Abstract
Heterotrimeric G proteins are critical regulators of growth and asexual and sexual development in the filamentous fungus Neurospora crassa. Three Gα subunits (GNA-1, GNA-2, and GNA-3), one Gβ subunit (GNB-1), and one Gγ subunit (GNG-1) have been functionally characterized, but genetic epistasis relationships between Gβ and Gα subunit genes have not been determined. Physical association between GNB-1 and FLAG-tagged GNG-1 has been previously demonstrated by coimmunoprecipitation, but knowledge of the Gα binding partners for the Gβγ dimer is currently lacking. In this study, the three N. crassa Gα subunits are analyzed for genetic epistasis with gnb-1 and for physical interaction with the Gβγ dimer. We created double mutants lacking one Gα gene and gnb-1 and introduced constitutively active, GTPase-deficient alleles for each Gα gene into the Δgnb-1 background. Genetic analysis revealed that gna-3 is epistatic to gnb-1 with regard to negative control of submerged conidiation. gnb-1 is epistatic to gna-2 and gna-3 for aerial hyphal height, while gnb-1 appears to act upstream of gna-1 and gna-2 during aerial conidiation. None of the activated Gα alleles restored female fertility to Δgnb-1 mutants, and the gna-3Q208L allele inhibited formation of female reproductive structures, consistent with a need for Gα proteins to cycle through the inactive GDP-bound form for these processes. Coimmunoprecipitation experiments using extracts from the gng-1-FLAG strain demonstrated that the three Gα proteins interact with the Gβγ dimer. The finding that the Gβγ dimer interacts with all three Gα proteins is supported by epistasis between gnb-1 and gna-1, gna-2, and gna-3 for at least one function.
INTRODUCTION
Eukaryotic cells sense extracellular stimuli such as hormones, neurotransmitters, odorants, chemoattractants, light, and nutrients using G protein-coupled receptors (GPCRs) (29). GPCRs are integral plasma membrane proteins that interact with heterotrimeric G proteins (19). G proteins consist of three subunits, α, β, and γ, which are associated with the plasma membrane (19). Gα proteins bind guanine nucleotides (GTP and GDP) and hydrolyze GTP to GDP and inorganic phosphate. Association with ligand-bound receptor leads to GDP-GTP exchange on the Gα protein and dissociation of Gα-GTP from the Gβγ dimer. Gα-GTP and the Gβγ dimer can both regulate downstream effectors (19, 37, 42). Subsequent GTP hydrolysis by the Gα subunit results in reassociation of GDP-bound Gα with the Gβγ dimer and the GPCR.
Heterotrimeric G proteins are important for growth, development, and pathogenesis in yeasts and filamentous fungi (reviewed in reference 37). In the yeast Saccharomyces cerevisiae, G proteins are required for critical processes, including the pheromone response and carbon sensing (reviewed in references 12 and 62). In filamentous fungi, heterotrimeric G proteins were first identified in the saprobe Neurospora crassa, where they regulate growth, carbon sensing, asexual sporulation, sexual fertility, and stress resistance (30, 53, 59). G proteins were later found to be essential for disease in numerous plant and animal pathogens, including Magnaporthe grisea, Ustilago maydis, Fusarium oxysporum, and Cryptococcus neoformans (1, 25, 38, 47). In Trichoderma species, G proteins are essential for mycoparasitism (48). G proteins regulate production of secondary metabolites, including toxins, in Aspergillus and Penicillium spp. (16, 20). Finally, G proteins are required for proper development in Aspergillus spp., Sordaria macrospora, Podospora anserina, and numerous other species (27, 40–41, 61).
Unlike yeasts, which have two Gα proteins, ascomycete filamentous fungi possess three conserved Gα subunits, which have been termed Group I, Group II, and Group III (6). In the species N. crassa, the corresponding proteins are GNA-1, GNA-2, and GNA-3 (3, 31, 53). One Gβ protein (GNB-1) and one Gγ protein (GNG-1) have also been characterized in N. crassa (34, 60). Phenotypes of single gene deletion mutants are summarized in Table 1. Loss of gna-1 leads to defects in extension of basal hyphae (tubelike cellular structures that form the body of the organism) on normal and hyperosmotic media, decreased growth on poor carbon sources, premature asexual sporulation (conidiation), and the inability to form female reproductive structures (22, 24, 36). Mutation of gna-2 intensifies the phenotypes of mutants also lacking gna-1 or gna-3 and reduces mass accumulation on poor carbon sources (3, 30, 36). Δgna-3 strains form very short aerial hyphae (a developmental structure that gives rise to conidia), conidiate prematurely, and accumulate less mass on poor carbon sources (30–31, 36). Finally, analysis of strains lacking both gna-1 and gna-3 revealed that these two Gα genes independently regulate growth and conidiation in N. crassa (30).
Table 1.
Phenotypes of mutants lacking a single G protein subunit genea
| Mutation | Submerged culture conidiationb | Aerial hyphal height | Aerial conidiation | Female fertilityc |
|---|---|---|---|---|
| Δgna-1 | None | Slight reduction | Slight increase | Sterile (no perithecia or ascospores) |
| Δgna-2 | None | Normal | Normal | Normal |
| Δgna-3 | Yes | Greatly reduced | Greatly increased | Normal |
| Δgnb-1 | Yes | Reduced | Greatly increased | Sterile (no perithecia or ascospores) |
| Δgng-1 | Yes | Reduced | Greatly increased | Sterile (no perithecia or ascospores) |
Phenotypes known prior to this study. All are relative to the wild type.
Inoculated at 106 cells/ml. The wild type does not conidiate under these conditions.
Fertility in heterozygous crosses with wild-type males.
In addition to deletion mutations, Gα gene functions have also been analyzed using alleles with specific mutations that lead to loss of GTPase activity, resulting in greater GTP occupancy and constitutive signaling (26). In mammals, a mutation in a conserved arginine (201 in Gαs or 178 in Gαii) or a glutamine (227 in Gαs or 204 in Gαi) results in constitutive signaling (10, 14, 17), due to ∼100-fold-lower GTPase activity (14, 17). We have previously analyzed strains containing GTPase-deficient alleles of gna-2 and gna-1 (3, 59). The gna-2R179C and gna-2Q205L alleles were expressed in a wild-type background. The transformants produced more-abundant aerial hyphae but were otherwise normal in appearance (3). In the second study, strains with GTPase-deficient, activated gna-1 alleles (R178C or Q204L) as the only source of GNA-1 protein were analyzed (59). Constitutive signaling of the Gα protein led to several observed phenotypes. In the sexual cycle, the activated allele strains were female fertile but produced fewer and larger perithecia than did the wild type. During asexual development, the activated allele strains displayed longer aerial hyphae, fewer conidia, and lower levels of carotenoid pigments than did wild-type strains. The results of this study suggested that GNA-1 possessed several Gβγ-independent functions in N. crassa (59).
Strains with deletion of the Gβ gene gnb-1, the Gγ gene gng-1, or both subunits have identical phenotypes, consistent with GNB-1 and GNG-1 functioning as a unit during signaling (34). Δgnb-1 and Δgng-1 mutants have asexual sporulation defects similar to those of gna-3 mutants and a female sterility phenotype that is shared with Δgna-1 strains (34, 60). Previous studies in N. crassa have shown that Gβ is required for normal Gγ protein levels (34). Furthermore, Gβ is essential for maintaining Gα protein amounts in both N. crassa and Cryphonectria parasitica (28, 34, 60). In N. crassa, levels of all three Gα proteins are nearly undetectable in nitrogen-starved sexually differentiated cultures (60). GNA-1 cannot be detected, the amount of GNA-2 is greatly reduced, and GNA-3 levels are normal in submerged liquid cultures (60). In vegetative plate (aerial) cultures, amounts of GNA-1 and GNA-2 follow the same trend as that observed in liquid cultures, while GNA-3 levels are reduced approximately 50% (60).
In N. crassa, a physical interaction between the Gβ and Gγ subunits has been demonstrated using coimmunoprecipitation experiments (34). However, no direct evidence has been provided to confirm an in vivo physical association between components of the Gβγ dimer and all Gα proteins in N. crassa or any other filamentous fungal species. In addition, few studies have utilized epistasis analysis to probe genetic interactions between Gα and Gβ or Gγ genes. The results from epistasis analysis would indicate whether the Gα and Gβ/Gg subunit operate in the same linear pathway or are in different signaling cascades. The observation of genetic epistasis between gnb-1 and a Gα gene would be consistent with the two encoded proteins being found in a heterotrimeric complex with GNG-1 during signaling.
In this study, we explored the relationship between the three Gα subunits and components of the Gβγ dimer in N. crassa. We investigated epistatic interactions between each of the three Gα genes and gnb-1 by comparing phenotypes of (i) ΔGα and Δgnb-1 single mutants and Δgnb-1 ΔGα double mutants and (ii) Δgnb-1 mutants and Δgnb-1 strains carrying GTPase-deficient, constitutively activated Gα alleles. Formation of a complex including the Gβγ dimer and Gα proteins was explored in coimmunoprecipitation experiments using N. crassa strains expressing epitope-tagged GNG-1, which has previously been shown to interact tightly with GNB-1.
MATERIALS AND METHODS
Strains and media.
N. crassa strains used in the experiments are listed in Table 2. For propagation of asexual spores (conidia) and growth of vegetative liquid or solid culture, strains were cultured in Vogel's minimal medium (VM) (54), with 1% agar added for solid medium (BBL; Becton, Dickinson and Co., Franklin Lakes, NJ). Sorbose medium (FGS) (9) was used to induce colony formation on plates. Synthetic crossing medium (SCM) containing 1% agar was used to induce female reproductive structures (protoperithecia) during sexual differentiation (56). Medium was supplemented with hygromycin at 200 μg/ml or histidine at 100 μg/ml when indicated.
Macroconidia were propagated in VM agar medium flasks (11) by incubation at 30°C for 3 days in the dark, followed by 5 days at room temperature in constant light. Conidia were harvested as previously described (11). Escherichia coli strain DH5α was used to maintain all plasmids (18).
Strain construction.
Δgnb-1 Δgna-1, Δgnb-1 Δgna-2, and Δgnb-1 Δgna-3 double mutants were made using genetic crosses between single mutants (11) (Table 2). In cases where both single mutants in the cross were female sterile (Δgnb-1 and Δgna-1), the strain used as the female was a heterokaryon with the am1 helper strain (45). The presence of the mutations in the progeny was verified by diagnostic PCR (for the gna-2 gnb-1 double mutant) using gene-specific and hph cassette-specific primers or by Southern analysis (3, 23, 31, 60).
Table 2.
N. crassa strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| 74 A-OR23-1A (74A) | Wild type, mat A | FGSCa 987 |
| 74 a-OR8-1a (74a) | Wild type, mat a | FGSC 988 |
| am1 | am1 cyh-1 ad3B | FGSC 4564 |
| his-3a | his-3 mata | 34 |
| 1B4 | Δgna-1::hph+ mat A | 24 |
| 1B8 | Δgna-1::hph+ mat a | 24 |
| Δ2 | Δgna-2::hph+ mat a | FGSC 12377 |
| 3lc2 | Δgna-3::hph+ mat A | 31 |
| hβJ | Δgnb-1::hph+ his-3 mat a | 31 |
| 42-5-11 | Δgnb-1::hph+ mat A | This study |
| 42-5-18 | Δgnb-1::hph+ mat A | 60 |
| 5A | Δgng-1::hph+ FLAG-gng-1+::his-3+ mat a | 34 |
| G1-F | Δgnb-1::hph+ his-3+::gna-1Q204L mat a | This study |
| G2-C | Δgnb-1::hph+ his-3+::gna-2Q205L mat a | This study |
| G2-D | Δgnb-1::hph+ his-3+::gna-2Q205L mat a | This study |
| G3-C | Δgnb-1::hph+ his-3+::gna-3Q208L mat a | This study |
| G1-23 | Δgna-1::hph+ Δgnb-1::hph+ mat a | This study |
| G2-5 | Δgna-2::hph+ Δgnb-1::hph+ mat A | This study |
| G3-21 | Δgna-3::hph+ Δgnb-1::hph+ mat A | This study |
FGSC, Fungal Genetics Stock Center, Kansas City, MO.
Vectors containing the GTPase-deficient, constitutively activating mutations gna-1Q204L, gna-2Q205L, and gna-3Q208L were previously made using site-directed mutagenesis (3, 59) (A. M. Kays and K. A. Borkovich, unpublished data). The 1.2- to 1.3-kb gna-1, gna-2, or gna-3 open reading frames (ORFs) (including introns) were PCR amplified from vectors containing the respective activated allele (pQY20 [59], pSR5 [3], and pAK21 [Kays and Borkovich, unpublished]), using primers that added XbaI and EcoRI sites to the ends of the fragments. The fragments were subcloned into pGEM-T (Promega Corporation, Madison, WI) and sequenced. The inserts were excised from pGEM-T using XbaI and EcoRI and cloned into the pMF272 vector (15) digested with the same enzymes (with eviction of green fluorescent protein [GFP]). pMF272 contains the highly expressed ccg-1 promoter and is used to target constructs to the his-3 locus in N. crassa (2). The final targeting plasmids were pSVK51 (gna-1Q204L), pSVK52 (gna-2Q205L), and pSVK53 (gna-3Q208L).
Electroporation of N. crassa with 1 to 2 μg of pSVK51, pSVK52, or pSVK53 was as previously described (22), using Δgnb-1 his-3 strain hβJ (Table 2) as the recipient. Transformants were selected by plating on sorbose medium lacking histidine. Genomic DNA was extracted from primary transformants (34) and subjected to Southern analysis for gna-1 and gna-2 as described previously (3, 59). gna-3Q208L was checked by digesting genomic DNA with HindIII and EcoRI and probing with the HindIII insert from pRAUW122 (contains the his-3 gene [2]). Under these conditions, the wild type has 3.6- and 6.0-kb hybridizing bands, while strains with gna-3 integrated at the his-3 locus have 3.6-, 3.5-, and 2.4-kb hybridizing fragments.
Transformants with a single integration event at the his-3 locus were purified to homokaryons using microconidiation (13). Homokaryons were checked for integration of the activated allele at the his-3 locus using Southern analysis, as described above.
Phenotypic analysis.
All cultures were inoculated using conidia, except for the Δgnb-1 gna-3Q208L G3-C strain, which produces very few conidia; in this case, cultures were inoculated with a small amount of aerial hyphae. To determine aerial hyphal height, 13- by 100-mm glass tubes containing 2 ml of VM liquid medium were inoculated with the strains and then incubated for 5 days in the dark and 1 day in light at room temperature. To analyze conidiation in submerged cultures, 30 ml of liquid medium was inoculated with conidia at a concentration of 106 cells/ml (except for strain G3-C, which was inoculated using ∼120 mg of packed aerial hyphae) and incubated with shaking at 200 rpm for 16 h at 30°C. Assessment of significant differences between strains with regard to the number of conidia and the height of aerial hyphae was determined using Student's t test (P < 0.05) (see Tables S1 and S2 in the supplemental material) with StatView v 5.0.1 (SAS Institute Inc., Cary, NC).
Cultures were viewed and photographed at ×60 magnification, using a differential interference contrast (DIC) oil immersion objective (N.A., 1.42) with an Olympus IX71 inverted microscope (Olympus America, Center Valley, PA) and a QIClick CCD camera (QImaging, Surrey, British Columbia, Canada). Images were analyzed using Metamorph software (Molecular Devices Corporation, Sunnyvale, CA). For fertility assays, strains were inoculated onto SCM plates and incubated for 6 days in constant light at 25°C. Cultures were fertilized with males (macroconidia) of opposite mating type and incubated for six more days before being photographed using an SZX9 stereomicroscope (Olympus) with a Powershot G10 camera (Canon USA, Lake Success, NY) at a magnification of ×57.
Western analysis.
Since some of the strains did not produce a significant amount of conidia, submerged cultures to be used for isolation of the plasma membrane fraction were inoculated using aerial hyphae harvested from 1-day-old plate cultures using a sterile wooden stick. A mass of packed hyphae corresponding to 0.5 ml (200 mg) of tissue was added to a 125-ml flask containing 50 ml of liquid VM. The flasks were incubated for 16 h at 30°C in the dark with shaking at 200 rpm. Tissue was collected on filter paper using a vacuum system, and cell pads were flash-frozen in liquid nitrogen prior to storage at −80°C. Tissue was pulverized in liquid nitrogen using a mortar and pestle and then transferred into a medium-sized Bead-Beater chamber (Biospec Products, Bartlesville, OK) containing glass beads and extraction buffer (10 mM HEPES [pH 7.5], 0.5 mM EDTA, 1 M sorbitol, 0.5 mM phenylmethylsulfonyl fluoride [PMSF] and 0.1% fungal protease inhibitor cocktail; catalog no. P8215; Sigma-Aldrich, St. Louis, MO). The tissue was homogenized for 45 s (repeated twice) at 4°C. The homogenate was centrifuged in a JA-25.50 rotor (Beckman-Coulter, Inc., Brea, CA) at 15,000 × g for 30 min at 4°C. The supernatant was removed, and the protein concentration was determined using the Bradford protein assay (Bio-Rad). Samples were adjusted to the same total protein concentration using extraction buffer and centrifuged at 46,000 × g for 60 min at 4°C using the JA-25.50 rotor (Beckman-Coulter). The supernatant was collected and its volume measured. The pellet was gently homogenized using a thin glass rod and resuspended in the same volume as the supernatant using a buffer containing 50 mM Tris-Cl (pH 7.5), 1 mM dithiothreitol (DTT), 0.5 mM PMSF, 10% glycerol, and 0.1% fungal protease inhibitor cocktail (Sigma-Aldrich). Samples containing 100 μg of total protein were subjected to SDS-PAGE and then transferred to a nitrocellulose membrane as described previously (34).
The membranes for Western analysis were probed using previously described primary antibodies for GNA-1, GNA-2, and GNB-1 at a final dilution of 1:1,000 (3, 22, 34, 57, 60). A previously described GNA-3 antibody (see Fig. 4) was used at a dilution of 1:500 (60). A second GNA-3 polyclonal antibody was produced in rabbits by a commercial source (Thermo Scientific Pierce Protein Research, Rockford, IL) and used at a 1:2,000 dilution (see Fig. 3). The antigen for this newer antiserum was a GNA-3-maltose binding protein fusion protein expressed in E. coli using the pMAL-c4X vector (New England BioLabs, Inc., Ipswich, MA). The fusion protein was purified from E. coli according to the manufacturer's recommendations (pMAL Protein Fusion and Purification System Manual; New England BioLabs). The antiserum reacted with a protein of the predicted size of GNA-3 in wild-type but not Δgna-3 strain extracts during Western analysis (see Fig. 3).
Fig 4.
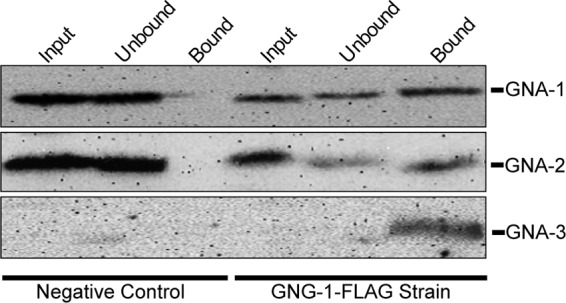
Neurospora Gα proteins physically interact with Gβγ in vivo. Wild-type (74a) and GNG-1-FLAG (5A) strains were grown for 16 h in submerged VM cultures. The particulate fraction was isolated and solubilized using lauryl maltoside and immunoprecipitated using anti-FLAG antibody coupled to agarose beads. Samples of the solubilized particulate fraction (Input), flowthrough (Unbound), and bound (Bound) fractions were subjected to Western analysis using GNA-1, GNA-2, and GNA-3 antibodies as described in Materials and Methods.
Fig 3.
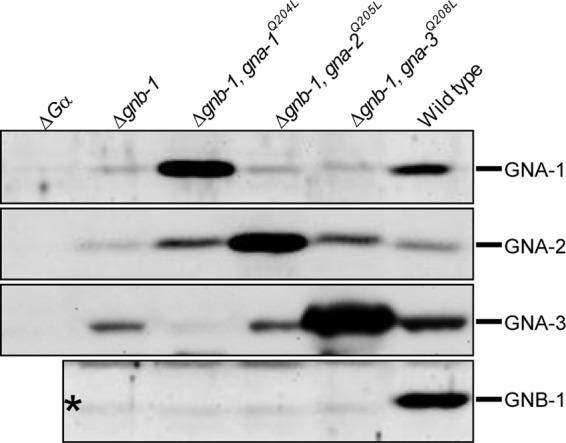
Analysis of Gα and Gβ protein levels. Strains are wild type (74a) and Δgnb-1 (42-5-11), Δgnb-1 gna-1Q204L (G1-F), Δgnb-1 gna-2Q205L (G2-D), Δgnb-1 gna-3Q208L (G3-C), Δgna-1 (1B8), Δgna-2 (Δ2), and Δgna-3 (31C2) mutants. The last three strains were used as negative controls for the corresponding Gα antibody (ΔGα lanes). Strains were cultured in liquid VM medium with shaking at 200 rpm for 16 h in the dark at 30°C. The plasma membrane protein fraction was isolated, and 100 μg of protein subjected to Western analysis using GNA-1, GNA-2, GNA-3, and GNB-1 antibodies as described in Materials and Methods.
The secondary antibody for all Western analysis was an antirabbit-horseradish peroxidase conjugate (Bio-Rad) used at a 1:5,000 dilution. Chemiluminescent detection was as described previously (34). Identical Coomassie-stained gels served as the loading controls (data not shown).
Coimmunoprecipitation experiments.
Submerged cultures (500 ml of liquid VM) were inoculated with conidia to a final concentration of 1 × 107 cells/ml and grown as described above for Western analysis. Tissues were pulverized in liquid nitrogen using a mortar and pestle and then transferred into a large bead-beater chamber (Biospec) with glass beads and cold extraction buffer (50 mM Tris-Cl [pH 8.0], 1 mM EDTA, 6 mM MgCl2, 0.1% fungal protease inhibitor cocktail [Sigma], 2.5 mM PMSF, and 1 mM GDP). The tissue was homogenized three times for 30 s and then centrifuged using a JA-17 rotor (Beckman) at 2,500 × g for 10 min at 4°C to remove cell debris. The supernatant was transferred to an L5-50 ultracentrifuge rotor (Beckman), and samples were centrifuged at 46,000 × g for 1 h at 4°C. The supernatant was aspirated and discarded. The pellet (particulate fraction) was gently homogenized and resuspended in 1.5 to 2.0 ml of extraction buffer using a glass Dounce homogenizer. From this solution, 900 μl was combined with 100 μl of 10% lauryl maltoside in a microcentrifuge tube and the mixture incubated with rotation for 2 to 3 h at 4°C. The mixture was spun down twice at 15,000 rpm in a microcentrifuge at 4°C for 20 min. The supernatant (input) was transferred to a new microcentrifuge tube. If the volume was less than 800 μl, extraction buffer was added to reach a final volume of 800 μl.
A volume containing 100 μl of packed anti-FLAG M2-agarose beads (Sigma) was pelleted for 1 min in a microcentrifuge at 6,000 rpm. The supernatant was discarded, and the beads were washed three times using 1 ml of a solution containing 10 mM Tris-Cl (pH 7.5) and 150 mM NaCl. A volume of input extract containing 400 μl was combined with 400 μl of a solution containing 20 mM Tris-Cl (pH 7.5), 300 mM NaCl, 1 mM GDP, and 6 mM MgCl2 to which 1 μl of fungal protease inhibitor cocktail (Sigma) and 2.5 μl of 100 mM PMSF had been added. Subsequently, 200 μl of the washed anti-FLAG agarose beads was added to each tube, followed by incubation at 4°C with rotation overnight. Tubes were centrifuged at 10,000 rpm in a microcentrifuge for 6 to 7 s at 4°C to collect the beads. The supernatant was discarded, and 1 ml of cold 1× Tris-buffered saline (TBS) supplemented with 6 mM MgCl2 and 1 mM GDP was added, followed by centrifugation at 15,000 rpm in a microcentrifuge for 6 to 7 s at 4°C. This step was repeated once. Immunoprecipitated proteins were eluted using 35 to 70 μl of 2× sample buffer (125 mM Tris-Cl [pH 6.8], 4% SDS, 20% glycerol, and 0.005% bromphenol blue) by heating to 95°C and then separated from the agarose beads by centrifuging for 5 min at 3,000 rpm in a microcentrifuge. Supernatants were analyzed using SDS-PAGE and Western analysis with GNA-1, GNA-2, and GNA-3 antisera as described above.
RESULTS
GNA-3 and GNB-1 may operate in a linear pathway to negatively regulate submerged conidiation, while GNA-1 and GNA-2 are independent of GNB-1.
Previous work has demonstrated that heterotrimeric G proteins are essential for normal asexual and sexual development of N. crassa (22, 32, 34, 60). The goal of this study was to identify the Gα subunits that interact with the GNB-1/GNG-1 Gβγ dimer in N. crassa during regulation of different cellular functions. We first utilized a genetic approach to determine whether there was evidence for an epistatic relationship between gnb-1 and the three Gα genes that would support a physical interaction between the encoded proteins in a heterotrimeric complex. Double mutants containing Δgnb-1 and one Gα deletion mutation were produced by using sexual crosses between single mutants. We also created strains expressing GTPase-deficient, constitutively activated Gα alleles in the Δgnb-1 background using an N. crassa gene-targeting system that directs DNA sequences to the his-3 locus (2). All three activated alleles were expressed using the ccg-1 promoter (4, 39) in order to eliminate the effects of different protein expression levels.
The results of the genetic analysis were interpreted as follows (see the diagram in Fig. S1 in the supplemental material). The observation that the Δgnb-1 ΔGα double mutant has the same phenotype as the Δgnb-1 single mutant and that introduction of the constitutively activated Gα allele does not suppress the Δgnb-1 phenotype shows that gnb-1 is epistatic to the Gα gene and supports GNB-1 acting downstream of Gα in a linear pathway (see Fig S1A in the supplemental material). Conversely, the finding that the Δgnb-1 ΔGα mutant has the same phenotype as the ΔGα mutant and that the activated Gα allele at least partially bypasses the Δgnb-1 phenotype indicates that the Gα gene is epistatic to gnb-1 and suggests that the Gα protein acts downstream of GNB-1 in a linear pathway (see Fig. S1B in the supplemental material). The interpretation of any other combination of results from genetic analysis was that the Gβ and Gα are at least partially independent and likely operate in different signaling pathways to regulate the cellular function being assayed.
We began by assessing several phenotypes during asexual growth and development in the strains. During the asexual phase of the life cycle, N. crassa grows by extension, branching, and fusion of hyphae to form the network structure called the mycelium (52). The asexual sporulation pathway, macroconidiation/conidiation, begins with differentiation of aerial hyphae from the mycelium, followed by constriction of the aerial hyphal tips to form the mature conidia (52). Since conidiation is induced by exposure to air, wild-type cultures submerged in liquid do not normally produce conidia (52). We have previously demonstrated that Δgna-1, Δgna-3, and Δgnb-1 single mutants produce conidiophores in submerged cultures (23, 30, 60). Δgna-1 mutants do so in a cell density-dependent manner, forming conidiophores only at relatively high inoculation densities (3 × 106 cells/ml or greater [23]). In order to increase the stringency of our screen, we assessed submerged cultures for the presence of conidiophores at an inoculation density of 1 × 106 cells/ml, a condition that leads to conidiation in Δgna-3 and Δgnb-1, but not Δgna-1, mutants.
As previously shown (31, 60), the only single mutants that produce conidiophores in submerged culture are those lacking gnb-1 or gna-3 (Fig. 1A). Further deletion of any of the three Gα genes in the Δgnb-1 background leads to a phenotype identical to that conferred by Δgnb-1 (Fig. 1A). Introduction of the constitutively activated gna-1Q204L or gna-3Q208L alleles into the Δgnb-1 background abolished inappropriate conidiation (Fig. 1A). In addition, hyphae in the Δgnb-1 gna-3Q208L strain were wider than in the other strains. The gna-2Q205L allele yielded a partial correction, with some conidiophores present among the normal hyphae (Fig. 1A). These results suggest that gna-3 is epistatic to gnb-1, since single and double mutants conidiate in submerged culture and gna-3Q208L corrects the conidiation defect in the Δgnb-1 background. This is consistent with GNB-1 acting upstream of GNA-3 in a linear pathway to negatively regulate submerged conidiation. In contrast, the two genetic epistasis assays gave opposite results for gna-1 and gna-2; the findings from double mutant analysis suggest that gnb-1 is epistatic to gna-1 and gna-2, while those from strains carrying the gna-1Q204L or gna-2 Q205L allele support gna-1 and gna-2 being epistatic to gnb-1. Observation of opposite results for the two epistasis assays is most consistent with at least partial independence of GNA-1 and GNA-2 from GNB-1 during negative control of submerged conidiation.
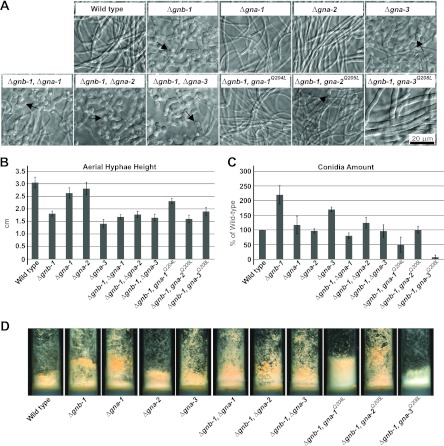
Fig 1.
Phenotypes during asexual growth and development. Strains are wild type (74a) and Δgnb-1 (42-5-18), Δgna-1 (1B4), Δgna-2 (Δ2), Δgna-3 (31c2), Δgna-1 Δgnb-1 (G1-23), Δgna-2 Δgnb-1 (G2-5), Δgna-3 Δgnb-1 (G3-21), Δgnb-1 gna-1Q204L (G1-F), Δgnb-1 gna-2Q205L (G2-C), and Δgnb-1 gna-3Q208L (G3-C) mutants. (A) Submerged cultures. Strains were cultured in VM liquid medium for 16 h with shaking at 200 rpm in the dark at 30°C. The arrows indicate conidiophores. (B) Aerial hyphal height. To measure aerial hyphal height, liquid VM tube cultures were inoculated with the indicated strains and incubated statically for 5 days in the dark and 1 day in light at room temperature. Values are from six replicates, with error calculated as the standard error of the mean. (C) Amount of conidia. VM agar flasks were inoculated with the indicated strains and incubated for 3 days in the dark at 30°C and 5 days in light at room temperature. Conidia were harvested from flasks and quantitated using a hemacytometer. Values are expressed as percentages of the wild type from three independent experiments, with the error calculated as the standard error of the mean. (D) Strain morphology in VM agar tube cultures. Strains were cultured in tubes containing 2 ml of VM agar medium for 3 days in the dark at 30°C and 5 days in light at room temperature.
Aerial hyphal height and production of conidia on solid medium involve different epistatic relationships.
We next investigated epistatic relationships between gnb-1 and the three Gα genes during aerial hypha production in standing liquid cultures and conidiation on solid medium (Fig. 1B). Student's t test was used to identify statistical differences between strains with regard to these two quantitative traits (see Tables S1 and S2 in the supplemental material). As previously determined, Δgnb-1 and Δgna-3 strains have significantly shorter aerial hyphae than those of the wild type (31, 60) (Fig. 1B), while the Δgna-2 mutant resembles the wild type in its aerial hyphal height (3) (Fig. 1B). In contrast to previous results (22, 24, 59), we did not detect a statistically significant difference between aerial hyphal height in wild-type and Δgna-1 strains (see Table S1 in the supplemental material). Deletion of a single Gα gene in the Δgnb-1 background results in aerial hyphal heights similar to those of the Δgnb-1 mutant (Fig. 1B). Δgnb-1 strains carrying the gna-2- or gna-3-activated Gα alleles have aerial hyphal heights similar to those of the Δgnb-1 mutant (Fig. 1B). Aerial hyphae are also denser in these strains (Fig. 1D and data not shown). These results are consistent with gnb-1 being epistatic to (acting downstream of) gna-2 and gna-3 with respect to aerial hyphal height, as double mutants and Δgnb-1 strains carrying the activated gna-2 or gna-3 allele resemble the Δgnb-1 single mutant. In contrast, the relationship between gnb-1 and gna-1 is more consistent with at least partial functional independence, as the Δgnb-1 gna-1Q204L strain has an aerial hyphal height significantly greater than that of Δgnb-1 (see Table S1 in the supplemental material).
Δgnb-1 strains produced the greatest amount of conidia in plate cultures in our study, followed by Δgna-3 mutants (Fig. 1C). Δgnb-1 strains carrying any of the three activated Gα genes resulted in significantly less conidium production than Δgnb-1 (see Table S2 in the supplemental material), with levels in the Δgnb-1 gna-3Q208L strain near zero (Fig. 1C). This indicates that all three activated Gα genes can negatively regulate conidium production in the Δgnb-1 background. With regard to epistatic relationships between gnb-1 and gna-1 and gna-2, the observation that double mutants have levels of conidia similar to those of Gα single mutants and that Δgnb-1 strains carrying gna-1Q204L or gna-2 Q205L have levels significantly lower than Δgnb-1 mutants is most consistent with gna-1 and gna-2 being epistatic to gnb-1. In contrast, the epistatic relationship between gnb-1 and gna-3 is less clear, as conidium levels are significantly lower in Δgnb-1 Δgna-3 double mutants than in either single mutant and lower still in Δgnb-1 gna-3Q208L strains (see Table S2 in the supplemental material).
We also observed differences in orange pigmentation, indicative of carotenoid production, in the strains (Fig. 1D). The intensity of pigmentation roughly correlated with the extent of conidiation, with Δgnb-1 gna-1Q204L and Δgnb-1 gna-3Q208L strains much lighter than the wild type. In the case of the Δgnb-1 gna-3Q208L strain, the suppression yielded a white hyphal mass (Fig. 1D).
Constitutive activation of Gα proteins does not restore sexual fertility to Δgnb-1 mutants.
Nitrogen starvation induces the sexual cycle by leading to production of female reproductive structures (protoperithecia) (46). Protoperithecia produce specialized chemotropic hyphae (trichogynes) that grow toward male cells of opposite mating type (5, 46). During the course of cell fusion, fertilization, and meiosis, the protoperithecium enlarges to form the perithecium. Approximately 2 weeks after fertilization, sexual spores (ascospores) are ejected and can germinate to form vegetative hyphae (46).
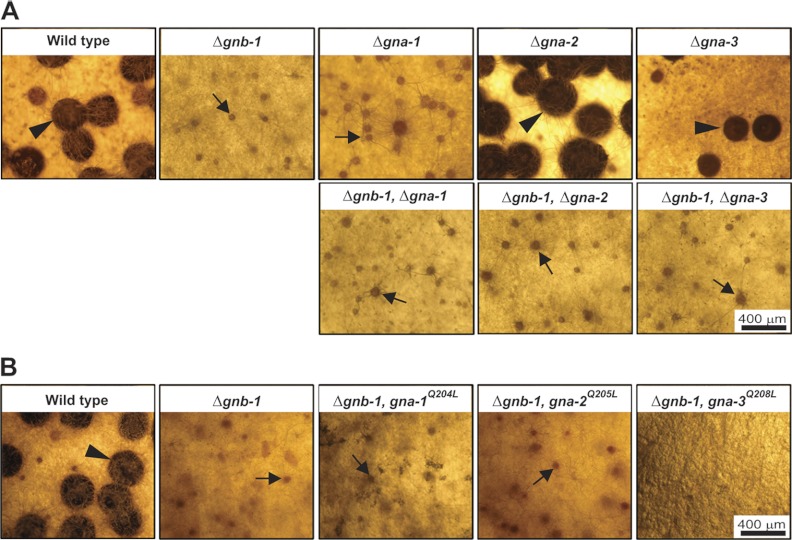
During the sexual cycle, Δgnb-1 and Δgna-1 mutants are male fertile but female sterile (60) (Fig. 2A). Although these strains produce protoperithecia and trichogynes, their trichogynes have a defect in chemotropism and are not attracted by male cells (22, 32, 34, 60). In contrast, Δgna-2 and Δgna-3 mutants produce protoperithecia and develop perithecia after fertilization with wild-type males (3, 31). In this study, we observed that all Gα Δgnb-1 double mutants resembled Δgnb-1 single mutants, forming protoperithecia but not perithecia after fertilization (Fig. 2A). The sexual cycle phenotypes of Δgnb-1 gna-1Q204L and Δgnb-1 gna-2Q205L strains were similar to those of the Δgnb-1 strain (Fig. 2B), with the Δgnb-1 gna-1Q204L strain exhibiting a delay in protoperithecial development (data not shown). In contrast, the gna-3Q208L allele completely inhibited protoperithecial formation in the Δgnb-1 background (Fig. 2B).
Fig 2.
Sexual phase phenotypes. Strains were inoculated onto SCM plates to induce production of female reproductive structures (protoperithecia) and incubated for 6 days in constant light at 25°C. Cultures were then fertilized with males (macroconidia) of opposite mating type and incubated for six more days before being photographed. Protoperithecia are indicated by black arrows, while perithecia are marked by black arrowheads. (A) Single and double mutants. Strains are wild type (74a) and Δgnb-1 (42-5-11), Δgna-1 (1B4), Δgna-2 (Δ2), Δgna-3 (31c2), Δgnb-1 Δgna-1 (G1-23), Δgnb-1 Δgna-2 (G2-5), and Δgnb-1 Δgna-3 (G3-21) mutants. (B) Strains carrying Gα-activated alleles. Strains are wild type (74a) and Δgnb-1 (42-5-11), Δgnb-1 gna-1Q204L (G1-F), Δgnb-1 gna-2Q205L (G2-C), and Δgnb-1 gna-3Q208L (G3-C) mutants.
We have previously hypothesized that the block in fertility of Δgnb-1 mutants may be a consequence of low GNA-1 protein levels (60). The findings from the double mutant analysis presented here are consistent with this idea, as Δgnb-1 is epistatic to Δgna-2 and Δgna-3 and has the same phenotype as Δgna-1. Furthermore, the results with the gna-1Q204L allele corroborate those from a previous study showing that although GNA-1 is required for chemotropism of female trichogynes toward male cells, constitutive activation of gna-1 cannot rescue the defect in chemotropism caused by loss of the pheromone receptor (32). The delayed perithecial development of Δgnb-1 gna-1Q204L strains and the complete inhibition of protoperithecial development by gna-3Q208L suggest that GNA-1 and GNA-3 must cycle through inactive and active forms during fertilization and protoperithecial development, respectively.
Introduction of activated Gα alleles into the Δgnb-1 background restores Gα protein levels.
We have previously shown that loss of gnb-1 leads to reduced levels of Gα proteins under various conditions in N. crassa (34, 60). Therefore, we used Western analysis to measure levels of GNA-1, GNA-2, GNA-3, and GNB-1 proteins in a plasma membrane fraction isolated from submerged liquid cultures (Fig. 3). Strains with deletion of a Gα gene (30) were included as negative controls for the reactions with each Gα antibody (ΔGα lanes in Fig. 3).
As expected, GNB-1 protein could be detected only in wild-type preparations (Fig. 3). Levels of GNA-1, GNA-2, and GNA-3 are decreased in Δgnb-1 strains relative to the wild type, with GNA-1 showing the greatest effect (Fig. 3). The decreased level of GNA-3 in submerged cultures is in contrast to results from a previous study using a different antibody for detection (60). Introduction of gna-1Q204L, gna-2Q205L, or gna-3Q208L alleles resulted in elevated production of the encoded proteins (Fig. 3), consistent with overexpression of the encoded proteins from the ccg-1 promoter. The gna-1Q204L and gna-3Q208L alleles also influenced protein levels of GNA-2 in the Δgnb-1 background.
The three Gα proteins coimmunoprecipitate with tagged Gγ.
In a previous study, we demonstrated coimmunoprecipitation of a FLAG-tagged GNG-1 protein with GNB-1 from a solubilized membrane particulate fraction isolated from submerged cultures of N. crassa (34). These results confirmed a physical association between the Gβ and Gγ protein in N. crassa (34). However, to our knowledge, no studies have reported testing for a physical interaction between a Gβγ dimer and all three Gα proteins in a single species of filamentous fungi. Therefore, we conducted a systematic analysis, testing for complex formation between GNG-1 and GNA-1, GNA-2, and GNA-3. A solubilized particulate fraction extract was prepared from the strain carrying FLAG-tagged GNG-1 and then immunoprecipitated using FLAG antibody coupled to agarose beads. An extract from a wild-type strain lacking the FLAG-gng-1 construct was used as a negative control.
GNA-1 and GNA-2 can be detected in greater quantity than GNA-3 in solubilized particulate fractions (Fig. 4, input lanes), consistent with previous observations based on mRNA amounts for the encoded proteins that suggested GNA-3 is present in smaller amounts than the other two Gα subunits (Kays and Borkovich, unpublished). GNA-1 and GNA-2 could be detected in the flowthrough from the FLAG antibody column (Fig. 4, unbound lanes). However, significant amounts of GNA-1, GNA-2, and GNA-3 proteins did bind to the FLAG antibody column (Fig. 4, bound lanes). Importantly, no Gα proteins were detected in the bound fraction from the control strain (Fig. 4), suggesting that the binding detected using the GNG-1-FLAG strain was specific. Consistent with previous results (34), GNB-1 was present in the immunoprecipitates that included the Gα proteins (data not shown). These results are consistent with all three Gα proteins associating with the Gβγ dimer in N. crassa. The observation of low GNA-3 amounts in input fractions coupled with high levels in the immunoprecipitated material suggests that GNA-3 has a relatively high affinity for the Gβγ dimer, compared to the other two Gα proteins.
DISCUSSION
We have previously demonstrated that GNB-1 is essential for the correct functioning of Gα and Gγ protein subunits in N. crassa (34, 60). In the absence of GNB-1, levels of the three Gα proteins and GNG-1 are severely reduced (60). In this study, we explored the relationship between the GNB-1/GNG-1 dimer and the three Gα proteins in signal transduction using genetic and biochemical approaches.
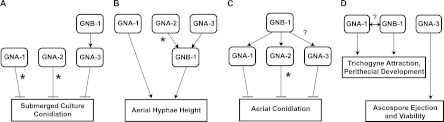
The genetic approach involved analysis of Gα-gnb-1 single and double mutants and Δgnb-1 strains carrying activated Gα alleles. Models that summarize the different epistatic relationships are presented in Fig. 5. The results indicate that gna-3 is epistatic to gnb-1 for negative regulation of submerged culture conidiation (Fig. 5A), while gna-1 and gna-2 are epistatic to gnb-1 with regard to negative control of aerial conidiation (Fig. 5C). These observations suggest that GNB-1 is necessary for activation of the Gα proteins, which then regulate conidiation. In contrast, gnb-1 appears to act downstream of gna-2 and gna-3 to regulate aerial hyphal height (Fig. 5B), consistent with GNA-2 and GNA-3 as positive regulators of GNB-1 for this trait. A caveat to these conclusions is the reduced levels of Gα proteins in a mutant lacking the Gβ or Gγ subunit; this scenario complicates the interpretation of the results from genetic analysis (34, 60). However, all Δgnb-1 mutant phenotypes cannot be explained by reduced levels of Gα proteins, as a mutant lacking all three Gα genes has much more severe defects in asexual and sexual growth and development than does the Δgnb-1 strain (30). Finally, the observation that constitutive activation of any Gα suppresses the hyperconidiation defects of Δgnb-1 mutants suggests that all three proteins are negative regulators of conidium production (Fig. 5A and C). This observation complements previous results establishing synergy between Gα gene deletion mutations during regulation of conidiation in N. crassa (30). However, our previous finding that gna-2 acts in a compensatory fashion to gna-1 and gna-3 to regulate conidiation suggests that the role of gna-2 is relatively minor compared to the roles of the other two Gα genes.
Fig 5.
Models for interactions between Gα proteins and the Gβγ dimer in Neurospora. (A) Submerged culture conidiation. GNB-1 acts upstream of GNA-3 to suppress conidiation in submerged cultures. The independent action of GNA-1 and GNA-2 is observed only in Δgnb-1 strains that express constitutively activated gna-1 or gna-2 alleles (denoted by asterisks). (B) Aerial hyphal height. GNA-2 and GNA-3 operate upstream of GNB-1 to positively modulate aerial hyphal height. The asterisk indicates that for gna-2, a phenotype is seen only upon constitutive activation in the Δgnb-1 background. GNA-1 positively regulates aerial hyphal height independently of GNB-1. (C) Aerial conidiation. GNA-1 and GNA-2 are downstream of GNB-1 during negative regulation of conidiation on solid medium. The role for gna-2 is revealed only by the presence of the gna-2Q205L allele in the Δgnb-1 background (denoted by the asterisk). GNA-3 is a strong negative regulator of aerial conidiation, but the epistatic relationship between gna-3 and gnb-1 is not clear from our analysis (denoted by a question mark). (D) Female fertility. Although GNB-1 and GNA-1 regulate trichogyne attraction and perithecial development, their epistatic relationship is unclear and the role of GNB-1 may be to maintain GNA-1 protein levels (denoted by the question mark above the double-ended arrow). GNA-3 is required for ascospore ejection and viability, while GNA-2 has no obvious function in regulating female fertility.
There was no clear epistatic relationship between gnb-1 and gna-3 with regard to aerial conidiation (Fig. 1B). Loss of gna-3 leads to increased conidiation, while constitutive activation of gna-3 in the Δgnb-1 background reduces conidiation to nearly zero, consistent with gna-3 as a strong negative regulator of conidiation. However, conidium levels are lower in the Δgnb-1 Δgna-3 double mutant than in either single mutant. The last observation may reflect the presence of low levels of constitutively active Gα proteins in the strains lacking gnb-1. These strains lack the Gβγ tether that prevents the Gα proteins from signaling in the absence of an activated GPCR. In this scenario, the recently discovered cytosolic guanine nucleotide exchange factor (GEF) RIC8 may function to load GTP on the remaining free Gα proteins. We have shown that RIC8 binds to and regulates GDP/GTP exchange on GNA-1 and GNA-3 in N. crassa (57).
Our results demonstrate that the three Gα genes are epistatic to gnb-1 for submerged (gna-3) or aerial (gna-1 and gna-2) conidiation (Fig. 5A and C). This indicates that the Gα proteins are likely to interact directly with downstream effector proteins. Since elevated cyclic AMP (cAMP) levels correlate with suppression of conidiation in N. crassa (50), one probable effector is adenylyl cyclase (CR-1), which converts ATP to cyclic AMP. GTP (Gα)-dependent adenylyl cyclase activity can be assayed in submerged liquid cultures of N. crassa (51). We have shown that loss of gna-1 leads to reduced GTP-dependent adenylyl cyclase (CR-1) activity (but normal CR-1 protein levels) in extracts from submerged liquid cultures, supporting adenylyl cyclase as a downstream effector of GNA-1 (24). Δgnb-1 mutants have normal CR-1 protein levels but reduced GTP-stimulatable adenylyl cyclase activity, consistent with a decreased GNA-1 protein amount in these strains (60). In contrast, mutation of gna-3 leads to reduced CR-1 protein levels but does not influence GTP-dependent activity (31), and gna-2 has no apparent effect on adenylyl cyclase protein levels or activity (24, 30). The last point implies that GNA-2 and GNA-3 interact with another/additional downstream effector(s). These observations, in combination with the known conidiation defects of N. crassa mitogen-activated protein (MAP) kinase mutants (35, 43, 44), suggest that additional effectors (such as MAP kinase modules) operate downstream of GNA-1, GNA-2, GNA-3, and GNB-1 to regulate conidiation.
We observed differences in pigmentation in the strains during this study. Previous work in our laboratory and others has established an inverse relationship between levels of carotenoid pigments and cAMP (7, 33, 59). For example, loss of gna-1 leads to increased carotenoid amount and lowered cAMP, while constitutive activation results in reduced levels of carotenoids and elevated cAMP (59). Furthermore, our previous work indicates that gna-3 has a more profound positive influence on cAMP levels than gna-1 does (24, 31). Taken together, these observations are consistent with our results showing that gna-3Q208L strains are less pigmented than gna-1Q204L mutants. They also reinforce the notion that regulation of cAMP levels is an important downstream function of heterotrimeric G proteins in N. crassa.
Regulation of female fertility likely involves GNB-1 regulating protein levels of GNA-1, the active subunit during mating (Fig. 5D). GNA-3 plays a role in ascospore ejection and viability, as few ascospores are ejected and are viable from homozygous Δgna-3 crosses (31) (Fig. 5D). Female fertility was not restored by transformation of the Δgnb-1 mutant with any activated Gα alleles. The observation that activation of GNA-1 is not sufficient to restore full mating in Δgnb-1 mutants is consistent with results from a similar experiment conducted in a strain background lacking a pheromone receptor gene (32). The inability to restore female fertility by expressing a GTPase-deficient gna-1 allele may stem from a need for both GNA-1-GDP and GNA-1-GTP at different times during protoperithecial development, mating, meiosis, and/or ascospore formation. Alternatively, GNB-1 may be absolutely necessary for normal sexual development and this requirement cannot be bypassed by activation of gna-1 or the other two Gα genes. The gna-3Q208L allele completely inhibited development of female reproductive structures, suggesting that GNA-3 needs to be in the inactive GDP-bound form during at least some critical stages of this process in N. crassa. This observation is consistent with the relatively normal functioning of Δgna-3 mutants (which completely lack GNA-3 protein) as females during crosses with wild-type males (31).
Our results can be compared to those of studies in Aspergillus nidulans and C. neoformans, where a single constitutively activated Gα allele has been tested for its ability to suppress defects of a Gβ mutant (21, 49). The results showed that an activated Group I Gα allele bypassed defects resulting from mutation of the Gβ gene in A. nidulans (49) but not in C. neoformans (21). In A. nidulans, the authors conclude that the Gα can function independently of Gβ to regulate proliferative growth. In contrast, the findings from C. neoformans suggest interdependence of the Gα and Gβ for downstream signaling.
We have proposed that a protein turnover mechanism leads to low levels of the Gα proteins in the Δgnb-1 and Δgng-1 backgrounds, as (i) mRNA levels for the three genes are normal in Δgnb-1 mutants (34, 60), (ii) proteolysis of Gα proteins is a regulatory mechanism observed in other fungi (8, 28, 55, 58), and (iii) results from preliminary experiments analyzing chemicals that influence the proteasomal or vacuolar protein turnover pathways are consistent with proteolysis of at least one Gα protein (GNA-1 [data not shown]). Importantly, we were able to restore levels of the three Gα proteins through overexpression of the activated alleles. This suggests either that the proteolysis machinery activated in the Δgnb-1 background cannot keep pace with the level of Gα protein being produced or that the GTP-bound form of the Gα proteins is resistant to the turnover mechanism. We have recently made similar observations after expression of activated Gα alleles in a mutant lacking the ric8 GEF (57). Taken together, these results point to critical functions for the Gβ subunit and the nonreceptor GEF RIC8 in regulation of Gα protein levels in N. crassa.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ann Kays for construction of the original gna-3Q208L plasmid and Qi Yang for construction of Δgnb-1 strain 42-5-11. We are indebted to Jacqueline Servin, Asharie Campbell, Patrick Schacht, and Ilva Cabrera for comments on the manuscript.
This work was supported by National Institutes of Health grants GM048626 and GM086565 to K.A.B.
Footnotes
Published ahead of print 17 August 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alspaugh JA, Perfect JR, Heitman J. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11:3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aramayo R, Metzenberg RL. 1996. Gene replacements at the his-3 locus of Neurospora crassa. Fungal Genet. Newsl. 43:9–13 [DOI] [PubMed] [Google Scholar]
- 3.Baasiri RA, Lu X, Rowley PS, Turner GE, Borkovich KA. 1997. Overlapping functions for two G protein α subunits in Neurospora crassa. Genetics 147:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell-Pedersen D, Dunlap JC, Loros JJ. 1992. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 6:2382–2394 [DOI] [PubMed] [Google Scholar]
- 5.Bistis GN. 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73:959–975 [Google Scholar]
- 6.Bolker M. 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25:143–156 [DOI] [PubMed] [Google Scholar]
- 7.Bramley PM, Mackenzie A. 1988. Regulation of carotenoid biosynthesis. Curr. Top. Cell Regul. 29:291–343 [DOI] [PubMed] [Google Scholar]
- 8.Cappell SD, Baker R, Skowyra D, Dohlman HG. 2010. Systematic analysis of essential genes reveals important regulators of G protein signaling. Mol. Cell 38:746–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Case ME, Schweizer M, Kushner SR, Giles NH. 1979. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc. Natl. Acad. Sci. U. S. A. 76:5259–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman DE, et al. 1994. Structures of active conformations of Gi α1 and the mechanism of GTP hydrolysis. Science 265:1405–1412 [DOI] [PubMed] [Google Scholar]
- 11.Davis RH, deSerres FJ. 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 71A:79–143 [Google Scholar]
- 12.Dohlman HG, Slessareva JE. 2006. Pheromone signaling pathways in yeast. Sci. STKE 2006:cm6 doi:10.1126/stke.3642006cm6 [DOI] [PubMed] [Google Scholar]
- 13.Ebbole DJ, Sachs MS. 1990. A rapid and simple method for isolation of Neurospora crassa homokaryons using microconidia. Fungal Genet. Newsl. 37:17–18 [Google Scholar]
- 14.Freissmuth M, Gilman AG. 1989. Mutations of GS α designed to alter the reactivity of the protein with bacterial toxins. Substitutions at ARG187 result in loss of GTPase activity. J. Biol. Chem. 264:21907–21914 [PubMed] [Google Scholar]
- 15.Freitag M, Hickey PC, Raju NB, Selker EU, Read ND. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:897–910 [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Rico RO, et al. 2008. The heterotrimeric Gα protein pga1 regulates biosynthesis of penicillin, chrysogenin and roquefortine in Penicillium chrysogenum. Microbiology 154:3567–3578 [DOI] [PubMed] [Google Scholar]
- 17.Graziano MP, Gilman AG. 1989. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs α. J. Biol. Chem. 264:15475–15482 [PubMed] [Google Scholar]
- 18.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 19.Hewavitharana T, Wedegaertner PB. 2012. Non-canonical signaling and localizations of heterotrimeric G proteins. Cell. Signal. 24:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks JK, Yu JH, Keller NP, Adams TH. 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 16:4916–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsueh YP, Xue C, Heitman J. 2007. G protein signaling governing cell fate decisions involves opposing Gα subunits in Cryptococcus neoformans. Mol. Biol. Cell 18:3237–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivey FD, Hodge PN, Turner GE, Borkovich KA. 1996. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7:1283–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivey FD, Kays AM, Borkovich KA. 2002. Shared and independent roles for a Gαi protein and adenylyl cyclase in regulating development and stress responses in Neurospora crassa. Eukaryot. Cell 1:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivey FD, Yang Q, Borkovich KA. 1999. Positive regulation of adenylyl cyclase activity by a Gαi homolog in Neurospora crassa. Fungal Genet. Biol. 26:48–61 [DOI] [PubMed] [Google Scholar]
- 25.Jain S, Akiyama K, Mae K, Ohguchi T, Takata R. 2002. Targeted disruption of a G protein alpha subunit gene results in reduced pathogenicity in Fusarium oxysporum. Curr. Genet. 41:407–413 [DOI] [PubMed] [Google Scholar]
- 26.Johnson GL, et al. 1994. How does the G protein, Gi2, transduce mitogenic signals? J. Cell. Biochem. 54:415–422 [DOI] [PubMed] [Google Scholar]
- 27.Kamerewerd J, Jansson M, Nowrousian M, Poggeler S, Kuck U. 2008. Three α-subunits of heterotrimeric G proteins and an adenylyl cyclase have distinct roles in fruiting body development in the homothallic fungus Sordaria macrospora. Genetics 180:191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasahara S, Wang P, Nuss DL. 2000. Identification of bdm-1, a gene involved in G protein β-subunit function and α-subunit accumulation. Proc. Natl. Acad. Sci. U. S. A. 97:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katritch V, Cherezov V, Stevens RC. 2012. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 33:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kays AM, Borkovich KA. 2004. Severe impairment of growth and differentiation in a Neurospora crassa mutant lacking all heterotrimeric Gα proteins. Genetics 166:1229–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kays AM, Rowley PS, Baasiri RA, Borkovich KA. 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20:7693–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Borkovich KA. 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52:1781–1798 [DOI] [PubMed] [Google Scholar]
- 33.Kritsky MS, Sokolovsky TA, Belozerskaya TA, Chernysheva EK. 1982. Relationship between cyclic AMP level and accumulation of carotenoid pigments in Neurospora crassa. Arch. Microbiol. 133:206–208 [Google Scholar]
- 34.Krystofova S, Borkovich KA. 2005. The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gbetagamma dimer required for normal female fertility, asexual development, and Gα protein levels in Neurospora crassa. Eukaryot. Cell 4:365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Borkovich KA. 2006. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot. Cell 5:1287–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Wright SJ, Krystofova S, Park G, Borkovich KA. 2007. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61:423–452 [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Dean RA. 1997. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant Microbe Interact. 10:1075–1086 [DOI] [PubMed] [Google Scholar]
- 39.Loros JJ, Dunlap JC. 1991. Neurospora crassa clock-controlled genes are regulated at the level of transcription. Mol. Cell. Biol. 11:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loubradou G, Begueret J, Turcq B. 1999. MOD-D, a Galpha subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics 152:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mah JH, Yu JH. 2006. Upstream and downstream regulation of asexual development in Aspergillus fumigatus. Eukaryot. Cell 5:1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neves SR, Ram PT, Iyengar R. 2002. G protein pathways. Science 296:1636–1639 [DOI] [PubMed] [Google Scholar]
- 43.Pandey A, Roca MG, Read ND, Glass NL. 2004. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park G, Pan S, Borkovich KA. 2008. Mitogen-activated protein kinase cascade required for regulation of development and secondary metabolism in Neurospora crassa. Eukaryot. Cell 7:2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkins D. 1985. Advantages of using the inactive-mating-type am1 strain as a helper component in heterokaryons. Neurospora Newsl. 31:41–42 [Google Scholar]
- 46.Raju NB. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96:241–262 [Google Scholar]
- 47.Regenfelder E, et al. 1997. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 16:1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha-Ramirez V, Omero C, Chet I, Horwitz BA, Herrera-Estrella A. 2002. Trichoderma atroviride G-protein α-subunit gene tga1 is involved in mycoparasitic coiling and conidiation. Eukaryot. Cell 1:594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosen S, Yu JH, Adams TH. 1999. The Aspergillus nidulans sfaD gene encodes a G protein beta subunit that is required for normal growth and repression of sporulation. EMBO J. 18:5592–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg G, Pall ML. 1979. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J. Bacteriol. 137:1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg GB, Pall ML. 1983. Characterization of an ATP-Mg2+-dependent guanine nucleotide-stimulated adenylate cyclase from Neurospora crassa. Arch. Biochem. Biophys. 221:243–253 [DOI] [PubMed] [Google Scholar]
- 52.Springer ML. 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays 15:365–374 [DOI] [PubMed] [Google Scholar]
- 53.Turner GE, Borkovich KA. 1993. Identification of a G protein α subunit from Neurospora crassa that is a member of the Gi family. J. Biol. Chem. 268:14805–14811 [PubMed] [Google Scholar]
- 54.Vogel HJ. 1964. Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat. 98:435–446 [Google Scholar]
- 55.Wang Y, Marotti LA, Jr, Lee MJ, Dohlman HG. 2005. Differential regulation of G protein α subunit trafficking by mono- and polyubiquitination. J. Biol. Chem. 280:284–291 [DOI] [PubMed] [Google Scholar]
- 56.Westergaard M, Mitchell HK. 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573–577 [Google Scholar]
- 57.Wright SJ, Inchausti R, Eaton CJ, Krystofova S, Borkovich KA. 2011. RIC8 is a guanine-nucleotide exchange factor for Gα subunits that regulates growth and development in Neurospora crassa. Genetics 189:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu BE, Kurjan J. 1997. Evidence that mating by the Saccharomyces cerevisiae gpa1Val50 mutant occurs through the default mating pathway and a suggestion of a role for ubiquitin-mediated proteolysis. Mol. Biol. Cell 8:1649–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q, Borkovich KA. 1999. Mutational activation of a Gαi causes uncontrolled proliferation of aerial hyphae and increased sensitivity to heat and oxidative stress in Neurospora crassa. Genetics 151:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Q, Poole SI, Borkovich KA. 2002. A G-protein beta subunit required for sexual and vegetative development and maintenance of normal Gα protein levels in Neurospora crassa. Eukaryot. Cell 1:378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu JH, Wieser J, Adams TH. 1996. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 15:5184–5190 [PMC free article] [PubMed] [Google Scholar]
- 62.Zaman S, Lippman SI, Zhao X, Broach JR. 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42:27–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.