Abstract
In Candida albicans, Upc2 is a zinc-cluster transcription factor that targets genes, including those of the ergosterol biosynthesis pathway. To date, three documented UPC2 gain-of-function (GOF) mutations have been recovered from fluconazole-resistant clinical isolates that contribute to an increase in ERG11 expression and decreased fluconazole susceptibility. In a group of 63 isolates with reduced susceptibility to fluconazole, we found that 47 overexpressed ERG11 by at least 2-fold over the average expression levels in 3 unrelated fluconazole-susceptible strains. Of those 47 isolates, 29 contained a mutation in UPC2, whereas the remaining 18 isolates did not. Among the isolates containing mutations in UPC2, we recovered eight distinct mutations resulting in putative single amino acid substitutions: G648D, G648S, A643T, A643V, Y642F, G304R, A646V, and W478C. Seven of these resulted in increased ERG11 expression, increased cellular ergosterol, and decreased susceptibility to fluconazole compared to the results for the wild-type strain. Genome-wide transcriptional analysis was performed for the four strongest Upc2 amino acid substitutions (A643V, G648D, G648S, and Y642F). Genes commonly upregulated by all four mutations included those involved in ergosterol biosynthesis, in oxidoreductase activity, the major facilitator efflux pump encoded by the MDR1 gene, and the uncharacterized ATP binding cassette transporter CDR11. These findings demonstrate that gain-of-function mutations in UPC2 are more prevalent among clinical isolates than previously thought and make a significant contribution to azole antifungal resistance, but the findings do not account for ERG11 overexpression in all such isolates of C. albicans.
INTRODUCTION
Candida albicans is an opportunistic fungal pathogen that causes mucosal, cutaneous, and systemic infections, including oropharyngeal candidiasis (OPC), the most frequent infection in people with AIDS (9, 13). In the United States, Candida is the fourth-most-common organism isolated from nosocomial bloodstream infections and is associated with a mortality rate approaching 40% (24). Fluconazole and other azole antifungal agents have proven effective in the management of OPC; however, with increased use of these agents, treatment failures have occurred that have been associated with the emergence of azole-resistant strains of C. albicans (25a). The azole class of antifungals work by inhibiting the cytochrome P450 enzyme lanosterol demethylase, a critical enzyme in the synthesis of ergosterol which is encoded by the ERG11 gene (14). The efficacy of fluconazole is decreased in clinical isolates of C. albicans by the interplay of several mechanisms of resistance (17, 21, 23, 32). Overexpression of the efflux transporter genes CDR1, CDR2, and MDR1 is a common mechanism of drug resistance in this organism (10, 17, 26). Point mutations in the ERG11 gene result in reduced binding affinity of azoles to their target without precluding enzymatic function (31). In addition to point mutations, overexpression of ERG11 has also been shown to decrease fluconazole susceptibility. ERG11 gene amplification by chromosome 5 duplication or the presence of a chr5L isochromosome is known to contribute to azole resistance (29). Alternately, the zinc-cluster transcription factor Upc2 has been shown to regulate the expression of ERG11 and other genes involved in ergosterol biosynthesis (19, 30). Previous studies show that activating mutations in Upc2 result in increased expression of ERG11 and decreased fluconazole susceptibility; however, only three substitutions (G648D, A643T, and A643V) in Upc2 have been identified in clinical isolates that contribute to azole resistance (7, 11, 12). In all cases, these substitutions have been identified in fluconazole-resistant clinical isolates of genetically matched isolate pairs.
In this study, we examined the prevalence of overexpression of ERG11, as well as the overexpression of efflux pump genes CDR1, CDR2, and MDR1, in a group of 63 unrelated fluconazole-resistant clinical isolates. We also investigated the prevalence of UPC2 mutations among isolates that overexpress ERG11. We determined which UPC2 mutations, both previously described and novel, result in increased ERG11 expression, altered cellular ergosterol content, and increased resistance to azoles, as well as to terbinafine. Finally, we identified genes that are coordinately differentially expressed in four strains expressing unique UPC2 gain-of-function mutations.
MATERIALS AND METHODS
Strains and growth conditions.
All C. albicans strains (Table 1) were stored as frozen stock in 20% glycerol at −80°C and cultured on YPD (1% yeast extract, 2% peptone, and 1% dextrose) agar plates at 30°C. YPD liquid medium was used for routine growth of strains. For selection of strains containing the SAT1-flipper cassette (25), nourseothricin (200 μg/ml) was added to YPD agar plates. One Shot Escherichia coli TOP10 chemically competent cells (Invitrogen, Carlsbad, CA) were used as the host for plasmid construction and propagation. These strains were grown in Luria-Bertani (LB) broth or on LB agar plates supplemented with 100 μg/ml ampicillin (Sigma) or 50 μg/ml kanamycin (Fisher BioReagents, Fair Lawn, NJ) when required.
Table 1.
C. albicans strains used in this study
| Strain | Strain background | Relevant characteristics or genotype | Source or reference |
|---|---|---|---|
| SC5314 | N/A | UPC2-1/UPC2-2 | ATCC |
| Clinical isolates | |||
| 1–10 | N/A | Azole susceptible | University of Iowa |
| 11–72 | N/A | Azole resistant | University of Iowa |
| Constructed laboratory strains | |||
| UPC2M4A | SC5314 | upc2-1Δ::FRT/upc2-2Δ::FRT | 6 |
| 11A8A2A | UPC2M4A | UPC2G648S::FRT/UPC2G648S::FRT | This study |
| SC11A1A | SC5314 | UPC2/UPC2G648S::FRT | This study |
| 22A1A13A | UPC2M4A | UPC2G304R::FRT/UPC2G304R::FRT | This study |
| SC22A3A | SC5314 | UPC2/UPC2G304R::FRT | This study |
| YFA3A2K1 | UPC2M4A | UPC2Y642F::FRT/UPC2Y642F::FRT | This study |
| SCYFA2A | SC5314 | UPC2/UPC2Y642F::FRT | This study |
| AVA1A16A | UPC2M4A | UPC2A646V::FRT/UPC2A646V::FRT | This study |
| SCAVA4 | SC5314 | UPC2/UPC2A646V::SAT1-FLIP | This study |
| 25B2D1 | UPC2M4A | UPC2W478C::FRT/UPC2W478C::FRT | This study |
| SC25A1 | SC5314 | UPC2/UPC2W478C::FRT | This study |
| 28A7A10A | UPC2M4A | UPC2A643V::FRT/UPC2A643V::FRT | This study |
| SC28A3A | SC5314 | UPC2/UPC2A643V::FRT | This study |
| SCUPC2R12A | SC5314 | UPC2G648D::FRT/UPC2-2 | 11 |
| SCUPC2R14A | SC5314 | UPC2G648D::FRT/UPC2G648D::FRT | 11 |
| SCUPC2R32A | SC5314 | UPC2A643T::FRT/UPC2-2 | 11 |
| SCUPC2R34A | SC5314 | UPC2A643T::FRT/UPC2A643T::FRT | 11 |
RNA isolation.
RNA was isolated using a small-scale version of the hot phenol method of RNA isolation described by Schmitt et al. (27). Briefly, overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.2 and then incubated at 30°C with shaking for an additional 3 or 6 h to mid-log phase. Cells were collected by centrifugation, resuspended in sodium acetate-EDTA buffer, and then transferred to a 2-ml microcentrifuge tube containing acid phenol (pH 4.3) with 1% SDS. Cells were incubated at 65°C for 10 min, and then lysates were clarified by centrifugation. The supernatant was then transferred into a new tube containing 900 μl of chloroform and mixed. The sample was then subjected to centrifugation again, and the top aqueous layer was transferred to a new tube containing 1 volume of isopropanol and 0.1 volume of 2 M sodium acetate. The RNA pellet was subsequently washed with 500 μl of 70% ethanol and collected by centrifugation. The RNA pellet was resuspended in DNase/RNase-free H2O. Quantity and purity were determined spectrophotometrically at absorbances of A260 and A280.
qRT-PCR.
First-strand cDNAs were synthesized separately from 1 μg of total RNA in a 21-μl reaction mixture volume using the SuperScript first-strand synthesis system for reverse transcription (Invitrogen). Quantitative PCRs (qRT-PCRs) were performed in triplicate as technical replicates using the 7000 sequence detection system (Applied Biosystems). PCRs were performed, independently amplifying 18S rRNA or the ACT1 gene (normalizing genes) and the genes of interest (GOI) from the same cDNA, using SYBR green PCR master mix (Applied Biosystems). Gene-specific primers were designed using Primer Express software (Applied Biosystems) synthesized by Integrated DNA Technologies (Coralville, IA) and are listed in Table 2. The PCR conditions consisted of AmpliTaq Gold activation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C. Determination of the dissociation curve and detection of SYBR green fluorescence was performed by using software provided with the 7000 sequence detection system. The cycle threshold (CT) value of the normalizing gene was subtracted from the GOI to obtain the ΔCT value. The ΔCT value of a calibrator was subtracted from the sample ΔCT value to obtain the ΔΔCT value. The change in fold expression was obtained by calculating 2−ΔΔCT. The standard error was calculated from ΔCT values as previously described (7).
Table 2.
Primers used in this study
| Purpose, primer | Sequencea |
|---|---|
| qRT-PCR | |
| ACT1-F | 5′-ACGGTGAAGAAGTTGCTGCTTTAGTT-3′ |
| ACT1-R | 5′-CGTCGTCACCGGCAAAA-3′ |
| 18S-F | 5′-CACGACGGAGTTTCACAAGA-3′ |
| 18S-R | 5′-CGATGGAAGTTTGAGGCAAT-3′ |
| ERG11-F | 5′-CCCCTATTAATTTTGTTTTCCCTAATTTAC-3′ |
| ERG11-R | 5′-CACGTTCTCTTCTCAGTTTAATTTCTTTC-3′ |
| CDR1-F | 5′-ATTCTAAGATGTCGTCGCAAGATG-3′ |
| CDR1-R | 5′-AGTTCTGGCTAAATTCGTAATGTTTTC-3′ |
| CDR2-F | 5′-TAGTCCATTCAACGGCAACAAT-3′ |
| CDR2-R | 5′-CACCCAGTATTTGGCATTGAAA-3′ |
| BMR1-F | 5′-ACATAAATACTTTGCCCATCCAGAA-3′ |
| BMR1-R | 5′-AAGAGTTGGTTTGTAATCGGCTAAA-3′ |
| UPC2 mutant construction | |
| UPC2-A | 5′-GGGCCCGAGATCTTGATGTCATTAG-3′ |
| UPC2-B | 5′-CTCGAGCTATATCTTCAATGAACTG-3′ |
| UPC2-E | 5′-CTCGAGCACCACAGTAACGAATCAC-3′ |
| UPC2-C | 5′-CCGCGGACAGGTCAATACCGCGTAG-3′ |
| UPC2-D | 5′-GAGCTCGTTCCTCTAGTATCACTCTT-3′ |
| UPC2 sequencing | |
| UPC2seqA | 5′-CTGCAGAGAATCACAGTGAAGTTC-3′ |
| UPC2seqB | 5′-CTCAGCCGGTGATTCCTCCA-3′ |
| UPC2seqC | 5′-CGGTCAAACCTCAATATGCTTGAC-3′ |
| UPC2seqD | 5′-GTTTCCAGTGCTTTTGGACTCTCC-3′ |
| UPC2seqZ | 5′-CCTATCATCTACGCGGTATTGACC-3′ |
| UPC2seqF | 5′-TGGAGGAATCACCGGCTGAG-3′ |
| UPC2seqG | 5′-GTCAAGCATATTGAGATTTGACCG-3′ |
| UPC2seqH | 5′-GGAGAGTCCAAAAGCACTGGAAAC-3′ |
Underlined sequence reflects the introduction of a restriction site sequence.
Plasmid construction for allele sequencing.
C. albicans UPC2 coding sequences were amplified by PCR (Pfu DNA polymerase; Stratagene) from C. albicans genomic DNA using the primers UPC2-A and UPC2-E (Table 2). Products were cloned into pCR-BLUNTII-TOPO using a Zero Blunt TOPO PCR cloning kit (Invitrogen) and transferred into Escherichia coli TOP10 cells with selection on LB agar plates containing 50 μg/ml kanamycin. Plasmid DNA was purified (QIAprep; Qiagen, Germantown, MD) and sequenced on an ABI model 3130XL genetic analyzer using the UPC2 sequencing primers (Table 2), resulting in a full-length sequence from both strands of the C. albicans UPC2 gene. The sequencing was performed using six sets of clones derived from three independent PCRs for each strain/isolate sequenced.
Sequenced plasmids containing the UPC2 open reading frame (ORF) whose predicted translation indicated an amino acid substitution were digested with restriction enzymes ApaI and XhoI, which excised the full-length ORF from the plasmid, and the UPC2 alleles were cloned upstream of the SAT1-flipper cassette into the ApaI and XhoI sites of plasmid pSFS2 (25). The UPC2 downstream segments were amplified with Ex Taq (TaKaRa) using primers UPC2C and UPC2D and cloned downstream of the SAT1-flipper cassette in pSFS2 using the SacI and SacII sites. This process generated plasmids pUPC2-G648S, pUPC2-M597I, pUPC2-G304R, pUPC2-A643V, pUPC2-Y642F, and pUPC2-A646V.
Construction of UPC2 allele strains.
C. albicans strains UPC2M4A (7) and SC5314 were transformed by electroporation with gel-purified inserts from plasmids pUPC2-G648S, pUPC2-M597I, pUPC2-G304R, pUPC2-A643V, pUPC2-Y642F, pUPC2-W467C, and pUPC2-A646V derived from the plasmid pSFS2. pSFS2 contains the SAT1-flipper disruption cassette developed by Reuss et al. (25), consisting of the SAT1 selectable marker which confers resistance to nourseothricin and the FLP flipper recombinase gene, both flanked by FRT sites (flipper recombinase target sequences). Nourseothricin-resistant transformants were selected as previously described (25). Upon induction of the FLP gene, the cassette is excised such that only the UPC2 allele with a downstream FRT is left in the UPC2 locus. Integration of constructs was confirmed by Southern hybridization.
Fluconazole susceptibility testing.
MICs were obtained by using a modified CLSI protocol outlined in CLSI document M27-A3 using RPMI or YPD medium (1). Overnight cultures grown at 30°C were streaked onto Sabouraud's agar. Plated cultures were grown for 24 h at 30°C. Individual colonies were suspended in sterile water until an OD600 of 0.1 was reached. The working colony concentration was made by making a 1:50 dilution and a 1:20 dilution sequentially in medium. Aliquots of 100 μl from the working stock were used to inoculate a series of fluconazole-YPD medium dilutions, the highest being 64 μg/ml. Similar procedures were used for terbinafine and amphotericin B dilutions; however, the highest concentration used for these agents was 8 μg/ml. Cultures were incubated at 35°C for 48 h, and MICs were recorded. Azole MICs for constructed strains were obtained by Etest (bioMérieux) following the manufacturer's instructions. Briefly, yeast cultures were grown in YPD broth medium overnight at 30°C in a shaking incubator and plated to Sabouraud dextrose agar at 18 h. Isolated colonies were then selected from these plated cultures, suspended in deionized water to achieve 0.5 McFarland turbidity, and streaked evenly across YPD plates by rotating 90° in three directions. Etest strips were then applied to the inoculated agar surface, and MICs were read at both 24 and 48 h.
Ergosterol quantification analysis.
Ergosterol was extracted and quantified as described previously (16). Briefly, a single colony of C. albicans from a fresh YPD plate was used to inoculate 50 ml of RPMI 1640 plus 2% glucose (Sigma, St. Louis, MO) and incubated for 16 h with shaking at 35°C. Stationary-phase cells were collected by centrifugation for 5 min at 2,700 rpm and washed twice with sterile distilled water. The net weight of the pellet was determined. To each pellet, 3 ml of 25% alcoholic potassium hydroxide solution (25 g of KOH, 35 ml of sterile distilled water, with final volume adjusted to 100 ml with 100% ethanol) was added, and then the pellet was vortexed for 1 min. Cell suspensions were transferred to a sterile borosilicate glass screw-cap tube and incubated at 85°C for 1 h. Sterols were then extracted from cooled tubes by the addition of a mixture of 3 ml n-heptane and 1 ml sterile distilled water followed by vortexing for 3 min. The heptane layer was transferred to a clean sterile borosilicate glass tube and stored at −20°C for up to 24 h. A 100-μl amount of the sterol-heptane mixture was scanned spectrophotometrically between 240 nm and 300 nm with a DU530 life science UV spectrophotometer (Beckman Coulter, La Brea, CA). The presence of ergosterol in the extracted sample resulted in a characteristic four-peaked curve. The absence of detectible ergosterol was indicated by a flat line. All samples were blanked to n-heptane. A decrease in the height of the absorbance peaks correlates to a decrease in ergosterol content. Statistical significance was determined by using Student's t test (P < 0.05).
Transcriptional profiling.
Gene expression profiles were obtained by hybridizing labeled cRNAs generated from C. albicans total RNA onto Affymetrix C. albicans custom expression arrays (CAN07, 49-5241 array format), which have been described previously (22). Total RNA was isolated as described earlier and subsequent cRNA synthesis/labeling, as well as probe hybridization, array scanning, and data analysis, were performed as previously described (22).
Among direct comparisons between strains, genes were considered to be differentially expressed if their change in expression was at least 1.5-fold greater (for upregulated genes) or at least 1.5-fold less (for downregulated genes) in both independent experiments of each comparison. Cells that were empty were called “absent” by the Affymetrix criteria for the corresponding comparison/experiment, and thus, their expression values were not valid. Examination of gene ontology (GO) classification enrichment of microarray data was performed using the CGD gene ontology term finder (http://www.candidagenome.org/cgi-bin/GO/goTermFinder).
Accession numbers.
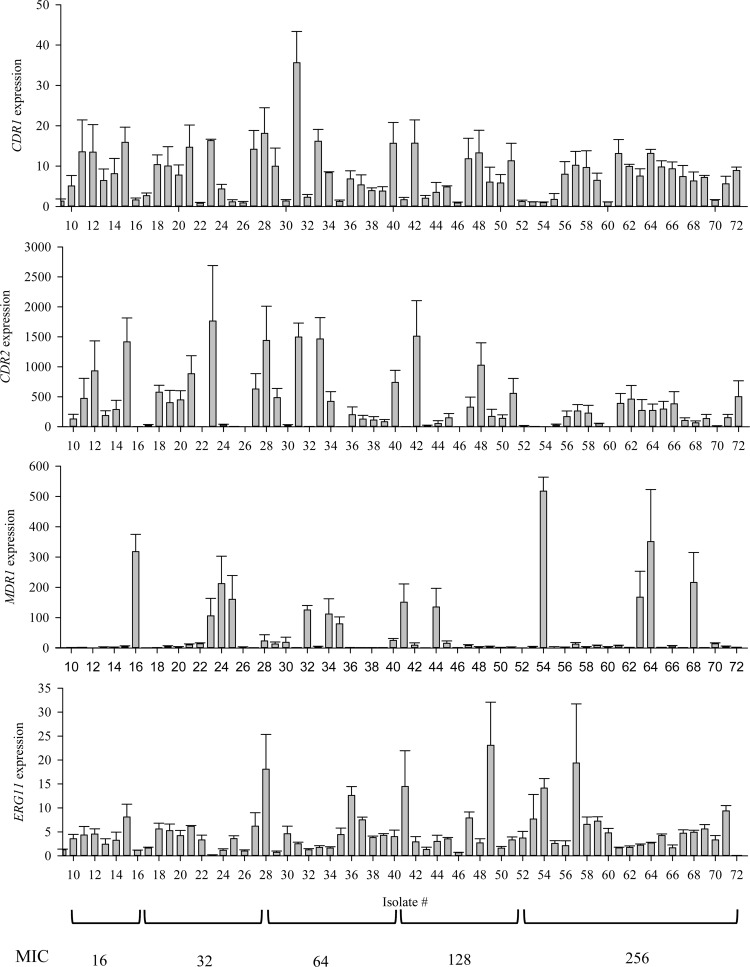
The coding sequences of the UPC2 alleles described in this study have been deposited in GenBank with the following accession numbers: JX494823, JX494820, JX494821, JX494822, JX494818, JX494819). We defined overexpression as an increase in expression of at least 2-fold. We observed that 77% (n = 49) of resistant isolates overexpressed both CDR1 and CDR2. MDR1 overexpression was increased by at least 2-fold in 76% (n = 48) of resistant isolates, but only 21% (n = 13) expressed MDR1 at levels consistent with the levels observed in isolates with MDR1-mediated fluconazole resistance, in which MDR1 expression is substantially increased. For example, in a study by Morschhäuser et al., the clinical isolates examined exhibited 400- to 1,200-fold increases in MDR1 expression (22). Surprisingly, ERG11 was found to be overexpressed in 75% (n = 47) of fluconazole-resistant isolates in this collection.
Most but not all isolates that overexpress ERG11 carry a GOF mutation in UPC2.
Twenty-nine of the 47 isolates that overexpressed ERG11 possessed one or both UPC2 alleles with a mutation that resulted in a predicted amino acid substitution. Although many silent mutations were observed in the UPC2 alleles tested, eight distinct single UPC2 mutations were also recovered, three of which had been described previously: G648D, A643T, and A643V (Table 3). Eighteen clinical isolates were found to overexpress ERG11 yet contained no amino acid substitution in Upc2. These data suggest an alternate mechanism of activating ERG11 expression in these isolates.
Table 3.
Occurrence of Upc2 amino acid substitutions in predicted translated sequence in fluconazole-resistant clinical ERG11-overexpressing isolates
| Upc2 substitution | Isolate | Genotype | Fluconazole MIC (μg/ml) | Expression (fold) of efflux transporter gene (±SE)a |
|||
|---|---|---|---|---|---|---|---|
| ERG11 | CDR1 | CDR2 | MDR1 | ||||
| G648D | 21 | Heterozygous | 32 | 6.2 (±0.2) | 14.7 (±5.5) | 883.3 (±301.9) | 10.4 (±3.1) |
| 38 | Heterozygous | 64 | 3.8 (±0.3) | 3.9 (±0.7) | 111.0 (±57.1) | 1.4 (±0.2) | |
| 47 | Heterozygous | 128 | 7.9 (±1.2) | 11.8 (±5.1) | 327.0 (±166.3) | 8.2 (±3.1) | |
| 52 | Heterozygous | 256 | 3.7 (±1.4) | 1.2 (±0.3) | 17.1 (±3.2) | 0.3 (±0.1) | |
| 59 | Heterozygous | >256 | 7.2 (±1.0) | 6.5 (±0.3) | 48.5 (±0.3) | 8.0 (±0.2) | |
| 71 | Heterozygous | >256 | 9.4 (±1.1) | 5.6 (±1.9) | 149.8 (±56.5) | 4.6 (±1.8) | |
| G648S | 25 | Heterozygous | 64 | 3.6 (±0.6) | 1.1 (±0.5) | 2.8 (±1.1) | 160.5 (±78.9) |
| 35 | Heterozygous | >256 | 4.4 (±1.3) | 1.2 (±0.4) | 0.7 (±0.3) | 79.9 (±22.8) | |
| 55 | Homozygous | >256 | 2.6 (±0.6) | 1.7 (±1.5) | 26.2 (±17.4) | 0.9 (±3.2) | |
| 56 | Homozygous | >256 | 2.1 (±1.0) | 8.0 (±3.1) | 167.8 (±94.0) | 2.5 (±0.9) | |
| 57 | Homozygous | >256 | 19.4 (±12.4) | 10.2 (±3.5) | 263.3 (±106.0) | 12.9 (±5.2) | |
| 58 | Homozygous | >256 | 6.6 (±1.5) | 9.7 (±4.2) | 226.6 (±131.7) | 3.8 (±1.2) | |
| 65 | Heterozygous | >256 | 4.2 (±0.3) | 9.8 (±1.5) | 294.9 (±128.4) | 2.4 (±0.1) | |
| 67 | Heterozygous | >256 | 4.7 (±0.7) | 7.4 (±2.8) | 105.1 (±43.3) | 1.2 (±0.5) | |
| 69 | Heterozygous | >256 | 5.6 (±0.9) | 7.2 (±0.5) | 136.8 (±69.3) | 1.4 (±0.5) | |
| A643T | 13 | Homozygous | 16 | 2.4 (±1.1) | 6.4 (±2.9) | 185.9 (±80.9) | 2.4 (1.6) |
| A643V | 30 | Heterozygous | 64 | 4.6 (±1.6) | 1.4 (±0.3) | 18.0 (±15.7) | 18.9 (±16.7) |
| 68 | Heterozygous | >256 | 4.9 (±0.4) | 6.3 (±2.2) | 64.5 (±31.8) | 216.3 (±98.9) | |
| A646V | 45 | Heterozygous | 128 | 3.5 (±0.3) | 4.8 (±0.3) | 147.8 (±71.7) | 15.5(±7.7) |
| 67 | Heterozygous | >256 | 4.7 (±0.7) | 7.4 (±2.8) | 105.1 (±43.3) | 1.2 (±0.5) | |
| Y642F | 33 | Homozygous | 36 | 12.6 (±1.8) | 6.8 (±2.0) | 201.5 (±129.0) | 1.5 (±0.5) |
| 34 | Homozygous | 37 | 7.5 (±0.6) | 5.4 (2.5) | 126.8 (±62.6) | 1.2 (±0.5) | |
| W478C | 15 | Heterozygous | 16 | 8.1 (±2.7) | 15.9 (±3.8) | 1415.1 (±400.1) | 4.0 (±3.0) |
| 27 | Heterozygous | 64 | 6.2 (±2.8) | 14.2 (±4.7) | 629.7 (±255.3) | 1.1 (±0.1) | |
| 28 | Heterozygous | 64 | 18.1 (±7.3) | 18.1 (±6.3) | 1439.7 (±570.8) | 23.2 (±20.6) | |
| G304R | 18 | Heterozygous | 32 | 5.6 (±1.2) | 10.4 (±2.4) | 575.0 (±117.2) | 1.0 (±0.3) |
| 19 | Heterozygous | 32 | 5.2 (±1.4) | 10.0 (±4.8) | 401.3 (±204.5) | 4.6 (±3.0) | |
qRT-PCR was performed in triplicate.
GOF mutations in UPC2 result in increased expression of ERG11.
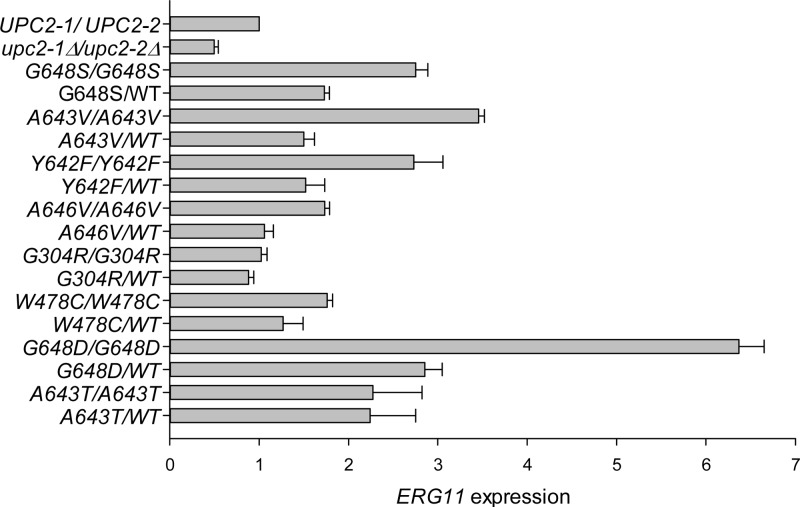
In order to assess the contribution of each individual mutant UPC2 allele to fluconazole resistance, we expressed each mutant allele alone, with the other UPC2 allele disrupted (data not shown), as a heterozygote with a wild-type UPC2 allele, and as a homozygote. ERG11 mRNA abundance was measured by qRT-PCR (Fig. 2). The homozygous null upc2Δ strain showed a significant decrease in ERG11 expression compared to that of its parental wild-type strain. Seven of the eight mutations tested resulted in increased ERG11 expression, with the strongest expression observed among homozygous strains. Interestingly, different mutations elicited different levels of ERG11 expression, with the G648D substitution resulting in the highest level of expression.
Fig 2.
ERG11 transcription levels in strains carrying mutant UPC2 alleles are represented as averages from three independent qRT-PCR assays. Error bars show standard errors. ERG11 expression was quantified for each mutant strain listed and is compared to that of wild-type strain SC5314.
Fig 1.
Expression levels of CDR1, CDR2, MDR1, and ERG11 in 63 fluconazole-resistant clinical isolates were measured by qRT-PCR. All gene expression levels were measured in triplicate, and fold expression of genes in resistant isolates was compared to the average of the expression levels in three susceptible isolates. Results for 63 isolates with reduced susceptibility to fluconazole are represented, but only even-numbered isolates' results are labeled. Error bars show standard errors.
GOF mutations in UPC2 result in elevated cellular ergosterol content.
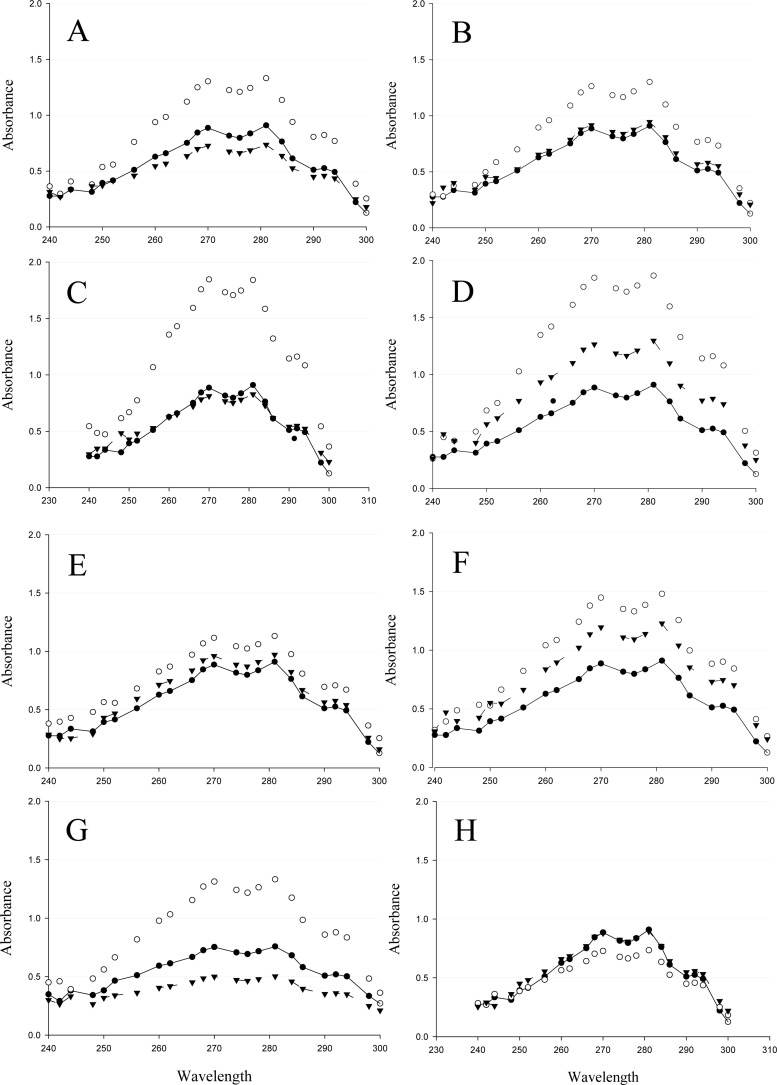
In order to confirm that activation of Upc2 results in a global increase of ergosterol biosynthesis, we compared the cellular ergosterol content in the wild type to the amounts in derivatives that were heterozygous or homozygous for various UPC2 mutations. We found that strains carrying UPC2 GOF alleles generally contained larger amounts of ergosterol than the wild-type strain (Fig. 3). For many isolates, increased ergosterol levels correlated with the increased ERG11 transcript levels resulting from each individual UPC2 mutation; however, this was not the case for the A643V allele, which resulted in strong ERG11 expression but a nonsignificant increase in cellular ergosterol content. UPC2 alleles containing the G648D, W478C, and Y642F amino acid substitutions showed statistically significant increases in ergosterol content relative to the amount in the wild-type strain, while strains carrying other UPC2 GOF alleles trended toward larger amounts of cellular ergosterol.
Fig 3.
Ergosterol quantification in C. albicans laboratory strains expressing mutant UPC2 alleles. Increased ergosterol content was shown for strains expressing 2 UPC2 mutant alleles compared to the expression levels in heterozygote and wild-type strains. The heptane extraction layer from 16-h cultures was scanned spectrophotometrically between 240 and 300 nm. The presence of ergosterol in an extracted sample resulted in a 4-peak curve. A decrease in absorbance peaks correlates to a decrease in ergosterol content. Each panel represents the results for a different Upc2 GOF mutation, as follows: G648S (A), A643V (B), Y642F (C), G648D (D), A646V (E), A643T (F), W478C (G), and G304R (H). UPC2 mutant alleles were expressed as homozygotes with the same GOF mutation (○) or as heterozygotes with the wild-type allele (▲). Their absorbencies were compared to those of the wild-type strain SC5314 (●). The results for the homozygous revertants shown in panels C, D, and G showed statistically significant greater ergosterol contents than were found in the wild type (P < 0.05).
GOF mutations in UPC2 influence susceptibility to antifungals.
To examine the impact of distinct UPC2 mutations on antifungal resistance, we determined the susceptibilities of strains constructed to carry mutant UPC2 alleles to a panel of antifungals (Table 4). Included in this panel were agents in the azole class (fluconazole, itraconazole, and voriconazole). Also included were terbinafine, which inhibits another ergosterol biosynthesis enzyme (squalene epoxidase), and amphotericin B, which targets ergosterol in the fungal cell membrane. All strains constructed were compared to the background strain, strain SC5314, and the upc2Δ/upc2Δ strain UPC2M4A. Strain UPC2M4A was highly susceptible to terbinafine, as well as all the azoles tested. Interestingly, this strain exhibited a 4-fold-higher MIC of amphotericin B than SC5314 at 48 h. Strains expressing UPC2 alleles carrying the G648D, G648S, A643T, A643V, Y642F, W478C, and A646V substitutions in the predicted protein sequence all exhibited increased resistance to fluconazole compared to that of strain SC5314, irrespective of the additional presence of a wild-type allele. Strains expressing the G304R mutant allele did not show a decrease in susceptibility beyond that of the wild-type, indicating that this mutation does not result in gain of function. Correlating with increased ERG11 expression, fluconazole susceptibility also decreased, with the most prominent change occurring in the homozygous strains. A similar trend was exhibited for the other azole agents and also for terbinafine susceptibilities. For amphotericin B, strains homozygous for an activated UPC2 mutant allele generally demonstrated a reduction in MIC at 48 h compared to that of the parental wild-type strain. However, all strains tested were susceptible to this agent. These results demonstrate that seven of the eight mutations in UPC2 confer increased resistance to ergosterol biosynthesis inhibitors.
Table 4.
MICs of a panel of antifungals in YPD against strains expressing UPC2 mutant alleles
| Strain | Relevant genotype | MIC (μg/ml) after indicated incubation (h)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCZ |
ICZ |
VCZ |
TBF |
AMB |
|||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | ||
| SC5314 | UPC2-1/UPC2-2 | 0.5 | 4 | 0.016 | 0.023 | 0.008 | 0.032 | 0.5 | 2 | 0.0313 | 0.125 |
| UPC2M4A | upc2-1Δ::FRT/upc2-2Δ::FRT | 0.023 | 0.032 | 0.002 | 0.004 | 0.002 | 0.002 | <0.016 | <0.016 | 0.0313 | 0.5 |
| 11A8A2A | UPC2G648S::FRT/UPC2G648S::FRT | 4 | >256 | 0.5 | 0.75 | 0.25 | >32 | 2 | 4 | 0.016 | 0.125 |
| SC11A1A | UPC2/UPC2G648S::FRT | 1 | >256 | 0.032 | 0.19 | 0.047 | >32 | 2 | 4 | 0.0625 | 0.25 |
| 28A7A10A | UPC2A643V::FRT/UPC2A643V::FRT | 2 | >256 | 0.125 | >32 | 0.25 | >32 | 1 | 4 | 0.016 | 0.125 |
| SC28A3A | UPC2/UPC2A643V::FRT | 1 | 2 | 0.023 | 0.047 | 0.047 | >32 | 1 | 2 | 0.0313 | 0.25 |
| YFA3A2K1 | UPC2Y642F::FRT/UPC2Y642F::FRT | 2 | >256 | 0.047 | 0.5 | 0.064 | >32 | 1 | 4 | 0.0625 | 0.5 |
| SCYFA2A | UPC2/UPC2Y642F::FRT | 0.75 | 1 | 0.023 | 0.032 | 0.023 | 0.19 | 1 | 2 | 0.0313 | 0.25 |
| AVA1A16A | UPC2A646V::FRT/UPC2A646V::FRT | 1.5 | >256 | 0.064 | 0.25 | 0.064 | >32 | 1 | 4 | 0.0313 | 0.25 |
| SCAVA4 | UPC2/UPC2A646V::SAT1-FLIP | 0.5 | 1.5 | 0.016 | 0.032 | 0.016 | 0.064 | 1 | 2 | 0.0313 | 0.5 |
| 22A1A13A | UPC2G304R::FRT/UPC2G304R::FRT | 0.19 | 0.75 | 0.012 | 0.023 | 0.008 | 0.032 | 1 | 2 | 0.0313 | 0.5 |
| SC22A3A | UPC2/UPC2G304R::FRT | 0.75 | >256 | 0.012 | 0.094 | 0.023 | >32 | 1 | 4 | 0.0313 | 0.25 |
| 25B2D1 | UPC2W478C::FRT/UPC2W478C::FRT | 1 | 2 | 0.047 | 0.047 | 0.016 | 0.5 | 0.25 | 1 | 0.0313 | 0.25 |
| SC25A1 | UPC2/UPC2W478C::FRT | 0.5 | 1 | 0.016 | 0.016 | 0.008 | 0.032 | 0.5 | 1 | 0.016 | 0.125 |
| SCUPC2R14A | UPC2G648D::FRT/UPC2G648D::FRT | 8 | >256 | >32 | >32 | 0.064 | >32 | 2 | 4 | 0.016 | 0.125 |
| SCUPC2R12A | UPC2G648D::FRT/UPC2-2 | 1.5 | >256 | 0.25 | >32 | 0.047 | >32 | 1 | 2 | 0.0313 | 0.25 |
| SCUPC2R34A | UPC2A643T::FRT/UPC2A643T::FRT | 1.5 | >256 | 2 | >32 | 0.064 | >32 | 2 | 2 | 0.016 | 0.125 |
| SCUPC2R32A | UPC2A643T::FRT/UPC2-2 | 2 | >256 | 0.016 | 0.032 | 0.012 | >32 | 1 | 2 | 0.016 | 0.125 |
Susceptibility was tested at 24 and at 48 h. Azole MICs were determined by Etest method, while terbinafine and amphotericin B MICs were determined by broth microdilution methods. FCZ, fluconazole; ICZ, itraconazole; VCZ, voriconazole; TBF, terbinafine; AMB, amphotericin B.
Genome-wide transcriptional-profile analysis of strains carrying activated UPC2 alleles.
We identified genes whose expression is influenced by Upc2 by comparing the transcriptional profiles of strains that were engineered to carry one of the four GOF UPC2 alleles with the strongest effects on ERG11 expression. The 11A8A2A (UPC2G648S/UPC2G648S) constructed strain expressed 170 upregulated genes and 9 downregulated genes compared to the expression profile of SC5314. Strain SCUPC2R14A (UPC2G648D/UPC2G648D) expressed 520 upregulated and 292 downregulated genes. There were 119 genes upregulated and 81 genes downregulated for strain YFA3A2K1 (UPC2Y642F/UPC2Y642F), while strain 28A7A10A (UPC2A643V/UPC2A643V) expressed 352 upregulated and 169 downregulated genes. There were 61 commonly upregulated genes and 5 commonly downregulated genes among the four strains carrying unique UPC2 gain-of-function alleles (Tables 5 and 6). Genes commonly upregulated in all four mutant strains included those involved in ergosterol biosynthesis and in oxidoreductase activity, the major facilitator efflux pump encoded by the MDR1 gene, and the uncharacterized ATP-binding cassette transporter CDR11.
Table 5.
Genes upregulated by at least 1.5-fold in strains carrying UPC2 GOF alleles
| Functiona | CGD name | orf19 designation | Change in expression (fold)b in indicated experiment |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G648S |
G648D |
Y642F |
A643V |
|||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Lipid metabolic process | ERG1 | orf19.406 | 1.8 | 1.8 | 2.7 | 2.3 | 2.2 | 2.0 | 2.3 | 2.3 |
| ERG2 | orf19.6026 | 1.7 | 1.5 | 2.3 | 1.9 | 1.9 | 1.9 | 2.1 | 2.0 | |
| ERG24 | orf19.1598 | 1.7 | 1.7 | 2.3 | 2.1 | 1.7 | 1.8 | 2.1 | 2.3 | |
| ERG27 | orf19.3240 | 1.8 | 2.1 | 3.3 | 3.4 | 2.2 | 2.2 | 3.1 | 3.2 | |
| ERG7 | orf19.1570 | 1.5 | 1.6 | 2.8 | 2.1 | 1.8 | 1.6 | 2.7 | 2.4 | |
| NCP1 | orf19.2672 | 1.6 | 1.7 | 2.6 | 2.0 | 1.7 | 1.9 | 1.9 | 1.9 | |
| orf19.3483 | 2.0 | 2.4 | 5.3 | 4.7 | 2.9 | 2.2 | 3.5 | 3.3 | ||
| orf19.1881 | 1.5 | 1.6 | 2.4 | 2.2 | 1.5 | 1.5 | 2.4 | 2.1 | ||
| orf19.6025 | 2.2 | 2.1 | 3.9 | 5.2 | 3.8 | 3.1 | 5.2 | 5.2 | ||
| Transcription factor activity | orf19.5210 | 1.5 | 2.1 | 2.7 | 2.7 | 1.8 | 1.5 | 2.9 | 2.3 | |
| Transport | MDR1 | orf19.5604 | 5.9 | 5.5 | 15.1 | 8.0 | 6.6 | 6.1 | 5.9 | 5.7 |
| orf19.5535 | 1.5 | 1.6 | 2.2 | 2.7 | 1.5 | 1.9 | 2.7 | 3.2 | ||
| FRP1 | orf19.5634 | 5.4 | 5.7 | 17.3 | 17.5 | 8.1 | 5.9 | 16.3 | 14.7 | |
| FTH1 | orf19.4802 | 2.6 | 2.6 | 5.3 | 5.2 | 2.3 | 1.9 | 6.6 | 5.2 | |
| GYP1 | orf19.3811 | 1.6 | 1.6 | 3.7 | 3.5 | 1.8 | 1.5 | 3.7 | 3.4 | |
| orf19.2350 | 1.7 | 1.7 | 3.4 | 4.4 | 2.0 | 1.9 | 3.2 | 3.0 | ||
| HXT5 | orf19.4384 | 1.8 | 2.1 | 2.6 | 2.0 | 1.8 | 1.5 | 2.3 | 1.7 | |
| Protein modification process | orf19.7329 | 1.6 | 2.1 | 3.1 | 3.0 | 1.7 | 1.8 | 2.5 | 2.3 | |
| orf19.6025 | 2.2 | 2.1 | 3.9 | 5.2 | 3.8 | 3.1 | 5.2 | 5.2 | ||
| orf19.7547 | 1.8 | 1.5 | 2.8 | 2.7 | 2.0 | 1.5 | 3.1 | 2.3 | ||
| orf19.2285 | 1.7 | 2.5 | 1.8 | 1.5 | 1.8 | 2.4 | 2.1 | 2.1 | ||
| Biosynthetic Process | COQ4 | orf19.3008 | 1.5 | 1.5 | 2.3 | 2.8 | 1.9 | 1.6 | 2.6 | 2.5 |
| HEM14 | orf19.4747 | 2.1 | 1.6 | 3.6 | 3.0 | 2.2 | 1.8 | 2.9 | 2.3 | |
| Pathogenesis | SLD1 | orf19.260 | 1.8 | 1.5 | 2.2 | 1.8 | 1.7 | 1.6 | 2.0 | 1.9 |
| PHO100 | orf19.4424 | 1.5 | 3.4 | 4.8 | 2.7 | 1.6 | 1.5 | 2.0 | 2.3 | |
| SET3 | orf19.7221 | 2.8 | 2.7 | 6.3 | 5.7 | 4.4 | 3.6 | 4.8 | 4.8 | |
| Metabolic process | orf19.3617 | 1.7 | 1.9 | 1.7 | 2.2 | 1.7 | 1.5 | 1.8 | 1.5 | |
| orf19.329 | 2.1 | 2.3 | 2.4 | 2.6 | 2.0 | 1.6 | 2.5 | 2.5 | ||
| orf19.4031 | 1.8 | 1.8 | 2.5 | 2.5 | 1.6 | 1.5 | 2.9 | 2.5 | ||
| orf19.6025 | 2.2 | 2.1 | 3.9 | 5.2 | 3.8 | 3.1 | 5.2 | 5.2 | ||
| orf19.1865 | 2.4 | 2.2 | 3.7 | 2.9 | 2.4 | 2.2 | 3.4 | 3.1 | ||
| orf19.496 | 2.4 | 2.3 | 4.4 | 3.8 | 2.5 | 2.0 | 3.6 | 3.1 | ||
| ARO9 | orf19.1237 | 1.5 | 3.4 | 5.8 | 4.2 | 1.5 | 1.9 | 2.1 | 2.8 | |
| IDP2 | orf19.3733 | 2.2 | 1.6 | 3.3 | 2.8 | 2.6 | 2.0 | 3.2 | 2.5 | |
| Response to stress | DDR48 | orf19.4082 | 2.2 | 4.3 | 5.8 | 4.7 | 2.7 | 2.9 | 3.4 | 4.3 |
| FMA1 | orf19.6837 | 1.8 | 3.2 | 2.7 | 5.3 | 1.5 | 3.3 | 1.7 | 3.0 | |
| YMX6 | orf19.5713 | 1.6 | 2.6 | 2.9 | 2.0 | 1.6 | 1.6 | 1.7 | 1.8 | |
| orf19.288 | 1.8 | 1.5 | 3.0 | 2.5 | 2.1 | 1.5 | 2.5 | 2.0 | ||
| Biological activity unknown | TEF4 | orf19.2652 | 16.4 | 16.6 | 55.8 | 90.2 | 29.9 | 32.7 | 65.5 | 67.8 |
| orf19.3627 | 4.0 | 1.9 | 7.0 | 4.0 | 4.4 | 1.9 | 5.1 | 1.9 | ||
| PGA7 | orf19.5635 | 2.3 | 3.7 | 5.7 | 7.4 | 2.7 | 3.8 | 4.8 | 6.1 | |
| BUL1 | orf19.5094 | 1.9 | 1.9 | 4.1 | 3.1 | 2.5 | 1.8 | 2.1 | 1.9 | |
| PGA45 | orf19.2451 | 1.7 | 1.5 | 2.1 | 2.2 | 1.7 | 1.9 | 2.0 | 2.3 | |
| orf19.344 | 5.1 | 6.2 | 20.2 | 16.2 | 7.2 | 6.6 | 9.6 | 9.0 | ||
| orf19.5777 | 1.7 | 2.4 | 4.8 | 3.8 | 1.9 | 1.5 | 3.1 | 2.5 | ||
| orf19.5799 | 1.7 | 2.0 | 3.4 | 2.4 | 1.5 | 1.5 | 2.4 | 2.6 | ||
| orf19.7456 | 1.7 | 2.6 | 4.4 | 4.1 | 2.2 | 2.1 | 3.9 | 3.5 | ||
| orf19.7043 | 2.5 | 1.9 | 5.2 | 3.6 | 2.8 | 2.3 | 4.9 | 3.0 | ||
| orf19.4013 | 2.6 | 2.2 | 4.2 | 3.5 | 3.1 | 2.8 | 4.8 | 3.8 | ||
| orf19.4014 | 1.5 | 2.1 | 2.3 | 2.4 | 1.7 | 1.6 | 2.6 | 2.6 | ||
| orf19.6840 | 2.0 | 2.1 | 4.1 | 4.4 | 2.6 | 2.4 | 3.1 | 2.6 | ||
| orf19.286 | 2.1 | 2.5 | 2.3 | 2.2 | 1.9 | 1.9 | 3.5 | 1.9 | ||
| orf19.7504 | 2.1 | 1.8 | 3.0 | 3.3 | 1.9 | 2.0 | 2.2 | 2.1 | ||
| orf19.3737 | 1.6 | 1.9 | 2.9 | 3.4 | 2.0 | 1.8 | 3.1 | 2.6 | ||
| orf19.1964 | 2.0 | 1.8 | 2.5 | 2.2 | 1.7 | 1.6 | 2.2 | 1.6 | ||
| orf19.1800 | 2.3 | 2.2 | 4.6 | 3.9 | 2.5 | 2.2 | 4.5 | 3.4 | ||
| orf19.7263 | 1.8 | 1.9 | 2.9 | 2.8 | 1.6 | 1.7 | 2.1 | 1.9 | ||
| orf19.2496 | 1.7 | 1.8 | 2.6 | 2.5 | 1.8 | 1.7 | 2.0 | 1.5 | ||
| ATO9 | orf19.3261 | 1.8 | 2.2 | 2.7 | 3.4 | 1.6 | 2.3 | 4.5 | 4.5 | |
Descriptions of genes are from the Candida Genome Database (http://www.candidagenome.org).
Fold change is defined as the average ratio of gene expression levels in the isolates compared in two independent microarray experiments.
Table 6.
Genes downregulated by at least 1.5-fold in strains carrying UPC2 GOF alleles
| Functiona | CGD name | orf19 designation | Change in expression (fold)b in indicated experiment |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G648S |
G648D |
Y642F |
A643V |
|||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Transport | HGT10 | orf19.5753 | 0.6 | 0.5 | 0.2 | 0.2 | 0.5 | 0.6 | 0.2 | 0.3 |
| Pathogenesis | SAP7 | orf19.756 | 0.4 | 0.4 | 0.3 | 0.2 | 0.4 | 0.4 | 0.3 | 0.4 |
| Biological activity unknown | orf19.716 | 0.4 | 0.5 | 0.4 | 0.4 | 0.6 | 0.6 | 0.4 | 0.4 | |
| orf19.3621 | 0.3 | 0.5 | 0.3 | 0.3 | 0.6 | 0.4 | 0.5 | 0.5 | ||
| orf19.4873 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | ||
Descriptions of genes are from the Candida Genome Database (http://www.candidagenome.org).
Fold change is defined as the average ratio of gene expression levels in the isolates compared in two independent microarray experiments.
DISCUSSION
In Candida albicans, it is well established that activating mutations in transcription factors regulating genes encoding efflux pumps mediate resistance to azole antifungals. It has been shown that specific mutations in the transcription factor gene TAC1 mediate the expression of the genes encoding ABC transporters CDR1 and CDR2, resulting in increased azole resistance in C. albicans (2, 3). Likewise, Mrr1 has been identified as the regulator of the major facilitator superfamily (MFS) transporter Mdr1 in azole-resistant isolates, and specific mutations in this transcriptional regulator result in its constitutive activation, leading to the overexpression of Mdr1 (6, 22). Fluconazole-resistant isolates that overexpress MDR1 have become homozygous for the mutated MRR1 allele and work in a semidominant fashion if expressed with a wild-type allele (6). Overexpression of these transporters is known to occur among azole-resistant clinical isolates and contributes significantly to this process.
Considerably less is known about the prevalence of constitutive ERG11 overexpression and its clinical impact on azole resistance. ERG11 encodes the azole target, lanosterol demethylase, a key enzyme in the ergosterol biosynthesis pathway. ERG11 is transcriptionally regulated by the zinc cluster transcription factor Upc2. In C. albicans, Upc2 is orthologous to two transcription factors in Saccharomyces cerevisiae, Upc2 and Ecm22, which regulate ergosterol biosynthesis and uptake of exogenous sterols (4, 5). The expression of these genes is affected by sterol depletion, but the exact mechanism of activation is currently unknown. Previous work in S. cerevisiae shows that ScUpc2 and ScEcm22 are localized to intracellular membranes outside the nucleus, and under sterol-depleting conditions, perinuclear localization is increased (20). Gain-of-function mutations in the activation domain located near the C terminus have been recovered from both ScUpc2 and ScEcm22; however, mediation of ERG11 expression during hypoxic conditions or when chemically treated with azole antifungals is mediated by ScUpc2 (5).
In C. albicans, only three mutations that increase the expression of ERG11 and result in decreased susceptibility to fluconazole have been described in Upc2. In the present study, we examined a large collection of unrelated azole-resistant C. albicans clinical isolates for possible UPC2 GOF mutations. As fluconazole resistance in C. albicans has most often been associated with OPC, the opportunity to examine resistant isolates cultured from both oral and nonoral sources was somewhat unique. Among the isolates studied here were 16 documented nonoral isolates, 12 of which were observed to exhibit reduced susceptibilities to fluconazole. Although our analysis cannot define the clinical implications for this observation, these data underscore that fluconazole resistance extends beyond oral manifestations of candidiasis.
We further delineated the mechanisms of resistance in these isolates by determining the relative levels of expression of CDR1, CDR2, MDR1, and ERG11. Not surprisingly, CDR1 and CDR2 overexpression was generally coordinately regulated and quite prevalent among these isolates. Of those isolates that did overexpress MDR1, even fewer isolates expressed MDR1 to the levels previously observed in azole-resistant isolates (22). ERG11 was found to be upregulated in almost three-fourths of the fluconazole-resistant isolates examined. This suggests that ERG11 overexpression is a common contributor to fluconazole resistance in C. albicans.
Among the independent azole-resistant, ERG11-overexpressing isolates studied here, we repeatedly recovered eight distinct single-nucleotide substitutions in UPC2, of which the G648S substitution occurred in nine isolates and the G648D substitution occurred in six isolates. Five of these substitutions in UPC2 have not been described previously. Of the five novel mutations, four mutations resulted in increased ERG11 expression and increased resistance to fluconazole but to various degrees. We observed that the homozygous strain carrying the G304R UPC2 allele was more susceptible to azoles than the strain carrying the G304R substitution heterozygously; however, neither strain resulted in any decrease in antifungal susceptibility or increase in ERG11 expression. As has been previously observed in UPC2 GOF mutations, amino acid substitutions resulting from activating mutations were localized near the C terminus, where the activation domain of zinc-cluster transcription factors is found (18). Mutations in this region of the protein are theorized to work by one of two mechanisms: (i) mutations in this domain could relieve the transcription factor from a repressor that would otherwise keep Upc2 inactive in the absence of an activating signal or (ii) a mutation in this area could interfere with the transmembrane region of the protein, causing localization to the nucleus and constitutive activation of its target promoters.
In total, seven of the eight Upc2 mutations observed resulted in increased expression of ERG11, but each mutation increased transcription to a different degree. For many strains, increased expression of ERG11 was directly correlated with the amount of ergosterol within the fungal cell, and this trend was also observed in the decreased azole susceptibility seen in strains that overexpressed ERG11. Unlike TAC1 GOF mutations, UPC2 GOF mutations were found to occur with a wild-type allele in many isolates. We observed that strains constructed to express two mutant alleles had increased levels of ERG11 expression and decreased levels of azole susceptibility compared to the levels in strains containing both an activated Upc2 and a wild-type allele. As seen with TAC1 or MRR1 mutations, a loss-of-heterozygosity event that results in two GOF alleles, either by a mitotic recombination event between homologous chromosomes or by a loss of a chromosome combined with a duplication of the homologous chromosome, would provide an advantage when challenged with antifungals (2, 22). Interestingly, we saw an opposite trend in the amphotericin B susceptibility of strains expressing activated UPC2 alleles, in which UPC2 GOF homozygous strains with higher ERG11 expression had increased susceptibility to this agent compared to that of the heterozygous strains or the homozygous null upc2Δ strain. This is probably due to higher levels of cellular ergosterol, which is the target of amphotericin B.
An important observation to note is the discrepancy between the levels of ERG11 expression of the clinical isolate containing a UPC2 mutation and the laboratory strain constructed to carry the same mutant UPC2 allele. This difference may be due to additional levels of ERG11 regulation that are developed in a clinical isolate, such as alternate transcriptional regulations or alternate single-nucleotide polymorphisms that influence ergosterol biosynthesis. Furthermore, not all ERG11 overexpression in clinical isolates was a result of a GOF mutation in UPC2; therefore, it is likely that other regulators of ergosterol biosynthesis are contributing factors. Alternatively, instances of aneuploidy, specifically, chromosome 5 duplication or chr5L isochromosome formation, are known mechanisms of ERG11 overexpression in C. albicans (29). However, the ERG11 expression levels we observed in many clinical isolates in this study exceed what would be expected from an extra copy of ERG11 due to the presence of a chr5L isochromosome. It is possible that some isolates that do not carry mutations in UPC2 actually overexpress UPC2 instead, due to a mutation in the UPC2 promoter region, gene duplication, or a trans-acting mutation. Whether this has occurred is being explored in ongoing investigations.
As azole resistance is mechanistically multifactorial in clinical isolates, we expected the fluconazole susceptibility of strains constructed to express specific UPC2 mutations to reflect the contribution of increased expression of genes of the ergosterol biosynthesis pathway, including ERG11, as a result of an activated UPC2 allele. In these clinical isolates, we have observed a high prevalence of mutations in the ERG11 gene itself (data not shown). It is likely that in clinical isolates, activated forms of Upc2 occur in conjunction with mutant ERG11 alleles and that its subsequent overexpression results in a combinatorial effect on azole susceptibility. The combinatorial effects of activated UPC2 alleles and mutant ERG11 alleles on fluconazole susceptibility have not been explored.
Although microarray analysis of the G648D UPC2 GOF mutation has been performed previously, we expanded this analysis with four of the seven strongest UPC2 GOF mutations (G648D, G648S, Y642F, and A643V) for a more comprehensive examination (7). All four mutations caused the coordinate upregulation of 61 genes. As expected, many of the ergosterol biosynthesis genes (ERG1, ERG2, ERG7, ERG24, ERG26, and ERG27) were upregulated by at least 1.5-fold over their expression in SC5314 in two separate experiments. ERG11 was upregulated in three of the four laboratory strains carrying mutations G648D, Y642F, and A643V, but elevated transcription of ERG11 could only be detected in one of the two experiments with the strain carrying the G648S allele (see Table S2 in the supplemental material). Interestingly, UPC2 was not found to be upregulated in any of the four strains analyzed by microarray analysis, although Upc2 is thought to autoregulate and has been shown to bind its own promoter in experiments using chromatin immunoprecipitation with microarray technology (ChIP-chip) (33).
Aside from genes involved in the ergosterol biosynthesis pathway, other core sets of target genes upregulated by Upc2 included those in the gene ontology (GO) functional grouping of oxidoreductase activity. Additionally, iron ion binding was a commonly found GO category identified by the GO term finder. Not surprisingly, MDR1 was overexpressed in all four strains with independent Upc2 GOF mutations; however, gene expression levels were not increased to those observed in fluconazole-resistant isolates containing an MRR1 GOF mutation. Upc2 has previously been shown to bind to the promoter of the drug efflux pump MDR1 but was not a cause of Mdr1-mediated drug resistance (28, 33). In the present study, CDR11 expression was increased in all independent UPC2 GOF mutations, and it increased 4.8-fold when two alleles of UPC2 carrying the predicted G648D substitution in its translated sequence were expressed. Interestingly, the gene encoding an uncharacterized ABC transporter, CDR11, was previously shown to be upregulated by 1.5- to 3.5-fold in a laboratory strain carrying a single UPC2 allele containing a G648D amino acid substitution; this gene has been hypothesized to play a role in sterol transport, because of its similarity to sterol transporters in S. cerevisiae (7).
In conclusion, we found overexpression of ERG11 to be prevalent among a collection of clinical isolates with reduced susceptibility to fluconazole and have identified and characterized novel UPC2 gain-of-function mutations that contribute to azole resistance in clinical isolates of C. albicans. Overexpression of ERG11 could be explained by a gain-of-function mutation in UPC2 in many but not all cases. Gain-of-function mutations led to increased resistance to azole antifungals and terbinafine, to increased cellular ergosterol levels, and to increased expression of genes involved in ergosterol biosynthesis and oxidoreductase activity, as well as the transporter genes MDR1 and CDR11. The mechanism by which ERG11 is upregulated in the absence of UPC2 gain-of-function mutations and the potential contribution of sterol uptake to Upc2-mediated azole resistance are under investigation.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH NIAID grant R01AI058145 (to P.D.R.) and by the Children's Foundation Research Center of Memphis at Le Bonheur Children's Medical Center.
We thank Qing Zhang for her assistance in the laboratory. We are grateful to Daniel Diekema for providing the clinical isolates used in this study.
We have no financial or commercial conflicts of interest to declare.
Footnotes
Published ahead of print 24 August 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved method M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 2.Coste AT, et al. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley JH, Leak FW, Jr, Shianna KV, Tove S, Parks LW. 1998. A mutation in a purported regulatory gene affects control of sterol uptake in Saccharomyces cerevisiae. J. Bacteriol. 180:4177–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies BS, Rine J. 2006. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunkel N, Blass J, Rogers PD, Morschhauser J. 2008. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkel N, et al. 2008. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot. Cell 7:1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Feigal DW, et al. 1991. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS 5:519–525 [DOI] [PubMed] [Google Scholar]
- 10.Franz R, Ruhnke M, Morschhauser J. 1999. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses 42:453–458 [DOI] [PubMed] [Google Scholar]
- 11.Heilmann CJ, Schneider S, Barker KS, Rogers PD, Morschhauser J. 2010. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 54:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoot SJ, Smith AR, Brown RP, White TC. 2011. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 55:940–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein RS, et al. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354–358 [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis DP, Lewis RE. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135–1144 [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Lin HX, White KA. 2004. A complex network of RNA-RNA interactions controls subgenomic mRNA transcription in a tombusvirus. EMBO J. 23:3365–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Ribot JL, et al. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacPherson S, Larochelle M, Turcotte B. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70:583–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacPherson S, et al. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 49:1745–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie C, Leyde S, White TC. 2008. Cytoplasmic localization of sterol transcription factors Upc2p and Ecm22p in S. cerevisiae. Fungal Genet. Biol. 45:1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morschhauser J. 2002. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta 1587:240–248 [DOI] [PubMed] [Google Scholar]
- 22.Morschhauser J, et al. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3:e164 doi:10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perea S, et al. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangel-Frausto MS, et al. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253–258 [DOI] [PubMed] [Google Scholar]
- 25.Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 25a.Rex JH, Rinaldi MG, Pfaller MA. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard D, et al. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt ME, Brown TA, Trumpower BL. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18:3091–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert S, et al. 2011. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob. Agents Chemother. 55:2212–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selmecki A, Forche A, Berman J. 2006. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313:367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver PM, Oliver BG, White TC. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 3:1391–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warrilow AG, et al. 2010. Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51). Antimicrob. Agents Chemother. 54:4235–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White TC, Marr KA, Bowden RA. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Znaidi S, et al. 2008. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot. Cell 7:836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.