Abstract
Septins were identified for their role in septation in Saccharomyces cerevisiae and were subsequently implicated in other morphogenic processes. To study septins in Candida albicans hyphal morphogenesis, a temperature-sensitive mutation was created that altered the C terminus of the essential Cdc12 septin. The cdc12-6 cells grew well at room temperature, but at 37°C they displayed expected defects in septation, nuclear localization, and bud morphogenesis. Although serum stimulated the cdc12-6 cells at 37°C to form germ tube outgrowths, the mutant could not maintain polarized hyphal growth and instead formed chains of elongated cell compartments. Serum also stimulated the cdc12-6 mutant to induce a hyphal reporter gene (HWP1-GFP) and a characteristic zone of filipin staining at the leading edge of growth. Interestingly, cdc12-6 cells shifted to 37°C in the absence of serum gradually displayed enriched filipin staining at the tip, which may be due to the altered cell cycle regulation. A striking difference from the wild type was that the cdc12-6 cells frequently formed a second germ tube in close proximity to the first. The mutant cells also failed to form the diffuse band of septins at the base of germ tubes and hyphae, indicating that this septin band plays a role in preventing proximal formation of germ tubes in a manner analogous to bud site selection. These studies demonstrate that not only are septins important for cytokinesis, but they also promote polarized morphogenesis and selection of germ tube sites that may help disseminate an infection in host tissues.
INTRODUCTION
The human fungal pathogen Candida albicans is capable of causing severe systemic infections. Although immunocompromised patients are particularly at risk, immunocompetent individuals are susceptible to infection when the inoculum is high, which can occur under circumstances such as biofilm formation on medical devices. The major pathogenic effects of C. albicans are due to invasive growth into tissues, which is facilitated in part by the ability of C. albicans to switch between different morphologies (29, 36). C. albicans can grow as budding cells, chains of elongated cells termed pseudohyphae, or as long filamentous cells with parallel walls called hyphae (36). The filamentous morphology promotes invasive growth into agar in vitro and has been linked to invasive growth into tissues in vivo (20, 32). Previous studies also indicated that C. albicans must be able to switch between different morphologies to be fully pathogenic. Mutants that are locked in either the hyphal or budding form have been shown to be less virulent in models of hematogenously disseminated systemic candidiasis (23, 32, 44).
The septin family of cytoskeletal filament-forming proteins has been shown to contribute to morphogenesis in C. albicans (16, 28, 34, 40). The septins were first identified in the yeast Saccharomyces cerevisiae as proteins that are needed for septum formation and cytokinesis (15, 25). The septins localize to the bud neck, where they form a scaffold to recruit other proteins that promote septum formation. The septins also act as a boundary domain to restrict proteins involved in septum formation to the proper position in the bud neck and also restrict certain proteins to the daughter cells (9, 30). Deletion analysis of the seven different septin genes in C. albicans revealed that their relative importance is similar to the orthologous genes in S. cerevisiae. CDC3 and CDC12 are essential, whereas CDC10, CDC11, and SEP7 are not essential but contribute to proper septation and morphogenesis (16, 40). Mutation of the C. albicans orthologs of the SPR3 and SPR28 septin genes that are expressed during sporulation in S. cerevisiae did not detectably affect C. albicans septation or morphogenesis (40).
Septins have also been implicated in other morphogenic events. For example, S. cerevisiae septins promote proper pheromone-induced morphogenesis, spore formation, and selection of the site of future bud formation (12). The possibility that the septins may also play special roles during C. albicans hyphal growth was suggested by studies that detected septins at sites distal to septum formation, including the base of the initial hyphal outgrowths (know as germ tubes) and at the growing hyphal tips (35, 40). Consistent with this, cdc10Δ and cdc11Δ mutants exhibited partial defects in hyphal morphogenesis, including a greater frequency of curved hyphae, and slight inconsistencies in cell wall deposition (40). Significantly, the cdc10Δ and cdc11Δ mutants displayed defects in morphogenesis and invasive growth in a mouse model of systemic C. albicans infection (39). These mutants also had a related defect in the morphogenesis of the filamentous cells that produce chlamydospores (26).
In order to obtain a fuller understanding of the role of the C. albicans septins in hyphal growth, a new approach was necessary to study the essential septin genes CDC3 and CDC12. Therefore, a temperature-sensitive CDC12 mutant strain was constructed that was patterned after the well-studied cdc12-6 S. cerevisiae mutant. The C. albicans cdc12-6 strain grew well at room temperature but not at 37°C, the temperature at which hyphal growth is induced. It also showed temperature-sensitive defects in septation and bud morphogenesis, similar to those reported for the analogous mutation of S. cerevisiae (1, 17). Interestingly, the C. albicans cdc12-6 mutant also revealed important new roles for septins in maintaining highly polarized hyphal growth and for selection of the second site of germ tube formation.
MATERIALS AND METHODS
Strains and media.
The C. albicans strains used in the present study are described in Table 1. The cells were propagated in rich YPD medium (2% glucose, 1% peptone, 2% yeast extract) or SD (yeast nitrogen base synthetic medium with dextrose) as described previously (33). Uridine at 80 mg/liter was added to cultures of ura3Δ cells.
Table 1.
Strains used in this study
| C. albicans strain | Parent strain | Genotype |
|---|---|---|
| BWP17 | Sc5314 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| DIC185 | BWP17 | ura3Δ::λ imm434/URA3 his1::hisG/HIS1 arg4::hisG/ARG4 |
| YLS685 | BWP17 | ura3Δ::λ imm434/URA3 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YAW12 | BWP17 | cdc12::ARG4/CDC12 ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| CZ10 | YAW12 | cdc12-6::URA3/cdc12Δ::ARG4 ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| CZ11 | CZ10 | cdc12-6 strain CZ10, except CDC10-GFPγ::HIS1 |
| CZ14 | CZ10 | cdc12-6 strain CZ10, except NOP1-GFPγ::HIS1 |
| LLF003 | BWP17 | BWP17, except CDC10-GFPγ::HIS1 |
| LLF006 | BWP17 | BWP17, except NOP1-GFPγ::URA3 |
| LLF009 | LLF003 | CDC10-GFPγ strain LLF003, except URA3/ura3::λimm434 |
| LLF010 | CZ10 | cdc12-6 strain CZ10, except HWP1-GFPγ::HIS1 |
| LLF012 | YLS685 | YLS685, except HWP1-GFPγ::HIS1 |
| LLF016 | CZ10 | cdc12-6 strain CZ10, HIS1/his1::hisG |
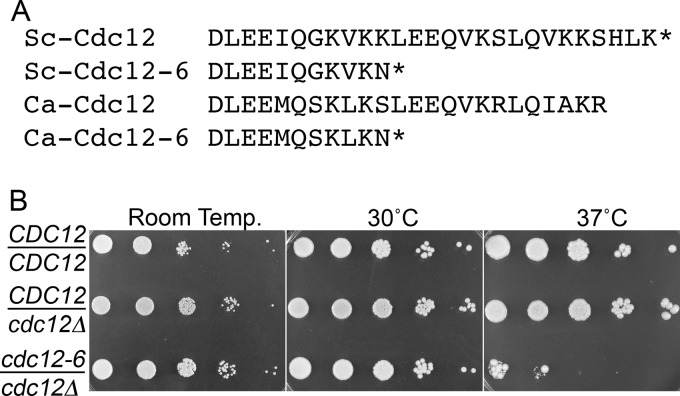
A C. albicans cdc12Δ/CDC12 heterozygous mutant was created in strain BWP17 by homologous recombination as described previously (40, 42). In brief, a deletion cassette was constructed by PCR using primers that contain sequence homology to the 5′ and 3′ ends of the CDC12 open reading frame to amplify a cassette containing the ARG4 gene for use as a selectable marker to delete one copy of CDC12. The C-terminal coding sequences of the remaining CDC12 allele were then replaced by an altered sequence that was patterned after the changes found in the S. cerevisiae cdc12-6 mutant allele (Brian Haarer, unpublished data). The S. cerevisiae cdc12-6 allele contains mutations that result in a Lys-to-Asn change at position 391, followed immediately by a TAG stop codon, truncating the mutant protein by 16 amino acids. Amino acid sequence alignments indicated that the Lys-391 of Sc-Cdc12 corresponds to Ser-384 of Ca-Cdc12. Therefore, the analogous mutation was constructed by mutating one allele of CDC12 to substitute Ser at position 384 with Asn and converting Leu at position 385 to the stop codon TAG (Fig. 1A). Oligonucleotide primers carrying the indicated changes were used to amplify the URA3 gene using pGEM-URA3 as a template (42), and then the resulting cassette was used to select for integration of the cdc12-6 allele into C. albicans. The primer sequences were 5′-GACTAATATTATTAATGAAAGAAATAGATTGAATCAAGACTTGGAAGAAATGCAATCGAAGTTGAAGAATTAGTTCTTGAGTTTGTAACAGCTGC-3′ and 5′-GTAACCCAAACAACAGAAATTAAGTGGAAGTAATATATTGCTAGCAAAAAAAAACTAAAAAAATGTTAATCTCTCCCCCTTCACATTTATAAT-3′. Similar temperature-sensitive phenotypes for budding and hyphal morphogenesis were observed for independent transformants that were cdc12::ARG4/cdc12-6::URA3, so one strain (CZ10) was then used for all subsequent studies. Strain CZ10 was transformed with a HIS1 gene to complement all of the auxotrophies and create strain LLF016. The HIS1 DNA fragment was isolated by PCR amplification using genomic DNA from C. albicans strain SC5314 as a template.
Fig 1.
The C. albicans cdc12-6 septin mutant is temperature sensitive for growth. (A) Alignment of the C-terminal sequences of the S. cerevisiae and C. albicans Cdc12 proteins. (B) Tenfold dilutions of the wild-type control CDC12/CDC12 (DIC185), cdc12-6/cdc12Δ (LLF016), and heterozygote CDC12/cdc12Δ (YAW12) strains were spotted onto rich-medium YPD plates. The plates were incubated for 48 h at the indicated temperature and then photographed.
CDC10-GFP and NOP1-GFP fusion genes were created using PCR primers that contained 80 bp of sequence homologous to the region immediately upstream and downstream of the stop codon to amplify a GFPγ::HIS1 cassette (43). This cassette contains the CaGFPγ version of green fluorescent protein (GFP) that is more photostable (43). Purified PCR product was transformed into the indicated strains, and then the resulting colonies were screened to identify strains that produced the appropriate GFP fusion protein. Similar methods were also used to create a strain carrying a hyphal reporter gene HWP1-GFP, except that GFPγ sequences were used to replace the entire open reading frame of the hypha-induced gene HWP1. The successful transformants were confirmed by microscopic examination of cells to demonstrate that that GFP expression was elevated in hyphal inducing condition.
Phenotypic analysis of the cdc12-6 mutant.
Temperature-sensitive growth properties of the cdc12-6 mutant were demonstrated by spotting 3 μl of a series of 10-fold dilutions of cells onto solid agar YPD medium, starting at a concentration of ∼107 cells/ml. The plates were then incubated at the indicated temperature for 2 days. Cells were also analyzed in liquid culture after shorter times of incubation at 37°C. Liquid cultures were grown overnight to log phase at room temperature in rich YPD medium. The cultures were split, and growth was then continued for the indicated times at room temperature or at 37°C. Cell morphology was examined using differential interference contrast (DIC) optics. Viability was determined by mixing the cells with an equal volume of 0.4% trypan blue (Sigma-Aldrich Corp.) and incubating them for 5 min, and then the cells were examined microscopically with visible light to detect intracellular staining indicate of plasma membrane lysis. GFP was analyzed directly in live cells without further processing by using a fluorescence microscope equipped with a fluorescein isothiocyanate (FITC) filter set. This filter set was used to detect GFP, since it was easier to recognize the autofluorescence of dead cells as a different color from the true GFP signal. Chitin staining was performed by incubating cells with 10 μg of Calcofluor White/ml in phosphate-buffered saline (PBS) for 5 min, followed by two washes with PBS, and then viewing the cells without fixation using a UV filter set. Filipin staining was carried out essentially as described previously (2, 27). The cells were induced with or without serum for 2 h at 37°C, stained with 10 μg of filipin/ml, and then analyzed immediately by fluorescence microscopy using a UV filter set. The cells were viewed on an Olympus BH-2 microscope, and images were captured with an AxioCam digital camera (Carl Zeiss, Thornwood, NY) operated with Axiovision software. The fluorescence signal intensity for cells expressing HWP1-GFP was quantified using Axiovision software. The results represent the average of three independent experiments in which at least 50 cells were analyzed each time.
Confocal microscopy was used to analyze septin ring structure in cells producing Cdc10-GFP. The cells were cultured overnight in log phase at room temperature, and then aliquots were incubated for 2 h at 23 or 37°C. The aliquot incubated at 37°C was further divided into two tubes. One tube was incubated in rich YPD medium to promote growth of budding cells. Bovine calf serum was added to the other tube to a final concentration of 20% to induce hyphal growth. The cells were then analyzed by fluorescence microscopy using a Zeiss LSM510 META NLO two-photon laser scanning confocal microscope at the Stony Brook University Central Microscopy Imaging Center.
RESULTS
Construction of a C. albicans temperature-sensitive cdc12-6 mutant.
The essential septin gene CDC12 was examined by creating a mutant allele based on the changes found in the well-studied temperature-sensitive S. cerevisiae cdc12-6 mutant (Haarer, unpublished). The mutation alters the C-terminal sequences of Cdc12 as depicted in Fig. 1A. These alterations may alter septin function because the C-terminal region of Cdc12 contributes to the stability of septin structures by promoting interaction between two septin filaments (4, 5, 37). The other copy of CDC12 was deleted so that only the mutant cdc12-6 allele remained. The C. albicans cdc12-6 strain displayed a strong temperature-sensitive phenotype (Fig. 1B). The cdc12-6 strain grew as well as the wild-type control strain or a cdc12Δ/CDC12 heterozygote when spotted onto solid medium agar plates at temperatures up through 30°C, but it was not viable at 37°C. Thus, although the C termini of Cdc12 from S. cerevisiae and C. albicans are not identical, introduction of the analogous alterations of the Sc-cdc12-6 allele into Ca-CDC12 still resulted in a strong temperature-sensitive phenotype.
cdc12-6 mutant phenotypes during budding.
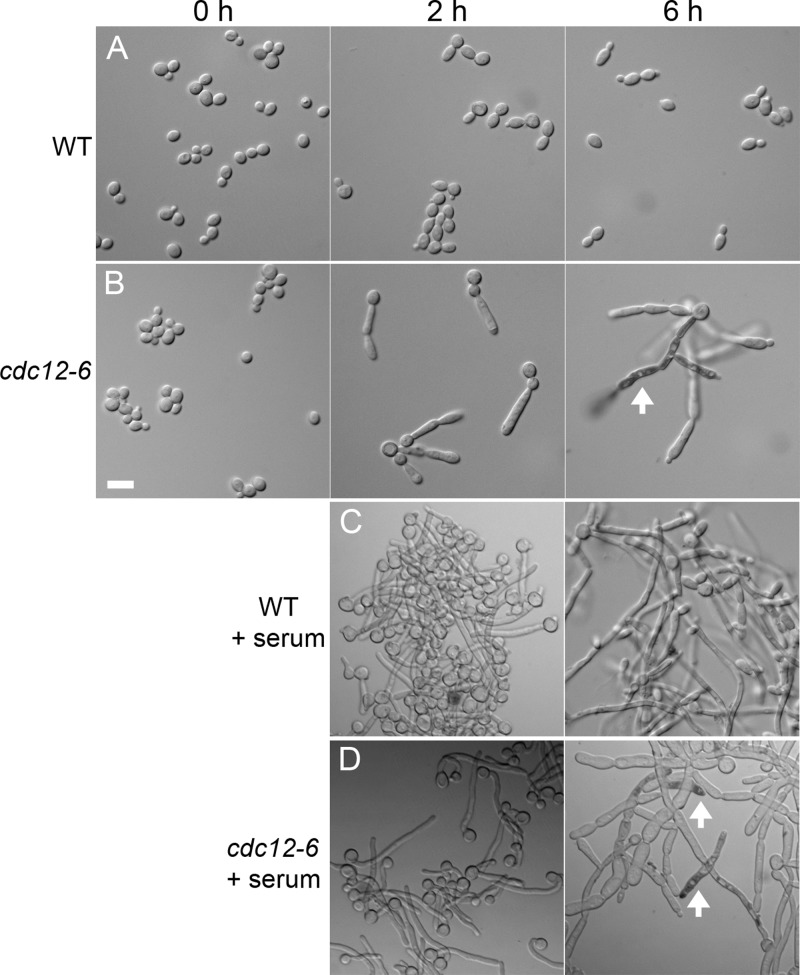
The effects of the cdc12-6 mutation on C. albicans morphogenesis were examined at room temperature (0 h) and at different times after shifting cells to 37°C (Fig. 2). When grown at room temperature, the cdc12-6 cells formed buds, albeit some with a slightly abnormal shape. Many cells were also present in clusters, indicating a partial defect in septation and cytokinesis. After 2 h at 37°C, cdc12-6 cells formed elongated buds (Fig. 2B). After 6 h, continued growth of the mutant cells resulted in highly elongated filamentous cells. At around 6 h the cdc12-6 cells began to lyse near the tips, as evidenced by intracellular staining with trypan blue (Fig. 2D). The highly elongated buds formed by the cdc12-6 mutant are similar to the morphology of septin mutants in S. cerevisiae, which are thought to form due to activation of a cell cycle checkpoint pathway that prolongs apical growth of cells (30).
Fig 2.
Altered morphology and viability of cdc12-6 cells after a shift to 37°C. Wild-type (DIC185) and cdc12-6 (LLF016) cells were cultured overnight in log phase in YPD medium at room temperature. The cells were then shifted to 37°C in the absence (A and B) or presence (C and D) of 20% serum and then incubated for the indicated times. Cells were stained with trypan blue and then examined by light microscopy with DIC optics. Examples of lysed cells that stain dark with trypan blue are indicated by an arrow. Bar, 10 μm. WT, wild type.
A hallmark of S. cerevisiae septin mutants is their defect in septation. To assay this in the C. albicans cdc12-6 mutant, log-phase cells shifted to 37°C were stained with Calcofluor White to detect the ring of cell wall chitin that forms at the septum (31). At room temperature, both the wild type and the cdc12-6 mutant showed typical Calcofluor White staining at bud necks (Fig. 3A). Similar results were observed for the wild-type strain at 37°C. In contrast, sites of septation were rarely detected in the cdc12-6 mutant shifted to 37°C (Fig. 3B). This was most evident after 6 h of incubation at 37°C; the cdc12-6 cells formed chains of elongated pseudohyphal-type cells with a few obvious septae (Fig. 3C). There were, however, patches of Calcofluor White staining that could represent aberrant attempts to form septae.
Fig 3.
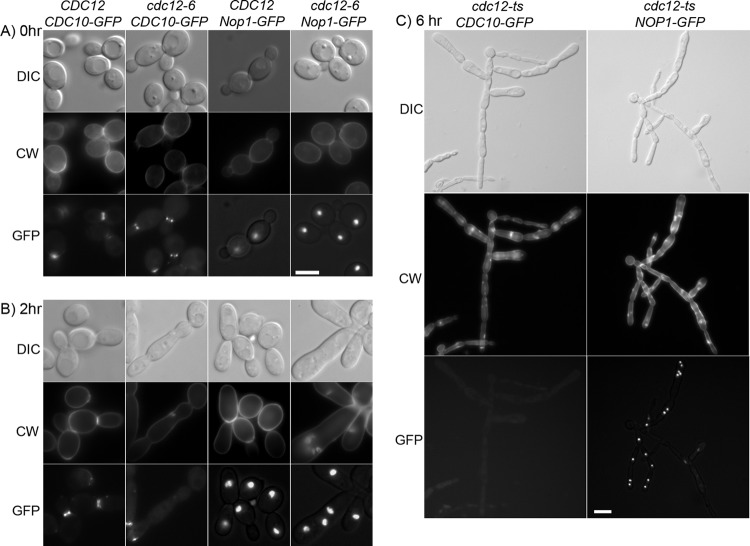
Altered localization of septins and nuclei in cdc12-6 cells at 37°C. Wild-type control and cdc12-6 cells engineered to produce either Cdc10-GFP or Nop1-GFP were analyzed as indicated at the top of each column of panels. Cells were grown overnight to log phase at room temperature (A) or were shifted to 37°C for 2 h (B) or 6 h (C). As indicated on the left, cells were analyzed (i) using DIC optics to detect morphology and (ii) fluorescence microscopy to detect Calcofluor White (CW) staining of the cell wall chitin and the localization of GFP-tagged proteins (GFP). The strains used were wild-type cells carrying CDC10-GFP (LLF009) or NOP1-GFP (LLF006) and cdc12-6 cells carrying CDC10-GFP (CZ11) or NOP1-GFP (CZ14). Photos were taken at 0 and 2 h using a ×100 objective lens, while those at 6 h were taken with a ×40 objective lens because of the larger cell size. Bars, 10 μm.
Septins also contribute to proper nuclear segregation in S. cerevisiae by interacting with microtubules to orient nuclear migration into the bud (21). The distribution of nuclei in C. albicans was therefore examined by monitoring the nuclear-localized protein Nop1-GFP. Wild-type cells typically contained one nucleus per cell, as expected. In contrast, the cdc12-6 cell compartments frequently contained multiple nuclei or they lacked a nucleus, especially after the longer 6 h of incubation at 37°C, indicating a defect in nuclear segregation (Fig. 3C).
Septin localization in the cdc12-6 mutant was analyzed by studying the Cdc10 septin fused to GFP. Wild-type cells grown at either room temperature or 37°C exhibited the expected localization of Cdc10-GFP to rings at the bud neck (Fig. 3A). The cdc12-6 cells grown at room temperature also showed bud neck localization of Cdc10-GFP (Fig. 3A). However, after a shift to 37°C for 2 h, approximately half of the cdc12-6 cells lacked detectable Cdc10-GFP, and the others primarily contained faint patches or rings of Cdc10-GFP toward the growing end of the elongated cell cluster (Fig. 3B). Thus, the septins are still capable of forming a complex at 37°C, but it primarily appears near the leading edge of growth and does not stabilize at the pinched zones that correspond to bud necks. Cdc10-GFP was still showed a similar distribution in the cdc12-6 mutant after 6 h at 37°C (Fig. 3C). Although a majority of cells appeared to lack Cdc10-GFP, a patch or ring of septins was frequently detected at the leading edge of growth or at sites of budding off the main filamentous cell clusters.
The three-dimensional structure of the Cdc10-GFP septin rings that formed in the cdc12-6 mutants was analyzed by confocal microscopy (Fig. 4). Wild-type cells grown at room temperature or 37°C showed the expected Cdc10-GFP ring at the bud neck. In contrast, the Cdc10-GFP structures in the cdc12-6 mutant were abnormal. At room temperature, Cdc10-GFP localized in a spectrum of patterns ranging from typical ring structures, to partial rings with a break in the continuity, and very faint rings (Fig. 4). Shifting the cdc12-6 cells to 37°C for 2 h resulted in much more severe defects in Cdc10-GFP localization. Cdc10-GFP most frequently appeared as a series of bars and did not form a contiguous ring. The Cdc10-GFP rings in cdc12-6 cells showed similar defects for cells grown in the presence or absence of serum. Some of these structures appeared to be similar to the types of septin rings seen in S. cerevisiae cells induced with mating pheromone or carrying a mutation in GIN4 (6, 24) and in C. albicans mutants with hyperactive Cdc42 (11).
Fig 4.
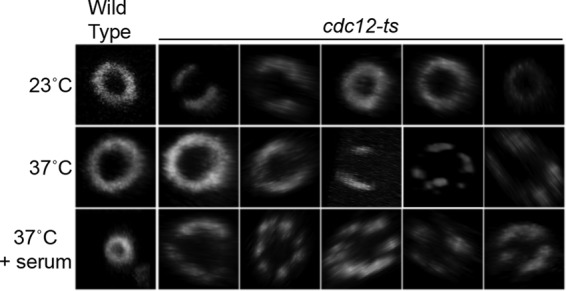
Defects in septin ring formation by the cdc12-6 mutant. Wild-type (LLF009) and cdc12-6 (CZ11) cells engineered to produce Cdc10-GFP were grown at room temperature or shifted to 37°C for 2 h in YPD medium in the presence or absence of 20% serum as indicated. Cdc10-GFP was analyzed by confocal microscopy to determine the three-dimensional shape of the rings.
Hyphal morphogenesis defects in cdc12-6 mutant cells.
The ability to undergo hyphal morphogenesis was examined by treating cells with 20% bovine calf serum at 37°C. As expected, wild-type cells efficiently formed the initial polarized outgrowths termed germ tubes that continued to elongate in a highly polarized manner to form filamentous cells with multiple cell compartments termed hyphae (Fig. 2 and 5). Serum also induced cdc12-6 cells to form germ tubes at 2 h that were generally similar to the wild-type cells (Fig. 2 and 5). Serum clearly induced a distinct morphogenesis pathway in the cdc12-6 mutant; most cell walls grew parallel and did not display the curvature that was seen in the absence of serum (Fig. 2 and 3). However, Cdc10-GFP localization was abnormal in the cdc12-6 cells induced with serum. About half of the cell clusters lacked detectable Cdc10-GFP, and the Cdc10-GFP structures that were present were typically fainter (Fig. 5A). In addition, the septin rings that formed in cdc12-6 cells had a wider diameter than those detected in the germ tubes and hyphae of wild-type cells (Fig. 4). This is likely due in part to the continued expansion of the width of the cdc12-6 germ tubes (see below). In addition, the Cdc10-GFP rings in cdc12-6 cells typically had breaks in their continuity and some appeared as a series of bars, as was seen for cells grown in the absence of serum (Fig. 4).
Fig 5.
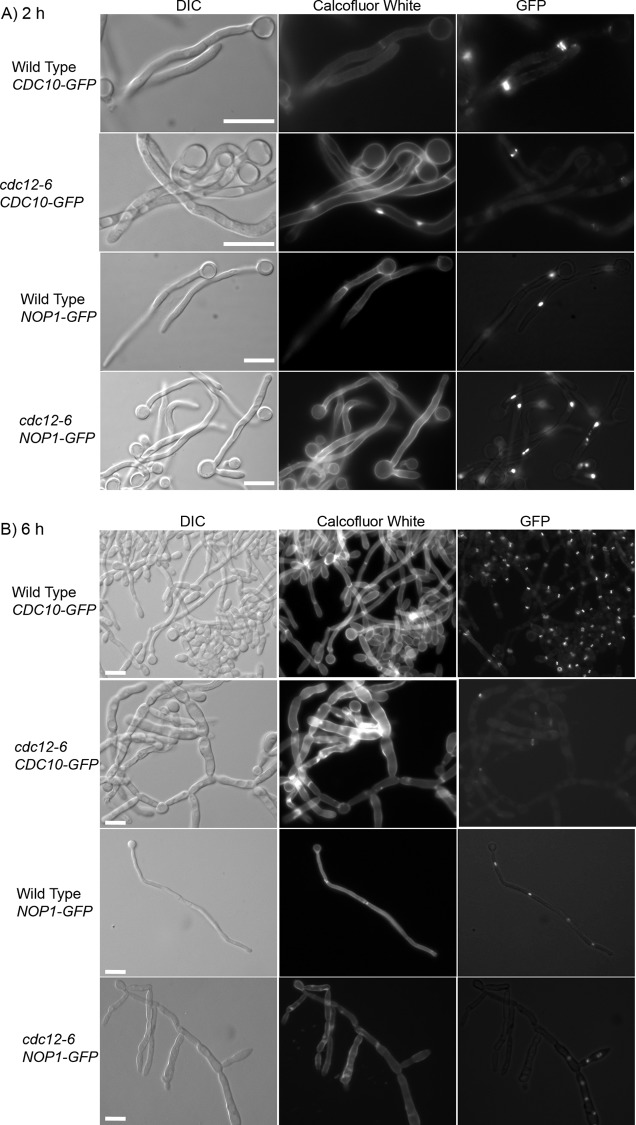
Cell morphology, septin ring, and nuclear localization are altered in cdc12-6 cells under hypha-inducing conditions. Cells were grown to log phase at room temperature, serum was added to 20% final concentration, and then the cultures were shifted to 37°C for 2 h (A) or 6 h (B). As labeled at the top of each column of photos, the cells were analyzed by light microscopy to detect morphology (DIC) or fluorescence microscopy to detect Calcofluor White staining of chitin (CW) or the GFP fusion protein (GFP) as indicated on the left. The strains used were wild-type cells carrying CDC10-GFP (LLF009) or NOP1-GFP (LLF006) and cdc12-6 cells carrying CDC10-GFP (CZ11) or NOP1-GFP (CZ14). Bars, 10 μm.
After 6 h of incubation at 37°C with 20% serum, the morphology of the cdc12-6 cells was very distinct from the wild type (Fig. 5B). The cdc12-6 cells formed filaments that were wider and curved, indicating the original germ tubes continued to grow in width, whereas new growth in wild-type cells is restricted to the apical tip. The hyphal inducing conditions did not appear to alter the viability of cells at 37°C. Trypan blue staining revealed that dead cells still began to accumulate by 6 h of incubation (Fig. 2). The cdc12-6 cells also showed frequent branching of new offshoots of filamentous outgrowth that was not seen for the wild type. Analysis of Nop1-GFP localization showed that many of these branched regions did not contain nuclei, whereas other regions contained multiple nuclei (Fig. 5). This indicates that nuclear division continued in the absence of septation but that the nuclei were not segregating into the different cell compartments. The cdc12-6 cells rarely formed septae that could be detected by Calcofluor White staining, even at the sites where Cdc10-GFP was localized. Instead, patches of Calcofluor White staining were commonly detected in the new filamentous growth that may represent aberrant attempts to initiate septum formation. After 6 h there were patches or rings of Cdc10-GFP detected in ca. 30% of the cells. Interestingly, the Cdc10-GFP structures in the cdc12-6 mutant were frequently detected in the middle of the elongating germ tube, as seen for wild-type cells, and not at the tip of the filamentous cell as was seen for cdc12-6 cells grown at 37°C in the absence of serum. This suggests that septin localization is affected by the altered cell morphogenesis or by distinct signaling pathways activated in hyphae.
Hypha-induced responses.
The ability of the mutant cells to induce hyphal genes was assayed by quantifying the expression of a HWP1-GFP gene fusion. This reporter gene was constructed by placing GFP expression under the control of the hypha-induced HWP1 promoter. Cells carrying this reporter gene were grown in the presence or absence of the hyphal inducers serum or GlcNAc, and the relative induction was assessed by quantifying the signal intensity of GFP using fluorescence microscopy. Although the wild-type and mutant cells strongly induced HWP1-GFP (Fig. 6A), the cdc12-6 cells were slightly less efficient than the wild type (P < 0.003). Thus, septin function is not essential for induction hyphal genes.
Fig 6.
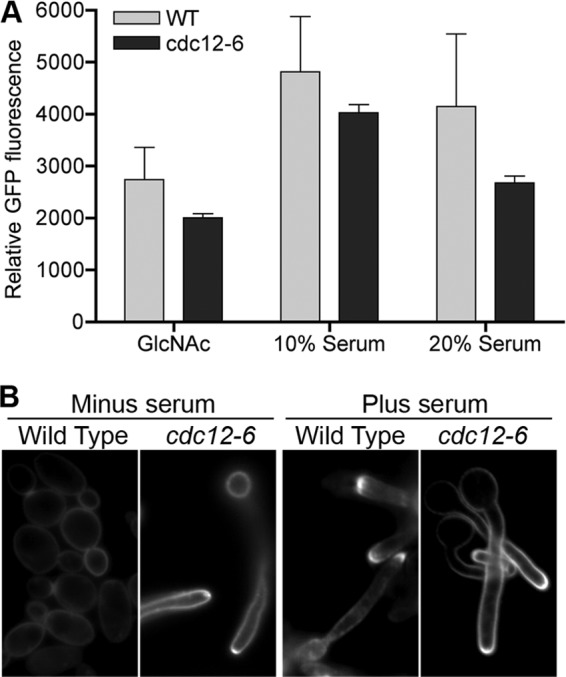
Serum induces cdc12-6 cells to express HWP1-GFP and to display increased filipin staining at hyphal tips. (A) Expression of hyphal reporter gene HWP1-GFP in budding and hyphal conditions for wild-type (LLF012) or cdc12-6 cells (LLF010). Cells were grown in the presence or absence of the hyphal inducer serum for 2 h or GlcNAc for 3.5 h. The level of GFP was quantified by fluorescence microscopy. Cells incubated in the absence of serum or GlcNAc did not show GFP levels above the background. (B) Wild-type (LLF009) and cdc12-6 (CZ11) cells were incubated with or without serum for 2 h at 37°C. The cells were then stained with 10 μg of filipin/ml and analyzed by fluorescence microscopy. WT, wild type.
Another hallmark of hyphal cells is that the apical region stains more readily with the ergosterol-binding agent filipin (27). As expected, essentially 100% of the wild-type cells induced with serum showed increased staining with filipin at hyphal tips (Fig. 6B). Similar results were observed for cdc12-6 cells induced with serum. Surprisingly, control studies showed that ca. 31.4% (n = 191) of the cdc12-6 cells shifted to 37°C for 1.5 h in the absence of serum also showed stronger filipin staining at the tips. This increased over time to 41.8% at 2 h (n = 110) and 63.3% at 3 h (n = 128). These results for filipin staining contrasted with the expression of HWP1-GFP, which required serum to be induced. Thus, this characteristic of hyphae could be induced in cdc12-6 cells in the absence of serum.
Altered position of second germ tubes in cdc12-6 mutant.
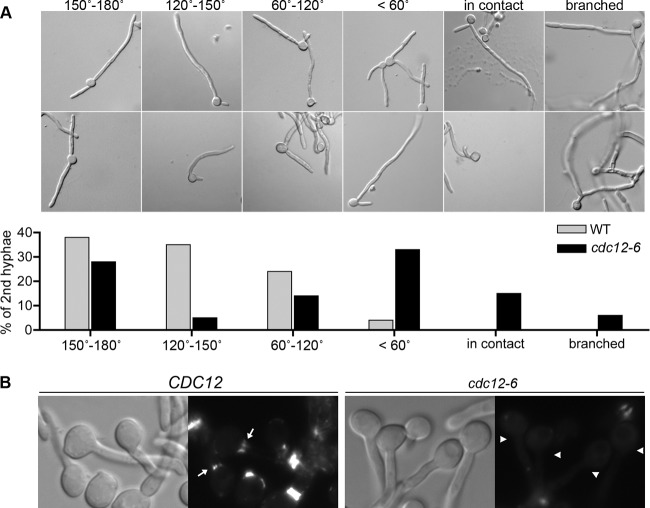
The cdc12-6 cells induced to form hyphae for an extended time frequently formed a second germ tube very close to the first one, which was rarely observed in the wild type (Fig. 7) (10). To quantify the difference, the relative positions of the sites where the first and second germ tubes initiated were scored as one of six patterns: 150° to 180° apart, 120° to 150° apart, 60 to 120° apart, <60° apart, two germ tubes in contact, or a second germ tube that emerged from the first germ tube rather than the mother cell. Interestingly, cdc12-6 cells showed significantly increased frequency of cells forming a second germ tube proximal to the first (Fig. 7A). The majority of cdc12-6 cells formed a second germ tube within a 60° angle of the first, whereas essentially all of the wild-type cells formed a second germ tube that was more than 60° from the first. The cdc10Δ and cdc11Δ mutants occasionally formed proximal germ tubes (40), but this defect was more extreme in the cdc12-6 cells.
Fig 7.
Position of the second germ tube is altered in cdc12-6 cells. (A) Wild-type control (DIC185) and cdc12-6 (LLF016) were shifted to 37°C and induced with 20% bovine calf serum for 3 h. Representative images of cells are shown with different positions of second germ tubes. The degree of separation between the first and second germ tubes was quantified, as shown in the table below. A total of 200 cells were counted from three independent hyphal induction experiments. (B) Wild-type control (LLF009) or cdc12-6 (CZ11) cells engineered to produce Cdc10-GFP were induced with 20% serum for 1 h at 37°C and then analyzed by fluorescence microscopy to detect the basal septin band at the junction between the mother cell and germ tube. Arrows point to the presence of the basal band in the wild type. Arrowheads point to the absence of the basal band in the cdc12-6 mutant. Note that basal septin band is more diffuse than the tight septin ring seen at sites of septation. WT, wild type.
The altered site selection for germ tube outgrowth in the cdc12-6 mutant indicates that septins influence this process. An interesting possibility is that the basal band of septins may play a role in determining the site of the second germ tube (Fig. 7B). The basal band is a more diffuse type of septin ring that is located at the junction between the mother cell and the germ tube (35, 40). The function of this basal band of septins is not clear, since cytokinesis does not occur at this site. However, its location suggests that the basal septin band may function in germ tube site selection analogous to the role of the septin ring in bud site section (24). Consistent with this, the basal band of septins was not detected in cdc12-6 cells (Fig. 7B). Thus, proper Cdc12 function is required to form both the basal septin band and the septin rings that form at sites of cytokinesis.
A relationship between bud site and germ tube site selection is also supported by previous studies which showed that the bud sites of wild-type C. albicans were clustered at an axial site at room temperature but were primarily not adjacent at 30 and 37°C (10). The budding mode of cdc12-6 cells could not be assessed at an elevated temperature, which is not permissive for this mutant. Therefore, we analyzed the nonessential cdc10Δ, cdc11Δ, and sep7Δ septin mutants. These mutants also showed defects at 37°C that prevented accurate assessment of bud site selection but could be examined at 30°C. Interestingly, all three nonessential septin mutants budded primarily at a cluster of axial sites at room temperature (>90%; n = >200), similar to the wild type. In contrast, at 30°C only 33% of wild-type cells budded in an axial manner (n = 202), whereas the cdc10Δ, cdc11Δ, and sep7Δ mutants all still budded primarily in an axial manner (>60%; n = >144). The effect was most obvious for the cdc11Δ mutant (76% axial budding at 30°C), a finding consistent with the cdc11Δ mutant having the morphogenesis phenotype of the three. This suggests that the temperature-related switch in bud-site selection underlies the mechanisms that promote germ tube formation at distal rather than axial sites.
DISCUSSION
Temperature-sensitive septin mutants have played a valuable role in S. cerevisiae for identifying the function of septins in septation and other morphogenic events, including mating and sporulation (12, 25). Although hyphal morphogenesis could not be examined in S. cerevisiae, studies of the nonessential septin genes CDC10, CDC11, and SEP7 indicated that they are important for normal hyphal morphogenesis in C. albicans (16, 34, 40) and in filamentous fungi (7, 14, 22). Therefore, in the present study a temperature-sensitive cdc12-6 mutant was created to carry out the first analysis of an essential septin gene in C. albicans. Shifting the C. albicans cdc12-6 strain to 37°C caused a rapid defect in morphogenesis and septation, similar to those seen in S. cerevisiae. The cdc12-6 mutation likely causes a temperature-sensitive phenotype because it alters the C-terminal region of Cdc12 that is important for stabilizing connections between two septin filaments (4, 5, 37). Temperature-sensitive mutants are well suited for the study of C. albicans hyphal morphogenesis because cells can be grown at room temperature and then shifted to a nonpermissive temperature of 37°C, the optimal temperature for hyphal morphogenesis. The rapid inactivation of Cdc12 function at a high temperature is therefore expected to reveal better insight into septin function than the use of a regulated promoter, which would require a longer incubation period to deplete the stable septin proteins. Thus, the new cdc12-6 mutant represents an important new tool for C. albicans research.
Induction and maintenance of hyphal morphogenesis.
The cdc12-6 cells formed buds at room temperature and could be stimulated with serum to form germ tubes at 37°C (Fig. 2 and 5). The initial germ tube outgrowths in the cdc12-6 mutant cells at 2 h did not appear to be significantly more defective than the wild type, whereas bud morphogenesis was clearly affected by 2 h (Fig. 2). This suggests that septin function is not required to initiate germ tubes. Previous studies showed that the C. albicans cdc10Δ and cdc11Δ mutants frequently formed curved germ tube necks (26, 40), but this phenotype was not exacerbated in the cdc12-6 mutant.
Although the cdc12-6 mutant formed germ tubes, highly polarized hyphal morphogenesis was not maintained. The filamentous outgrowths became wider over time and took on the characteristics of pseudohyphal cells. The cdc12-6 mutant phenotype was more extreme than the mutants lacking the nonessential septin genes CDC10 and CDC11, which showed subtler defects in maintaining polarized morphogenesis (40). This indicates that the septins have a special function in maintaining highly polarized growth. Altered septin localization is therefore likely to contribute to the abnormal hyphal morphogenesis of mutants that display defects in targeting septins to appropriate sites (11, 18, 41). However, some phenotypes of cdc12-6 cells may be due to the activation of stress pathways. The defects of cdc12-6 cells in septation and cell wall biogenesis (Fig. 2 and 5) should activate cell wall stress pathways that could indirectly affect actin localization and morphogenesis. Activation of stress pathways could also account from some of the altered patches of Calcofluor White staining in cdc12-6 cells at 37°C (Fig. 3 and 5). Unusual patches of Calcofluor White staining were also detected after treatment of cells with caspofungin, an inhibitor of cell wall β-glucan synthesis (3).
Regulation of the Cdc11 septin by phosphorylation has also been implicated in proper C. albicans hyphal morphogenesis. Mutation of a site in Cdc11 to prevent phosphorylation by Cdc28 (S394A) caused a defect in maintaining highly polarized hyphal growth (34). This defect in maintaining polarized growth was more extreme than the defects seen for cdc12-6 cells. Mutation of a different site in Cdc11 to prevent phosphorylation by Gin4 (S395A) caused cells to sequentially initiate multiple short germ tubes, suggesting a role for septins in stabilizing the active site of polarized morphogenesis (34). However, the cdc10Δ, cdc11Δ, and cdc12-6 mutants rarely produce the multiple germ tube protuberances seen in the wild type or that were seen so frequently in the cdc11-S395A mutant (34). Thus, some phenotypes caused by mutating Cdc11 phosphorylation sites are likely due to the dominant activity of a misregulated septin rather than the absence of septin function.
The cdc12-6 mutant at 37°C also showed more frequent branching of the filamentous outgrowths. This phenotype likely relates to altered cell cycle regulation due to the failure of the germ tubes to undergo septation to form hyphae with different cell compartments. In wild-type cells, the mother cell vacuole swells to force most of the cytoplasmic constituents to the daughter cell compartment at the leading edge of growth (38). Consequently, the mother cells and subsequent subapical cells are delayed in initiating second germ tubes or branches until they can restore the cytoplasmic components. Consistent with this, septin rings persist in the hyphal cells at sites of septation and do not disassemble quickly after septation as they do in budding cells (35, 40). More frequent branching was also seen for septin in mutants of the filamentous fungus Aspergillus nidulans (19, 22).
Hypha-induced gene expression and filipin staining.
The ability of the cdc12-6 mutant to induce hyphal responses was confirmed by showing that serum and GlcNAc also induced expression of the HWP1-GFP reporter gene (Fig. 6A). In addition, serum also induced a domain at the tips of the germ tubes in both the wild type and the cdc12-6 mutant that stained more readily with the ergosterol binding agent filipin (Fig. 6B). The increased filipin staining is indicative of an altered lipid content in the plasma membrane at hyphal tips (27). Surprisingly, a high proportion (63%) of the cdc12-6 cells shifted to 37°C in the absence of serum for 3 h also showed increased tip staining. This was unexpected because previous studies indicated that this filipin-staining domain was only detected in hyphal and not pseudohyphal cells (27). These results could suggest that cdc12-6 cells induce this response in the absence of hyphal inducers. However, enriched filipin staining is also transiently observed at sites of cytokinesis (2, 27). Thus, a likely alternative possibility is that the filipin staining relates to altered cell cycle regulation in cdc12-6 cells. In support of this latter possibility, enriched filipin staining of the tips of cdc12-6 cells increased over time rather than coinciding with the induction of the initial germ tube outgrowth as seen with serum induction (13, 27).
Site of second germ tube formation.
A striking defect of the cdc12-6 mutant is that the second germ tube usually formed proximal to the first germ tube (Fig. 7). This contrasts with wild-type cells in which the second germ tube emerges at a distal position. The basal band of septins that forms at the junction between the mother cell and the germ tube is therefore implicated in this process. The role of the basal band of septins is not well understood; septation does not occur at this site, and the septins are detected in a more diffuse pattern than the septin ring seen at sites of septation (18, 35, 40). This raises the possibility that the function of the basal band is to prevent the initiation of second germ tubes proximal to the first. Consistent with this, the basal septin band is stably maintained after the germ tube has undergone cytokinesis and turned into a hyphal cell. Thus, it is stably maintained until later stages when the second gem tube initiates. The formation of the second germ tube at a distal site is significant, since it would help disseminate an infection by promoting growth in a new direction.
This role for septins in germ tube site selection is likely related to their role in bud site selection. In C. albicans, wild-type cells form buds in an axial manner at room temperature but switch to forming buds at nonadjacent bipolar sites at 37°C (10). In contrast, the C. albicans septin mutants primarily budded in an axial manner at 30°C, where wild-type cells had mostly switched to budding at bipolar sites. Thus, septins play roles in preventing the formation of a new site morphogenesis adjacent to an existing bud or germ tube in C. albicans. In contrast, the S. cerevisiae septins are needed for proper axial budding of haploid cells (8), which means they act in a distinct manner to recruit the morphogenesis machinery to an adjacent site.
Altogether, these studies demonstrate that, in addition to their essential role in septum formation, the septins are needed for maintenance of the highly polarized morphology and proper selection of sites of germ tube formation. Both of these are important for dissemination of an infection in host tissues. These conclusions are supported by the defect of cdc10Δ and cdc11Δ mutants in invasive growth into tissues and virulence in a mouse model of candidiasis (39).
ACKNOWLEDGMENTS
This research was supported by a grant awarded to J.B.K. from the National Institutes of Health (RO1 AI47837).
We gratefully acknowledge Brian Haarer for providing unpublished data regarding the specific DNA sequence changes present in the S. cerevisiae cdc12-6 mutation. We also thank the members of our labs for their helpful advice and comments on the manuscript.
Footnotes
Published ahead of print 10 August 2012
REFERENCES
- 1. Adams AEM, Pringle JR. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98: 934–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez FJ, Douglas LM, Konopka JB. 2007. Sterol-rich plasma membrane domains in fungi. Eukaryot. Cell 6: 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badrane H, et al. 2012. Rapid redistribution of phosphatidylinositol-(4,5)-bisphosphate and septins during the Candida albicans response to caspofungin. Antimicrob. Agents Chemother. 56: 4614–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertin A, et al. 2008. Saccharomyces cerevisiae septins: supramolecular organization of hetero-oligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. U. S. A. 105: 8274–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertin A, et al. 2012. Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae. Mol. Biol. Cell 23: 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bharucha JP, Larson JR, Konopka JB, Tatchell K. 2008. Saccharomyces cerevisiae Afr1 protein is a protein phosphatase 1/Glc7-targeting subunit that regulates the septin cytoskeleton during mating. Eukaryot. Cell 7: 1246–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyce KJ, Chang H, D'Souza CA, Kronstad JW. 2005. An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize. Eukaryot. Cell 4: 2044–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casamayor A, Snyder M. 2002. Bud-site selection and cell polarity in budding yeast. Curr. Opin. Microbiol. 5: 179–186 [DOI] [PubMed] [Google Scholar]
- 9. Caudron F, Barral Y. 2009. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16: 493–506 [DOI] [PubMed] [Google Scholar]
- 10. Chaffin WL. 1984. Site selection for bud and germ tube emergence in Candida albicans. J. Gen. Microbiol. 130: 431–440 [Google Scholar]
- 11. Court H, Sudbery P. 2007. Regulation of Cdc42 GTPase activity in the formation of hyphae in Candida albicans. Mol. Biol. Cell 18: 265–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douglas LM, Alvarez FJ, McCreary C, Konopka JB. 2005. Septin function in yeast model systems and pathogenic fungi. Eukaryot. Cell 4: 1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douglas LM, Martin SW, Konopka JB. 2009. BAR domain proteins Rvs161 and Rvs167 contribute to Candida albicans endocytosis, morphogenesis, and virulence. Infect. Immun. 77: 4150–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gladfelter AS. 2010. Guides to the final frontier of the cytoskeleton: septins in filamentous fungi. Curr. Opin. Microbiol. 13: 720–726 [DOI] [PubMed] [Google Scholar]
- 15. Gladfelter AS, Pringle JR, Lew DJ. 2001. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4: 681–689 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez-Novo A, et al. 2008. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol. Biol. Cell 19: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haarer BK, Pringle JR. 1987. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol. Cell. Biol. 7: 3678–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hausauer DL, Gerami-Nejad M, Kistler-Anderson C, Gale CA. 2005. Hyphal guidance and invasive growth in Candida albicans require the Ras-like GTPase Rsr1p and its GTPase-activating protein Bud2p. Eukaryot. Cell 4: 1273–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernandez-Rodriguez Y, Hastings S, Momany M. 2012. The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation. Eukaryot. Cell 11: 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumamoto CA. 2008. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat. Rev. Microbiol. 6: 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kusch J, Meyer A, Snyder MP, Barral Y. 2002. Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 16: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindsey R, Cowden S, Hernandez-Rodriguez Y, Momany M. 2010. Septins AspA and AspC are important for normal development and limit the emergence of new growth foci in the multicellular fungus Aspergillus nidulans. Eukaryot. Cell 9: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo HJ, et al. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949 [DOI] [PubMed] [Google Scholar]
- 24. Longtine MS, Bi E. 2003. Regulation of septin organization and function in yeast. Trends Cell Biol. 13: 403–409 [DOI] [PubMed] [Google Scholar]
- 25. Longtine MS, et al. 1996. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8: 106–119 [DOI] [PubMed] [Google Scholar]
- 26. Martin SW, Douglas LM, Konopka JB. 2005. Cell cycle dynamics and quorum sensing in Candida albicans chlamydospores are distinct from budding and hyphal cells. Eukaryot. Cell 4: 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin SW, Konopka JB. 2004. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell 3: 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin SW, Konopka JB. 2004. SUMO modification of septin-interacting proteins in Candida albicans. J. Biol. Chem. 279: 40861–40867 [DOI] [PubMed] [Google Scholar]
- 29. Odds FC. 1988. Candida and candidosis. Bailliere Tindall, Philadelphia, PA [Google Scholar]
- 30. Oh Y, Bi E. 2011. Septin structure and function in yeast and beyond. Trends Cell. Biol. 21: 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pringle JR. 1991. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 194: 732–735 [DOI] [PubMed] [Google Scholar]
- 32. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherman F. 1991. Getting started with yeast. Methods Enzymol. 194: 3–21 [DOI] [PubMed] [Google Scholar]
- 34. Sinha I, et al. 2007. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev. Cell 13: 421–432 [DOI] [PubMed] [Google Scholar]
- 35. Sudbery PE. 2001. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol. Microbiol. 41: 19–31 [DOI] [PubMed] [Google Scholar]
- 36. Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9: 737–748 [DOI] [PubMed] [Google Scholar]
- 37. Versele M, et al. 2004. Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol. Biol. Cell 5: 4568–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veses V, Richards A, Gow NA. 2009. Vacuole inheritance regulates cell size and branching frequency of Candida albicans hyphae. Mol. Microbiol. 71: 505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warenda AJ, Kauffman S, Sherrill TP, Becker JM, Konopka JB. 2003. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 71: 4045–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warenda AJ, Konopka JB. 2002. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 13: 2732–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wightman R, Bates S, Amornrrattanapan P, Sudbery P. 2004. In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization. J. Cell Biol. 164: 581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181: 1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Konopka JB. 2010. A photostable green fluorescent protein variant for analysis of protein localization in Candida albicans. Eukaryot. Cell 9: 224–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng X, Wang Y. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 23: 1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]