Abstract
The fungal pathogen Cryptococcus neoformans can grow as a biofilm on a range of synthetic and prosthetic materials. Cryptococcal biofilm formation can complicate the placement of shunts used to relieve increased intracranial pressure in cryptococcal meningitis and can serve as a nidus for chronic infection. Biofilms are generally advantageous to pathogens in vivo, as they can confer resistance to antimicrobial compounds, including fluconazole and voriconazole in the case of C. neoformans. EDTA can inhibit biofilm formation by several microbes and enhances the susceptibility of biofilms to antifungal drugs. In this study, we evaluated the effect of sublethal concentrations of EDTA on the growth of cryptococcal biofilms. EDTA inhibited biofilm growth by C. neoformans, and the inhibition could be reversed by the addition of magnesium or calcium, implying that the inhibitory effect was by divalent cation starvation. EDTA also reduced the amount of the capsular polysaccharide glucuronoxylomannan shed into the biofilm matrix and decreased vesicular secretion from the cell, thus providing a potential mechanism for the inhibitory effect of this cation-chelating compound. Our data imply that the growth of C. neoformans biofilms requires the presence of divalent metals in the growth medium and suggest that cations are required for the export of materials needed for biofilm formation, possibly including extracellular vesicles.
INTRODUCTION
The fungus Cryptococcus neoformans is a major problem in immunocompromised individuals, specifically AIDS patients. Although usually contained within the lungs of immunocompetent individuals, in the setting of immune impairment, the fungus can disseminate to other organs such as the brain, where it may manifest itself as cryptococcal meningitis. Cryptococcal infections are notorious for their chronicity and the difficulty in eradicating them with antifungal drugs. The pathogenesis of cryptococcal infections involves the shedding of large amounts of the capsular polysaccharide glucuronoxylomannan (GXM) into tissue and body fluids, where it causes many deleterious effects on host immune responses (4, 6, 29).
Many pathogens, including a wide range of bacteria and fungi, have the ability to grow as biofilms (8, 10, 16), communities of microbes that attach to and colonize surfaces and are surrounded by an extracellular matrix often consisting of polysaccharide. Biofilms have the ability to form on a wide range of prosthetic materials, such as catheters, dentures, and shunts (1, 7, 23), and C. neoformans has been reported to form biofilms on ventroatrial shunts, constituting a threat to patients with drainage devices used for the treatment of the elevated intracerebral pressure, often associated with lethal cryptococcosis (31). Biofilms are advantageous to microbes in vivo, as they exhibit increased resistance to a range of antimicrobial drugs and peptides and the host immune system, compared to their planktonic cells (3, 11, 28), a property that makes the pathogen harder to kill and thus provides the opportunity for development into a chronic infection. Growth as biofilms provides Cryptococcus resistance to a range of antimicrobial drugs and molecules, including fluconazole and voriconazole (13, 15). Amphotericin B and caspofungin inhibit biofilm growth, but this inhibition is less than that observed with planktonic cryptococcal cells (15). Consequently, it is important to understand cryptococcal biofilm formation and to identify potential strategies for inhibiting this process. The divalent cation chelator EDTA was previously shown, when used either alone or in combination with other drugs, to reduce biofilm colonization by a range of bacterial and fungal pathogens, including candidal and staphylococcal species (2, 9, 20, 22, 25). Indeed, when used in combination with minocycline or minocycline plus ethanol, biofilms were completely eradicated, with no return of pathogen growth when used with the latter (21, 22).
In this study, we evaluated the effect of cation chelators on C. neoformans biofilms. The study of the effects of the chelator EDTA on C. neoformans was motivated by our initial findings and by the previously reported observation that divalent cations are essential for capsular architecture (17). Our choice of EDTA as an antibiofilm agent is strengthened by studies demonstrating the prevention of biofilm growth by EDTA in both bacteria and fungi. Hence, we reasoned that cations could also be essential for cryptococcal biofilm formation. This study shows that the chelator EDTA inhibits the growth of C. neoformans biofilms and implicates divalent cations in cryptococcal biofilm formation. We propose that the chelator may reduce the secretion rate of polysaccharide-containing vesicles, thus interrupting normal biofilm formation.
MATERIALS AND METHODS
Strains and growth conditions.
C. neoformans var. grubii strain H99 was obtained from Mauricio del Poeta (Charleston, SC) and was used in all experiments. C. neoformans cultures were grown in Sabouraud dextrose broth (SDB) at 30°C with shaking at 150 rpm. Cells were killed by heating in a 65°C water bath for 60 min, where required. Biofilm formation was induced by incubating cells in inducing medium (10% SDB diluted in 50 mM MOPS [morpholinepropanesulfonic acid] [pH 7.5]) at 37°C on the desired surface with no shaking.
Chemicals.
All chemicals were obtained from Sigma-Aldrich and were prepared as follows. EDTA, EGTA, deferoxamine mesylate salt (DFO), and triethylenetetramine (TETA) were dissolved in double-distilled water (ddH2O). 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) was dissolved to 50 mg/ml in 0.2 M sodium bicarbonate. Biofilm-inducing medium was incubated overnight with Chelex 100 resin (Bio-Rad) to remove divalent cations and subsequently filtered to remove the remaining resin.
XTT reduction assay.
Sterile 96-well plates were coated with 100 μl monoclonal antibody (MAb) 18B7 (10 μg/ml), as previously described (26), and incubated for 1 h at room temperature. Wells were subsequently washed three times with sterile phosphate-buffered saline (PBS). C. neoformans cells were harvested by centrifugation at 10,600 × g, washed 3 times with PBS, and resuspended to a concentration of 1 × 107 cells/ml in inducing medium, and a total of 100 μl of the cell suspension was added to the appropriate wells, in triplicate. As negative controls, wells containing heat-killed C. neoformans cells or medium only were included. Plates were incubated at 37°C for the durations described in Results. After incubation, wells were washed 3 times with PBS to remove any unbound planktonic cells; 200 μl fresh inducing medium containing various concentrations of EDTA (0 to 250 mM), EGTA (0 to 25 mM), BAPTA (0 to 25 mM), DFO (0 to 25 mM), or TETA (0 to 25 mM) was added; and the cells were incubated for an additional 24 h. Except for the highest concentration of EDTA used (250 mM), the addition of this chelator had no effect on the pH of the fungal medium. When required, the inducing medium also contained 0.01 mM to 1 M magnesium chloride and/or calcium chloride in addition to EDTA. When necessary, Chelex-treated inducing medium containing exogenously supplemented magnesium chloride, calcium chloride, or zinc chloride (0.01 mM to 1 M) was added to preformed (7 h) biofilms, and the mixtures were subsequently incubated at 37°C for 24 h. A semiquantitative measurement of biofilm formation was obtained by using a 2,3,-bis[2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium-hydroxide (XTT) reduction assay. Fifty microliters of XTT salt solution (1 mg/ml in PBS) and 4 μl menadione (1 mM in acetone) were added to each well. Plates were incubated at 37°C for 1 h, and the colorimetric change was measured by recording the absorbance at 492 nm.
Addition of antifungals to EDTA-treated biofilms.
Multiwell plates were coated with MAb 18B7 as described above. C. neoformans biofilms were washed, as described above, and resuspended to 1 × 107 cells/ml in biofilm-inducing medium containing 0.025 mM EDTA and either fluconazole (4 to 32 μg/ml) or voriconazole (2 to 64 μg/ml). Samples were incubated for 24 h at 37°C, washed, and quantified by using an XTT assay. Wells that contained C. neoformans in inducing medium supplemented with either antifungal drug, but containing no EDTA, were also included.
Time-lapse microscopy.
MatTek plates were coated with MAb 18B7 (10 μg/ml), as described above. A total of 2 × 105 C. neoformans cells were added to the plates in 2 ml inducing medium and grown at 37°C for 9 h. Fresh inducing medium containing 0.025 mM EDTA was then added, and incubation was resumed for another 24 h, after which point wells were washed and new EDTA-free inducing medium was added for an additional 24 h. An untreated control was included. Live imaging was performed by using a 10× objective, with images being taken at 4-min intervals. An Axiovert 200 M inverted microscope with a Hamamatsu Orca ER cooled charge-coupled-device (CCD) camera was used and controlled by Axio Vision 4.6 software (Carl Zeiss Micro Imaging, New York, NY).
Spot enzyme-linked immunosorbent assay (ELISA).
C. neoformans cells were harvested as described above and resuspended in either inducing medium or inducing medium containing 0.025 mM EDTA. A total of 500 cells in 200 μl medium were added to the wells of a sterile 96-well plate, in triplicate under each condition, and incubated at 37°C for 24 h. Heat-killed cells were also included as a negative control. After incubation, wells were washed 3 times with PBS and blocked by using 100 μl PBS containing 1% bovine serum albumin (PBS-B) at 4°C overnight or at 37°C for 1 h. Subsequent washing steps were performed in triplicate with PBS, and incubations were done at 37°C for 1 h. After washing, 50 μl of PBS containing 10 μg/ml secondary MAb 2D10 (GXM-specific immunoglobulin M [IgM]) was added to each well, and the wells were incubated and then washed. Subsequently, 50 μl of 10 μg/ml biotin-labeled goat anti-mouse IgM in PBS-B was added, followed by 1 h of incubation and a washing step and the addition of 50 μl Vectastain ABC mix (Vector Laboratories, CA), which was followed by another incubation at room temperature for 30 min. Fifty microliters of 1 mg of 5-bromo-4-chloro-3-indolyl phosphate (BCIP; Amresco, Solon, OH) per ml in AMP buffer (0.2 g MgCl2 · 6H2O, 0.1 ml Triton X-405, and 95.8 ml 2-amino-2-methyl-1-propanol in 800 ml distilled H2O [pH 9.8]) was added to each well, and the mixture was incubated at room temperature for up to 3 h. Plates were subsequently washed in triplicate with PBS and once with distilled water. Spots were imaged by using an enzyme-linked immunosorbent spot (ELISPOT) plate reader (AID GmbH).
Vesicle isolation.
Planktonic C. neoformans cultures were grown to the mid-log phase in 15 ml minimal medium (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, 3 μM thiamine-HCl [pH 5.5]) and pulsed with 2 μCi [1-14C]palmitic acid for 4 h. Cells were collected by centrifugation at 2,500 × g, washed with 5 ml deionized water, and resuspended in 15 ml cold minimal medium. After 72 h of incubation at 30°C, vesicles were purified by using the following protocol: cells were removed through two successive centrifugations at 2,500 × g, followed by filtration through an 0.8-μm-pore-size filter. The cell-free supernatant was concentrated by using centrifugal filtration units with a 100-kDa cutoff (Millipore). The concentrate was then centrifuged at 100,000 × g at 4°C for 1 h. The high-speed pellet was washed with 1 ml PBS, centrifuged at high speed again, and resuspended in 0.25 ml PBS. Samples of the cell pellet, flowthrough, concentrate supernatant, and concentrate pellet were taken to trace all radioactivity within the sample. The radioactive signal was read by using a Packard Bioscience Tricarb A2900 liquid scintillation counter. Vesicles, found in the high-speed pellet, are represented as a percentage of the radioactive signal of the total sample radioactivity.
Urease activity.
Planktonic C. neoformans cells were grown at 30°C for 24 h in either SDB containing 0.025 mM EDTA or medium alone. Cells were then washed and resuspended to 1.5 × 107 cells/ml in PBS, and 1 ml of this suspension was added to 1 ml 2× Roberts urea broth (4 g urea, 0.02 g yeast extract, 0.002 g phenol red, 0.273 g KH2PO4, and 0.285 g Na2HPO4 in 100 ml distilled water [dH2O]). Cells were incubated at 37°C with shaking for 6 h, after which the absorbance of the samples was measured at 560 nm.
Induction of capsule growth.
Planktonic C. neoformans cells were inoculated into 5 ml capsule-inducing medium (Dulbecco's modified Eagle's medium [DMEM] with 1% NCTC-109 medium [Life Technologies] and 10% inactivated fetal bovine serum) and incubated at 37°C with 10% CO2 for 48 h, according to established protocols for capsule induction (33). Capsule-inducing medium was supplemented with 0.025 mM EDTA both at the time of inoculation and 24 h later. Capsule size was measured with a 40× bright-field objective.
Statistical analysis.
GraphPad Prism software (version 5.0a) was used for statistical analysis.
RESULTS
Divalent Mg2+ and Ca2+ ions are required for biofilm formation by C. neoformans.
XTT assays were performed on cells that had been incubated with a variety of divalent cation chelators, namely, EGTA and EDTA, which chelate predominantly Mg2+ and Ca2+, and DFO and TETA, which chelate Fe2+ and Cu2+, respectively (Fig. 1). We observed similar concentration-dependent decreases in biofilm formation when either EDTA or EGTA was used, with the onset of effects at micromolar concentrations, and noted that cation chelation using EDTA reduced biofilm growth more efficiently than did cation chelation using EGTA. DFO had no effect on biofilm growth, whereas TETA reduced growth only at millimolar concentrations (2.5 and 25 mM).
Fig 1.
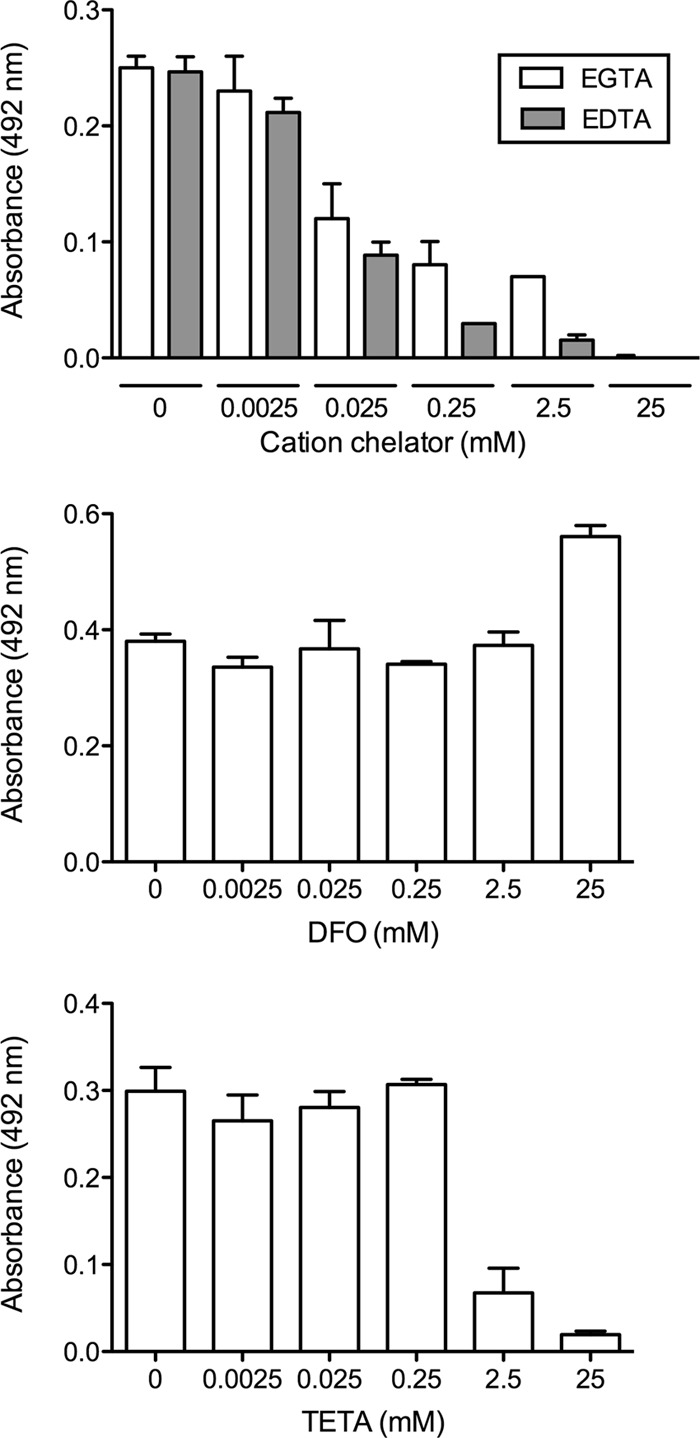
Biofilm formation measured by XTT reduction assays. C. neoformans biofilms were grown in biofilm-inducing medium containing one of the following cation chelators at concentrations of 0 to 25 mM (with major target cations in parentheses): EDTA (Ca2+ and Mg2+), EGTA (Ca2+ and Mg2+), DFO (Fe2+), or TETA (Cu2+). Cells were incubated with the chelator for 24 h, and XTT assays were subsequently performed.
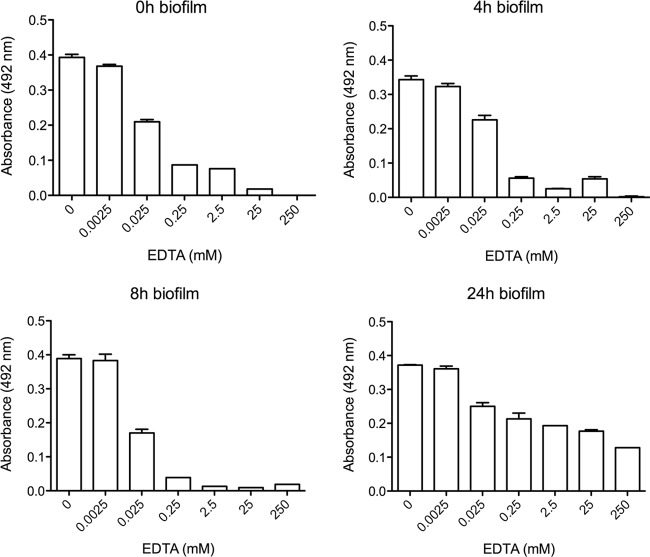
C. neoformans was then grown under biofilm-forming conditions for 0, 4, 8, and 24 h, after which EDTA (0 to 250 mM) was added and the plates were incubated for an additional 24 h (Fig. 2). EDTA concentrations of 0.0025 mM or lower had no effect on biofilm formation, as determined by XTT viability assays, whereas concentrations ranging between 0.025 mM and 250 mM reduced biofilm growth in a concentration-dependent manner, compared to the growth in untreated wells. EDTA concentrations of 0.025 mM and higher all had similar effects on growth when added to immature and intermediate (0-, 4-, and 8-h) biofilms. Similar concentrations also reduced the growth of mature (24-h) communities although to a lesser extent than when the chelator was added at earlier time points. Based on the results shown in Fig. 1 and 2 and the results described below, we used 0.025 mM EDTA to treat cells in subsequent experiments, as it decreased the rate of biofilm formation, while the cells within the biofilm community remained viable.
Fig 2.
EDTA affects various stages of biofilm development. Biofilms were grown in inducing medium for 0 h, 4 h, 8 h, or 24 h. After this time, the medium was removed and replaced with fresh inducing medium containing various concentrations of EDTA (0 to 250 mM). After an additional 24 h of incubation, cell viability was measured by an XTT assay. Measurements were performed in triplicate under each condition, and error bars represent standard deviations.
Removal of EDTA from cells, and saturation of EDTA with Mg2+ and Ca2+ ions, resumes normal biofilm growth.
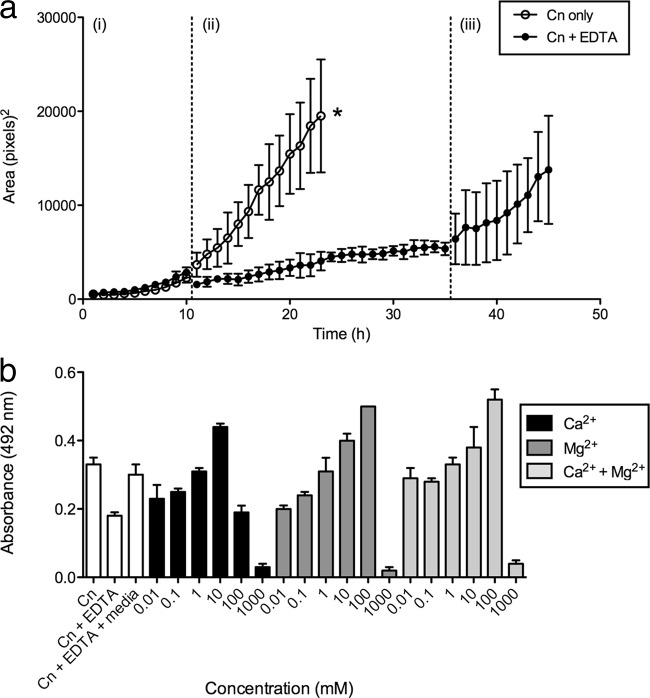
Next, we investigated whether the effect of EDTA was attributed directly to the molecule or caused by the EDTA-mediated depletion of divalent cations. We first studied the consequences of the removal of EDTA on biofilm formation by using time-lapse microscopy to monitor biofilm formation (Fig. 3a). Two independent sets of biofilms were grown in inducing medium for 9 h, which exhibited almost identical growth patterns during this time (Fig. 3ai). Medium alone or medium containing 0.025 mM EDTA was then added to one of the biofilm sets, and the growth of each set was recorded for another 24 h (Fig. 3aii). When medium alone was added (Fig. 3a, open circles), biofilm growth continued to increase steadily. However, when EDTA was added (Fig. 3a, closed circles), there was a visible reduction in biofilm formation, as determined by microcolony sizes. The biofilms were then thoroughly washed to remove EDTA, fresh EDTA-free inducing medium was added, and the plates were incubated for an additional 9 h (Fig. 3aiii). Upon the removal of EDTA, normal biofilm growth quickly resumed, as shown by the increase in microcolony sizes, indicating that the removal of the cation chelator can reverse its effects and also that EDTA had not killed the cells within the biofilm.
Fig 3.
Removal of EDTA or EDTA saturation with divalent cations resumes biofilm growth. (a) Measurement of biofilm growth by time-lapse microscopy. (i) Two independent sets of C. neoformans biofilms were grown in inducing medium for 9 h. (ii) Cells were subsequently incubated with medium containing 0.025 mM EDTA or medium alone for an additional 24 h. (iii) Cells were thoroughly washed to remove EDTA and grown again in EDTA-free inducing medium for another 9 h. Biofilm formation was recorded by using time-lapse microscopy, the areas of three biofilm microcolonies were measured, and the averages were plotted (error bars indicate standard errors of the means). The asterisk indicates that untreated cells had grown to confluence and, therefore, that individual microcolonies could not be measured after this stage. (b) Measurement of biofilm growth by XTT assays. Biofilms were grown for 9 h in inducing medium and then incubated with 0.025 mM EDTA for 24 h. Various concentrations of Mg2+ and/or Ca2+ (0 to 1 M) were added to fresh EDTA-containing inducing medium, and cells were incubated for an additional 24 h. XTT viability assays were used to measure biofilm growth. The averages of data from three wells were plotted, and standard deviations are shown. Controls consisting of untreated biofilms (C. neoformans only [Cn]), biofilms treated with 0.025 mM EDTA (Cn + EDTA), and EDTA-treated biofilms washed and subsequently grown in EDTA-free medium (Cn + EDTA + media) were included.
We then examined the effect of the saturation of EDTA by adding an excess of cations (Fig. 3b). We treated 9-h-old biofilms with 0.025 mM EDTA for 24 h, after which calcium chloride or magnesium chloride (0.01 mM to 1 M) was added for an additional 24 h. Compared to control wells not given exogenous cations, there was a concentration-dependent increase in biofilm growth with the addition of either magnesium or calcium, suggesting that these cations were able to saturate EDTA and thus prevent its effect on biofilm formation. Growth rates appeared to improve when cations were added up to a point where very high concentrations of either cation became toxic to the cells. The addition of both cations did not appear to have a significant synergistic effect on the growth rate. A second set of controls was included, where EDTA-treated samples were washed and the medium was replaced with EDTA-free inducing medium for 24 h. In these cases, biofilm formation also resumed, up to levels comparable to those in untreated cells, again suggesting that the cation at this concentration was not toxic to the cells. We additionally noted that at higher concentrations of cations (10 to 100 mM), growth rates actually exceeded those seen for the control, suggesting that the cations may promote biofilm formation. To confirm that Mg2+ and Ca2+ ions promote biofilm formation, we removed all divalent cations from biofilm-inducing medium using Chelex resin. We added increasing concentrations of magnesium chloride, calcium chloride, or zinc chloride (0.01 mM to 1 M) to the chelated inducing medium. Both magnesium chloride and calcium chloride promoted growth, but there was no significant effect of the addition of zinc chloride (data not shown).
The addition of EDTA does not make biofilms more susceptible to antifungal drugs.
As expected, when fluconazole (4, 16, and 32 μg/ml) or voriconazole (2, 8, and 64 μg/ml) was added to cells, the cells formed biofilms to the same extent as untreated cells, as determined by an XTT assay (see Fig. S1 in the supplemental material), thus confirming that C. neoformans cells within biofilms are resistant to antifungal drugs. When we added these two drugs at the above-mentioned concentrations to biofilms that were treated with 0.025 mM EDTA, we did not observe any synergistic effect on biofilm development compared to the biofilm development in cells that were grown in the presence of EDTA alone, suggesting that EDTA does not make cryptococcal biofilms more susceptible to antifungal therapy.
EDTA reduces polysaccharide shedding into the biofilm extracellular matrix and increases soluble GXM concentrations.
To determine how EDTA prevents biofilm formation, we studied the effect of the chelator on the shedding of the polysaccharide GXM into the extracellular matrix by using spot ELISAs (Fig. 4). C. neoformans biofilms were grown in 96-well plates for 24 h in biofilm-inducing medium with or without 0.025 mM EDTA. In the presence of EDTA, there was a significant reduction in the quantity of GXM shed relative to that in untreated cells, as measured by a reduction in the spot area labeled with GXM-binding MAb, suggesting that EDTA affects the ability of the biofilm matrix to retain GXM. Heat-killed cells did not attach to the polystyrene support, consistent with the need to establish a matrix for attachment.
Fig 4.
EDTA affects GXM polysaccharide shedding. GXM polysaccharide shedding was determined by spot ELISAs. Biofilm formation was induced in both the presence and absence of 0.025 mM EDTA, and cells were grown for 24 h. A colorimetric spot ELISA that stains GXM polysaccharide blue was used to visualize GXM shedding. Spot areas were measured, and bars represent the averages of data for 46 microcolonies, with the standard errors of the means indicated. The asterisk indicates that the number of heat-killed C. neoformans (HK Cn) cells bound to the well surface was zero.
EDTA prevents extracellular vesicle transport in C. neoformans.
Planktonic C. neoformans cells were pulse-chased with radioactively labeled palmitic acid and incubated in either inducing medium alone or inducing medium containing 0.025 mM EDTA. After 72 h of incubation, cells were removed by centrifugation, and vesicles were collected by supernatant concentration and ultracentrifugation. The radioactive signal from all sample fractions was measured, and vesicle-associated radioactivity is presented as a percentage of the total sample radioactivity (Fig. 5a). In samples incubated without EDTA, the vesicles comprised 0.26% of the total sample radioactivity, consistent with the range of previously observed results (our unpublished data). In samples incubated with EDTA, the vesicles comprised 0.01% of the total sample radioactivity, representing a significant reduction in the vesicle-associated radioactive signal (P < 0.02), suggesting that EDTA affects vesicle secretion in C. neoformans. We ruled out an effect of EDTA on vesicle stability, as purified radiolabeled vesicles incubated with either PBS or EDTA remained pelletable upon ultracentrifugation, while vesicles incubated with 10% SDS did not (data not shown).
Fig 5.
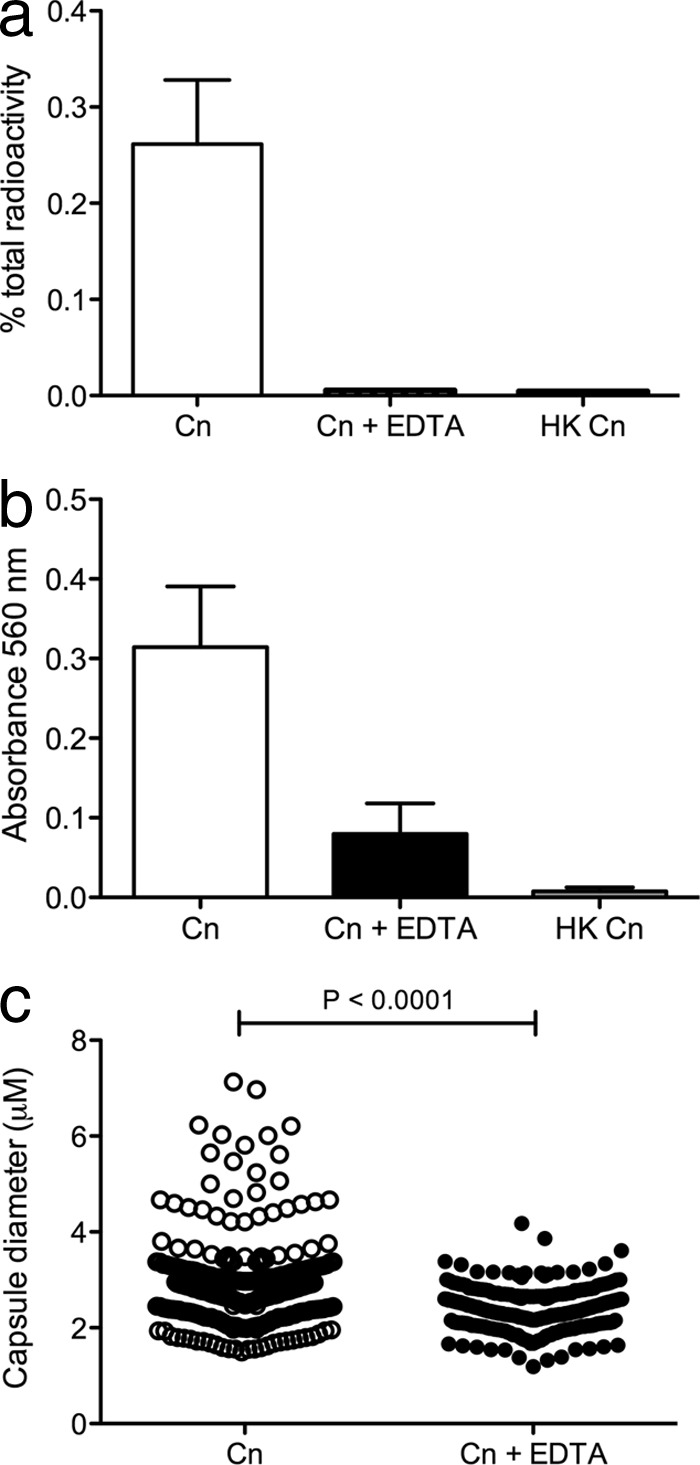
Radioactive labeling of membranes to measure vesicle secretion suggests that EDTA affects processes dependent on vesicle secretion. (a) Planktonic cultures of C. neoformans were pulse-chased with [1-14C]palmitic acid and incubated in inducing medium with 0.025 mM EDTA for 72 h. Both untreated and heat-killed (HK) controls were included. Vesicles were purified from cell-free supernatants by concentration and ultracentrifugation. The vesicle radioactive signal is represented as a percentage of the total sample radioactivity. Statistics were performed by using Mann-Whitney t tests, and error bars represent standard errors of the means. (b) EDTA reduces C. neoformans urease activity. Cultures of planktonic cells of C. neoformans grown overnight (with or without 0.025 mM EDTA) were added to Roberts urea broth and incubated for 6 h. Urease-positive reactions were determined to be those with an OD560 greater than 0.3. Results from three independent experiments were combined, and error bars represent standard errors of the means. (c) EDTA inhibits polysaccharide capsule growth. Planktonic cryptococcal cells were grown in capsule-inducing medium (open circles) or capsule-inducing medium containing 0.025 mM EDTA (closed circles) for 48 h, and capsule sizes were measured. The results of three individual experiments were combined (n = 300), and statistical analyses were performed by using unpaired t tests.
In addition to the secretion of GXM-containing vesicles, we analyzed the effect of EDTA on other processes that require vesicle secretion, including urease activity and capsule growth. After growth in medium containing 0.025 mM EDTA, either planktonic cells were added to Roberts urea broth and urease activity was quantified by spectrophotometry, or planktonic cells were negatively stained with India ink and their capsules were measured. We observed a reduction in urease activity when cells were incubated with EDTA (Fig. 5b): the urease activity of treated cells (mean optical density at 560 nm [OD560] of 0.08) did not completely abate to the background levels observed for the heat-killed controls (mean OD560 of 0.01), but it was reduced to less than a third of those recorded for untreated cells (mean OD560 of 0.31). It should be noted, however, that the difference between treated and untreated cells barely missed statistical significance at a level of a P value of 0.05 (P value of 0.0516). In addition, significant reductions in the capsule diameters of untreated and treated cells were observed (Fig. 5c), where the mean capsule sizes were 2.9 μm and 2.4 μm, respectively, and the largest capsule diameters were 7.1 μm and 4.2 μm, respectively.
DISCUSSION
The foundations for the experimental work reported in this paper were laid upon a number of factors: the ease and speed at which C. neoformans can form biofilms and its use as a model for studies of other characteristics of C. neoformans (26), the knowledge that divalent cations are essential for biofilm formation in other fungi and can be inhibited by the chelator EDTA (2, 20, 25), and the continued interest in GXM synthesis and secretion in C. neoformans.
Our initial question was whether EDTA inhibited cryptococcal biofilm formation and, if so, what role the divalent cations were essential for in biofilm growth. To quantify biofilm growth, metabolic activity within the biofilm was measured by using XTT reduction assays, which are known to correlate with biofilm CFU (12). Wells were washed prior to incubation with XTT, to remove planktonic cells and ensure that only cells within biofilms were assayed. Throughout the study, we used the tetrasodium salt form, as it has been shown to have a better range of activity than the disodium form of EDTA on biofilm formation in other biofilm models (19). ELISA plates were initially coated with MAb 18B7, as it was shown previously to aid in initial biofilm formation and thus provide results faster than with uncoated wells (26). We coincubated cryptococcal biofilms with a range of concentrations of EDTA and observed a concentration-dependent inhibition of growth, consistent with the trend observed for EDTA-treated Candida albicans biofilms (25). To determine whether the chelator affected certain phases of biofilm formation, we tested the effects of EDTA on biofilms at various stages of development: early (4-h) biofilms, where cells adhere to surfaces and form a monolayer of budding cells; intermediate (8-h) stages, where cell numbers increase and microcolonies are formed; and mature (24-h) biofilms, where there is a dense cell volume that is embedded in the extracellular matrix (14). The ability of EDTA to affect multiple biofilm stages suggests that its mechanism may be multifactorial, in that it prevents both attachment and subsequent growth phases of biofilm formation. It was important to ensure that although biofilm growth was impeded, the concentration of EDTA used for the remainder of the experiments (0.025 mM) did not actually kill the cells. We established this by using two different methods: time-lapse microscopy and an XTT assay. In both experiments, when EDTA was removed and fresh inducing medium was added to the cells, biofilm formation resumed, suggesting that the cells within the biofilm were still alive. This regrowth was also observed previously for EDTA-treated candidal biofilms, where the drug was removed and fresh medium was added (22, 25).
For C. albicans, treatment with EDTA prevents cell filamentation, a process that is required for biofilm formation in this pathogen (10, 24, 25). Although Cryptococcus neoformans is not a filamentous fungus, the shedding of GXM was shown previously to be essential for cryptococcal biofilm formation (14), and we hypothesized that divalent cations might be involved in this process. Indeed, spot ELISAs showed that in the presence of EDTA, less GXM was shed into the biofilm extracellular matrix, as represented by the smaller spot area. It was described previously that the aggregation of GXM and the subsequent capsule growth are also dependent on divalent cations (17), such that capsule assembly appears to involve the inclusion of divalent metals binding two GXM chains via their negatively charged GlcA units. It seems likely, therefore, that GXM is retained within the C. neoformans biofilm in a similar manner by the binding of GXM with divalent cations within the extracellular matrix; thus, the removal of cations prevents the retention of GXM within the matrix.
One mechanism for GXM secretion by C. neoformans is as cargo of vesicles that are transported to the extracellular space (27). Consequently, we hypothesized that the EDTA-dependent decrease in the amount of GXM shed was due to a decrease in vesicle secretion, and we tested this hypothesis by measuring vesicle production using a radioactively labeled palmitic acid technique that we recently described (32). It should be noted that the reason for performing this experiment, as well as the subsequent urease assays and capsule measurements, on planktonic cells is because it is technically very difficult to remove cells from biofilms due to their strong attachment to the extracellular matrix; the successful removal of cells often requires the processing of biofilms with harsh chemical or physical treatments to disassociate cells from the matrix, and we did not want to introduce these factors into this study. Three possibilities could explain the decrease in the vesicle signal with EDTA treatment. First, EDTA may somehow prevent vesicles from being physically secreted from the cell, and in this regard, it is possible that vesicle transport through the cell wall requires molecular motors that are dependent on divalent metal ions. Second, EDTA may interfere with a signal necessary to generate vesicles for secretion, although electron microscopy studies revealed vesicles at the cell perimeter, suggesting that vesicle synthesis was maintained (data not shown). Finally, EDTA may affect vesicle stability such that vesicles are secreted normally but were not detected by our assay due to lysis; however, we found no effect of EDTA on vesicle stability (data not shown). In addition, this information, combined with the observed EDTA-dependent decrease in urease activity, refutes this possibility, since a decrease in stability should affect the vesicle radioactive signal but not cargo secretion. Our data therefore favor the former possibility, that EDTA affects and blocks the physical secretion of vesicles, including those containing GXM, thus reducing biofilm formation by C. neoformans.
It was described previously that cells within C. neoformans biofilms are more resistant than planktonic cells to a range of antimicrobial drugs (15). Using XTT assays, we have shown that the addition of EDTA has no effect on the efficacy of these antimicrobials, as the rate of biofilm growth was not reduced. Our results are in contrast to what has been described for candidal species, where EDTA makes the cells within the biofilm community more susceptible to drugs such as amphotericin B and fluconazole (20, 30). It is conceivable, however, that the polysaccharide capsule surrounding the cryptococcal cell may make it less penetrable to EDTA/antifungal agents, thus helping to explain why we did not see a synergistic effect, which is in contrast to what has been observed for candidal biofilms. Despite the fact that cation chelation did not make C. neoformans biofilms more sensitive to antimicrobials, the use of EDTA to slow down biofilm formation may still be of medical interest: previous reports described that the use of EDTA in combination with other antimicrobial agents was effective in catheter antimicrobial lock solutions (5, 19, 22), and it is conceivable that it may find a similar use in the management of Cryptococcus-infected shunts. In addition, antibiotic-impregnated shunts were shown to decrease the incidence of cerebrospinal fluid shunt infections in hydrocephalus patients (18), although it should be noted that EDTA treatment was not included in these studies. It is therefore feasible that EDTA impregnation could be utilized for ventroatrial shunts or lumbar drains used for cryptococcosis patients.
In conclusion, the cation chelator EDTA reduces biofilm formation by Cryptococcus neoformans, an effect that that appears to result from the inhibition of the secretion of GXM-containing vesicles. By blocking vesicle transportation, soluble GXM is not deposited into the extracellular matrix, a step that is essential for C. neoformans biofilm formation; thus, the biofilm does not form. In addition to highlighting the role of divalent cations in the processes of extracellular vesicle secretion and biofilm formation, our study again emphasizes the benefits of the use of biofilms as tools to study other characteristics of C. neoformans.
Supplementary Material
Footnotes
Published ahead of print 31 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Bosio S, et al. 2012. Mycobacterium fortuitum prosthetic valve endocarditis: a case for the pathogenetic role of biofilms. Cardiovasc. Pathol. 21: 361–364 [DOI] [PubMed] [Google Scholar]
- 2. Devine DA, et al. 2007. Inhibition of biofilms associated with dentures and toothbrushes by tetrasodium EDTA. J. Appl. Microbiol. 103: 2516–2524 [DOI] [PubMed] [Google Scholar]
- 3. Donlan RM. 2000. Role of biofilms in antimicrobial resistance. ASAIO J. 46: S47–S52 doi:10.1097/00002480-200011000-00037 [DOI] [PubMed] [Google Scholar]
- 4. Fries BC, et al. 2005. Phenotypic switching of Cryptococcus neoformans can produce variants that elicit increased intracranial pressure in a rat model of cryptococcal meningoencephalitis. Infect. Immun. 73: 1779–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghannoum MA, Isham N, Jacobs MR. 2011. Antimicrobial activity of B-Lock against bacterial and Candida spp. causing catheter-related bloodstream infections. Antimicrob. Agents Chemother. 55: 4430–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graybill JR, et al. 2000. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups Clin. Infect. Dis. 30: 47–54 [DOI] [PubMed] [Google Scholar]
- 7. Guggenbichler JP, Assadian O, Boeswald M, Kramer A. 2011. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials—catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg. Interdiszip. 6: Doc18 doi:10.3205/dgkh000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karunakaran E, Mukherjee J, Ramalingam B, Biggs CA. 2011. “Biofilmology”: a multidisciplinary review of the study of microbial biofilms. Appl. Microbiol. Biotechnol. 90: 1869–1881 [DOI] [PubMed] [Google Scholar]
- 9. Kite P, Eastwood K, Sugden S, Percival SL. 2004. Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J. Clin. Microbiol. 42: 3073–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez-Ribot JL. 2005. Candida albicans biofilms: more than filamentation. Curr. Biol. 15: R453–R455 doi:10.1016/j.cub.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 11. Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9: 34–39 [DOI] [PubMed] [Google Scholar]
- 12. Martinez LR, Casadevall A. 2007. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 73: 4592–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez LR, Casadevall A. 2006. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect. Immun. 74: 6118–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez LR, Casadevall A. 2005. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73: 6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez LR, Casadevall A. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50: 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moscoso M, Garcia E, Lopez R. 2009. Pneumococcal biofilms. Int. Microbiol. 12: 77–85 [PubMed] [Google Scholar]
- 17. Nimrichter L, et al. 2007. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot. Cell 6: 1400–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker SL, Anderson WN, Lilienfeld S, Megerian JT, McGirt MJ. 2011. Cerebrospinal shunt infection in patients receiving antibiotic-impregnated versus standard shunts. J. Neurosurg. Pediatr. 8: 259–265 [DOI] [PubMed] [Google Scholar]
- 19. Percival SL, et al. 2005. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect. Control Hosp. Epidemiol. 26: 515–519 [DOI] [PubMed] [Google Scholar]
- 20. Raad II, et al. 2008. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int. J. Antimicrob. Agents 32: 515–518 [DOI] [PubMed] [Google Scholar]
- 21. Raad I, et al. 2003. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother. 47: 3580–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. 2007. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramage G, Martinez JP, Lopez-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 6: 979–986 [DOI] [PubMed] [Google Scholar]
- 24. Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214: 95–100 [DOI] [PubMed] [Google Scholar]
- 25. Ramage G, Wickes BL, Lopez-Ribot JL. 2007. Inhibition on Candida albicans biofilm formation using divalent cation chelators (EDTA). Mycopathologia 164: 301–306 [DOI] [PubMed] [Google Scholar]
- 26. Robertson EJ, Casadevall A. 2009. Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation. Appl. Environ. Microbiol. 75: 2528–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodrigues ML, et al. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6: 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358: 135–138 [DOI] [PubMed] [Google Scholar]
- 29. Vecchiarelli A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38: 407–417 [DOI] [PubMed] [Google Scholar]
- 30. Venkatesh M, Rong L, Raad I, Versalovic J. 2009. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 58: 936–944 [DOI] [PubMed] [Google Scholar]
- 31. Walsh TJ, Schlegel R, Moody MM, Costerton JW, Salcman M. 1986. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery 18: 373–375 [DOI] [PubMed] [Google Scholar]
- 32. Wolf JM, Rivera J, Casadevall A. 2012. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell. Microbiol. 14: 762–773 [DOI] [PubMed] [Google Scholar]
- 33. Zaragoza O, Casadevall A. 2004. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 6: 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.