Abstract
The autonomic nervous system provides both cholinergic and noncholinergic neural inputs to end organs within the airways, which includes the airway and vascular smooth muscle. Heightened responsiveness of the airways to bronchoconstrictive agents is a hallmark feature of reactive airways diseases. The mechanisms underpinning airways hyperreactivity still largely remain unresolved. In this paper we summarize the substantial body of evidence that implicates dysfunction of the autonomic nerves that innervate smooth muscle in the airways and associated vasculature as a prominent cause of airways hyperresponsiveness in asthma.
1. Introduction
With the exception of airway smooth muscle, perhaps no other group of cells has as clear a role in the pathogenesis of asthma as the neurons comprising the afferent and efferent innervation of the airways and lungs. The symptoms of asthma—wheezing, dyspnea, chest tightness, cough, reversible airways obstruction, mucus hypersecretion, and airways hyperresponsiveness—all inextricably link the nervous system to this disease. It is thus remarkable that in the 440 pages of the National Heart, Lung and Blood Institute (NHLBI) guidelines on asthma, nerves are mentioned in just one sentence [1]. Nerves are not mentioned at all in the British Thoracic Society (BTS) guidelines for asthma [2]. Even the recent and potentially landmark study by Peters et al. [3], in which the anticholinergic tiotropium was found to be at least as good as steroids or β-agonists (perhaps better) for treatment of asthma, nerves are not mentioned in the article itself nor in the accompanying editorial [4]. In this brief review we summarize the large body of evidence supporting a primary role for airway autonomic nerve dysfunction in the hyperresponsiveness of the airway smooth muscle in asthma.
2. The Understated Role of Nerves in Asthma
Guidelines such as those produced by the NHLBI and BTS, in which immune cells including eosinophils are given a central role in asthma pathogenesis appropriately highlight the prominent feature of inflammation in the asthmatic lung. Inflammation may precipitate airways hyperresponsiveness [1, 5–8]. But the association between inflammation and airways hyperresponsiveness has probably been overemphasized [9–13]. The bias towards inflammation in asthma guidelines reveals the disproportionate influence immunologists, and allergists have had overdefining this disease for national and international medical organizations as well as their influence over the direction of asthma-related research. As asthma prevalence and asthma mortality rates have remained largely unchanged in the decades where inflammation has become a central theme in asthma research and therapy, it may be time for a new perspective on old concepts of the pathogenesis of reactive airways disease.
Given the strong case for neural dysfunction in asthma, it is surprising how little attention airway nerves receive in the published literature. The under emphasis on nerves in asthma and the exaggerated influence of inflammation in asthma can be illustrated by comparing the scant references to neural mechanisms in this disease with the incessant discussions of eosinophils in asthma guidelines and in all of asthma-related literature. This is especially surprising, given how strong the evidence is in favor of neural mechanisms in asthma and how comparatively weak the evidence is supporting a role for eosinophils in this disease. Even the most ardent proponents might struggle to make a strong case for a role of eosinophils in asthma. There is no increase in the risk of asthma for patients with hypereosinophilic syndrome [14]. Nonasthmatic atopic patients develop a profound eosinophilia of the airways upon exposure to allergen but develop few if any of the symptoms of asthma [15, 16]. Many asthmatics have eosinophil levels in their airways and air spaces that are comparable to that of nonasthmatics [5, 6, 17]. Experimental therapies such as anti-IL-5 and recombinant IL-12 have a profound effect on circulating and airway eosinophil numbers and on allergen-induced recruitment of eosinophils to the airways but little or no effect on asthma symptoms in most patients and no effect on airways reactivity [18–21]. Even steroids, which markedly inhibit eosinophil function, survival and recruitment to the airways, have only modest effects on airways hyperresponsiveness [22]. And yet, in spite of what seems to be a clear role for the nervous system in asthma and at best a debatable role for eosinophils, nerves are mentioned in one sentence combined in the NHLBI and BTS guidelines while these same guidelines, cite eosinophils 85 and 43 times, respectively. This imbalance is pervasive in the published literature as well. Since 2001, several years after Leckie and colleagues reported their disappointing results with anti-IL-5, there have been over 4600 papers published with the keywords of “asthma” and “eosinophil” but less than 450 papers with the keywords “asthma” and “nerve”. Indeed, there have been more papers published with the keywords “eotaxin” and “asthma” over the past 10 years than papers with the keywords “asthma” or “COPD” and “nerves” combined.
The mechanisms of airways hyperresponsiveness are poorly understood. It seems all but certain that smooth muscle is central to regulating airways reactivity. However, studies of airways smooth muscle contractility in vitro, conducted using airways obtained from asthmatic and nonasthmatic lung donors, yield results that are somewhat varied [23–28]. Thus, an argument can be made that neither smooth muscle contractility (efficacy) nor responsiveness (potency) differs to any great extent between airways obtained from diseased or nondiseased patients. Bronchodilators are also largely equally effective in smooth muscle from asthmatics and nonasthmatics [29, 30]. The defect in asthma may therefore manifest only within the context of the intact body and lung.
If, however, we accept the evidence in support of the hypothesis that airway smooth muscle accounts in large part for the most defining pathophysiological features of asthma (reversible airways obstruction and airways hyperresponsiveness) it is then critical to determine what ultimately regulates airway smooth muscle contraction. Airway smooth muscle generates little myogenic tone and so contraction depends upon the actions of contractile agonists. Despite an extensive list of autacoids and neurotransmitters that can contract human airway smooth muscle, a survey of the published literature suggests that only 3 endogenously released ligands, acetylcholine, histamine, and the cysteinyl-leukotrienes, reliably contract human airway smooth muscle to any significant extent and in physiologically relevant conditions in the airways of asthmatics. What so clearly defines the role of the nervous system in regulating the airways hyperresponsiveness in asthma is the indisputable source of the acetylcholine that regulates airway smooth muscle tone and the profound effects of anticholinergics on the airways obstruction and airways reactivity that define this disease.
3. Autonomic Innervation of Human Airway Smooth Muscle
The autonomic nervous system plays a primary role in regulating airway smooth muscle tone. The highly regulated activity of these nerves allows ongoing input to the airway smooth muscle such that basal tone is regulated on a breath by breath basis. The origin of this ongoing drive depends upon centrally (i.e., brainstem) mediated activity established by both respiratory and reflexive inputs [31–34]. In most animals and in humans, stimulation of airway autonomic nerves evokes near maximal constrictions of the airways through the actions of acetylcholine released from postganglionic parasympathetic nerves. Alternatively, activation of airway autonomic nerves can reverse completely spasmogen-evoked bronchoconstriction through the actions of noncholinergic neurotransmitters such as nitric oxide (NO) and vasoactive intestinal peptide (VIP) and related peptides. It follows logically that dysfunction or dysregulation of airway autonomic nerves is likely to contribute to the pathogenesis of asthma and COPD (reviewed in [35]).
For years it had been widely assumed that noncholinergic neurotransmitters mediating relaxations of the airways were coreleased with acetylcholine from a single population of postganglionic parasympathetic nerves. It was further speculated that these noncholinergic cotransmitters served as a brake on the parasympathetic nervous system, preventing excessive constriction during periods of elevated autonomic tone. Our studies have revealed, however, that anatomically and physiologically distinct parasympathetic nerves mediate cholinergic contractions and noncholinergic (nitrergic) relaxations of the airways [36–38]. Importantly, reflexes differentially regulate these distinct parasympathetic pathways [34, 39]. The existence of two parasympathetic pathways with opposing actions on the bronchial musculature changes entirely how autonomic nerve-dependent regulation of airway caliber should be viewed. Bronchospasm could be evoked by increases in cholinergic nerve activity or withdrawal of nitrergic neural activity. Conversely, increased nitrergic nerve activity or decreased cholinergic tone could elicit bronchodilatation. The role of the autonomic nervous system in disease must also now be viewed differently. With distinct neuronal pathways mediating contractions and relaxations of airway smooth muscle, dysfunction or dysregulation of either parasympathetic pathway could account for the alterations in airway tone associated with asthma and COPD (Figure 1).
Figure 1.
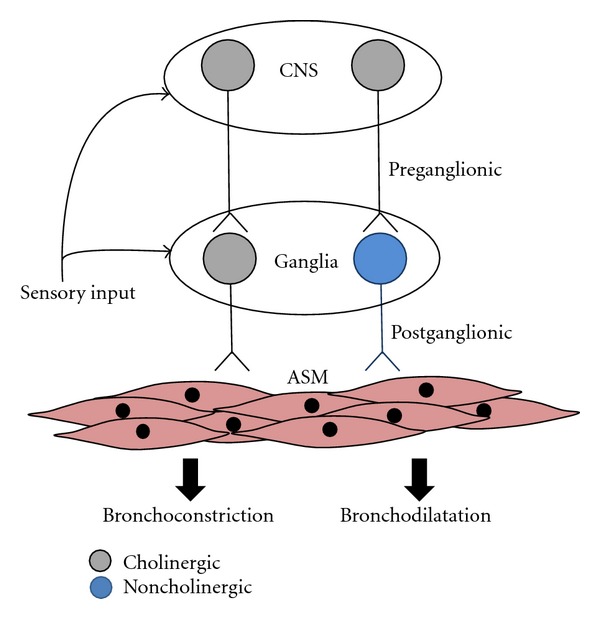
Two distinct vagal parasympathetic pathways regulate airway tone. Cholinergic preganglionic neurons originate in the brainstem and provide cholinergic drive to airway autonomic ganglia. Cholinergic postganglionic neurons are the major contractile input to the airways, whereas noncholinergic neurons expressing nitric oxide and vasoactive intestinal peptide provide the relaxant innervation to the airways. Airway sensory nerves contribute differential reflex regulation over cholinergic and non-cholinergic vagal pathways at the level of the brainstem and/or the airway ganglia. Dysfunction in ganglionic neurotransmission, neuromuscular transmission, or sensory reflexive control will precipitate changes in airway smooth muscle reactivity.
4. Autonomic Dysfunction and Asthma
There is indisputable evidence supporting the hypothesis that dysregulation of airway cholinergic nerves contributes to the pathogenesis of airways obstruction and airways hyperresponsiveness. Cholinergic nerve-mediated obstruction of the airways is increased in asthma and COPD [28, 40]. Airways hyperresponsiveness is also associated with alterations in cholinergic nerve function. Anticholinergics markedly reduce (10–20-fold) or abolish airways reactivity to a wide variety of spasmogens and stimuli including prostanoids, histamine, bradykinin, capsaicin, hyperpnea, exercises and allergen (reviewed in [35]; Table 1). Airways hyperresponsiveness associated with extrapulmonary disorders may also be dependent upon alterations in airway autonomic control. Bronchospasm initiated by gastroesophageal reflux or airways obstruction associated with allergic rhinitis is prevented by anticholinergics. Similarly, in patients with upper respiratory tract infections, the marked increases in airways reactivity precipitated by the infection are reversed by atropine [41]. More recent studies suggest anticholinergics might be highly effective in treating asthma. Soon after the study of Peters et al. [3], which suggested that tiotropium was superior to steroids and β-agonists in controlling asthmatic airway function, 2 subsequent studies reported similar findings when using the ultra-potent and long acting anticholinergic [42, 43]. The reported effects of tiotropium on airway smooth muscle mass suggest that in addition to relieving functional obstruction, anticholinergics may play an important role in reversing airways remodeling [44].
Table 1.
Effect of anticholinergics on airways hyperresponsiveness in asthmaa.
| Provocation | Effect |
|---|---|
| Beta blockers | Abolished response |
| Bradykinin | 5-fold increase in PD35 |
| Capsaicin | 60% reduction in response |
| Distilled water | 50–100% reduction in response |
| Exercise | 30% reduction in response |
| Histamine | 10-fold increase in PC100 SRaw |
| Hyperpnea | Abolished response in children |
| Prostaglandin D2 | 12- to 22-fold increase in PC20 |
| Psychogenic stimulation | Abolished response |
| Reflux or esophageal acidification | Abolished response |
| Thromboxane A2 | 23-fold increase in PC20 |
aAnticholinergics used were either ipratropium bromide or atropine delivered via aerosol. Results reviewed in detail elsewhere [35]. Abbreviations: PC20 and PD35: provocative concentration (or dose) of agonist producing a 20% or 35% decrease, respectively, in forced expiratory volume in 1 sec (FEV1); PC100 SRaw: provocative concentration of agonist producing a 100% increase in specific airways resistance.
Evidence suggesting that nitrergic parasympathetic nerves are dysfunctional in airways disease is circumstantial but compelling. In humans and in many animal species, adrenergic nerves are sparse or absent in the airways and without apparent influence over airway smooth muscle tone [45]. Consequently, nitrergic parasympathetic nerves represent the only functional relaxant innervation of airway smooth muscle. Importantly, in asthma, an inability to dilate with deep inspiration and not excessive smooth muscle constriction may underlie the pathogenesis of airways hyperresponsiveness [46]. In a preliminary report, inhibitors of NO synthase (NOS), which can inhibit relaxations mediated by airway nitrergic parasympathetic nerves, prevent the bronchoprotective effects of deep inspiration in normal patients [47]. Perhaps airway nitrergic nerves regulate airways reactivity by counteracting the actions of spasmogens through tonic, ongoing effects in the airways or by subserving a compensatory role with increased activity following challenge. Consistent with these hypotheses, NO synthase inhibitors exacerbate airways responsiveness to bradykinin in mild asthmatics, a compensatory mechanism that is lost in severe asthmatics [48]. Although the source of the nitric oxide was not determined in these clinical studies, experiments using animals and studies of human airway preparations indicate that parasympathetic nerves are one potential source [33, 49–52]. Pathological and molecular biological studies are also consistent with the hypothesis that dysregulation of airway noncholinergic nerves contributes to the pathogenesis of asthma and COPD. For example, arginase (which competes with neuronal NOS for the substrate L-arginine) activity is increased in models of asthma, thereby leading to a reduced capacity to produce neuronal NO [53]. Mutations in the gene encoding the neuronal isoform of NOS have been associated with asthma [54, 55]. These mutations are associated with a decrease in exhaled nitric oxide in asthma [56]. In fatal asthma, VIP-containing nerves have been reported to be sparse in the airways [57]. VIP and NOS are colocalized to airway ganglia [51]. All of these observations indicate that dysregulation of nitrergic parasympathetic nerves might contribute to the pathogenesis of airways diseases.
5. Autonomic Regulation of Vascular Tone in Asthma
Vascular beds in the airways play an important role in basal airway obstruction through the regulation of airway wall volume [58]. Mucosal edema is a prominent feature in the asthmatic airways, and this contributes significantly to airflow limitations [59]. The airway vasculature, however, can also directly modulate airway smooth muscle reactivity by regulating the clearance of bronchoactive agents from the airway wall. For example, animal studies have shown that vasoconstriction or reduced vascular perfusion of the airways significantly potentiates airway smooth muscle responsiveness to a variety of bronchospastic agents [60–62] (Figure 2). In asthmatics, intravenous angiotensin II increases methacholine bronchoconstriction but does not alter bronchoconstriction evoked by endothelin, an autacoid that constricts airway vascular smooth muscle (while methacholine relaxes vascular smooth muscle; [63, 64]). The loop diuretic furosemide also reduces airways reactivity to exogenous stimuli in humans [27], perhaps via a vasodilatory action since it does not relax airway smooth muscle in vitro. Similarly, in vivo epinephrine (a nonselective adrenergic agonist that would evoke both bronchodilatation and vasoconstriction) has no effect on reactivity yet in vitro (where the vasculature is no longer intact), it is more potent and efficacious than the β-adrenergic agonist albuterol at preventing airway smooth muscle constriction [65].
Figure 2.
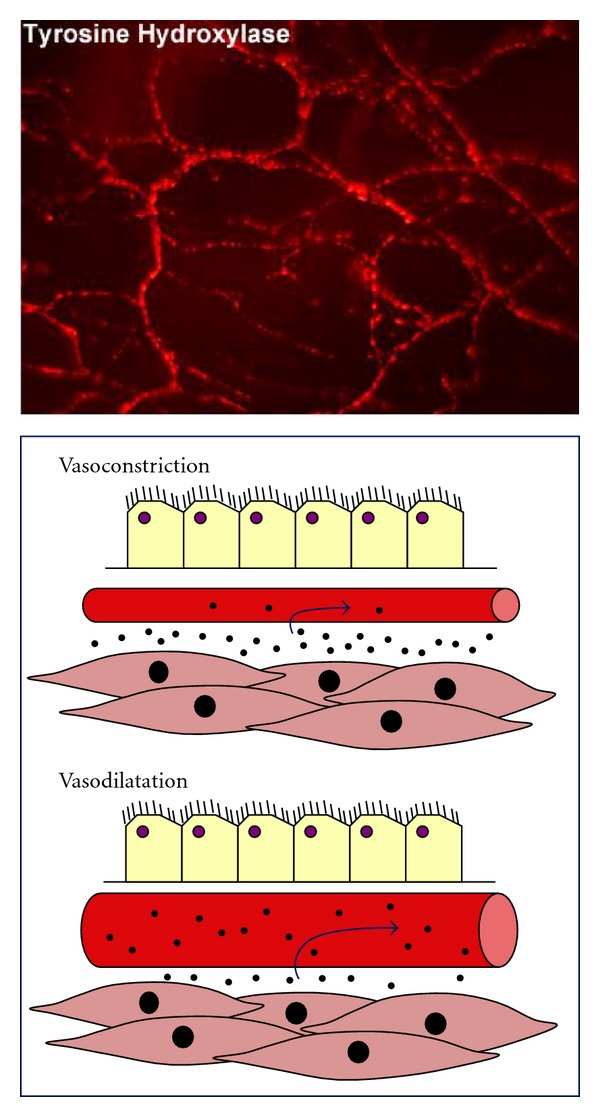
Airway vascular tone and blood flow regulate airway smooth muscle reactivity. The airway vasculature is densely innervated by sympathetic (tyrosine hydroxylase expressing) neurons which provide a basal level of adrenergic vascular tone. Soluble and insoluble particles in the airway wall are actively cleared by the submucosal vasculature. Increased blood flow is associated with increased clearance, and this can significantly modify airway smooth muscle reactivity to bronchoactive agents which are deposited onto, or generated within, the airway wall. See text for additional details.
As is the case with airway smooth muscle, vascular smooth muscle possesses a baseline level of tone that is dependent upon ongoing activity of the autonomic nervous system [61]. However, unlike the airway smooth muscle, vascular tone is heavily dependent upon adrenergic sympathetic nerves acting via alpha-adrenergic receptors (reviewed in [66]). Neuropeptide Y also constricts the vascular smooth muscle secondary to sympathetic nerve activation, whereas activation of parasympathetic nerves evokes vasodilatation following the release of acetylcholine or nitric oxide and vasoactive intestinal peptide. In some species neuropeptide expressing sensory nerves can mediate vasodilatation via axon reflexes, although this is not likely a prominent mechanism of vasoregulation in humans.
6. Mechanisms of Autonomic Dysfunction
While it is clear that the nervous system is essential to the reactivity of the airways in asthma, it is unclear precisely what drives dysfunction of airway nerves in asthma. The simplest explanation might be that inflammation alters airway autonomic function in asthma. Airways inflammation has been associated with enhanced cholinergic responses following altered prejunctional control mechanisms (such as the muscarinic M2 autoreceptor that normally prevents acetylcholine release from nerves) or by sensitizing neurotransmission through the parasympathetic autonomic ganglia (the synaptic relay between pre- and postganglionic neurons) (reviewed in [35]). Nitrergic relaxant nerve responses may also be diminished in the asthmatic airways. The synthesis or degradation of the peptidergic and nitrergic neurotransmitters (or their substrates) utilized by nitrergic nerves may be perturbed in asthma via the actions of peptidases, free radical scavengers, and arginase. An alternative explanation is that the effects of airway inflammation are indirectly linked to autonomic nerve dysfunction. For example, sensitization and altered activity of airway sensory nerves is a common feature in asthma. Sensory nerves provide direct inputs to airway autonomic pathways, both at the level of the brainstem and the autonomic ganglia, and it is therefore likely that altered sensory function contributes to changes in autonomic drive to the airways [35].
7. Conclusions and Future Directions
It seems possible that an overemphasis on the role of inflammation in models of asthma, and less attention to the central role of airways hyperresponsiveness, has contributed to the frequent failure to translate promising therapeutic strategies discovered in animals into patients with asthma [67, 68]. Important insights into the mechanisms of inflammation in asthma have been established. In clinical studies, both leukotrienes and IL-5 induce pulmonary eosinophilia [69, 70], whereas in asthmatics, leukotriene modifiers and anti-IL-5 reduce eosinophil and basophil recruitment to the airways [19, 20, 28, 71]. The Th2 cytokines IL-4 and IL-13 also seem to play a role in asthmatic inflammation [1], but therapies targeting IL-5 [19–21], IL-4 [72], or IL-13 [73, 74] have provided little or no relief of asthma symptoms and little relief from airways hyperresponsiveness. By contrast, anticholinergics have proven remarkably effective at reducing the acute responses to allergen challenge and consistently decrease airways obstruction and airways reactivity in asthmatics. There is much unknown about the innervation of the airways. Given its central role in the pathogenesis of asthma, efforts to fill the many gaps in our understanding of airway neural control are warranted.
References
- 1.National Heart, Lung and Blood Institute (NHLBI) Expert Panel Report 3. 07-4051. National Institutes of Health (NIH) Publication; 2007. Guidelines for the diagnosis and management of asthma. [Google Scholar]
- 2.British Thoracic Society Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax. 2008;63, supplement 4:iv1–iv121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 3.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. The New England Journal of Medicine. 2010;363(18):1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LJ. Anticholinergics for patients with asthma? The New England Journal of Medicine. 2010;363(18):1764–1765. doi: 10.1056/NEJMe1009429. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. American Journal of Respiratory and Critical Care Medicine. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 7.Hamid Q, Tulic M. Immunobiology of asthma. Annual Review of Physiology. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 8.Tillie-Leblond I, Montani D, Crestani B, et al. Relation between inflammation and symptoms in asthma. Allergy. 2009;64(3):354–367. doi: 10.1111/j.1398-9995.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 9.Baroffio M, Barisione G, Crimi E, Brusasco V. Noninflammatory mechanisms of airway hyper-responsiveness in bronchial asthma: an overview. Therapeutic Advances in Respiratory Disease. 2009;3(4):163–174. doi: 10.1177/1753465809343595. [DOI] [PubMed] [Google Scholar]
- 10.Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. The Lancet. 1989;1(8651):1346–1348. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- 11.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. American Journal of Respiratory and Critical Care Medicine. 2000;161(6):2086–2091. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- 12.Kermode JA, Brown NJ, Hardaker KM, et al. The effect of airway remodelling on airway hyper-responsiveness in asthma. Respiratory Medicine. 2011;105(12):1798–1804. doi: 10.1016/j.rmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. American Journal of Respiratory and Critical Care Medicine. 2003;168(8):983–988. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 14.Klion AD, Law MA, Riemenscheider W, et al. Familial eosinophilia: a benign disorder? Blood. 2004;103(11):4050–4055. doi: 10.1182/blood-2003-11-3850. [DOI] [PubMed] [Google Scholar]
- 15.Chakir J, Laviolette M, Turcotte H, Boutet M, Boulet LP. Cytokine expression in the lower airways of nonasthmatic subjects with allergic rhinitis: influence of natural allergen exposure. Journal of Allergy and Clinical Immunology. 2000;106(5):904–910. doi: 10.1067/mai.2000.110100. [DOI] [PubMed] [Google Scholar]
- 16.Shaver JR, O’Connor JJ, Pollice M, et al. Pulmonary inflammation after segmental ragweed challenge in allergic asthmatic and nonasthmatic subjects. American Journal of Respiratory and Critical Care Medicine. 1995;152(4):1189–1197. doi: 10.1164/ajrccm.152.4.7551369. [DOI] [PubMed] [Google Scholar]
- 17.McGrath KW, Icitovic N, Boushey HA, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. American Journal of Respiratory and Critical Care Medicine. 2012;185(6):612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan SA, O’Connor BJ, Matti S, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. The Lancet. 2000;356:2149–2153. doi: 10.1016/S0140-6736(00)03497-8. [DOI] [PubMed] [Google Scholar]
- 19.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. American Journal of Respiratory and Critical Care Medicine. 2007;176(11):1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 20.Leckie MJ, Ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. The Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 21.Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. The New England Journal of Medicine. 2009;360(10):985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 22.CAMP Study Investigators. Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. The New England Journal of Medicine. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 23.Armour CL, Black JL, Berend N, Woolcock AJ. The relationship between bronchial hyperresponsiveness to methacholine and airway smooth muscle structure and reactivity. Respiration Physiology. 1984;58(2):223–233. doi: 10.1016/0034-5687(84)90150-6. [DOI] [PubMed] [Google Scholar]
- 24.Cerrina J, Labat C, Haye-Legrande I, Raffestin B, Benveniste J, Brink C. Human isolated bronchial muscle preparations from asthmatic patients: effects of indomethacin and contractile agonists. Prostaglandins. 1989;37(4):457–469. doi: 10.1016/0090-6980(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JA, Raeburn D, Rodger IW, Thomson NC. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax. 1984;39(11):837–843. doi: 10.1136/thx.39.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Koppen CJ, Rodrigues de Miranda JF, Beld AJ, Van Ginneken CAM, Lammers JWJ, Van Herwaarden CLA. Muscarinic receptor sensitivity in airway smooth muscle of patients with obstructive airway disease. Archives Internationales de Pharmacodynamie et de Therapie. 1988;295:238–244. [PubMed] [Google Scholar]
- 27.Bianco S, Pieroni MG, Refini RM, Rottoli L, Sestini P. Protective effect of inhaled furosemide on allergen-induced early and late asthmatic reactions. The New England Journal of Medicine. 1989;321(16):1069–1073. doi: 10.1056/NEJM198910193211602. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RW, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. American Journal of Physiology. 1994;267(2):L218–L222. doi: 10.1152/ajplung.1994.267.2.L218. [DOI] [PubMed] [Google Scholar]
- 29.Goldie RG, Spina D, Henry PJ, et al. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, β-adrenoceptor agonists and theophylline. British Journal of Clinical Pharmacology. 1986;22(6):669–676. doi: 10.1111/j.1365-2125.1986.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whicker SD, Armour CL, Black JL. Responsiveness of bronchial smooth muscle from asthmatic patients to relaxant and contractile agonists. Pulmonary Pharmacology. 1988;1(1):25–31. doi: 10.1016/0952-0600(88)90007-5. [DOI] [PubMed] [Google Scholar]
- 31.Jammes Y, Mei N. Assessment of the pulmonary origin of bronchoconstrictor vagal tone. Journal of Physiology. 1979;291:305–316. doi: 10.1113/jphysiol.1979.sp012814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. Journal of Physiology. 1999;518(3):843–855. doi: 10.1111/j.1469-7793.1999.0843p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kesler BS, Mazzone SB, Canning BJ. Nitric oxide-dependent modulation of smooth-muscle tone by airway parasympathetic nerves. American Journal of Respiratory and Critical Care Medicine. 2002;165(4):481–488. doi: 10.1164/ajrccm.165.4.2004005. [DOI] [PubMed] [Google Scholar]
- 34.Mazzone SB, Canning BJ. Evidence for differential reflex regulation of cholinergic and noncholinergic parasympathetic nerves innervating the airways. American Journal of Respiratory and Critical Care Medicine. 2002;165(8):1076–1083. doi: 10.1164/ajrccm.165.8.2001121270c. [DOI] [PubMed] [Google Scholar]
- 35.Canning BJ. Reflex regulation of airway smooth muscle tone. Journal of Applied Physiology. 2006;101(3):971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 36.Canning BJ, Fischer A. Localization of cholinergic nerves in lower airways of guinea pigs using antisera to choline acetyltransferase. American Journal of Physiology. 1997;272(4):L731–L738. doi: 10.1152/ajplung.1997.272.4.L731. [DOI] [PubMed] [Google Scholar]
- 37.Canning BJ, Undem BJ. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. Journal of Physiology. 1993;471:25–40. doi: 10.1113/jphysiol.1993.sp019889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGovern AE, Mazzone SB. Characterization of the vagal motor neurons projecting to the guinea pig airways and esophagus. Frontiers in Neurology. 2010;1, article 153 doi: 10.3389/fneur.2010.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichinose M, Inoue H, Miura M, Yafuso N, Nogami H, Takishima T. Possible sensory receptor of nonadrenergic inhibitory nervous system. Journal of Applied Physiology. 1987;63(3):923–929. doi: 10.1152/jappl.1987.63.3.923. [DOI] [PubMed] [Google Scholar]
- 40.Gross NJ, Co E, Skorodin MS. Cholinergic bronchomotor tone in COPD: estimates of its amount in comparison with that in normal subjects. Chest. 1989;96(5):984–987. doi: 10.1378/chest.96.5.984. [DOI] [PubMed] [Google Scholar]
- 41.Empey DW, Laitinen LA, Jacobs L. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. American Review of Respiratory Disease. 1976;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- 42.Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. Journal of Allergy and Clinical Immunology. 2011;128(2):315–322. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Kerstjens HAM, Disse B, Schröder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. Journal of Allergy and Clinical Immunology. 2011;128(2):308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 44.Gosens R, Bos IST, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. American Journal of Respiratory and Critical Care Medicine. 2005;171(10):1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- 45.Richardson J, Beland J. Nonadrenergic inhibitory nervous system in human airways. Journal of Applied Physiology. 1976;41(5):764–771. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- 46.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. Journal of Clinical Investigation. 1995;96(5):2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gratziou C, Rovina N, Lignos M, et al. Attenuation of deep inspiration (DI)—induced bronchoprotection (BP) by an NO synthase inhibitor. American Journal of Respiratory and Critical Care Medicine. 2001;161A830 [Google Scholar]
- 48.Ricciardolo FLM, Geppetti P, Mistretta A, et al. Randomised double-blind placebo-controlled study of the effect of inhibition of nitric oxide synthesis in bradykinin-induced asthma. The Lancet. 1996;348(9024):374–377. doi: 10.1016/s0140-6736(96)04450-9. [DOI] [PubMed] [Google Scholar]
- 49.De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. Journal of Experimental Medicine. 1999;189(10):1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dey RD, Altemus JB, Rodd A, Mayer B, Said SI, Coburn RF. Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. American Journal of Respiratory Cell and Molecular Biology. 1996;14(3):207–216. doi: 10.1165/ajrcmb.14.3.8845170. [DOI] [PubMed] [Google Scholar]
- 51.Fischer A, Hoffmann B. Nitric oxide synthase in neurons and nerve fibers of lower airways and in vagal sensory ganglia of man: correlation with neuropeptides. American Journal of Respiratory and Critical Care Medicine. 1996;154(1):209–216. doi: 10.1164/ajrccm.154.1.8680682. [DOI] [PubMed] [Google Scholar]
- 52.Ward JK, Barnes PJ, Springall DR, et al. Distribution of human i-NANC bronchodilator and nitric oxide-immunoreactive nerves. American Journal of Respiratory Cell and Molecular Biology. 1995;13(2):175–184. doi: 10.1165/ajrcmb.13.2.7542897. [DOI] [PubMed] [Google Scholar]
- 53.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. British Journal of Pharmacology. 2009;158(3):652–664. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grasemann H, Yandava CN, Storm Van’s Gravesande K, et al. A neuronal NO synthase (NOS1) gene polymorphism is associated with asthma. Biochemical and Biophysical Research Communications. 2000;272(2):391–394. doi: 10.1006/bbrc.2000.2794. [DOI] [PubMed] [Google Scholar]
- 55.Grasemann H, Yandava CN, Drazen JM. Neuronal NO synthase (NOS1) is a major candidate gene for asthma. Clinical and Experimental Allergy, Supplement. 1999;29, supplement 4:39–41. [PubMed] [Google Scholar]
- 56.Wechsler ME, Grasemann H, Deykin A, et al. Exhaled nitric oxide in patients with asthma: association with NOS1 genotype. American Journal of Respiratory and Critical Care Medicine. 2000;162(6):2043–2047. doi: 10.1164/ajrccm.162.6.2003089. [DOI] [PubMed] [Google Scholar]
- 57.Ollerenshaw S, Jarvis D, Woolcock A, Sullivan C, Scheibner T. Absence of immunoreactive vasoactive intestinal polypeptide in tissue from the lungs of patients with asthma. The New England Journal of Medicine. 1989;320(19):1244–1248. doi: 10.1056/NEJM198905113201904. [DOI] [PubMed] [Google Scholar]
- 58.Widdicombe J. Why are the airways so vascular? Thorax. 1993;48(3):290–295. doi: 10.1136/thx.48.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson JW, Hii S. The importance of the airway microvasculature in asthma. Current Opinion in Allergy and Clinical Immunology. 2006;6(1):51–55. doi: 10.1097/01.all.0000200505.54425.47. [DOI] [PubMed] [Google Scholar]
- 60.Csete ME, Chediak AD, Abraham WM, Wanner A. Airway blood flow modifies allergic airway smooth muscle contraction. American Review of Respiratory Disease. 1991;144(1):59–63. doi: 10.1164/ajrccm/144.1.59. [DOI] [PubMed] [Google Scholar]
- 61.Mazzone SB, Lim LHK, Wagner EM, Mori N, Canning BJ. Sympathetic nerve-dependent regulation of mucosal vascular tone modifies airway smooth muscle reactivity. Journal of Applied Physiology. 2010;109(5):1292–1300. doi: 10.1152/japplphysiol.00632.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner EM, Foster WM. Importance of airway blood flow on particle clearance from the lung. Journal of Applied Physiology. 1996;81(5):1878–1883. doi: 10.1152/jappl.1996.81.5.1878. [DOI] [PubMed] [Google Scholar]
- 63.Chalmers GW, Millar EA, Little SA, Shepherd MC, Thomson NC. Effect of infused angiotensin II on the bronchoconstrictor activity of inhaled endothelin-1 in asthma. Chest. 1999;115(2):352–356. doi: 10.1378/chest.115.2.352. [DOI] [PubMed] [Google Scholar]
- 64.Myou S, Fujimura M, Kurashima K, Tachibana H, Watanabe K, Hirose T. Type 1 angiotensin II receptor antagonism reduces antigen-induced airway reactions. American Journal of Respiratory and Critical Care Medicine. 2000;162(1):45–49. doi: 10.1164/ajrccm.162.1.9907128. [DOI] [PubMed] [Google Scholar]
- 65.Baldwin DR, Sivardeen Z, Pavord ID, Knox AJ. Comparison of the effects of salbutamol and adrenaline on airway smooth muscle contractility in vitro and on bronchial reactivity in vivo. Thorax. 1994;49(11):1103–1108. doi: 10.1136/thx.49.11.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Widdicombe JG. Neural control of airway vasculature and edema. American Review of Respiratory Disease. 1991;143(3):S18–S21. doi: 10.1164/ajrccm/143.3_Pt_2.S18. [DOI] [PubMed] [Google Scholar]
- 67.Mullane K. Asthma translational medicine: report card. Biochemical Pharmacology. 2011;82(6):567–585. doi: 10.1016/j.bcp.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Mullane K. The increasing challenge of discovering asthma drugs. Biochemical Pharmacology. 2011;82(6):586–599. doi: 10.1016/j.bcp.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 69.Diamant Z, Hiltermann JT, Van Rensen EL, et al. The effect of inhaled leukotriene D4 and methacholine on sputum cell differentials in asthma. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):1247–1253. doi: 10.1164/ajrccm.155.4.9105062. [DOI] [PubMed] [Google Scholar]
- 70.Shi HZ, Xiao CQ, Zhong D, et al. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):204–209. doi: 10.1164/ajrccm.157.1.9703027. [DOI] [PubMed] [Google Scholar]
- 71.Calhoun WJ, Lavins BJ, Minkwitz MC, Evans R, Gleich GJ, Cohn J. Effect of zafirlukast (Accolate) on cellular mediators of inflammation: bronchoalveolar lavage fluid findings after segmental antigen challenge. American Journal of Respiratory and Critical Care Medicine. 1998;157(5):1381–1389. doi: 10.1164/ajrccm.157.5.9609014. [DOI] [PubMed] [Google Scholar]
- 72.Borish LC, Nelson HS, Lanz MJ, et al. Interleukin-4 receptor in moderate atopic asthma: a phase I/II randomized, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine. 1999;160(6):1816–1823. doi: 10.1164/ajrccm.160.6.9808146. [DOI] [PubMed] [Google Scholar]
- 73.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. The New England Journal of Medicine. 2011;365(12):1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 74.Gauvreau GM, Boulet LP, Cockcroft DW, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. American Journal of Respiratory and Critical Care Medicine. 2011;183(8):1007–1014. doi: 10.1164/rccm.201008-1210OC. [DOI] [PubMed] [Google Scholar]


