat varieties. It also demonstrates that Zn2+ uptake rates are lower in a durum wheat variety than in a bread wheat line, especially at low solution Zn2+ activities. Furthermore, the data show that Zn partitioning between root and shoot is similar in the two varieties. These results suggest that the rate of Zn uptake may be an important predictor of Zn efficiency. This information may be useful in breeding durum wheat lines that are more efficient in extracting Zn from soil solution. Alternatively, agronomic practices may be devised that increase Zn uptake in durum wheat by increasing the levels of available Zn in soils. Finally, the reduced Zn uptake rates in durum wheat measured in these experiments suggest that an investigation of the role of Zn-Cd interactions at the root surface may help in understanding Cd accumulation in low-Zn soils.
ACKNOWLEDGMENT
We thank the North Central Research Center (Minot, ND) for generously supplying the seeds of cvs Grandin and Renville.
LITERATURE CITED
- Bobilya DJ, Briske-Anderson M, Reeves PG. Zinc transport into endothelial cells is a facilitated process. J Cell Physiol. 1992;151:1–7. doi: 10.1002/jcp.1041510102. [DOI] [PubMed] [Google Scholar]
- Bowen JE. Absorption of copper, zinc and manganese by sugarcane leaf tissue. Plant Physiol. 1969;44:255–261. doi: 10.1104/pp.44.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen JE. Kinetics of active uptake of boron, zinc, copper and manganese in barley and sugarcane. J Plant Nutr. 1981;3:215–223. [Google Scholar]
- Bowen JE. Kinetics of zinc uptake by two rice cultivars. Plant Soil. 1986;94:99–107. [Google Scholar]
- Budd K. A high-affinity system for the transport of zinc in Neocosmospora vasinfecta. Exp Mycol. 1988;12:195–202. [Google Scholar]
- Cakmak S, Gulut KY, Marschner H, Graham RD. Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency. J Plant Nutr. 1994;17:1–17. [Google Scholar]
- Chaudhry FM, Loneragan JF. Zinc absorption by wheat seedlings and the nature of its inhibition by alkaline earth cations. J Exp Bot. 1972;23:552–560. [Google Scholar]
- Codex Alimentarius Commission (1993) Risk assessment procedures used by the Codex Alimentarius Commission, and its subsidiary and advisory bodies. In Joint FAO/WHO Food Standards Programme, Rome, Twentieth Session, June 28–July 7, 1993. International Conference Centre, Geneva, Switzerland, pp 1–21
- DiTomaso JM, Hart JJ, Kochian LV. Transport kinetics and metabolism of exogenously applied putrescine in roots of intact maize seedlings. Plant Physiol. 1992;98:611–620. doi: 10.1104/pp.98.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Rengel Z, Graham RD. Effects of herbicide chlorsulfuron on growth and nutrient uptake parameters of wheat genotypes differing in Zn-efficiency. Plant Soil. 1995;173:275–282. [Google Scholar]
- Gadd GM, White C, Mowll JL. FEMS Microbiol Ecol. 1987;45:261–267. [Google Scholar]
- Giordano PM, Noggle JC, Mortvedt JJ. Zinc uptake by rice, as affected by metabolic inhibitors and competing cations. Plant Soil. 1974;41:637–646. [Google Scholar]
- Graham RD, Ascher JS, Hynes SC. Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil. 1992;146:241–250. [Google Scholar]
- Graham RD, Rengel Z. Genotypic variation in zinc uptake and utilization by plants. In: Robson AD, editor. Zinc in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 107–118. [Google Scholar]
- Halvorson AD, Lindsay WL. The critical Zn2+ concentration for corn and the nonabsorption of chelated zinc. Soil Sci Soc Am J. 1977;41:531–534. [Google Scholar]
- Hart JJ, DiTomaso JM, Linscott DL, Kochian LV. Characterization of the transport and cellular compartmentation of paraquat in roots of intact maize seedlings. Pestic Biochem Physiol. 1992;43:212–222. [Google Scholar]
- Hart JJ, DiTomaso JM, Linscott DL, Kochian LV. Investigations into the cation specificity and metabolic requirements for paraquat transport in roots of intact maize seedlings. Pestic Biochem Physiol. 1993;45:62–71. [Google Scholar]
- Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998;116:1413–1420. doi: 10.1104/pp.116.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. Zinc absorption from hydroponic solutions by plant roots. In: Robson AD, editor. Zinc in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 45–57. [Google Scholar]
- Lasat MM, Baker AJM, Kochian LV. Physiological characterization of root Zn2+ absorption and translocation to shoots in Zn hyperaccumulator and nonaccumulator species of Thlaspi. Plant Physiol. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay WL (1991) Inorganic equilibria affecting micronutrients in soils. In JJ Mortvedt, FR Cox, LM Shuman, RM Welch, eds, Micronutrients in Agriculture, Ed 2. Soil Science Society of America, Madison, WI, pp 89–112
- Mullins GL, Sommers LE. Cadmium and zinc influx characteristics by intact corn (Zea mays L.) seedlings. Plant Soil. 1986;96:153–164. [Google Scholar]
- Norvell WA, Welch RM. Growth and nutrient uptake by barley (Hordeum vulgare L. cv. Herta). Studies using an N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid-buffered nutrient solution technique. Plant Physiol. 1993;101:619–625. doi: 10.1104/pp.101.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1994) GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models. Special publication No. 42. Soil Science Society of America, Madison, WI, pp 253–269
- Ramani S, Kannan S. An examination of zinc uptake patterns by cultivars of sorghum and maize: differences amongst hybrids and their parents. J Plant Nutr. 1985;8:1199–1210. [Google Scholar]
- Rengel Z, Graham RD. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. I. Growth. Plant Soil. 1995a;176:307–316. [Google Scholar]
- Rengel Z, Graham RD. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. II. Nutrient uptake. Plant Soil. 1995b;176:317–324. [Google Scholar]
- Sabie FT, Gadd GM. Effect of zinc on the yeast-mycelium transition of Candida albicans and examination of zinc uptake at different stages of growth. Mycol Res. 1990;94:952–958. [Google Scholar]
- Santa Maria GE, Cogliatti DH. Bidirectional Zn-fluxes and compartmentation in wheat seedling roots. J Plant Physiol. 1988;132:312–315. [Google Scholar]
- Schmid WE, Hang HP, Epstein E. Absorption of zinc by excised barley roots. Physiol Plant. 1965;18:860–869. [Google Scholar]
- Tacnet F, Watkins DW, Ripoche P. Studies of zinc transport into brush-border membrane vesicles isolated from pig small intestine. Biochim Biophys Acta. 1990;1024:323–330. doi: 10.1016/0005-2736(90)90361-q. [DOI] [PubMed] [Google Scholar]
- Tagaki S, Nomoto K, Takemoto T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr. 1984;7:469–477. [Google Scholar]
- Veltrup W. Characteristics of zinc uptake by barley roots. Physiol Plant. 1978;42:190–194. [Google Scholar]
- Welch RM. Zinc concentrations and forms in plants for humans and animals. In: Robson AD, editor. Zinc in Soil and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 183–195. [Google Scholar]
- Welch RM. Micronutrient nutrition of plants. Crit Rev Plant Sci. 1995;14:49–82. [Google Scholar]
- White C, Gadd GM. The uptake and cellular distribution of zinc in Saccharomyces cerevisiae. J Gen Microbiol. 1987;133:727–737. [Google Scholar]
- Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, Bonnin E. Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn and wheat. J Agric Food Chem. 1983;31:1240–1244. doi: 10.1021/jf00120a024. [DOI] [PubMed] [Google Scholar]