Abstract
Cryptosporidium parvum is a zoonotic protozoan parasite that causes cryptosporidiosis, an infectious diarrheal disease primarily affecting humans and neonatal ruminants. Understanding the transmission dynamics of C. parvum, particularly the specific contributions of zoonotic and anthroponotic transmission, is critical to the control of this pathogen. This study used a population genetics approach to better understand the transmission of C. parvum in the Upper Midwest United States. A total of 254 C. parvum isolates from cases of human cryptosporidiosis in Minnesota and Wisconsin and diarrheic calves in Minnesota, Wisconsin, and North Dakota were genotyped at eight polymorphic loci. Isolates with a complete profile from all eight loci (n = 212) were used to derive a multilocus genotype (MLT), which was used in population genetic analyses. Among the 94 MLTs identified, 60 were represented by a single isolate. Approximately 20% of isolates belonged to MLT 2, a group that included both human and cattle isolates. Population analyses revealed a predominantly panmictic population with no apparent geographic or host substructuring.
INTRODUCTION
Cryptosporidium is a protozoan parasite that causes cryptosporidiosis, a diarrheal disease that can become chronic and life threatening in immunocompromised persons. Although a number of species have been reported to cause cryptosporidiosis, C. hominis and C. parvum are responsible for most human disease (26, 27). Humans appear to be the only major host for C. hominis, and consequently transmission is almost exclusively anthroponotic. C. parvum has a broader host range that includes a number of mammalian species, particularly neonatal ruminants, in addition to humans (26, 28). Given that an infected calf can shed 107 C. parvum oocysts per gram of feces (2), the potential for zoonotic transmission is high. Interestingly, it appears that not all C. parvum subtypes are zoonotic, since human-restricted subtypes have been identified (14).
Water plays a significant role in the transmission of Cryptosporidium oocysts, and waterborne outbreaks continue to occur worldwide. Recent data from the U.S. Centers for Disease Control and Prevention show a 79.9% increase in the number of cryptosporidiosis cases throughout the United States during the period from 2006 to 2008 (30). This increase was attributed in part to a number of large recreational water outbreaks.
Minnesota and Wisconsin consistently have among the highest incidences of cryptosporidiosis in the United States (29, 30). Cryptosporidium parvum was identified as the most frequent cause of cryptosporidiosis in Wisconsin, suggesting that zoonotic transmission may predominate in the area (7). Further supporting zoonotic transmission, the GP60 (60-kDA glycoprotein) genotype of Wisconsin human isolates was previously identified in cattle from neighboring Michigan and other locations in the United States (19).
It has been suggested that the C. parvum epidemiology varies under the influence of regional transmission factors. Cryptosporidium parvum has been variously described as having a clonal, panmictic, or epidemically clonal population structure (13–15, 17, 24). A global comparison of Cryptosporidium populations indicates that the relative importance of genetic recombination varies according to prevalence, such that in countries where transmission rates are high, the opportunity for recombination between distinct genotypes is high and linkage equilibrium among markers is observed (24).
Our goal in this study was to better understand C. parvum transmission in the Upper Midwest United States. We used a population genetic approach to analyze human isolates from Minnesota and Wisconsin and bovine isolates from North Dakota, Minnesota, and Wisconsin. Our findings indicate that the C. parvum population in this region is predominantly panmictic with no apparent geographic or host substructuring.
MATERIALS AND METHODS
Isolate selection.
A total of 254 C. parvum isolates of human (n = 199) or bovine (n = 55) origin were included in this study. Human isolates were obtained from clinical cryptosporidiosis cases detected by the Minnesota Department of Health and Wisconsin State Laboratory of Hygiene during the period 2003 to 2007. Bovine isolates were obtained from clinical cryptosporidiosis cases identified by the North Dakota Veterinary Diagnostic Laboratory (ND-VDL) during the period 2004 to 2009 and directly from cattle on farms in Wisconsin in 2009. Cases identified by the ND-VDL were from North Dakota and Minnesota. Cryptosporidium parvum was identified using a nested PCR-restriction fragment length polymorphism (PCR-RFLP) approach targeting an approximately 830-bp fragment of the small subunit rRNA gene (8).
Multilocus typing.
C. parvum isolates were typed at eight micro- and minisatellite loci. Details of the amplification primers used and the chromosome on which each locus is found are provided in Table 1. With the exception of GRH, markers have been described previously (see references in Table 1). The GRH locus was identified in the present study using the software program Tandem Repeats Finder (http://tandem.bu.edu/trf/trf.html). The locus contains a six-nucleotide repeat (TTCTGA) and is part of a gene (cgd1_470) on chromosome 1 that encodes a hypothetical secreted protein. The GRH locus was amplified using a nested PCR approach. Primary and secondary PCRs were carried out in 50-μl volumes with 2.5 μl of template DNA, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate (dNTP), 25 pmol of each primer, and 1.5 U of Taq DNA polymerase (Promega, Madison, WI) in 1× PCR buffer. Amplification conditions for primary and secondary reactions consisted of an initial denaturation of 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 60°C for 45 s, and 72°C for 60 s, with a final extension of 72°C for 7 min.
Table 1.
Primers used in this study
| Name of target locus | Chromosome | Primera | Sequence | Reference |
|---|---|---|---|---|
| TP14 | 8 | For-1 | 5′-CTAACGTTCACAGCCAACAGTACC | 13 |
| Rev–1 | 5′–GTACAGCTCCTGTTCCTGTTG | |||
| For–2 | 5′–GTTCACAGCCAACAGT | |||
| Rev–2 | 5′–CATTTTGATTTTGGGAGT | |||
| CP47 | 6 | For–1 | 5′–GCTTAGATTCTGATATGGATCTAT | 9 |
| Rev–1 | 5′–AGCTTACTGGTCCTGTATCAGTT | |||
| For–2 | 5′–ACCCCAGAAGGCGGACCAAGGTT | |||
| Rev–2 | 5′–GTATCGTGGCGTTCTGAATTATCAA | |||
| MS5 | 8 | For–1 | 5′–GCTTCCTTACTATCATTTCC | 13 |
| Rev-1 | 5′-GCTTCAGGCTATGACAAA | |||
| For-2 | 5′-ATGTAGTCGTATCCGGAA | |||
| Rev-2 | 5′-GTATGCTGGGTGAATATAG | |||
| MS9 | 5 | For-1 | 5′-GGACTAGAAATAGAGCTTTGGCTGG | 14 |
| Rev-1 | 5′-GTCTGAGACAGAATCTAGGATCTAC | |||
| For-2 | 5′-ACCTGGAGTGTGATTTGG | |||
| Rev-2 | 5′-GTTCTTGTTCAAAGTCA | |||
| MSC6-7 | 6 | For-1 | 5′-ATTGAACAAACGCCGCAAATGTACA | 9 |
| Rev-1 | 5′-CGATTATCTCAATATTGGCTGTTATTGC | |||
| For-2 | 5′-GCTATTTGCTATCGTCTCACATAACT | |||
| Rev-2 | 5′-CTACTGAATCTGATCTTGCATCAAGT | |||
| DZ-HRGP | 6 | For-1 | 5′-TGGTTGAGGTTGAAGGCCCAT | 4 |
| Rev-1 | 5′-CATTTCAGCTATTTTAGCTCAACC | |||
| For-2 | 5′-CATTAATCTTTTAGCAAGAGTAGCTGA | |||
| Rev-2 | 5′-AATGCGTTAAGCCTTAAAGCTGG | |||
| GRH | 1 | For-1 | 5′-AGATCTTCAGGTGGCCATCATCCT | This study |
| Rev-1 | 5′-TTGTCTCCTTTATCGTCACCGCCT | |||
| For-2 | 5′-TCAGGTGGCCATCATCCTCTTGAA | |||
| Rev-2 | 5′-TGTGACGGAAGGTATGGCAGCAAA | |||
| GP60 | 6 | For-1 | 5′-ATAGTCTCCGCTGTATTC | 1 |
| Rev-1 | 5′-GGAAGGAACGATGTATCT | |||
| For-2 | 5′-TCCGCTGTATTCTCAGCC | |||
| Rev-2 | 5′-GAGATA TATCTTGGTGCG |
For all loci, with the exception of GP60, the forward primer in the secondary PCR was fluorescently labeled with 6-carboxyfluorescein (6-FAM) (Integrated DNA Technologies, Coralville, IA).
With the exception of GP60, all loci were typed by fragment size analysis. Secondary PCR products were 6-carboxyfluorescein (FAM) labeled, separated by capillary electrophoresis (Prism 3100 DNA analyzer; Applied Biosystems), and sized relative to carboxy-X-rhodamine (ROX)-labeled size standards using commercially available fragment-sizing software (Peak Scanner v1.0; Applied Biosystems). In samples that contained more than one peak, the highest peak was used to assign the allele.
The GP60 locus (5, 21) was typed by direct sequencing. Electrophoretically separated secondary PCR products of expected sizes were excised, purified (Wizard SV; Promega, Madison, WI), and sequenced bidirectionally using secondary PCR primers as sequencing primers. Sequences were assembled and edited using the software program SeqMan Pro (DNAStar, Madison, WI). Allele codes proposed by Sulaiman et al. (22) were adopted.
Data analysis.
Linkage analysis was performed using the LIAN software program (http://adenine.biz.fh-weihenstephan.de/cgi-bin/lian/lian.cgi.pl), which tests the null hypothesis of complete panmixia. LIAN calculates the standardized index of association (IAS) (10, 15), a value that fluctuates randomly around zero in complete panmixia but moves further from zero as linkage disequilibrium increases.
Patterns of descent among multilocus types were inferred using the BURST algorithm and visualized as an eBURST diagram (6). In the eBURST diagram, MLTs are represented by a solid circle, the diameter of which is proportional to the prevalence of the MLT in the population. MLTs differing at a single locus (single-locus variants) are connected by a line. Circles representing MLT frequencies are color coded to proportionally represent the source (human or cattle) and geographic location (North Dakota, Minnesota, or Wisconsin).
For subpopulation analysis, Nei's genetic distance and a variation of Wright's index (FST) were calculated using the software program GenAlEx 6.2 (18). Nei's genetic distance measures the genetic relatedness of populations (16). FST, which is a measure of population subdivision (25), was calculated using analysis of molecular variance (AMOVA) in the GenAlEx 6.2 program (18) with 999 permutations. An FST of zero indicates no subdivision and random mating.
Principal coordinate analysis (PCA) was used to visualize the structure of the C. parvum population based on the 8 markers described above. Pairwise distances between MLTs were calculated with GenAlEx using the simple Hamming distance, where the distance between pairs of isolates is equal to the sum of loci with a different allele. A second measure of distance, dubbed SSR, was also used. The SSR distance was calculated as the sum of squared difference between pairs of alleles. The distance matrices based on the Hamming and SSR distance were used for PCA. The two axes representing the largest fraction of variation were used for plotting the MLTs of 212 C. parvum isolates.
Nucleotide sequence accession numbers.
GP60 sequences from this study are available in GenBank under the accession numbers JX575582 to JX575601.
RESULTS
Multilocus genotyping.
A complete eight-locus genotype was obtained from 212 isolates, including 166 isolates from humans and 46 from cattle (Table 2). The greatest number of alleles was detected at the GP60 locus (22 alleles), which is consistent with the fact that GP60 amplicons were sequenced. In order of number of detected alleles, GP60 was followed by GRH (9 alleles), MS9 (8 alleles), DZ-HRGP (6 alleles), and TP14, MSC6-7, CP47, and MS5 (3 alleles each). All 22 GP60 alleles were identified in human isolates; however, only two of these alleles (IIaA15G2R1 and IIaA15G2R2) were present in cattle isolates. MS9 (6 alleles) was the most variable locus among cattle isolates.
Table 2.
Allele sizes and types identified in this study
| Locus | Allele sizes (in bp) or GP60 types (allele no. assigned in this study) |
|---|---|
| TP14 | 212 (1), 221 (3), 189 (2) |
| MS9 | 192 (1), 186 (2), 198 (3), 180 (4), 174 (5), 189 (6), 204 (7), 222 (8) |
| CP47 | 414 (1), 417 (2), 411 (3) |
| MS5 | 326 (1), 276 (2), 349 (3) |
| GRH | 342 (1), 353 (2), 359 (3), 348 (4), 336 (5), 412 (6), 418 (7), 424 (8), 388 (9), 420 (10) |
| DZ-HRGP | 572 (1), 566 (2), 597 (3), 579 (4), 501 (5), 591 (6) |
| MSC6-7 | 554 (1), 524 (2), 570 (3) |
| GP60 | IIaA15G2R1 (1), IIaA15G2R2 (2), IIaA14G1R2 (3), IIaA13G1R1 (4), IIaA17G2R1 (5), IIaA16G2R3 (6), IIaA16G3R2 (7), IIaA17G3R1 (8), IIaA16G1R1 (9), IIaA14G2R1 (10), IIaA17G2R2 (11), IIaA16G2R1 (12), IIaA18G2R1 (13), IIaA17G4R2 (14), IIaA12G1R2 (15), IIaA16G3R1 (16), IIaA19G2R1 (17), IIaA19G4R2 (18), IIaA14G1R1 (19), IIaA16G2R2 (20), IIaA18G3R2 (21), IIaA13G2R1 (22) |
Ninety-four MLTs were identified among the 212 isolates with a complete profile. Sixty MLTs were represented by a single isolate. Nine MLTs were common to humans and cattle, and these MLTs were identified in 43% (91/212) of isolates. Among cattle isolates, 70% (32/46) had MLTs that were also found in humans. MLT 2 was the most prevalent genotype in humans (16%; 27/166) and cattle (33%; 15/46).
Population analyses.
Table 3 presents data from linkage analyses performed on the overall C. parvum population (all 212 isolates from humans and cattle) and populations defined by host and geographic origin. (IAS) did not differ from zero (i.e., P value = 0.11 based on Monte Carlo test with 1,000 permutations) for the overall population, which supports the null hypothesis of panmixia. Similarly, panmixia is supported for cattle, human, and Wisconsin subpopulations (P values > 0.05; Table 3). However, evidence for linkage disequilibrium was detected in both Minnesota and North Dakota subpopulations.
Table 3.
Linkage analysis of various human and bovine Cryptosporidium parvum populationsa
| Population (nb) | IAS | P value | VD > L? | LE or LD |
|---|---|---|---|---|
| All (212) | 0.010 | 0.11 | No | LE |
| Human (166) | 0.006 | 0.22 | No | LE |
| Cattle (46) | 0.021 | 0.11 | No | LE |
| Minnesota (116) | 0.028 | 0.01 | Yes | LD |
| Minnesota, repeat MLTs counted as one (48) | −0.033 | 0.99 | No | LE |
| North Dakota (15) | 0.067 | 0.03 | Yes | LD |
| North Dakota, repeat MLTs counted as one (8) | −0.003 | 0.50 | No | LE |
| Wisconsin (81) | 0.003 | 0.37 | No | LE |
The standardized index of association (IAS) was calculated from populations with complete MLT profiles. When the variance of pairwise differences (VD) is greater than the 95% critical value for VD (L), then the population is in linkage disequilibrium (LD). Conversely, when VD is less than L, the population is in linkage equilibrium (LE).
n, no. of isolates.
The extent of geographic and host substructuring was assessed by estimating Nei's genetic distance and FST values between different subpopulations (Table 4). Wright proposed that FST values in the ranges 0.0 to 0.05, 0.05 to 0.15, 0.15 to 0.25, and >0.25 are indicative of little, moderate, great, and very great differentiation, respectively (25). Using this guideline, there is little differentiation between the Minnesota and Wisconsin subpopulations (FST = 0.024), and based on a value for Nei's distance that is close to zero (0.008), they are genetically very similar. In comparison, moderate differentiation and a relatively greater genetic distance were detected between the Minnesota and North Dakota (FST = 0.125; Nei's distance = 0.049) and Wisconsin and North Dakota (FST = 0.086; Nei's distance = 0.044) subpopulations. However, the latter comparisons should be interpreted with care considering the small size of the data set from North Dakota (n = 15). Overall, there is little geographic substructuring in the population. Similarly, the relatively low values for FST (0.067) and Nei's distance (0.026) between cattle and human subpopulations indicate a lack of host substructuring.
Table 4.
Nei's genetic distance and FST for selected subpopulations
| Subpopulations compared (na) | Nei's distance | FST (95% CIb) |
|---|---|---|
| Human (166) and cattle (46) | 0.026 | 0.067 (0.009–0.113) |
| Minnesota (116) and Wisconsin (81) | 0.008 | 0.024 (0.010–0.037) |
| Minnesota (116) and North Dakota (15) | 0.049 | 0.125 (0.019–0.271) |
| Wisconsin (81) and North Dakota (15) | 0.044 | 0.086 (0.038–0.116) |
n, no. of isolates.
CI, confidence interval.
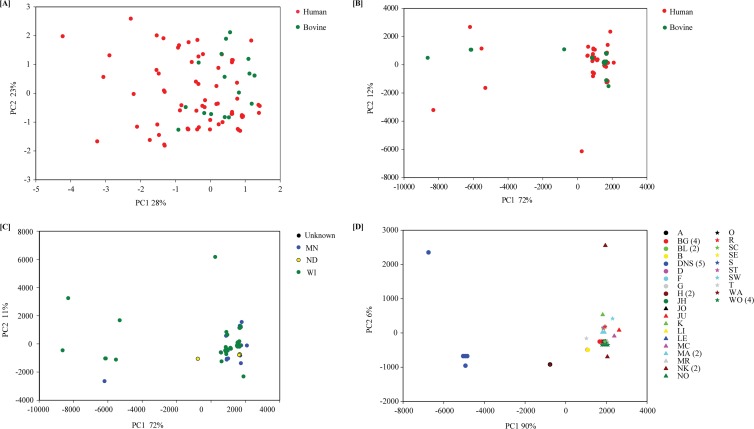
To further investigate the C. parvum population, PCA was applied to all isolates for which the 8 markers were successfully typed. PCA based on the Hamming distance and SSR distance did not reveal any segregation between C. parvum isolated from humans and that from cattle (Fig. 1A and B). Consistent with the large proportion of identical MLTs, PCA of the SSR distance (which explained more than 80% of the observed variation in SSR in components one and two) identified a large cluster of MLTs and eight genotypes that did not cluster with the majority of MLTs (Fig. 1B). No segregation according to geographic origin was apparent when MLTs were color coded according to state (Fig. 1C). We also coded MLTs according to farm, such that isolates from the same farm are displayed with the same symbol and color. As expected from previous farm-scale studies (23), most isolates originating at the same farm were identical (Fig. 1D). Interestingly, two Wisconsin farms, DNS and NK, each harbored two highly divergent MLTs, and all 5 DNS isolates were very distinct from the majority of MLTs. The origin of these divergent MLTs was not investigated further.
Fig 1.
PCA of the C. parvum population. (A) Hamming distance between MLTs defined by host. (B) SSR distance between MLTs defined by host. (C) SSR distance between MLTs defined by state. (D) SSR distance between MLTs defined by farm.
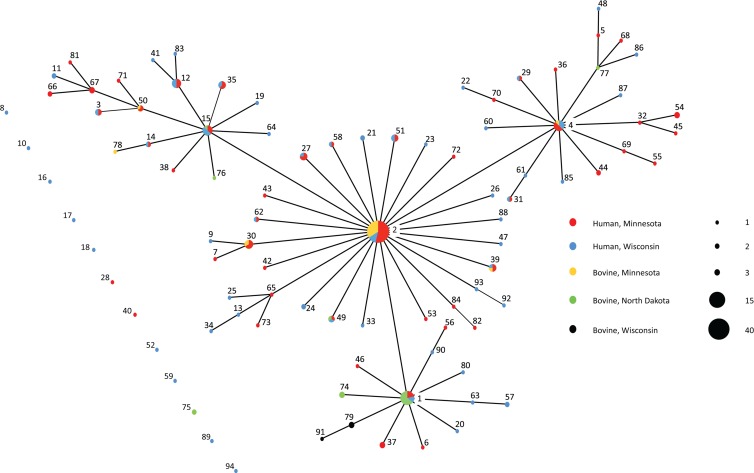
The eBURST diagram shows that MLT 2 is the predicted founder, with 100% bootstrap support, while MLTs 1, 4, and 15 are major clonal groups and single-locus variants of MLT 2 (Fig. 2). Confirming the PCA, color coding the eBURST diagram according to host and geography confirmed that most abundant MLTs were found in humans and cattle and in different states. However, there were geographic differences in the prevalences of MLT 1 and MLT 2. MLT 1 was the most prevalent genotype among isolates from North Dakota (40%; 6/15) but was less prevalent in the remaining population (4%; 8/197) (χ2 = 23.6; P < 0.001). MLT 2 was identified in 50% of isolates from Minnesota cattle but was absent in isolates from North Dakota and Wisconsin cattle. MLT 2 was also more prevalent in human isolates from Minnesota (23%; 22/94) than in those from Wisconsin (7%; 5/72) (P = 0.008). Counting MLT 1 and MLT 2 repeats as a single data point in the respective subpopulations was sufficient to change the structures from clonal to panmictic (data not shown).
Fig 2.
eBURST representation of MLT relationships.
DISCUSSION
We analyzed the population structure of C. parvum infecting humans and cattle in North Dakota, Minnesota, and Wisconsin in order to identify barriers, if any, to gene flow. Our rationale for the study was that knowledge of population structure would advance our understanding of transmission dynamics, including the extent of zoonotic transmission. This aspect of the epidemiology of cryptosporidiosis is not well understood, because with few exceptions (12), most surveys of genetic diversity have examined human or animal isolates but not both. Through linkage analysis, we found that IAS did not differ significantly from zero except for the Minnesota and North Dakota subpopulations. In complete panmixia, IAS stochastically fluctuates around zero (10). The P values we found are large and the variance of pairwise differences (VD) is consistently less than the 95% critical value of VD, which are evidence for panmixia. Panmixia may be the consequence of a high rate of C. parvum transmission, as was previously shown for Plasmodium and C. parvum populations (3, 24). Minnesota and Wisconsin consistently report among the highest incidences of human cryptosporidiosis in the United States (11, 29, 30), and we have shown previously that C. parvum predominates as a cause of cryptosporidiosis in Wisconsin (7).
Comparisons between different subpopulations revealed limited host or geographic substructuring, as might be expected in a predominantly panmictic population. The apparent absence of barriers to recombination suggests that C. parvum is transmitted freely between cattle and humans in the region. The finding that almost three quarters of cattle isolates had MLTs that were also found in humans further supports zoonotic transmission. In contrast, a study in France found little evidence for C. parvum transmission between cattle and humans. There was little overlap in MLTs between the hosts, and the population structures differed (clonal in humans and epidemically clonal in cattle) (17). In a series of studies, the C. parvum population infecting humans and cattle in Scotland was found to have a complex structure with panmictic, epidemic, and clonal subpopulations (13–15). A genetically distinct subset of C. parvum isolates in Scotland appeared to be restricted to humans, suggesting either that these isolates are circulating only in humans (anthroponotic) or that a source other than cattle was responsible for the infections (13).
Closer inspection of the Minnesota and North Dakota subpopulations in the present study suggests an epidemic outbreak of particular C. parvum genotypes passing through the subpopulations. The value for IAS does not differ from zero (and linkage equilibrium is detected) when repeat MLTs in each subpopulation are counted as a single data point in the linkage analysis, which is indicative of a clonal epidemic (20). Examining the eBURST representation of MLT frequencies and relationships, it is apparent that MLT 1 and MLT 2 are primarily responsible for the epidemic structure in North Dakota and Minnesota, respectively. However, given that the overall population is panmictic and lacks substructure, we are reluctant to conclude that these are truly epidemic subpopulations. An alternative explanation is that the observed eBURST topology is due to limited genetic diversity and a small ND sample.
In summary, data presented in this study support a predominantly randomly mating C. parvum population in the Upper Midwest United States with no apparent host or geographic substructuring. These findings are expected to advance our understanding of the complex population structures of C. parvum worldwide and the potential for zoonotic transmission in the region.
ACKNOWLEDGMENTS
J.M.M., M.E.C., E.K., and G.R.H. received funding from the USDA National Research Initiative (2008-35102-19260). J.M.M. received funding from the NIH National Institute of Allergy and Infectious Diseases (AI067284) and NIH National Center for Research Resources (2P20 RR015566). G.W. was partially supported by grant AI052781 from the NIAID. M.B. was supported by the University of Wisconsin—Eau Claire Faculty/Student Research Collaboration Program.
We are grateful to the Minnesota Department of Health, Wisconsin State Laboratory of Hygiene, and North Dakota Veterinary Diagnostic Laboratory for assistance in the collection of isolates.
Footnotes
Published ahead of print 14 September 2012
REFERENCES
- 1. Alves M, et al. 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41: 2744–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson BC. 1981. Patterns of shedding of cryptosporidial oocysts in Idaho calves. J. Am. Vet. Med. Assoc. 178: 982–984 [PubMed] [Google Scholar]
- 3. Anderson TJ, et al. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17: 1467–1482 [DOI] [PubMed] [Google Scholar]
- 4. Cama VA, Arrowood MJ, Ortega YR, Xiao L. 2006. Molecular characterization of the Cryptosporidium parvum IOWA isolate kept in different laboratories. J. Eukaryot. Microbiol. 53(Suppl 1): S40–S42 [DOI] [PubMed] [Google Scholar]
- 5. Cevallos AM, et al. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 68: 4108–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186: 1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feltus DC, et al. 2006. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 44: 4303–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng Y, et al. 2007. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 144: 1–9 [DOI] [PubMed] [Google Scholar]
- 9. Gatei W, et al. 2006. Development of a multilocus sequence typing tool for Cryptosporidium hominis J. Eukaryot. Microbiol. 53(Suppl 1): S43–S48 [DOI] [PubMed] [Google Scholar]
- 10. Haubold B, Hudson RR. 2000. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Linkage analysis. Bioinformatics 16: 847–848 [DOI] [PubMed] [Google Scholar]
- 11. Hlavsa MC, Watson JC, Beach MJ. 2005. Cryptosporidiosis surveillance—United States 1999–2002. MMWR Morb. Mortal. Wkly. Rep. 54: 1–8 [PubMed] [Google Scholar]
- 12. Learmonth JJ, Ionas G, Ebbett KA, Kwan ES. 2004. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 70: 3973–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mallon M, et al. 2003. Population structures and the role of genetic exchange in the zoonotic pathogen Cryptosporidium parvum. J. Mol. Evol. 56: 407–417 [DOI] [PubMed] [Google Scholar]
- 14. Mallon ME, MacLeod A, Wastling JM, Smith H, Tait A. 2003. Multilocus genotyping of Cryptosporidium parvum type 2: population genetics and sub-structuring. Infect. Genet. Evol. 3: 207–218 [DOI] [PubMed] [Google Scholar]
- 15. Morrison LJ, et al. 2008. The population structure of the Cryptosporidium parvum population in Scotland: a complex picture. Infect. Genet. Evol. 8: 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nei M, Roychoudhury AK. 1974. Sampling variances of heterozygosity and genetic distance. Genetics 76: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ngouanesavanh T, et al. 2006. Cryptosporidium population genetics: evidence of clonality in isolates from France and Haiti. J. Eukaryot. Microbiol. 53: S33–S36 [DOI] [PubMed] [Google Scholar]
- 18. Peakall R, Smouse PE. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6: 288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng MM, et al. 2003. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: implications for understanding the transmission dynamics. Parasitol. Res. 90: 175–180 [DOI] [PubMed] [Google Scholar]
- 20. Smith JM, Smith NH, O'Rourke M, Spratt BG. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. U. S. A. 90: 4384–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strong WB, Gut J, Nelson RG. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68: 4117–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sulaiman IM, et al. 2005. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 43: 2805–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanriverdi S, Blain JC, Deng B, Ferdig MT, Widmer G. 2007. Genetic crosses in the apicomplexan parasite Cryptosporidium parvum define recombination parameters. Mol. Microbiol. 63: 1432–1439 [DOI] [PubMed] [Google Scholar]
- 24. Tanriverdi S, et al. 2008. Inferences about the global population structures of Cryptosporidium parvum and Cryptosporidium hominis. Appl. Environ. Microbiol. 74: 7227–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright S. 1978. Evolution and the genetics of populations: variability within and among natural populations, vol 4 University of Chicago Press, Chicago, IL [Google Scholar]
- 26. Xiao L. 2010. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 124: 80–89 [DOI] [PubMed] [Google Scholar]
- 27. Xiao L, Ryan UM. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17: 483–490 [DOI] [PubMed] [Google Scholar]
- 28. Xiao L, Zhou L, Santin M, Yang W, Fayer R. 2007. Distribution of Cryptosporidium parvum subtypes in calves in eastern United States. Parasitol. Res. 100: 701–706 [DOI] [PubMed] [Google Scholar]
- 29. Yoder JS, Beach MJ. 2007. Cryptosporidiosis surveillance—United States, 2003–2005. MMWR CDC Surveill. Summ. 56: 1–10 [PubMed] [Google Scholar]
- 30. Yoder JS, Harral C, Beach MJ. 2010. Cryptosporidiosis surveillance—United States, 2006–2008. MMWR. Surveill. Summ. 59: 1–14 [PubMed] [Google Scholar]