Abstract
While anthrax is typically associated with bioterrorism, in many parts of the world the anthrax bacillus (Bacillus anthracis) is endemic in soils, where it causes sporadic disease in livestock. These soils are typically rich in organic matter and calcium that promote survival of resilient B. anthracis spores. Outbreaks of anthrax tend to occur in warm weather following rains that are believed to concentrate spores in low-lying areas where runoff collects. It has been concluded that elevated spore concentrations are not the result of vegetative growth as B. anthracis competes poorly against indigenous bacteria. Here, we test an alternative hypothesis in which amoebas, common in moist soils and pools of standing water, serve as amplifiers of B. anthracis spores by enabling germination and intracellular multiplication. Under simulated environmental conditions, we show that B. anthracis germinates and multiplies within Acanthamoeba castellanii. The growth kinetics of a fully virulent B. anthracis Ames strain (containing both the pX01 and pX02 virulence plasmids) and vaccine strain Sterne (containing only pX01) inoculated as spores in coculture with A. castellanii showed a nearly 50-fold increase in spore numbers after 72 h. In contrast, the plasmidless strain 9131 showed little growth, demonstrating that plasmid pX01 is essential for growth within A. castellanii. Electron and time-lapse fluorescence microscopy revealed that spores germinate within amoebal phagosomes, vegetative bacilli undergo multiplication, and, following demise of the amoebas, bacilli sporulate in the extracellular milieu. This analysis supports our hypothesis that amoebas contribute to the persistence and amplification of B. anthracis in natural environments.
INTRODUCTION
Anthrax is a serious and often fatal disease of most warm-blooded animals caused by the spore-forming bacterium Bacillus anthracis. Human infection with B. anthracis is typically zoonotic and initiates through one of four possible routes: ingestion, cutaneous exposure, parenteral injection, or inhalation, the last being the most lethal and of greatest concern when B. anthracis is weaponized. Anthrax spores germinate and multiply within macrophages (10–12, 19). Infection of hosts is mediated in large part by factors encoded on the two large virulence plasmids pX01 (GenBank accession number NC_012579) and pX02 (GenBank accession number NC_012577). The pX01 plasmid carries genes encoding a tripartite exotoxin, among others, while the pX02 plasmid carries the genes for encapsulation (19). B. anthracis produces six different germination receptors that sense amino acids and purine nucleosides (3, 4). The germination receptors GerH (chromosomal) and GerX (encoded on pX01) have been implicated in the germination of spores interacting with macrophages (13). GerH is the sole germination receptor that is sufficient to mediate full virulence in animal models of infection (4, 30).
Where anthrax is endemic, B. anthracis spores survive best in alkaline soils that are rich in organic matter, calcium, and other minerals (14, 28). It is generally believed that spores do not germinate and multiply in soils because vegetative bacilli, especially in laboratory simulations, compete poorly with indigenous microorganisms (18, 25, 26). However, repeated samplings of soil from spore-contaminated areas indicate a progressive loss of the pX02 plasmid over 5 to 8 years (27), suggesting, at a minimum, that a limited amount of growth must take place under these conditions. The debate over environmental cycling and spore amplification is traced to the studies of Van Ness and Stein (29), which connected ambient temperatures above 15.5°C with soil alkalinity in “incubator areas,” described as depressions which collect water, dead vegetation, calcium, minerals, and salts washed from the surrounding areas following rainfall (14). Alternatively, it has been suggested that spores, due to their hydrophobicity (2), are likely carried in runoff that collects and concentrates them in depressions. In either case, livestock become infected by grazing within these indentations where the grass is both greener and teeming with spores (14).
Since anthrax outbreaks correlate with warmer temperatures, is temperature a clue to spore amplification? In this regard, warm stagnant water interacting with moist soils, high in organics, is essentially a nutrient broth teeming with diverse microbial life. These environments are also reservoirs for other human pathogens, including nontuberculous species of mycobacteria and Legionella pneumophila (8, 21), which can multiply within amoebic hosts as well as human macrophages. Since B. anthracis also infects mammalian macrophages (5, 24), we considered the possibility that soil amoebas, active in warm weather, might serve as natural hosts and thereby contribute to spore amplification and persistence. As one of the most ubiquitous organisms found in natural environments, amoebas are the main predators controlling bacterial populations as well as being natural amplifiers of L. pneumophila (7, 15, 22).
Here, we show that under conditions simulating a stagnant water/moist soil environment, B. anthracis spores indeed germinate and multiply within the free-living amoeba Acanthamoeba castellanii in a pX01-dependent manner. Our studies provide evidence to suggest that soil amoebas and perhaps other protozoa contribute to the amplification and persistence of B. anthracis spores by providing an intracellular microenvironment that permits the completion of an entire bacterial life cycle from spore germination, to vegetative bacterial replication, to sporulation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains used in this study are Sterne 7702 (BEI Resources, Manassas, VA), Sterne 7702 triple toxin knockout (TKO) (16), 9131 (6), Sterne 34F2, 34F2 ΔgerH, 34F2 ΔgerX, and Ames (BEI Resources). Red fluorescent protein (RFP)-expressing B. anthracis bacteria were built by electroporating plasmid pRP1028, a kind gift from Scott Stibitz, Roger Plaut, and Tod Merkel, into B. anthracis Sterne strain. Strains 34F2, 34F2 ΔgerH, and 34F2 ΔgerX were a kind gift from Philip Hanna (4). B. anthracis spores were generated on nutrient broth-yeast extract (NBY)-agar plates, with subsequent purification on an Omnipaque (GE Healthcare, Inc., NJ) gradient, and stored in sterile distilled deionized water, as previously described (9).
Amoeba growth conditions.
The amoeba used in this study was Acanthamoeba castellanii (ATCC 30010). The amoebas were grown in tissue culture flasks in peptone-yeast-glucose (PYG) medium (ATCC medium 712) at 25°C. The trophozoites were maintained in exponential growth phase by subculture every 3 to 4 days and then harvested by tapping the flasks; this was followed by three washes with sterile distilled water and centrifugation at 500 × g for 5 min at 25°C to remove carried-over nutrients. Experiments were carried out in 12-well tissue culture plates when biosafety level 2 (BSL2) bacteria were used and in 15-ml screw-cap tubes when BSL3 bacteria were used. Amoebas were used at a final cell concentration of 105/ml in autoclaved creek water medium (CWM) from a stream located in a small nature preserve, The Dell at the University of Virginia at Charlottesville, VA. Modified CWM (CWM+) was supplemented with 0.4 M MgSO4 · 7H2O, 0.05 M CaCl2, 0.1 M sodium citrate · 2H2O, 0.25 M Na2HPO4 · 7H2O, 0.25 M KH2PO4, and 0.1 M glucose.
Coculture of B. anthracis with A. castellanii.
Spore suspensions in sterile distilled water were used to inoculate amoeba cultures at a multiplicity of infection (MOI) of 50. Low-speed centrifugation at 500 × g for 5 min was used to initiate physical interaction between bacteria and amoebas, and then the cocultures were incubated at 30 or 37°C in an incubator with 5% CO2 in all of the experiments describe. The presence or absence of 5% CO2 was found to have no effect on the bacteria-amoeba interactions described herein. Cultures were resuspended in sterile creek water, and the concentrations of A. castellanii and B. anthracis were determined at day 0 (D0). The cocultures were then analyzed at the times indicated in the figures. The number of amoebas (with or without bacteria) was determined by counting with a hemacytometer. Simultaneously, the growth of B. anthracis was determined from aliquots of the same experimental samples by lysing amoebas by passing the sample through a 26-gauge needle five to six times or by the addition of 0.5% saponin (Spectrum Chemicals), followed by vortexing in the BSL3 experiments (to avoid the generation of aerosols caused by needle passage). The total number of B. anthracis spores was established by performing serial 10-fold dilutions with sterile H2O of the coculture medium containing lysed amoebas and bacteria that were subsequently spread in triplicate on Luria-Bertani (LB) agar (Becton, Dickinson) and incubated at 37°C overnight. The B. anthracis spore count was established by heating the above samples to 65°C for 20 min such that all vegetative bacteria were destroyed, leaving only dormant spores to form CFU on the LB agar.
Cytotoxicity of B. anthracis.
The cytotoxicity of B. anthracis strains toward amoebas was studied with phase-contrast microscopy and quantified with a hemacytometer and trypan blue (Invitrogen) staining. The influence of bacteria on amoebal monolayer formation in 12-well plates containing 105 amoebas/well infected with the bacterium at an MOI of 50 was determined from photographs taken at the times indicated in the figures.
Fluorescence microscopy-based spore internalization assay.
Determination of spore internalization by A. castellanii was performed as previously described (1, 23). Briefly, spores were labeled with sulfosuccinimidyl-6-biotinamido-hexanoate (sulfo-NHS-LC-biotin) and NHS-Alexa 488 (DyLight; Thermo Scientific) prior to the infection of amoebas. After coculture of spores with amoebas for the times indicated in the figures, streptavidin labeled with rhodamine was added such that any external spores would be labeled with rhodamine-streptavidin and Alexa 488 and those within the amoebas were labeled with only Alexa 488.
Transmission electron microscopy.
Axenic cultures of A. castellanii were infected for 6 and 12 h with spores of Sterne strain Bacillus anthracis 7702 and its derivative 9131 strain at an MOI of 50. After the medium was decanted, amoebas were fixed at room temperature with 2.5% glutaraldehyde and 4% paraformaldehyde. The cells were washed and postfixed for 40 min with 1% OsO4 in 0.15 M sodium cacodylate buffer. After dehydration in ethanol solutions (at 40°, 60°, 80°, and twice at 100°), amoebas were embedded in Epon resin. Ultrathin sections were stained with uranyl acetate followed by lead citrate and examined using a JEOL 1200 CX electron microscope at 80 kV.
Statistical analysis.
The data were analyzed with a two-tailed Student's t test (Microsoft Excel 2007), with an alpha value of 0.05, under the assumption that data follow a normal distribution and have equal variances.
RESULTS
Low-nutrient creek water, which mimics wet terrestrial environments, was used as the medium (CWM) and is permissive for analyzing B. anthracis-A. castellanii interactions but does not induce spore germination or outgrowth in the absence of amoebas. In coculture with A. castellanii and tracked by time-lapse fluorescence microscopy, spores of a red fluorescent protein-expressing attenuated BSL2 B. anthracis Sterne 7702 strain (pX01+ pX02−) germinated and multiplied within the amoebas (Fig. 1A). Optimal growth of spores derived from the B. anthracis Sterne strain occurred at 37°C in coculture with A. castellanii, which permitted bacterial numbers to increase nearly 100-fold by 72 h (Fig. 1B). Similar results were also obtained with the amoeba Hartmannella vermiformis (data not shown), indicating that the observed phenomenon was not limited to a single amoebic species.
Fig 1.
Intracellular germination and growth of B. anthracis in coculture with A. castellanii. (A) Micrograph (magnification, ×100) of A. castellanii infected with RFP-expressing Sterne 7702 spores (red) after 6 h at 37°C. rp, replicative phagosome containing vegetative B. anthracis; sp, spores; vf, vegetative form; n, nucleus. (B) Growth of B. anthracis Sterne 7702, TKO, and 9131 with or without (spores only) A. castellanii (Ac) at the indicated times at 37°C. Data are the means ± standard errors of the means (n = 4 to 6; performed in triplicate). Statistical differences were determined by Student's t test comparing the indicated strain to the Sterne strain cultured in the absence of amoebas at 72 h (**; P1 = 0.000285; P2 = 0.00105). (C) Phase-contrast micrograph (magnification, ×100) after 6 h of culture at 37°C of A. castellanii with fluorescently labeled strain 9131(red spores). p, phagosome containing B. anthracis spores; sp, spores; n, nucleus. (D) Similar to the experiment described for panel B but with B. anthracis Sterne 34F2 as the parental strain control for mutants with either GerX or GerH eliminated. Data are the means ± standard errors of the means (n = 4). Statistical differences were determined by a Student's t test (**, P < 0.001). (E) Similar to the experiment described for panel B but with the B. anthracis Ames strain with or without A. castellanii. Data are the means ± standard errors of the means (n = 4). Statistical differences were determined by a Student's t test (**, P = 0.000356).
Since B. anthracis plasmid loss has been reported to occur in the environment (27), we tested whether the pX01 plasmid was required for germination and intracellular multiplication. As seen in Fig. 1B, not only did the plasmidless strain 9131 (pX01− pX02−) fail to multiply in A. castellanii, but bacterial numbers also dropped by over 2 logs by 72 h (Fig. 1B), yielding a count significantly less than that of the Sterne strain with or without amoebas. In contrast, the growth characteristics of a mutant Sterne strain containing deletions of the three exotoxin genes on plasmid pX01 (TKO strain) in A. castellanii were indistinguishable from those of the Sterne strain (Fig. 1B). To eliminate the possibility that the 9131 strain was not ingested by A. castellanii, we used inside-outside staining with fluorescent probes to show that the amoebas ingested 9131 and Sterne 7702 spores with equal efficiencies (see Fig. S1 in the supplemental material) and that 9131 spores were plentiful within the amoebas (Fig. 1C), suggesting that other plasmid functions beyond exotoxin production are required for germination and outgrowth.
To explore a possible connection between the defects observed in strain 9131 and germination in the presence of amoebas, we next cocultured Sterne strain mutants with GerH or GerX production eliminated since these two germination receptors had been previously implicated in spore germination in the presence of macrophages (10, 13). The pX01-encoded gerX knockout strain showed no difference in growth characteristics from its parental Sterne 34F2 strain, which grew similarly to Sterne 7702, while the chromosomally encoded gerH knockout strain demonstrated delayed growth but ultimately reached similar total numbers as its parental strain (Fig. 1D). Thus, the growth defect observed for the plasmidless strain 9131 cannot be explained by the lack of GerX production from pX01, yet it is clear from the result obtained with the gerH knockout that germination plays an important role in the timing of bacterial growth in the presence of amoebas.
Since initial BSL2 experiments were conducted with the Sterne strains, which lack pX02, we addressed whether the fully virulent Ames strain behaved similarly to the Sterne strain in A. castellanii and whether the pX02 plasmid contributed to the efficiency of germination and/or intracellular multiplication. As seen in Fig. 1E, the Ames strain also germinated and grew rapidly within the amoebas, with growth peaking by 24 h, but achieved a lower growth yield (∼15-fold) than the attenuated Sterne strain. Bacteria removed from these cultures and treated with India ink demonstrated the production of capsule (data not shown). Overall, these observations imply that pX02 increases the rate of bacterial growth and thereby amoebal lysis but is not necessary for spore germination and subsequent bacterial multiplication within amoebas.
We next evaluated the efficiency of conversion of vegetative bacilli to spores by taking advantage of the heat-resistant nature of the spore. From the results depicted in Fig. 1B, D, and E representing the total bacterial count (spores plus vegetative bacteria), we determined the proportion representing spores by heating the samples such that only heat-resistant viable spores produced a CFU. Over a 72-h incubation time (Fig. 2), Sterne, TKO, and Ames strains all showed increases in the spore populations, while the population in the plasmidless 9131 strain decreased more than 2 logs in comparison to controls (spores incubated with no amoebas). Since spores represented nearly 100% of the total number of CFU for the Ames strain at 72 h, versus ∼70% for Sterne and TKO strains, the apparent increase in sporulation efficiency by the Ames strain might result from an earlier lysis of the amoebas (yielding a lower total bacterial count than the Sterne strain) and therefore a longer period outside (24 to 72 h) to complete conversion to spores. Figure 3A shows that germination and intracellular multiplication of the Sterne and TKO strains in A. castellanii result in a decrease in amoebal counts, with few amoebas observed by 24 h. As seen by microscopic examination, extracellular filaments of B. anthracis are readily observed in both the Sterne and TKO strains at this time point but not in the 9131 strain (Fig. 3A). By 12 h Sterne spores can be seen emerging from extracellular mother cells (Fig. 3B).
Fig 2.
Quantification of B. anthracis spores in coculture with A. castellanii. Spore quantification was determined by heating samples from the indicated times to 65°C for 20 min and then plating for CFU. Ac, A. castellanii. Open squares, Sterne spores only. Data are the means ± standard errors of the means (n = 4 to 6; performed in triplicate). Statistical differences were determined by a Student's t test (**, P < 0.001).
Fig 3.
Effect of B. anthracis on amoeba viability. (A) Representative phase-contrast images (Axio Imager; 63× objective) of A. castellanii cultured with or without the indicated B. anthracis strains for 24 h at 37°C. Insets are ×10 enlargements of a subsection of the larger photograph (dashed box). (B) Representative phase-contrast image (Axio Imager; 63× objective) of B. anthracis filaments undergoing sporulation after 12 h of coculture with A. castellanii at 37°C. r, spores being released from the mother cell. (C) The number of viable A. castellanii amoebas cocultured without bacteria (amoeba only) or with Sterne 7702, TKO, or 9131 at the indicated times at 37°C was quantified using a hemacytometer and trypan blue staining.
Within 24 h the number of viable A. castellanii amoebas in coculture with Sterne or TKO had dropped significantly from those of uninfected amoebas or those cocultured with 9131 (Fig. 3C). However, during this period, the amoebas are also transitioning to cyst forms as the CWM is nutrient poor. We considered the possibility that cysts would not phagocytose the spores, and thus we might be underestimating the amplification potential of the amoebic population, or, in the case of the 9131 strain, any delay in germination might be completely missed in our model. Alternatively, we considered the possibility that the observed unexpectedly high rate at which B. anthracis overcomes and destroys amoebas might be exacerbated by physiologic stress on the amoebas. To address these possibilities, we modified the CWM (referred to as CWM+) by adding calcium and other salts and glucose as the carbohydrate source, improved the buffer capacity of the medium, and lowered the growth temperature to 30°C. The CWM+ formulation greatly improved the growth of A. castellanii while remaining nonpermissive to germination or growth of Sterne B. anthracis spores. As seen in Fig. 4A and C, growth of the Sterne strain 7702 and TKO was slightly improved in the CWM+ compared with that in CWM. Even in the improved CWM+, the 9131 plasmidless strain was not amplified by A. castellanii, further supporting the role of the pX01 plasmid in germination. It is noteworthy that in both media, the Sterne strains displayed a delay in germination at 30°C that was not observed in studies performed at 37°C. When these studies were performed with the Ames strain (Fig. 4B and D), there was no delay in germination in either medium at 30°C though the growth kinetics were slightly higher in the CWM+.
Fig 4.
Intracellular germination and growth of B. anthracis in coculture with A. castellanii at 30°C and in CWM+. (A) Growth of B. anthracis Sterne 7702, TKO, and 9131 with or without A. castellanii (Ac) (spores only) at the indicated times at 30°C in standard CWM. Data are the means ± standard errors of the means for experiments performed in triplicate. (B) Growth of B. anthracis Ames with or without A. castellanii (Ac) at the indicated times at 30°C in standard CWM. Data are the means ± standard errors of the means of experiments performed in triplicate. (C) Growth of B. anthracis Sterne 7702, TKO, and 9131 with or without A. castellanii (Ac) (spores only) at the indicated times at 30°C in CWM+. Data are the means ± standard errors of the means of experiments performed in triplicate. (D) Growth of B. anthracis Ames with or without (spores only) A. castellanii (Ac) at the indicated times at 30°C in CWM+. Data are the means ± standard errors of the means of experiments performed in triplicate.
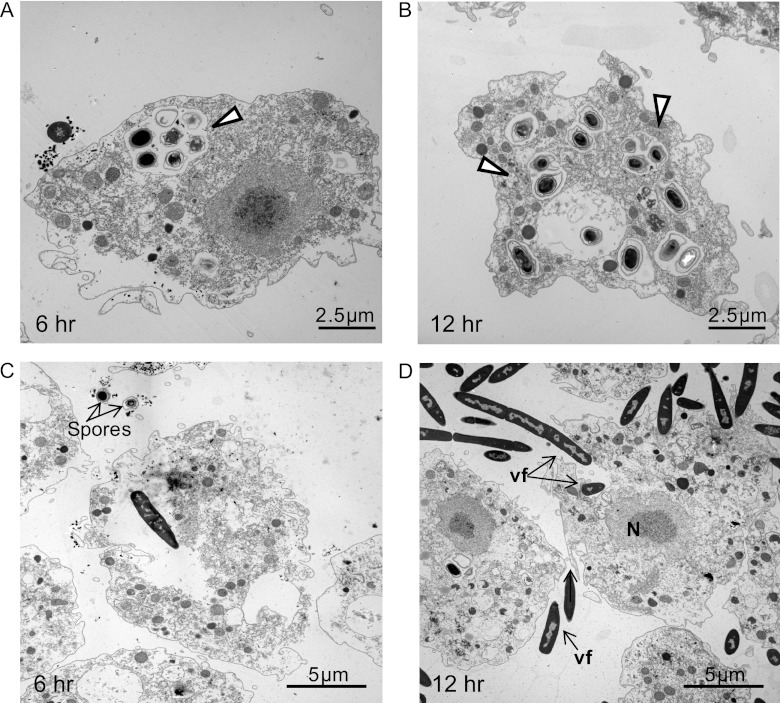
Considering that spores must germinate to become susceptible to killing by mammalian macrophages (23), we might similarly conclude that spores of strain 9131 partially germinated, as indicated by loss of both refractivity (Fig. 1C) and heat resistance (Fig. 2), but were incapable of forming vegetative filaments or lysing amoebas (Fig. 3A). When samples were examined at 6 and 12 h postinoculation by electron microscopy, it was found that strain 9131 remained within the infected A. castellanii phagosomes with little indication of outgrowth into vegetative bacilli (Fig. 5A and B). In parallel, at similar times, spores of the Sterne strain germinated within A. castellanii and had become elongated bacilli that on occasion could be seen rupturing the amoebal plasma membrane (Fig. 5C and D).
Fig 5.
Transmission electron microscopy analysis of B. anthracis-A. castellanii interactions. (A and B) Micrographs show spores of strain 9131 contained in A. castellanii phagosomes (open arrowheads) after 6 and 12 h of coculture at 37°C, respectively. (C) A vegetative Sterne spore within an A. castellanii trophozoite in a phagosome after 6 h of coculture. (D) Vegetative forms of Sterne inside and outside amoebas after 12 h of infection (black arrows). N, nucleus; vf, vegetative form.
DISCUSSION
We have developed an A. castellanii infection model that supports spore germination and intracellular growth of B. anthracis under conditions that simulate standing water/moist environments in which B. anthracis persists. The brief encounter between an amoeba and anthrax spores leads to the demise of the amoeba and a net gain of ∼50-fold more spores. As seen in Fig. 6, the transition, as tracked by phase-contrast microscopy, occurs quickly from attachment and internalization of spores (Fig. 6, frame i) within 2 h, through germination and destruction of the amoebas (Fig. 6, frame ii) as early as 6 h, and terminates in sporulation of exogenous bacilli (Fig. 6, frame iii) as early as 12 h, consistent with the spore data presented in Fig. 2. A similar scenario unfolds at 30°C or 37°C in CWM or in CWM+, suggesting that B. anthracis is amplified by amoebas under a broad range of conditions.
Fig 6.
Model for the B. anthracis amoebal life cycle illustrated with time-lapse phase-contrast micrographs. The following stages are shown: spore uptake within the first 2 h when cells were cocultured at 37°C (sp, spores) (frame i); germination and replication of B. anthracis with subsequent destruction of amoebas after ≈6 h (frame ii) (g, germinated spores); sporulation of B. anthracis with release of spores (r) from the mother cell after 12 h (frame iii).
The observed partial germination of 9131 spores suggests that a critical early step in the germination and outgrowth process must require a factor encoded by the pX01 plasmid, and the data in Fig. 1 demonstrate that this is independent from the exotoxins, similar to mammalian macrophages (5). However, unlike macrophages, the gerX gene on pX01 is dispensable for B. anthracis germination and growth in amoebas (10, 13), while the chromosomally encoded gerH contributes significantly to this process, as predicted from previous macrophage studies (4). This suggests that at least one uncharacterized gene on pX01 (for which the majority of genes are not characterized [20]) that is independent from the exotoxins and the gerX operon is vital for B. anthracis to multiply in the presence of amoebas. Suggestions that unrecognized genes on pX01 are relevant to pathogenesis are pervasive (17), and they may prove relevant to interactions with amoebas as well. Furthermore, our studies suggest that lower temperatures might favor amoebic amplification of B. anthracis strains that contain both plasmids and that this selection may be lost at 37°C or higher, implying that ambient temperatures may influence the retention or loss of the virulence plasmids. This may help explain why B. anthracis isolates lacking pX02 have been found in the environment, yet none lacking pX01 have been isolated; pX01 is vital for B. anthracis amplification in amoebas while pX02 is dispensable.
When applying our observations to real-world scenarios, one can envision repeated cycles of spore amplification each time environmental conditions promote amoebal growth, such as in warm summer months punctuated with rainfall, so that in the end the spore load within such soils could reach levels sufficient to infect warm-blooded hosts. Whether free-living amoebas represent the missing link in the incubator area hypothesis put forth by Van Ness will require direct field testing to identify indigenous amoebic hosts (28). Understanding how this organism persists in soil systems and interacts with the free-living amoebas will contribute to our understanding of the epidemiology and microbial ecology of B. anthracis and potentially permit the development of methods to aid in its eradication from contaminated pastures.
ACKNOWLEDGMENTS
We thank Scott Stibitz, Roger Plaut, and Tod Merkel at the Food and Drug Administration for supplying the RFP plasmid (pRP1028), Philip Hanna at the University of Michigan for the ger knockout strains, David Lowe for his aid in assay design, Olga Chertihin for her expertise on electron microscopy, and William Petri, Kodi Ravichandran, Erik Hewlett, Molly Hughes, and Alex Hoffmaster for their critical review of the manuscript.
These studies and R.D. were supported by NIH grant R01AI066058 to P.S.H. I.J.G. was supported by the University of Virginia School of Medicine Dean's allocated funds.
We have no financial, personal, or professional interests that have influenced the paper.
Footnotes
Published ahead of print 14 September 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Agerer F, Waeckerle S, Hauck CR. 2004. Microscopic quantification of bacterial invasion by a novel antibody-independent staining method. J. Microbiol. Methods 59:23–32 [DOI] [PubMed] [Google Scholar]
- 2. Brahmbhatt TN, et al. 2007. Bacillus anthracis exosporium protein BclA affects spore germination, interaction with extracellular matrix proteins, and hydrophobicity. Infect. Immun. 75:5233–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carr KA, Janes BK, Hanna PC. 2010. Role of the gerP operon in germination and outgrowth of Bacillus anthracis spores. PLoS One 5:e9128 doi:10.1371/journal.pone.0009128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr KA, Lybarger SR, Anderson EC, Janes BK, Hanna PC. 2010. The role of Bacillus anthracis germinant receptors in germination and virulence. Mol. Microbiol. 75:365–375 [DOI] [PubMed] [Google Scholar]
- 5. Dixon TC, Fadl AA, Koehler TM, Swanson JA, Hanna PC. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell Microbiol. 2:453–463 [DOI] [PubMed] [Google Scholar]
- 6. Etienne-Toumelin I, Sirard JC, Duflot E, Mock M, Fouet A. 1995. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J. Bacteriol. 177:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evstigneeva A, Raoult D, Karpachevskiy L, La Scola B. 2009. Amoeba co-culture of soil specimens recovered 33 different bacteria, including four new species and Streptococcus pneumoniae. Microbiology 155:657–664 [DOI] [PubMed] [Google Scholar]
- 8. Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. 2007. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 3:e76 doi:10.1371/journal.ppat.0030076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guidi-Rontani C, Weber-Levy M, Labruyere E, Mock M. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9–17 [DOI] [PubMed] [Google Scholar]
- 11. Gut IM, et al. 2011. Bacillus anthracis spore interactions with mammalian cells: relationship between germination state and the outcome of in vitro. BMC Microbiol. 11:46 doi:10.1186/1471-2180-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanna PC, Ireland JA. 1999. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 7:180–182 [DOI] [PubMed] [Google Scholar]
- 13. Hu H, Emerson J, Aronson AI. 2007. Factors involved in the germination and inactivation of Bacillus anthracis spores in murine primary macrophages. FEMS Microbiol. Lett. 272:245–250 [DOI] [PubMed] [Google Scholar]
- 14. Hugh-Jones M, Blackburn J. 2009. The ecology of Bacillus anthracis. Mol. Aspects Med. 30:356–367 [DOI] [PubMed] [Google Scholar]
- 15. Huws SA, Morley RJ, Jones MV, Brown MR, Smith AW. 2008. Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol. Lett. 282:258–265 [DOI] [PubMed] [Google Scholar]
- 16. Janes BK, Stibitz S. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 74:1949–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levy H, et al. 2012. Differential contribution of Bacillus anthracis toxins to pathogenicity in two animal models. Infect. Immun. 80:2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindeque PM, Turnbull PC. 1994. Ecology and epidemiology of anthrax in the Etosha National Park, Namibia. Onderstepoort J. Vet. Res. 61:71–83 [PubMed] [Google Scholar]
- 19. Mock M and Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671 [DOI] [PubMed] [Google Scholar]
- 20. Okinaka RT, et al. 1999. Sequence and organization of pX01, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509–6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Primm TP, Lucero CA, Falkinham JO., III 2004. Health impacts of environmental mycobacteria. Clin. Microbiol. Rev. 17:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez-Zaragoza S. 1994. Ecology of free-living amoebas. Crit. Rev. Microbiol. 20:225–241 [DOI] [PubMed] [Google Scholar]
- 23. Russell BH, Vasan R, Keene DR, Xu Y. 2007. Bacillus anthracis internalization by human fibroblasts and epithelial cells. Cell Microbiol. 9:1262–1274 [DOI] [PubMed] [Google Scholar]
- 24. Ruthel G, Ribot WJ, Bavari S, Hoover TA. 2004. Time-lapse confocal imaging of development of Bacillus anthracis in macrophages. J. Infect. Dis. 189:1313–1316 [DOI] [PubMed] [Google Scholar]
- 25. Saile E, Koehler TM. 2006. Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Appl. Environ. Microbiol. 72:3168–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuch R, Fischetti VA. 2009. The secret life of the anthrax agent Bacillus anthracis: bacteriophage-mediated ecological adaptations. PLoS One 4:e6532 doi:10.1371/journal.pone.0006532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turnbull PC, et al. 1992. Bacillus anthracis but not always anthrax. J. Appl. Bacteriol. 72:21–28 [DOI] [PubMed] [Google Scholar]
- 28. Van Ness GB. 1971. Ecology of anthrax. Science 172:1303–1307 [DOI] [PubMed] [Google Scholar]
- 29. Van Ness GB and Stein CD. 1956. Soils of the United States favorable for anthrax. J. Am. Vet. Med. Assoc. 128:7–12 [PubMed] [Google Scholar]
- 30. Weiner MA, Hanna PC. 2003. Macrophage-mediated germination of Bacillus anthracis endospores requires the gerH operon. Infect. Immun. 71:3954–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]