Abstract
The links between sexual violence, genitoanal injury, and HIV are understudied but potentially significant for understanding the epidemic's disproportionate impacts on young women and girls, particularly in sub-Saharan Africa, other hyperendemic areas, and conflict-affected regions. A Scientific Research Planning Meeting was convened by the Social Science Research Council at the Greentree Foundation in New York, March 19–20, 2012, bringing together an interdisciplinary group of researchers, clinicians, and policy makers to identify knowledge needs and gaps in three key areas: (1) the role of genitoanal injury on HIV transmission, acquisition, and pathogenesis; (2) the influence of sex and age-related anatomic characteristics on HIV transmission, acquisition, and pathogenesis; and (3) the role of heterosexual anal intercourse in HIV transmission. This article reflects the consensus that emerged from the Greentree Meeting regarding priority scientific research questions in these three areas, associated data collection and measurement challenges and opportunities, and implications for policy and practice.
Introduction
The physiological aspects of sexual violence (SV) are poorly studied and yet potentially significant in the overall expansion of the AIDS epidemic and its disproportionate impacts on young women, particularly in sub-Saharan Africa. A growing body of behavioral and social science research points to a significant and bidirectionala relationship between SV and HIV transmission risk, especially in conflict-affected situations and hyperendemic areas, as well as among populations at high risk, such as young adolescent women, sex workers, and widows.1–15 Although some exploratory mathematical modeling has shown the potential for increased risk at a population level in some settings,11,16 the biological and social cofactors of SV have yet to be incorporated systematically into model estimates of population level prevalence, in basic scientific and clinical research, or as a focus of HIV prevention efforts.
This White Paper reflects a consensus agenda for research, policy, and practice that resulted from a Scientific Research Planning Meeting on Sexual Violence and HIV, convened by the Social Science Research Council on March 19–20, 2012 at the Greentree Foundation in New York (hereafter referred to as the “Greentree Meeting”). The Greentree Meeting was sponsored by the National Institutes of Health, Office of AIDS Research; the Joint United Nations Programme on HIV/AIDS (UNAIDS), and UN Action Against Sexual Violence in Armed Conflict. The Greentree Meeting brought together basic, clinical, epidemiological, and social science researchers and policy makers with the goal of generating new insights about the physiology of sexual violence and its role in HIV transmission, particularly among women and girls.b The meeting objectives were to (1) examine what is known about the physiology of sexual violence and its role in HIV transmission, acquisition, and pathogenesis; (2) specify the sex and age-related anatomic and physiological factors that increase the risk of HIV transmission, acquisition, and pathogenesis during the maturation of the female genital and anal tracts throughout the reproductive cycle; and (3) develop a research agenda to explore unanswered questions. This article summarizes the consensus reached through the Greentree Meeting regarding priority research questions, associated data collection and measurement challenges, and implications for policy and practice. The summary Report of Proceedings and the review papers developed for the Greentree Meeting are in preparation for publication in a Special Issue of the American Journal of Reproductive Immunology.
Research Priorities
The role of genitoanal injury in HIV transmission, acquisition, and pathogenesis
The biology of HIV sexual transmission is not well understood.17 Most scientific studies of vaginal transmission use ex vivo cervicovaginal tissue models that cannot reproduce the cellular interactions that occur in vivo. Nor can primate models, which deliver HIV or SIV atraumatically through intravaginal and intrarectal inoculations, recreate the microabrasions and injuries that occur naturally from vaginal and anal intercourse, or more excessively from forced and violent sex.
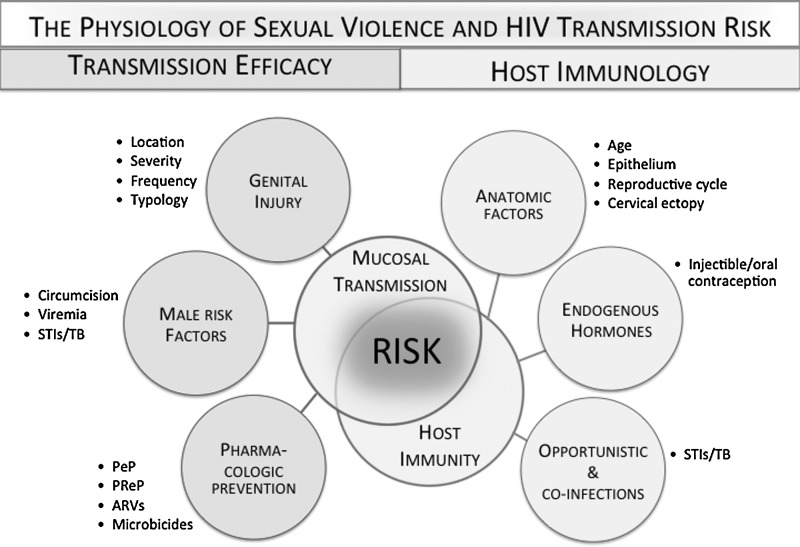
In creating a physiological pathway that facilitates viral transmission, genitoanal injury may significantly increase the per-act probabilities of heterosexual transmission in specific contexts (Fig. 1).18–20 Normally, the lower female reproductive tract, which constitutes the outer cervix and vaginal walls, is composed of a multilayer stratified epithelium that gives greater protection against injury and infection than the single-cell columnar epithelium lining the upper reproductive tract (endocervix and uterus). A number of factors can disrupt this otherwise protective barrier and immune protection in the female genital tract. These include ulcerations and inflammation due to sexually transmitted infections (STIs), as well as proinflammatory immune factors associated with exogenous21–23 and endogenous24–26 hormones and sex/semen.27,28 Cervical and vaginal lesions, microabrasions, inflammation, and immaturity of the female reproductive tract (cervical ectopy) may modulate susceptibility to HIV infection since damaged areas provide open access to the subepithelial structures where HIV-1 target cells predominantly reside.29 The columnar epithelium of the upper reproductive tract and that associated with ectopy may render these sites more susceptible to injury than the lower genital tract. Very little is known about the compounding effects of genital trauma in environments where multiple cofactors are present, such as STIs, cervical ectopy, and the use of hormonal contraception.
FIG. 1.
The physiology of sexual violence and HIV transmission risk.
Inflammation, abrasions, and injury can result from both forced and consensual sex,30,31 although most studies associate higher rates (up to three times higher) of injury with sexual assault.32–34 Rates of reported injury in cases of forced sex vary immensely, with studies estimating between 32%30 and 94%.34 Sexually assaulted prepubescent girls and adolescents may sustain more injuries than adults due to the hymeneal, vaginal, or cervical lacerations that occur naturally with sexual debut.35–37 The severity, frequency, location, and patterns of injury will vary in relation to many factors, including age, the degree of force or coercion, the use of objects, the perpetrator's characteristics and motivation, and exposure opportunities.c But injury reporting may not capture these differences. The absence of standard definitions, classifications, reporting, and collection protocols means that injury detection will vary according to the individual examiner, clinical capacity, legal requirements regarding sexual assault reporting, and the techniques used.d In many cases, clinical reports and related research do not specify anal injuries or inflammation, both significant risk factors for HIV transmission.38–40,e
The physiological effects of intimate partner violence (IPV) on the immune system are also underresearched. An emerging literature points to immune system effects that result from altered hypothalamic, pituitary, and adrenal gland interactions and the suppression of Th1 cell cytokine production (which fights bacteria and viruses).7 Posttraumatic stress disorder (PTSD) and depression—comorbidities associated with intimate partner violence—can also contribute to immune suppression, proinflammatory responses, and faster progression to AIDS.41–43
Priority scientific research questions identified by the participants included the following:
1. What is the impact of genitoanal injury on the female reproductive and anal tracts? How do these effects vary in relation to different developmental stages of the female reproductive and anal tracts?
2. How are genitoanal injuries defined and measured; and is there a “threshold” that can be associated with increased risk of HIV transmission, acquisition, and pathogenesis?
3. How do the immunological and other effects of intimate partner violence increase the risk of HIV transmission and progression?
4. What is the contribution of genitoanal injury to HIV transmission, acquisition, and pathogenesis when multiple cofactors are present, such as STIs and hormonal contraception?
The influence of sex and age-related anatomic characteristics on HIV transmission, acquisition, and pathogenesis
UNAIDS estimates that 40% of new adult infections each year are among those 15 to 24 years of age.44 Of all young people living with HIV—some five million—75% are in sub-Saharan Africa45 and 71% of these are female (Table 1).46 Yet epidemiological and behavioral models of HIV transmission risk have been unable to explain why young women between 15 and 24 years in sub-Saharan Africa, who average only 2.3 sex partners in their lifetime, are two to eight times more likely than men to be HIV positive.47–51 While HIV remains low in the United States, similar patterns emerge when examining the rates of other STIs among adolescent girls as compared to boys, particularly chlamydia, gonorrhea, and human papillomavirus (HPV). Although young people aged 15–24 years represent only 25% of the sexually experienced population in the United States, they acquire nearly half of all new STIs. In the United States, young women aged 15–19 experience the highest rates of STIs, followed closely by women ages 20–24.52 In 2010, young women between 15 and 19 years of age were three times more likely than boys to have chlamydia.53
Table 1.
HIV Prevalence Among Young Men and Women, Ages 15–24
| |
Prevalence young male (15–24) (%) |
Prevalence young female (15–24) (%) |
||
|---|---|---|---|---|
| Country | Estimate | [Low–high estimate] | Estimate | [Low–high estimate] |
| Haiti | 0.6 | [0.4–0.8] | 1.3 | [1.0–1.8] |
| Somalia | 0.4 | [0.3–0.7] | 0.6 | [0.4–1.1] |
| Sudan | 0.5 | [0.4–0.7] | 1.3 | [0.9–1.8] |
| Papua New Guinea | 0.3 | [0.2–0.5] | 0.8 | [0.6–1.2] |
| Angola | 0.6 | [0.4–0.9] | 1.6 | [1.1–2.2] |
| Burundi | 1.0 | [0.8–1.2] | 2.1 | [1.6–2.7] |
| CAF | 1.0 | [0.6–1.4] | 2.2 | [1.4–3.1] |
| Cameroon | 1.6 | [1.2–2.1] | 3.9 | [3.1–5.4] |
| Chad | 1.0 | [0.7–2.0] | 2.5 | [1.7–5.2] |
| Congo | 1.2 | [0.9–1.6] | 2.6 | [2.1–3.6] |
| Côte d'Ivoire | 0.7 | [0.5–1.1] | 1.5 | [1.1–2.3] |
| DRC | — | [0.4–0.6] | — | [0.9–1.5] |
| Eritrea | 0.2 | [0.1–0.3] | 0.4 | [0.2–0.7] |
| Kenya | 1.8 | [1.3–2.4] | 4.1 | [3.0–5.4] |
| Lesotho | 5.4 | [4.1–7.4] | 14.2 | [11.2–19.2] |
| Liberia | 0.3 | [0.1–0.5] | 0.7 | [0.2–1.2] |
| Malawi | 3.1 | [2.3–4.2] | 6.8 | [5.3–9.2] |
| Mozambique | 3.1 | [2.4–4.4] | 8.6 | [7.0–12.1] |
| Namibia | 2.3 | [1.3–3.6] | 5.8 | [3.7–8.6] |
| Rwanda | 1.3 | [0.9–1.6] | 1.9 | [1.3–2.3] |
| Sierra Leone | 0.6 | [0.3–1.0] | 1.5 | [0.9–2.5] |
| South Africa | 4.5 | [4.1–5.0] | 13.6 | [12.3–15.0] |
| Swaziland | 6.5 | [4.8–8.8] | 15.6 | [12.6–21.3] |
| Uganda | 2.3 | [1.8–2.8] | 4.8 | [4.0–6.4] |
| Zimbabwe | 3.3 | [2.5–4.4] | 6.9 | [5.3–9.3] |
UNAIDS: Report on the Global AIDS Epidemic, 2010.
Age-related anatomic, biological, and physiological risk factors amplify acquisition probabilities among young adolescent women. During adolescence, sex hormones play a central role in regulating immune protection against HIV acquisition throughout the course of the menstrual cycle and the maturation of the female reproductive tract. Adolescent and adult cervices differ strikingly in their epithelial composition; the corollary differences in mucosal immune function may increase adolescent susceptibility.54,55 Cervical ectopy, which occurs naturally in young women, may affect HIV risk by extending columnar cells that normally line the inside of the cervical canal to its outer surface, providing easier access to the submucosa where most of the HIV target cells reside. Additionally, sexually active healthy adolescents may undergo active epithelial maturation in the cervix over relatively short periods of time; this increased cell proliferation can enhance susceptibility to HPV infection, an STI that might also contribute to enhanced HIV risk.56 HSV-2, which increases HIV transmission, is another common STI often acquired during adolescence.57,58
Another understudied risk factor in women's susceptibility to HIV acquisition is female genital mutilation/cutting (FGM/C), which occurs among young girls and before puberty.f Although there is little evidence linking FGM/C and HIV transmission, the few studies that have been carried out point to the potential role of nonsterile/soiled equipment, transfusion with potentially contaminated blood following hemorrhage, reproductive tract infections that increase risk of HIV acquisition, and inflammation/abrasion of vaginal tissue from the physical injury of FGM/C.59 Other potential risk factors include earlier sexual debut, polygamous relationships, male preference for “uncut” women (therefore looking elsewhere for partners), and higher rates of anal intercourse (AI) (due to difficult and painful vaginal intercourse and as a strategy to prevent pregnancy).60
A major limitation in assessing age-related biological and physiological determinants of HIV susceptibility among young women and adolescent girls is the absence of comparative and age-disaggregated data. Gaps in data result from the exclusion of adolescents from trials for ethical and regulatory reasons and the significant underreporting of child sexual assault as a mode of transmission. Age categories used in data collection are also inconsistent across institutions within and across regions of the world, and most do not distinguish children, adolescents (ages 13–18), or young adults (ages 19–24). The World Health Organization (WHO) defines young people as between 10 and 24 and most AIDS data are reported among the age group 18–49. For the U.S. Centers for Disease Control and Prevention (CDC), the only reportable transmission categories for children are either perinatal or “other,” which includes hemophilia, blood transfusion, or risk not identified.61
Priority scientific research questions identified by the participants included the following:
1. How do changes in the cervical and vaginal epithelium affect immune responses to genital injury and HIV acquisition risk before, during, and after adolescence, throughout different stages of the reproductive cycle, and among postmenopausal women?
2. How do the physiological and social cofactors of forced and early sex contribute to adolescent vulnerability and increase the risk of HIV acquisition over the longer term?
3. What are direct and indirect pathways linking female genital mutilation/cutting and HIV transmission and acquisition, namely in relation to immune protection and the development of the female genital tract?
The role of heterosexual anal intercourse in HIV transmission
The role of heterosexual AI in the epidemiology of HIV is poorly understood and likely underestimated.62 In developed countries, transmission probability for unprotected receptive anal intercourse has been estimated at 1.4%, or about 18 times the risk for receptive vaginal intercourse.63,64 This is primarily due to the inherent fragility of the rectal mucosa, the immune environment and the chronic inflammatory state of that compartment.65 While AI has historically been considered a significant risk factor for men who have sex with men, its prevalence and frequency are rarely reported among heterosexual population and little is known about the age-related and anatomic, physiological, or hormonal differences between the male and female anal tracts and how these differences might influence transmission risk.
From the limited available data, forced and consensual AI occurs across populations, age groups, and countries with some studies reporting that up to 20% and more of selected populations in America, Africa, and elsewhere have ever engaged in AI.63 Condom use is often lower in heterosexual anal sex than in vaginal sex.66,67 Some studies show that women are coerced or forced to have anal sex by their partners and in the context of transactional sex.68,69 In cases of sexual assault, reports of forced anal penetration range between 13% and 22.5%.33,36,70 Rates of AI may also be higher among women who have undergone the most extreme form of female genital mutilation/cutting, which makes vaginal intercourse very difficult or painful.71,72
The potential contribution of heterosexual AI to HIV transmission among women is not known but could have significant implications for the usage, safety, and delivery mechanisms of vaginal and rectal microbicides and other HIV prevention modalities. A modeling exercise by Boily et al.73 shows that even low levels of unprotected AI (5%) can reduce the effectiveness of vaginal microbicide interventions in heterosexual populations by 17–39% over 25 years.
Priority scientific research questions identified by the participants included the following:
1. Are there age-related and anatomic, physiological, or hormonal differences between the male and female anal tracts and, if so, how do they influence transmission risk?
2. What is the prevalence, frequency, and relationship characteristics and dynamics of unprotected AI and its contribution to HIV acquisition? What proportion is forced and how does this influence the severity of injuries and the likelihood of unprotected intercourse?
3. What are the implications of heterosexual anal sex for dual usage, safety considerations, and delivery mechanisms for rectal and vaginal microbicides?
Data Collection and Measurement Challenges and Opportunities
The Greentree Meeting identified four key data collection and measurement challenges and opportunities that must be addressed in order to expand the knowledge base in the critical areas of research noted above:
▪ Improve age-disaggregated data collection and methodologies, particularly among adolescents and young adults. Develop and disseminate research guidelines and protocols for productively and ethically studying prepubescent girls and female genital mutilation/cutting. Investigate differences in the efficacy of reporting mechanisms of sexual violence, genital injury, and anal intercourse across age, geographic, and other sociocultural categories.
▪ Develop a common system for classifying, detecting, and reporting the patterns, severity, and frequency of genitoanal injuries, using consistent demographic categories, age disaggregation, and the inclusion of various types of anal, oral, digital, and nonsexual injuries. Develop a common approach and standard definitions and indicators of sexual violence and its cultural variants as well as associated relationship characteristics and dynamics.
▪ Strengthen clinical and research capacity and infrastructure, especially in low-resource settings, e.g., in relation to training, detection, and equipment for injury detection and documentation. Establish protocols, priorities, parameters, and timeliness for sample collection in the context of sexual violence, e.g., in relation to tissue vaginal swabs and cytobrushes.
▪ Increase collaboration across basic, clinical, epidemiological, behavioral, and social science research on sexual violence and HIV transmission, acquisition, and pathogenesis. Develop strategies to integrate clinical assessments and treatment of SV and genitoanal injury into HIV surveillance, clinical trials, and other studies, and to integrate HIV surveillance, prevention, and treatment into clinical services, screening, and counseling for sexual violence prevention interventions.
Sexual Violence, Genitoanal Injury, and HIV: Implications for Policy and Practice
Heterosexual transmission accounts for the largest proportion of HIV prevalence worldwide: approximately 23.5 of the 34.2 million people currently living with HIV are believed to have acquired it through heterosexual intercourse. But the shift of epidemic monitoring from prevalent infections to incidence has focused attention and resources on the smallest subpopulations with the highest proportion of new HIV cases. In epidemiological models, these “key populations” typically refer to sex workers, people who inject drugs, and men who have sex with men, although the term also applies to others at highest risk of HIV exposure in a specific setting.45,74–77,g In contrast, very little is known about the epidemiological or social risk factors that drive “heterosexual transmission,” a term that is often and misleadingly characterized as “low-risk.” In model estimates of incidence and prevalence, heterosexual transmission is in fact a highly aggregated category of risk and exposure that reveals very little about who is most at risk or why and often includes people who are bisexual in their practices (if not their identity). Epidemic models define the population sexual structure according to a number of behaviorally defined risk groups, e.g., “low-risk” heterosexual refers to men and women reporting only one sexual partner in the year preceding the survey from which size estimates were obtained.74,h In this context, risk is not differentiated by the type of sexual interaction (vaginal or anal), age-related characteristics, or age discrepancy in relationships (e.g., child sexual assault or early marriage), or the use of force (e.g., incest, gang rape, conflict-related rape), or relational typologies (e.g., survival sex, levirate marriage, polygamy, monogamy).
As a mode of exposure—i.e., a biological pathway for viral transmission—the genitoanal injuries that result from sexual transmission—forced and consensual—may help explain the significant underestimation of per-sex-act probabilities associated with heterosexual transmission and the disproportionate rates of infection among young women as compared to young men. Research, policy and practice must give greater attention to the interaction between the physiological and social cofactors that increase the vulnerability of young adolescent women, and to the potential impact of sexual violence prevention on reducing HIV incidence within and across different subpopulations.
The Greentree Meeting proposed three priority directions for policy and practice:
Young adolescent women: The cluster of physiological variables that place young adolescent women at disproportionate risk is further augmented by the social factors that determine the type of relationships they have and their likelihood of experiencing forced and early sex. Early coital debut (consensual, forced, and coerced) and age difference between partners are significant predictors of HIV infection12,78–82 and studies show that adolescents in early and forced marriages face a higher risk of HIV infection, other STIs, and obstetric fistula due to early childbearing.15,47,83,84 DHS data from 29 countries in Africa and Latin America indicate that more than 80% of married adolescent girls between the ages of 15 and 19 have unprotected sex with their partners and are pressured to become pregnant.47 Most have older husbands with an average of between 5 and 11 years age difference, and a large number are junior wives in polygamous unions. For these reasons, prevention focused on condoms and abstinence is likely to have little impact. Program and policy attention to the links between sexual violence and HIV needs to extend beyond intimate partner violence to include family violence, and especially child sexual assault, infant rape, and incest. Specialized strategies are needed to reach young adolescent women that take into account their particular risks and needs and the social and institutional contexts that may be protective or increase their vulnerability, including the longer term physiological, social, and behavioral effects of early sexual debut and forced sex.
Conflict-affected situations: In conflict situations, women and girls are at risk of sexual violence in their homes, in public spaces, marketplaces, at work, in the fields, in camps, and in so-called safe havens: schools, health facilities, and places of worship.85,86 Poverty and desperation may force them to exchange sex for survival, to cross borders, or even for protection. The same conditions that increase the risk of sexual and gender-based violence can also facilitate the spread of HIV, including the use of rape as an instrument of war, genocide, and/or displacement and the breakdown of protective family and community structures.87 Who is at risk and why will be shaped fundamentally by the risk environment, including overall HIV and SV prevalence, as well as the availability of and access to services, including condoms, emergency and reproductive health care, treatment for STIs and tuberculosis (TB), safe blood, and uninterrupted access to antiretroviral treatment.88 Greater understanding is also needed about the characteristics of perpetrators, including their likelihood of using condoms, and/or of being at greater risk of HIV and other STIs, and how their motivations may increase transmission risk. For example, in armed conflicts, rape may be used as an instrument of war or torture, and there is increased likelihood of multiple perpetrators and victims, trafficking, and injuries resulting from the use of weapons, objects, and multiple perpetrations in coerced sex. There is a great need to improve emergency reproductive health responses in the context of humanitarian, postconflict transitional, and peacekeeping environments.89
Epidemic modeling: Epidemiologists and other modelers are ideally placed to bridge the emerging questions in biomedical research with an already robust body of social and behavioral science on HIV, sexual violence, and gender. Introducing new assumptions and more precise parameter estimates into HIV epidemic modeling in real time—as the science evolves—will dramatically improve our understanding about epidemic drivers and the potential impact of interventions in different contexts. Collaboration is needed among basic science, clinical, social, and behavioral HIV researchers and epidemic modelers in the following areas: (1) to assess the likely relative contribution of different genitoanal injuries to the force of infection—i.e., the rate at which susceptible individuals become infected per unit time; (2) to assess the relative contribution of age-related anatomic and physiological factors that place young girls and adolescents at increased risk; (3) to assess the relative contribution of genitoanal injury on important predictive variables such as “risk behavior” and “modes of exposure,” and “high- and low- risk” heterosexual transmission; (4) to demonstrate the relative contribution of SV to HIV transmission, acquisition, and pathogenesis, and the intersection between different “typologies” of SV and the subpopulations that are predisposed to increased risk; and (5) to assess the design of tailored programs and combination prevention interventions that are prioritized to specific populations and are most likely to reduce the risk of SV and its potential contribution to HIV transmission. The results will also help identify the most critical areas of needed data collection that are likely to have a significant impact on the field and begin to signal directions for targeting interventions.
Conclusions
The contribution of sexual violence and genitoanal injury to HIV transmission and acquisition has not yet been fully understood or integrated into the global AIDS response, even though it plays a potentially significant role in the development of HIV epidemics, particularly among young women. Causal pathways between sexual violence and genitoanal injury and HIV infection are complex, and involve a range of biological, behavioral, and social factors that must be explored simultaneously. Participants at the Greentree Scientific Research Planning Meeting identified a robust agenda for interdisciplinary research, cognizant of its applications to program and policy, that should be pursued imminently.
Greentree Meeting Participants
Kathryn Anastos, Albert Einstein College of Medicine; Letitia Anderson, UN Action Against Sexual Violence in Conflict; Judy Auerbach, Independent Consultant; Marie-Claude Boily, Imperial College; Jacquelyn Campbell, Johns Hopkins University; Michele Decker, Johns Hopkins University; Khady Diouf, Brigham and Women's Hospital; Kristin Dunkle, Emory University; Anneka Ehrnst, Karolinska Institute; Aissatou Gaye-Diallo, Universite Cheikh Anta DIOP; Nancy Glass, Johns Hopkins University; Serigne Magueye Gueye, University Cheikh Anta DIOP; Catherine Hankins, Amsterdam Institute for Global Health and Development; Betsy Herold, Albert Einstein College of Medicine; Amelia Hoover Green, Drexel University; Thomas Hope, Northwestern University; Mazeda Hossain, London School of Hygiene and Tropical Medicine; Jantine Jacobi, UNAIDS and Global Coalition on Women and AIDS; Rowena Johnston, The American Foundation for AIDS Research; Charu Kaushic, McMaster University; Jennifer F. Klot, Social Science Research Council; Kathryn Laughon, University of Virginia; Souleymane Mboup, Universite Cheikh Anta Diop; Velda Mushangwe-Mtisi, University of Zimbabwe Medical School; Ragnhild Nordås, Peace Research Institute Oslo; Carel Pretorius, Futures Institute; Sengeziwe Sibeko, University of Oxford; Papa Salif Sow, University of Dakar; Christina Thobakgale, University of KwaZulu-Natal; Fulvia Veronese, National Institutes for Health; Charlotte Watts, London School of Hygiene and Tropical Medicine; Alex Welte, South African Centre for Epidemiological Modelling and Analysis, Stellenbosch University; Charles Wira, Dartmouth Medical School.
The authors would also like to recognize the following people who contributed to developing this agenda through personal communications:
Sally Blower, University of California, Los Angeles; Gina Brown, National Institutes for Health; Judith Bruce, Population Council; Cynthia Buckley, University of Illinois; Mardge Cohen, Stroger Hospital; Susan Cu-Uvin, Brown University; Berthilde Gahongayire, UNAIDS; Geoff Garnett, the Gates Foundation; Simon Gregson, Imperial College; Timothy Hallett, Imperial College; Gillian Holmes, UN Office for Disaster Risk Reduction; Quarraisha Abdool Karim, University of KwaZulu-Natal; Rupert Kaul, University of Toronto; Leo Kenny, UNAIDS; Alan Landay, Rush University Medical Center; Ahuka Ona Longombe, University of Kisangani and Panzi General Referral Hospital, Democratic Republic of Congo; Daniela Ligiero, President's Emergency Plan For AIDS Relief; Michele Moloney-Kitts, Together for Girls, UNAIDS; Nelly Mugo, International Clinical Research Center and Kenyatta National Hospital; Susan Newcomer, National Institutes for Health; Nawal M. Nour, Harvard Medical School; Melissa Robbiani, Population Council; Joseph Ruminjo, Engender Health; Samira Sami, (CDC); Barbara Shacklett, University of California, Davis; John Stover, Futures Institute; Nertila Tavanxhi, Marleen Temmerman, World Health Organization; Basia Tomczyk, CDC; Jim Turpin, National Institutes for Health; Ron Veazey, Tulane Medical School; Gilbert Wembodinga, Panzi General Referral Hospital, Democratic Republic of Congo.
Footnotes
This meeting explored the physiological cofactors of sexual violence that increase the risk of HIV acquisition and progression among women and girls. Violence—sexual, verbal, and physical assault—can also be a consequence of disclosing HIV status.
It is recognized that different forms of sexual violence and exploitation take place among men and women, boys and girls. The focus of this meeting, however, was given to understanding the implications of genitoanal injury for HIV transmission risk, acquisition, and pathogenesis among girls and women in the context of both consensual and nonconsensual sex.
For example, during the conflict in eastern Congo, so many cases of traumatic fistula caused by systematic, violent gang rape were reported that the destruction of the vagina is now considered a war injury and is recorded by doctors as a crime of combat.
For example, colposcopy and toluidine dye reveal more injuries than direct visualization.90 Digital photography, a much less expensive alternative, offers similarly high-quality imaging.91
Although there is no standardized reporting, most genital injuries identified in nonconsensual sex occur in the posterior fourchette, hymen, labia minora, cervix, urethrea/periurethra, anus, vagina, and rectum.
For the purpose of this discussion, FGM/C was considered as an anatomic risk factor (rather than a form of sexual violence) that might exacerbate risks resulting from genital trauma.
Key populations are those that are key to the dynamics of the HIV epidemic in a geographic area and key to the response. Effective HIV prevention with respect to key populations is built on a foundation of meaningful engagement and involvement of these populations.
High-risk heterosexual refers to men and women reporting more than one sexual partner in the year preceding a given survey. The efficacy of reporting mechanisms of sexual violence vary considerably. It is less likely that a forced sexual encounter—which may pose a high risk of exposure—is included among the number of sexual partners that are self-reported by sexual assault survivors
Acknowledgments
Funding for this meeting was provided by the National Institutes of Health, Office of AIDS Research; UNAIDS, UN Action Against Sexual Violence in Armed Conflict and the Greentree Foundation. The authors wish to thank SSRC research assistants and meeting organizers, Miranda Berry, Patience Mungwari, and April Pei.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andersson N. Cockcroft A. Shea B. Gender-based violence and HIV: Relevance for HIV prevention in hyperendemic countries of southern Africa. www.ncbi.nlm.nih.gov/pubmed/19033757. [Jan 23;2012 ];AIDS. 2008 22(Suppl 4):S73–S86. doi: 10.1097/01.aids.0000341778.73038.86. [DOI] [PubMed] [Google Scholar]
- 2.Maman S. Campbell J. Sweat MD. Gielena C. The intersections of HIV and violence: Directions for future research and interventions. Soc Sci Med. 2000;50(4):459–478. doi: 10.1016/s0277-9536(99)00270-1. [DOI] [PubMed] [Google Scholar]
- 3.Silverman JG. Key to prevent HIV in women: Reduce gender-based violence. Lancet. 2010;376(9734):6–7. doi: 10.1016/S0140-6736(10)60971-3. [DOI] [PubMed] [Google Scholar]
- 4.Jewkes R. Sikweyiya Y. Morrell R. Dunkle K. The relationship between intimate partner violence, rape and HIV amongst South African men: A cross-sectional study. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3173408&tool=pmcentrez&rendertype=abstract. [Nov 24;2011 ];PloS One. 2011 6(9):e24256. doi: 10.1371/journal.pone.0024256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunkle K. Jewkes R. Brown H. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. www.lancet.com/journals/lancet/article/PIIS0140-6736(04)16098-4. [May 29;2012 ];Lancet. 2004 363(9419):1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 6.Townsend L. Jewkes R. Mathews C, et al. HIV risk behaviours and their relationship to intimate partner violence (IPV) among men who have multiple female sexual partners in Cape Town, South Africa. AIDS Behav. 2011;15(1):132–141. doi: 10.1007/s10461-010-9680-5. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JC. Baty ML. Ghandour RM, et al. The intersection of intimate partner violence against women and HIV/AIDS: A review. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3274697&tool=pmcentrez&rendertype=abstract. [Nov 28;2011 ];Int J Inj Contr Saf Promot. 2008 15(4):221–231. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jewkes R. Dunkle K. Nduna M. Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: A cohort study. www.ncbi.nlm.nih.gov/pubmed/20557928. [Jul 14;2011 ];Lancet. 2010 376(9734):41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 9.Decker MR. Iii GRS. Hemenway D, et al. Intimate partner violence functions as both a risk marker and risk factor for women's HIV infection: Findings from Indian husband–wife dyads. J Acquir Immun Defic Syndr. 2009;51(5):593–600. doi: 10.1097/QAI.0b013e3181a255d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockman JK. Campbell JC. Celentano DD. Sexual violence and HIV risk behaviors among a nationally representative sample of heterosexual American women: The importance of sexual coercion. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2799543&tool=pmcentrez&rendertype=abstract. [Jun 14;2011 ];J Acquir Immun Defic Syndr. 2010 53(1):136–143. doi: 10.1097/QAI.0b013e3181b3a8cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts CH. Foss AM. Hossain M, et al. Sexual violence and conflict in Africa: Prevalence and potential impact on HIV incidence. http://sti.bmj.com.ezproxy.uct.ac.za/content/86/Suppl_3/iii93.full.pdf+html?sid=9503f514-cdde-41fb-9e32-3aa4f953f09f. [Sep 7;2011 ];Sex Trans Infect. 2010 86(Suppl 3):93–99. doi: 10.1136/sti.2010.044610. [DOI] [PubMed] [Google Scholar]
- 12.Ybarra ML. Bull SS. Kiwanuka J. Bangsberg DR. Korchmaros J. Prevalence rates of sexual coercion victimization and perpetration among Uganda adolescents. www.ncbi.nlm.nih.gov/pubmed/22299764. [Feb 16;2012 ];AIDS Care. 2012 Feb;:37–41. doi: 10.1080/09540121.2011.648604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvard School of Public Health: Program on International Health and Human Rights. HIV/AIDS and Gender-Based Violence (GBV) Literature Review. Boston: 2006. pp. 1–44. [Google Scholar]
- 14.Kayibanda JF. Bitera R. Alary M. Violence toward women, men's sexual risk factors, and HIV infection among women: Findings from a national household survey in Rwanda. www.ncbi.nlm.nih.gov/pubmed/22227491. J Acquir Immun Defic Syndr. 2012;59(3):300–307. doi: 10.1097/QAI.0b013e31823dc634. [DOI] [PubMed] [Google Scholar]
- 15.Undie C. Addressing sexual violence, HIV risk among married adolescent girls in rural Nyanza, Kenya. 2011. www.popcouncil.org/publications/serialsbrief/TABriefs.asp www.popcouncil.org/publications/serialsbrief/TABriefs.asp
- 16.Supervie V. Halima Y. Blower S. Assessing the impact of mass rape on the incidence of HIV in conflict-affected countries. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2978669&tool=pmcentrez&rendertype=abstract. [Sep 19;2011 ];AIDS. 2010 24(18):2841–2847. doi: 10.1097/QAD.0b013e32833fed78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merbah M. Introini A. Fitzgerald W, et al. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3184648&tool=pmcentrez&rendertype=abstract. [Jul 27;2011 ];Am J Reprod Immunol. 2011 65(3):268–278. doi: 10.1111/j.1600-0897.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boily MC. Baggaley RF. Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. www.sciencedirect.com/science/article/pii/s1473-3099(09)70021-0. [Feb 23;2012 ];Lancet Infect Dis. 2009 9(2):118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J. Fidler S. Sexual transmission of HIV-1. www.ncbi.nlm.nih.gov/pubmed/19874852. [Feb 29;2012 ];Antiviral Res. 2010 85(1):276–285. doi: 10.1016/j.antiviral.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Powers KA. Poole C. Pettifor AE. Cohen MS. Rethinking the heterosexual infectivity of HIV-1: A systematic review and meta-analysis. Lancet Infect Dis. 2009;8(9):553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeten JM. Benki S. Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21(13):1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 22.Heffron R. Donnell D. Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3266951&tool=pmcentrez&rendertype=abstract. [Oct 4;2011 ];Lancet Infect Dis. 2011 12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison CS. Chen P-L. Kwok C, et al. Hormonal contraception and HIV acquisition: Reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wira CR. Patel MV. Ghosh M. Mukura L. Fahey JV. Innate immunity in the human female reproductive tract: Endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65(3):196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahey JV. Wright JA. Shen L, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. www.nature.com.ezproxy.wesleyan.edu:7790/mi/journal/v1/n4/full/mi200820a.html. [Apr 26;2012 ];Mucos Immunol. 2008 1(4):317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wira CR. Fahey JV. A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. www.ncbi.nlm.nih.gov/pmc/articles/PMC2647143/ AIDS. 2008;22(15):1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharkey DJ. Tremellen KP. Jasper MJ. Gemzell-Danielsson K. Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. www.jimmunol.org/cgi/content/abstract/188/5/2445. [Apr 17;2012 ];J Immunol. 2012 188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 28.Sharkey DJ. Macpherson AM. Tremellen KP. Robertson S. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. www.ncbi.nlm.nih.gov/pubmed/17483528. [Apr 18;2012 ];Mol Hum Reprod. 2007 13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 29.Myer L. Wright TC. Denny L. Kuhn L. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Trans Dis. 2006;33(11):683–687. doi: 10.1097/01.olq.0000216026.67352.f9. [DOI] [PubMed] [Google Scholar]
- 30.Anderson S. McClain N. Riviello RJ. Genital findings of women after consensual and nonconsensual intercourse. www.ncbi.nlm.nih.gov/pubmed/17073065. J Forensic Nurs. 2006;2(2):59–65. doi: 10.1111/j.1939-3938.2006.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JC. Sheridan DJ. Female genital injury following consensual, nonconsensual sex: State of the science. http://dx.doi.org/10.1016/j.jen.2010.10.014. [Feb 20;2012 ];J Emerg Nurs. 2011 doi: 10.1016/j.jen.2010.10.014. in press. [DOI] [PubMed] [Google Scholar]
- 32.McLean I. Roberts SA. White C. Paul S. Female genital injuries resulting from consensual and non-consensual vaginal intercourse. http://dx.doi.org/10.1016/j.forsciint.2010.04.049. [Feb 20;2012 ];Forensic Sci Int. 2011 204(1–3):27–33. doi: 10.1016/j.forsciint.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Hilden M. Schei B. Sidenius K. Genitoanal injury in adult female victims of sexual assault. www.ncbi.nlm.nih.gov/pubmed/16182966. [Nov 18;2011 ];Forensic Sci Int. 2005 154(2–3):200–205. doi: 10.1016/j.forsciint.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Slaughter L. Brown CR. Crowley S. Peck R. Patterns of genital injury in female sexual assault victims. www.ncbi.nlm.nih.gov/pubmed/9077615. [Jun 16;2011 ];Am J Obstet Gynecol. 1997 176(3):609–616. doi: 10.1016/s0002-9378(97)70556-8. [DOI] [PubMed] [Google Scholar]
- 35.Jones JS. Rossman L. Wynn BN. Dunnuck C. Schwartz N. Comparative analysis of adult versus adolescent sexual assault: Epidemiology and patterns of anogenital injury. www.ncbi.nlm.nih.gov/pubmed/12896889. Acad Emerg Med. 2003;10(8):872–877. doi: 10.1111/j.1553-2712.2003.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 36.Sugar N. Fine D. Eckert L. Physical injury after sexual assault: Findings of a large case series. http://linkinghub.elsevier.com/retrieve/pii/S0002937803009128. [Apr 18;2012 ];Am J Obstet Gynecol. 2004 190(1):71–76. doi: 10.1016/s0002-9378(03)00912-8. [DOI] [PubMed] [Google Scholar]
- 37.Baker RB. Sommers MS. Relationship of genital injuries, age in adolescent, young adult rape survivors. www.ncbi.nlm.nih.gov/pubmed/18507599. [Jul 24;2012 ];J Obst Gynecol Neonat Nurs. 37(3):282–289. doi: 10.1111/j.1552-6909.2008.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones JS. Rossman L. Diegel R. Van Order P. Wynn BN. Sexual assault in postmenopausal women: Epidemiology and patterns of genital injury. http://dx.doi.org/10.1016/j.ajem.2008.07.010. [Aug 25;2011 ];Am J Emerg Med. 2009 27(8):922–929. doi: 10.1016/j.ajem.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Anderson SL. Parker BJ. Bourguignon CM. Predictors of genital injury after nonconsensual intercourse. www.ncbi.nlm.nih.gov/pubmed/20118876. [Feb 8;2012 ];Adv Emerg Nurs J. 2009 31(3):236–247. doi: 10.1097/TME.0b013e3181afd306. [DOI] [PubMed] [Google Scholar]
- 40.Anderson SL. McClain N. Riviello RJ. Genital findings of women after consensual and nonconsensual intercourse. www.ncbi.nlm.nih.gov/pubmed/17073065. J Forensic Nurs. 2006;2(2):59–65. doi: 10.1111/j.1939-3938.2006.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 41.Ickovics JR. Hamburger ME. Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 42.Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. http://linkinghub.elsevier.com/retrieve/pii/S0006322303003238. [Apr 18;2012 ];Biol Psychiatry. 2003 54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 43.Newton TL. Fernandez-Botran R. Miller JJ, et al. Markers of inflammation in midlife women with intimate partner violence histories. www.ncbi.nlm.nih.gov/pubmed/22044065. [Apr 18;2012 ];J Womens Health. 2011 20(12):1871–1880. doi: 10.1089/jwh.2011.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UNAIDS. Together we will end AIDS. 2012.
- 45.UNAIDS. Global AIDS epidemic facts and figures. 2012.
- 46.WHO, UNAIDS, UNICEF: Global HIV/AIDS Response. Epidemic update, health sector progress towards Universal Access. 2011.
- 47.Clark S. Bruce J. Dude A. Protecting young women from HIV/AIDS: The case against child and adolescent marriage. www.jstor.org/stable/10.2307/4147596. [Apr 25;2012 ];Int Fam Plan Perspect. 2006 32(2):79–88. doi: 10.1363/3207906. [DOI] [PubMed] [Google Scholar]
- 48.Bruce J. Temin M. Hallman K. Evidence-based approaches to protecting adolescent girls at risk of HIV. 2012. www.aidstar-one.com/focus_areas/gender/resources/spotlight/evidence_based_approaches_protecting_adolescent_girls_risk_hiv?utm_source=blog&utm_medium=social&utm_content=SPOTLIGHTADO&utm_campaign=Afro www.aidstar-one.com/focus_areas/gender/resources/spotlight/evidence_based_approaches_protecting_adolescent_girls_risk_hiv?utm_source=blog&utm_medium=social&utm_content=SPOTLIGHTADO&utm_campaign=Afro
- 49.Joint United Nations Programme on HIV/AIDS (UNAIDS) Joint United Nations Programme on HIV/AIDS: Agenda for accelerated country action for women, girls, gender equality, HIV: Operational plan for the UNAIDS action framework. Geneva, Switzerland: 2010. [Google Scholar]
- 50.Bruce J. Girls Left Behind: Redirecting HIV Interventions Toward the Most Vulnerable. Population Council; New York: 2007. [Google Scholar]
- 51.Pettifor AE. Hudgens MG. Levandowski B. Rees HV. Cohen MS. Highly efficient HIV transmission to young women in South Africa. www.ncbi.nlm.nih.gov/pubmed/17415041. AIDS. 2007;21(7):861–865. doi: 10.1097/QAD.0b013e3280f00fb3. [DOI] [PubMed] [Google Scholar]
- 52.Centers for DiseaseControl and Prevention. Sexually Transmitted Disease Surveillance. U.S. Department of Health and Human Services; Atlanta: 2009. 2010. [Google Scholar]
- 53.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance. U.S. Department of Health and Human Services; Atlanta: 2010. 2011. [Google Scholar]
- 54.Moscicki A-B. Singer A. The Cervix. Blackwell Publishing Ltd; Oxford, UK: 2009. The cervical epithelium during puberty and adolescence; pp. 81–101. [Google Scholar]
- 55.Hwang L. Scott M. Ma Y, et al. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3049722&tool=pmcentrez&rendertype=abstract. [Apr 18;2012 ];J Reprod Immunol. 2011 88(1):66–71. doi: 10.1016/j.jri.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang LY. Ma Y. Benningfield SM, et al. Factors that influence the rate of epithelial maturation in the cervix in healthy young women. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2662755&tool=pmcentrez&rendertype=abstract. [Mar 12;2012 ];J Adolesc Health. 2009 44(2):103–110. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández-Romero J. Abraham CJ. Rodriguez A, et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3256046&tool=pmcentrez&rendertype=abstract. [Sep 2;2012 ];Antimicrob Agents Chemother. 2012 56(1):358–368. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickson N. van Roode T. Herbison P, et al. Risk of herpes simplex virus type 2 acquisition increases over early adulthood: Evidence from a cohort study. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2598626&tool=pmcentrez&rendertype=abstract. [Sep 2;2012 ];Sex Trans Infect. 2007 83(2):87–90. doi: 10.1136/sti.2006.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monjok E. Essien EJ. Holmes L., Jr Female genital mutilation: Potential for HIV transmission in sub-Saharan Africa and prospect for epidemiologic investigation and intervention. Afr J Reprod Health. 2007;11(1):33–42. [PubMed] [Google Scholar]
- 60.Almroth L. Almroth-Berggren V. Hassanein OM, et al. Male complications of female genital mutilation. www.ncbi.nlm.nih.gov/pubmed/11710420. Soc Sci Med. 2001;53(11):1455–1460. doi: 10.1016/s0277-9536(00)00428-7. [DOI] [PubMed] [Google Scholar]
- 61.Gutman LT. Herman-Giddens ME. McKinney RE. Pediatric acquired immunodeficiency syndrome. Barriers to recognizing the role of child sexual abuse. www.ncbi.nlm.nih.gov/pubmed/8322751. [Jul 24;2012 ];Am J Dis Child. 1993 147(7):775–780. doi: 10.1001/archpedi.1993.02160310077023. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson A. Morris C. Assessing the role of anal intercourse in the epidemiology of AIDS in Africa. www.ncbi.nlm.nih.gov/pubmed/14678597. Int J STD AIDS. 2003;14(12):856. doi: 10.1258/095646203322556228. [DOI] [PubMed] [Google Scholar]
- 63.Baggaley RF. White RG. Boily M-C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2929353&tool=pmcentrez&rendertype=abstract. [Sep 19;2011 ];Int J Epidemiol. 2010 39(4):1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin F. Jansson J. Law M, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2852627&tool=pmcentrez&rendertype=abstract. [Jul 24;2012 ];AIDS. 2010 24(6):907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levy JA. The transmission of HIV and factors influencing progression to AIDS. http://linkinghub.elsevier.com/retrieve/pii/000293439390237J?showall=true. JAMA. 1993;95(1):86–100. doi: 10.1016/0002-9343(93)90237-j. [DOI] [PubMed] [Google Scholar]
- 66.Topping AA. Milhausen RR. Graham C, et al. A comparison of condom use errors and problems for heterosexual anal and vaginal intercourse. www.ncbi.nlm.nih.gov/pubmed/21515752. [Apr 30;2012 ];Int J STD AIDS. 2011 22(4):204–208. doi: 10.1258/ijsa.2011.010259. [DOI] [PubMed] [Google Scholar]
- 67.Baldwin JI. Baldwin JD. Heterosexual anal intercourse: An understudied, high-risk sexual behavior. www.ncbi.nlm.nih.gov/pubmed/10948725. Arch Sexual Behav. 2000;29(4):357–373. doi: 10.1023/a:1001918504344. [DOI] [PubMed] [Google Scholar]
- 68.Stadler JJ. Delany S. Mntambo M. Sexual coercion and sexual desire: Ambivalent meanings of heterosexual anal sex in Soweto, South Africa. www.ncbi.nlm.nih.gov/pubmed/18071961. [Apr 30;2012 ];AIDS Care. 2007 19(10):1189–1193. doi: 10.1080/09540120701408134. [DOI] [PubMed] [Google Scholar]
- 69.Tucker S. Krishna R. Prabhakar P. Panyam S. Anand P. Exploring dynamics of anal sex among female sex workers in Andhra Pradesh. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3326863&tool=pmcentrez&rendertype=abstract. [Apr 30;2012 ];Indian J Sex Trans Dis. 2012 33(1):9–15. doi: 10.4103/2589-0557.93787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams JA. Girardin B. Faugno D. Adolescent sexual assault: Documentation of acute injuries using photo-colposcopy. Pediatr Adolesc Gynecol. 2001;14(4):175–180. doi: 10.1016/s1083-3188(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 71.Yount KM. Abraham BK. Female genital cutting and HIV/AIDS among Kenyan women. Studies Fam Plan. 2007;38(2):73–88. doi: 10.1111/j.1728-4465.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 72.Brady M. Female genital mutilation: Complications and risk of HIV transmission. AIDS Patient Care STDS. 1999;13(12):709–716. doi: 10.1089/apc.1999.13.709. [DOI] [PubMed] [Google Scholar]
- 73.Boily M. Dimitrov D. Abdool Karim S. Masse B. The future role of rectal and vaginal microbicides to prevent HIV infection in heterosexual populations: Implications for product development and prevention. http://bmj-sti.highwire.org/content/87/7/646.abstract. [Feb 23;2012 ];Sex Transm Infect. 2011 87(7):646–653. doi: 10.1136/sextrans-2011-050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mishra S. Sgaier SK. Thompson LH, et al. HIV epidemic appraisals for assisting in the design of effective prevention programmes: Shifting the paradigm back to basics. http://dx.plos.org/10.1371/journal.pone.0032324. [Jul 18;2012 ];PloS One. 2012 7(3):e32324. doi: 10.1371/journal.pone.0032324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gouws E. Estimating the distribution of new HIV infections by mode of transmission. www.unaids.org/en/dataanalysis/tools/incidencebymodesoftransmission/ SACEMA Q. 2010;(April):1–3. [Google Scholar]
- 76.UNAIDS. Modes of Transmission Study: Guidelines for country teams. 2008. Version 12:60.
- 77.Colvin M. Gorgens-Albino M. Kasedde S. Analysis of HIV prevention response and modes of HIV transmission: The UNAIDS-GAMET supported synthesis process. Joint U.N. Programme on HIV/AIDS; Geneva: 2008. p. 9. [Google Scholar]
- 78.Erulkar BA. Ferede A. Social exclusion and early or unwanted sexual initiation among poor urban females in Ethiopia. Int Perspect Sex Reprod Health. 2009;35(4):186–193. doi: 10.1363/ipsrh.35.186.09. [DOI] [PubMed] [Google Scholar]
- 79.Pettifor AE. van der Straten A. Dunbar MS. Shiboski SC. Padian NS. Early age of first sex. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00002030-200407020-00010. [Apr 16;2012 ];AIDS. 2004 18(10):1435–1442. doi: 10.1097/01.aids.0000131338.61042.b8. [DOI] [PubMed] [Google Scholar]
- 80.Erulkar AS. The experience of sexual coercion among young people in Kenya. Int Fam Plan Perspect. 2004;30(4):182–189. doi: 10.1363/3018204. [DOI] [PubMed] [Google Scholar]
- 81.Lang DL. Sales JM. Salazar LF, et al. Rape victimization and high risk sexual behaviors: Longitudinal study of African-American adolescent females. www.biomedsearch.com/attachments/00/21/73/17/21731791/wjem12_3p0333.pdf. [Jan 10;2012 ];West J Emerg Med. 2011 12(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 82.Lalor K. Child sexual abuse and HIV transmission in sub-Saharan Africa. http://doi.wiley.com/10.1002/car.1020. [Feb 10;2012 ];Child Abuse Rev. 2008 17(2):94–107. [Google Scholar]
- 83.Mensch BS. Clark WH. Lloyd CB. Erulkar S. Premarital sex, schoolgirl pregnancy, and school quality in rural Kenya. www.ncbi.nlm.nih.gov/pubmed/11831048. Stud Fam Plann. 2001;32(4):285–301. doi: 10.1111/j.1728-4465.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 84.Nour NM. Health consequences of child marriage in Africa. www.ncbi.nlm.nih.gov/pubmed/17283612. Emerg Infect Dis. 2006;12(11):1644–9. doi: 10.3201/eid1211.060510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson BD. Collins L. VanRooyen MJ, et al. Impact of sexual violence on children in the Eastern Democratic Republic of Congo. http://dx.doi.org/10.1080/13623699.2011.645148. [Feb 20;2012 ];Med Confl Surviv. 2011 27(4):211–225. doi: 10.1080/13623699.2011.645148. [DOI] [PubMed] [Google Scholar]
- 86.Bartels S. Scott J. Mukwege D, et al. Patterns of sexual violence in Eastern Democratic Republic of Congo: Reports from survivors presenting to Panzi Hospital in 2006. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2883538&tool=pmcentrez&rendertype=abstract. Confl Health. 2010;4(May):9. doi: 10.1186/1752-1505-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.United Nations. United Nations General Assembly Security Council; 2012. Conflict-related sexual violence: Report of the Secretary General. [Google Scholar]
- 88.Klot J. Delargy P. Sexual violence and HIV/AIDS transmission. Force Migr Rev. 2010;(27):2. [Google Scholar]
- 89.Waal A de. Klot J. Mahajan M. Huber D. HIV/AIDS, Security, Conflict: New Realities, New Responses. SSRC; Brooklyn, NY: 2010. [Aug 22;2012 ]. [Google Scholar]
- 90.Zink T. Fargo JD. Baker RB, et al. Comparison of methods for identifying ano-genital injury after consensual intercourse. www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2917333&tool=pmcentrez&rendertype=abstract. [Feb 13;2012 ];J Emerg Med. 2010 39(1):113–118. doi: 10.1016/j.jemermed.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ernst EJ. Speck PM. Fitzpatrick JJ. Evaluation of image quality of digital photo documentation of female genital injuries following sexual assault. J Forensic Nurs. 2011;7(4):182–189. doi: 10.1111/j.1939-3938.2011.01103.x. [DOI] [PubMed] [Google Scholar]