Abstract
Cytokinin is required for the initiation of leguminous nitrogen fixation nodules elicited by rhizobia and the delay of the leaf senescence induced by drought stress. A few free-living rhizobia have been found to produce cytokinin. However, the effects of engineered rhizobia capable of synthesizing cytokinin on host tolerance to abiotic stresses have not yet been described. In this study, two engineered Sinorhizobium strains overproducing cytokinin were constructed. The tolerance of inoculated alfalfa plants to severe drought stress was assessed. The engineered strains, which expressed the Agrobacterium ipt gene under the control of different promoters, synthesized more zeatins than the control strain under free-living conditions, but their own growth was not affected. After a 4-week inoculation period, the effects of engineered strains on alfalfa growth and nitrogen fixation were similar to those of the control strain under nondrought conditions. After being subjected to severe drought stress, most of the alfalfa plants inoculated with engineered strains survived, and the nitrogenase activity in their root nodules showed no apparent change. A small elevation in zeatin concentration was observed in the leaves of these plants. The expression of antioxidant enzymes increased, and the level of reactive oxygen species decreased correspondingly. Although the ipt gene was transcribed in the bacteroids of engineered strains, the level of cytokinin in alfalfa nodules was identical to that of the control. These findings suggest that engineered Sinorhizobium strains synthesizing more cytokinin could improve the tolerance of alfalfa to severe drought stress without affecting alfalfa nodulation or nitrogen fixation.
INTRODUCTION
Cytokinin (CK) is a classical phytohormone. It regulates cell growth, cell differentiation, apical dominance, and leaf senescence. It can be classified into two types, adenine and phenylurea. Adenine-type CKs (zeatin, kinetin, and 6-benzylaminopurine) are synthesized mainly in roots by multiple enzymes, including an adenosine phosphate-isopentenyltransferase (IPT), which acts in the first biosynthetic reaction (13, 14, 16). Some plant-pathogenic bacteria (such as Agrobacterium) containing ipt genes can also synthesize CK and so affect their host plant's growth and development (1, 2). CK can also be produced by recycled tRNAs in plants and bacteria (25).
CK is required for the symbiotic interactions between leguminous plants and rhizobia (a group of mutualistic soil bacteria) and for the formation of nitrogen-fixing root nodules (20, 23, 27). The Lotus japonicus HIT1 (encoding a sensor kinase gene of CK) null mutant does not form root nodules (20). In a model rhizobium, Sinorhizbium meliloti, mutants lacking nodA and nodB failed to produce active nodulation factors (Nod factors), so no root nodules formed on the host alfalfa (Medicago sativa). However, overexpression of a foreign ipt gene in these strains was found to rescue this deficiency (8).
Drought tolerance is an important agronomic trait among land crops. This trait can be enhanced by constructing a transgenic plant that overexpresses an IPT gene. The plant then synthesizes more zeatins and experiences delayed leaf senescence (13, 19, 24, 31). However, it is unclear whether S. meliloti overexpressing ipt can produce more zeatins and thus improve the plant's drought tolerance. In this study, two S. meliloti strains expressing a foreign ipt gene (engineered S. meliloti) were constructed and inoculated onto alfalfa seedlings. After a 4-week inoculation and drought treatment period, the zeatin content, plant biomass, nodulation kinetics, nitrogenase activity, accumulation of reactive oxygen species (ROS), and expression of antioxidant genes were analyzed. The alfalfa plants inoculated with the engineered strains exhibited superior drought tolerance, and a preliminary study into elucidating a possible mechanism was performed.
MATERIALS AND METHODS
Bacterial strains and plant materials.
Sinorhizobium 1021 and derivative strains were grown in LB/MC (LB containing 2.5 mM CaCl2 and 2.5 mM MgSO4) medium supplemented with 500 μg/ml streptomycin and 100 μg/ml neomycin at 28°C. The cultures were shaken overnight at 200 rpm. Alfalfa (Medicago sativa cv. Xinjiang) plants inoculated with rhizobia were grown as described by Wang et al. (29).
Construction of engineered S. meliloti.
The ipt gene from Agrobacterium tumefaciens C58 was amplified with primers P1 and P2 (Table 1), digested with restriction enzymes NdeI and PstI (BioLab), ligated into pSRK-Km with T4 DNA ligase (TaKaRa, Dalian, China), and transformed into Escherichia coli DH5α competent cells (15). The colonies carrying the recombinant plasmid were screened on an LB agar plate containing kanamycin. The recombinant plasmids were extracted using a plasmid extraction kit (Transgen, Beijing, China) and identified using PCR, NdeI and PstI digestion, and DNA sequencing (Invitrogen, Shanghai, China). The ipt gene was under the control of a lac promoter called plac-ipt (18). The recombinant plasmid was transferred into S. meliloti 1021 by conjugation with the help of MT616/pRK600, resulting in strain LMG201 (17). The plasmid pTZS (controlled by a trp promoter) was transferred into Rm1021, resulting in strain LMG202; Rm1021 carrying an empty vector (pSRK-Km) was used as a negative control (8). These Sinorhizobium strains were inoculated onto alfalfa seedlings.
Table 1.
Primers used in PCR
| Gene | Primer sense | Primer sequencec |
|---|---|---|
| ipta | Forward | CGTCATATGTTACTCCATCTCATCTACGGACC |
| Reverse | TAGCTGCAGTCACCGAATTCGCGTCAGC | |
| actin | Forward | TGGCATCACTCAGTACCTTTCAAG |
| Reverse | ACCCAAAGCATCAAATAATAAGTCAACC | |
| SOD | Forward | AATGTCACCGTCGGTGATGATG |
| Reverse | GTTCATCCTTGCAAACCAATAATACC | |
| CAT | Forward | CCTATTTGATGATGTGGGTGTCC |
| Reverse | GTCTTGAGTAGCATGGCTGTGGT | |
| sAPX | Forward | ACCAACCTCGTTCAGTGTCCAT |
| Reverse | AGAGCGCTGTCTGCGTTCTATT | |
| thylAPX | Forward | TCATCCTCTTTTGATTCGTTTGG |
| Reverse | CTTTGATTGGCTGGAGAAGTTTC | |
| DHAR | Forward | GATTGGAGACTGCCCTTTTAGC |
| Reverse | CTGTAGCCTTTTCAGGTGGTGT | |
| MDHAR | Forward | AGCGTTCGTTTACGTGATTCTTG |
| Reverse | CATTTGGGAGTTAGCCTTTCCTC | |
| GR | Forward | TTTGAACAAAGGTGCAGAAGAAGG |
| Reverse | TGGGAACACAACCACGAATGAC | |
| GPX | Forward | TGGACAGGAGCCAGGATCTAGT |
| Reverse | ATTTTCAGAGGAGCGGTGGTAG | |
| iptb | Forward | TTCGGACGCCTTTCTCAC |
| Reverse | GCCGCCCTGCATCAATAT | |
| rpsF | Forward | CCTCGCTCGGCAGGACAT |
| Reverse | GCCTTGCGGTTCTTCTTGAT |
Primers used to clone the ipt open reading frame.
Primers used for analysis of the expression of ipt during RT-PCR.
Underlined bases indicate restriction enzyme (NdeI and PstI) sites.
Severe drought treatment.
Fifteen alfalfa plants inoculated with rhizobia were grown in barrels (50 cm in diameter) in a greenhouse (29). The plants were watered with 80 ml of Jensen's liquid medium per barrel per week. After 4 weeks, alfalfa plants were not watered with Jensen's medium; this represented extreme drought (24). The plants were photographed every day, and fresh and dry weights were measured.
ROS assay.
DAB (3,3′-diaminobenzidine) staining was used for ROS assays (26). Four weeks after inoculation, alfalfa plants were subjected to 4 days of severe drought. Then, their leaves were picked and immersed in a DAB solution (80 ml of Jemson's solution plus 10 ml of 100 mM menthol-NaOH plus 10 ml of 1% DAB-HCl) for 1 h. They were then washed 3 times with 95% boiled ethanol. The color of the leaves was observed under an optical microscope (Leica, Germany). Pictures were taken using a digital camera (Nikon, Japan).
RT-PCR assay of ipt and antioxidant enzyme genes.
Primers for A. tumefaciens C58 ipt, S. meliloti rpsF, and the Medicago sativa genes homologous to SOD, CAT, sAPX, thylAPX, DHAR, MDHAR, GR, GPX, and actin were designed and synthesized by Invitrogen (Shanghai, China) and used in reverse transcription (RT)-PCR (11, 24, 30). The total RNA from freshly collected rhizobia (optical density at 600 nm [OD600], 0.8) and 3-week-old root nodules collected 4 weeks after inoculation of roots and leaves were extracted using RNA extraction and purification kits (Transgen, Beijing, China). The total RNA was reverse transcribed as cDNA with a TaKaRa reverse transcription kit (TaKaRa, Dalian, China). RT-PCR was performed according to the protocol provided with a PrimeScript RT reagent kit (TaKaRa, Dalian, China). All primers are listed in Table 1.
Zeatin content assay.
An extraction and quantification protocol for CK was carried out as described by Pan et al. with some modifications (21). The supernatant of 50 ml rhizobial LB/MC cultures was prepared by centrifugation at 13,000 × g for 10 min at 4°C. Then, 0.5 g of fresh tissue per sample was ground in 5 ml of a mixture of 2-propanol, H2O, and concentrated HCl at a ratio of 2:1:0.002 by volume. After centrifugation, supernatants were subjected to quadrupole time of flight chromatography/mass spectrometry (Q-TOF LC/MS) analysis (6520 Accurate-Mass Q-TOF LC/MS; Agilent). Purification and analysis conditions were as follows: column, Zorbax Extend-C18, 4.6 mm (internal diameter) by 50 mm, 1.8-μm pore size; 5-μl injection; flow, 0.2 ml/min; flow phase, A = 0.1% FA H2O, B = 0.1% FA menthol; detection wavelength, DAD, 210, 254, 280, 320, 360, 226 nm; mass range, 50 to 400; nebulizer pressure, 40 psig, drying gas, N2 350°C, 9 liter/min, ESIV cap 3,500 V; capillary, fragmentor 160 V, skimmer 65 V, Oct RFV pp750V; and scanning mode, negative ms scan mode 2 GHz Ext Dyn (1700).
RESULTS
CK produced by engineered Sinorhizobium under free-living conditions.
The growth of engineered strains was analyzed in the complex medium. As shown in Fig. 1A, the growth curve of LMG201 was identical to that of the control strain. The growth of LMG202 increased after 36 h of incubation (Fig. 1A). These data suggest that the ipt gene on the plasmid does not suppress the growth of S. meliloti under free-living conditions.
Fig 1.
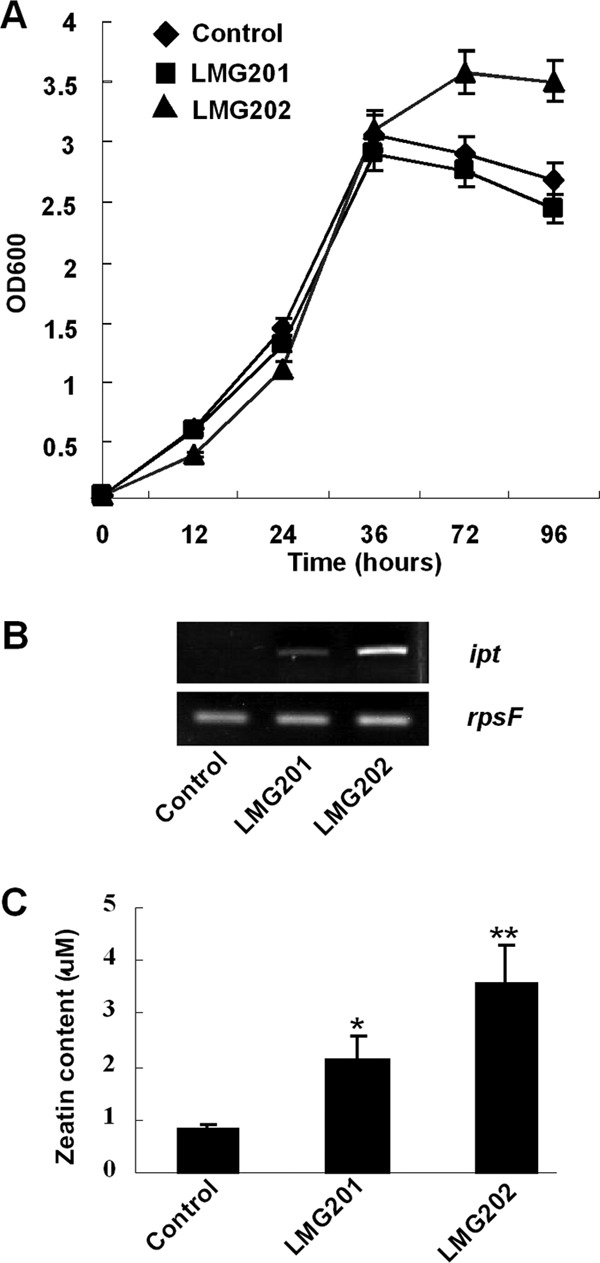
Effects of engineered S. meliloti strains on cytokinin production under free-living conditions. (A) Growth curve of S. meliloti strains in LB/MC medium. (B) Expression of ipt in free-living S. meliloti strains. (C) Cytokinin content in cultures of S. meliloti (OD600 ≈ 2.0). Data from three independent experiments. Control, S. meliloti 1021 carrying an empty plasmid; LMG201 and LMG202, S. meliloti 1021 expressing constructs of Plac-ipt and Ptrp-ipt, respectively. The t test was performed for the results in panel C; a single star indicates significant differences (P < 0.05); two stars indicate highly significant differences (P < 0.01).
To determine whether the ipt gene carried by the plasmid is expressed in free-living rhizobia, total RNA was extracted and RT-PCR was performed. No ipt transcript was detected in the control strain (Fig. 1B). This was consistent with the genomic data of S. meliloti 1021 (9). Unlike what was observed for the control, the introduced ipt gene was transcribed in the engineered rhizobia LMG201 and LMG202. The level of ipt transcription was lower in LMG201 than in LMG202 (Fig. 1B). This suggests that the introduced ipt gene is expressed in engineered S. meliloti strains.
To verify that the transcription of ipt is correlated with CK biosynthesis, zeatin content was assessed using Q-TOF LC-MS. Although S. meliloti 1021 does not contain a gene homologous to ipt, the control strain still synthesized and secreted a small amount of zeatin under free-living conditions (Fig. 1C). In contrast, the two engineered strains produced 2.6 and 4.3 times the zeatin produced by the control strain (Fig. 1C). This was consistent with the level of transcription of the ipt gene (Fig. 1B). From this, it can be concluded that the genetically modified S. meliloti synthesized more CK under free-living conditions than the control strain did.
Effects of genetically modified S. meliloti on alfalfa tolerance to extreme drought stress.
Because transgenic plants overexpressing an IPT gene are usually tolerant to severe drought, the tolerance of alfalfa plants to drought after a 4-week inoculation with engineered strains was evaluated (13, 19, 24, 31). After a 4-week inoculation and incubation period, the alfalfa plants were similar in appearance under ordinary conditions (Fig. 2A). From the beginning of the 5th week, these plants were subjected to severe drought (no watering). After 3 to 4 days, alfalfa plants inoculated with the control strain started to wilt. After 6 days, these plants were completely wilted. However, plants inoculated with the engineered strains were only partially wilted after 6 days (Fig. 2B). After the drought treatment, all plants were rewatered. Alfalfa plants inoculated with engineered strains regained full or partial turgor, but those inoculated with the control strain did not recover and died (Fig. 2C).
Fig 2.
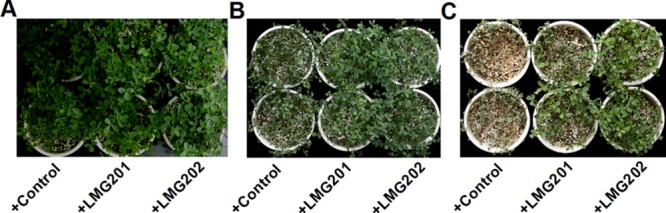
Tolerance of alfalfa plants to extreme drought stress after inoculation with engineered S. meliloti strains. (A and B) Alfalfa plants at 4 weeks after inoculation were either not subjected to drought stress treatment (A) or subjected to severe drought (absence of watering) for 3 to 4 days (B). (C) Alfalfa plants at 5 weeks after inoculation were rewatered after 6 days of drought treatment.
Before drought treatment, the biomass (fresh weight) showed no apparent difference across alfalfa plants (both shoots and roots) inoculated with each rhizobial strain (Fig. 3C and D). However, the fresh weight of alfalfa plants inoculated with LMG202 was significantly higher than that of plants inoculated with the control strain after drought treatment (Fig. 3C and D). The dry weights of all alfalfa plants were almost identical (whole plant, 0.041 ± 0.004, 0.044 ± 0.002, and 0.041 ± 0.002 g/plant; leaves, 0.026 ± 0.002, 0.028 ± 0.001, and 0.028 ± 0.001g/plant). Therefore, the water content of alfalfa plants with treatment of extreme drought was about 50%, 30%, and 5% after inoculation of the control and two engineered strains, respectively. These data suggest that alfalfa plants inoculated with engineered S. meliloti may maintain water content more effectively than control plants under drought stress conditions.
Fig 3.
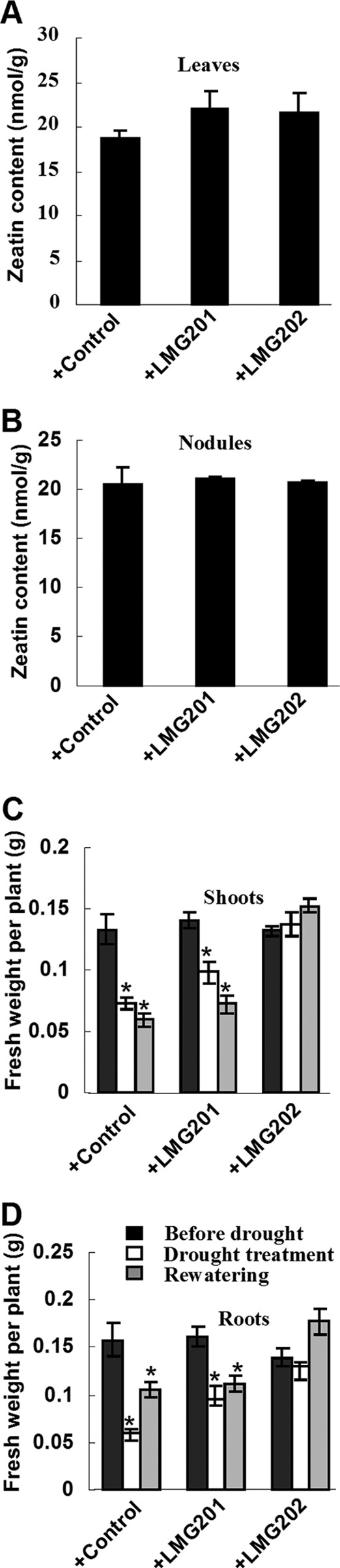
Effects of engineered S. meliloti strains on alfalfa growth. Cytokinin content in alfalfa leaves (A) and nodules (B). Fresh weight of alfalfa shoots (C) and roots (D). Data are means ± standard deviations (SD) from three independent experiments (n > 20). The t test was performed; a single star indicates a significant difference (P < 0.05).
Effects of engineered S. meliloti strains on nitrogen fixation.
The transcription of ipt in bacteroids was first analyzed by RT-PCR. The transcript of ipt was detected in bacteroids of both engineered strains, but no transcript was observed in the control bacteroids (Fig. 4A), indicating that the ipt gene carried by the plasmid is transcribed in the bacteroids of alfalfa nodules.
Fig 4.
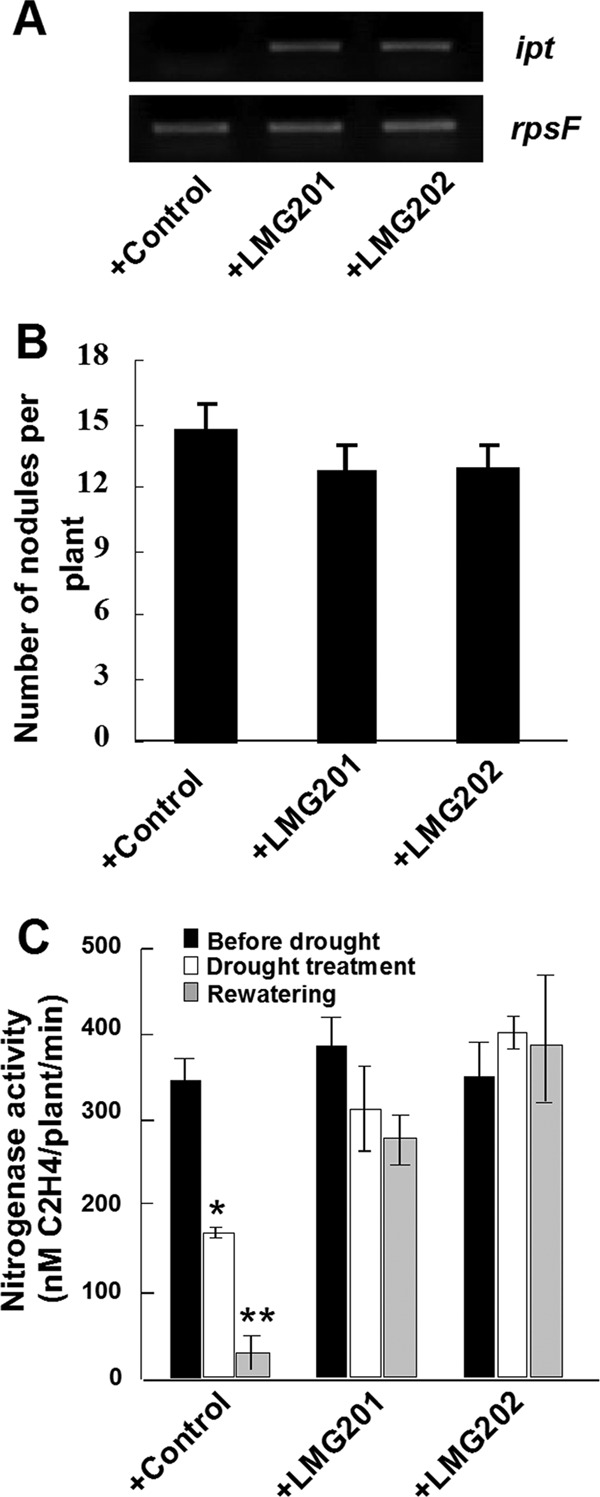
Effects of engineered S. meliloti strains on alfalfa nodulation and nitrogen fixation. (A) Expression of ipt in alfalfa nodules induced by Rhizobium; (B) number of nodules at 4 weeks after inoculation in alfalfa plants; (C) nitrogenase activity of alfalfa nodules induced by S. meliloti strains. Data are means ± standard deviations (SD) from three independent experiments (n > 20). The t test was performed for results shown in panels B and C; a single star indicates a significant difference (P < 0.05); two stars indicate a highly significant difference (P < 0.01).
To determine whether S. meliloti expressing an introduced ipt gene affects nodulation and nitrogen fixation in alfalfa, nodulation kinetics and nitrogenase activity were assessed. The number of nodules induced by the two engineered strains in alfalfa at 4 weeks postinoculation was a little lower than in controls (13.3% and 12.5%) (Fig. 4B). However, a few large nodules were observed on some alfalfa plants inoculated with both engineered strains, and the average fresh weight of nodules per plant induced by each strain was not significantly different (0.005 ± 0.002, 0.0052 ± 0.001, and 0.0049 ± 0.001 g/plant). Therefore, there was no significant difference in the nitrogen-fixing capacity of alfalfa nodules hosting the two rhizobium strains (Fig. 4C). This indicated that the transcription of the introduced ipt gene in S. meliloti does not affect the nitrogen-fixing capabilities of inoculated alfalfa plants.
Because drought stress induces premature senescence and suppresses nitrogen fixation in leguminous nodules, it seemed likely that engineered strains might delay this process (10). Alfalfa nodules hosting the control strain appeared pale pink or gray after drought treatment and during rewatering, but those exposed to the engineered strains were completely pink. The nitrogenase activity of alfalfa nodules elicited by the control strain was also significantly lower after drought stress and even after rewatering than in plants not subjected to drought (by about 50% and 90%, respectively) (Fig. 4C). The nitrogenase activity was only a little lower (by about 10% and 15%, respectively) in nodules containing LMG201, but it was not decreased in nodules containing LMG202 (Fig. 4C). This suggests that engineered S. meliloti strains delay the premature senescence that can be induced in alfalfa nodules by drought stress and maintain nitrogenase activity.
CK biosynthesis, accumulation of ROS, and expression of antioxidant genes in alfalfa leaves.
The drought tolerance of alfalfa plants inoculated with engineered strains inspired us to assay the level of CK in alfalfa nodules and leaves. The zeatin levels were found to be slightly increased (17.79% and 13.97%) in alfalfa leaves inoculated with engineered strains, but no difference was observed in root nodules (Fig. 3A and B).
It has been reported that CK delays leaf senescence by upregulating the expression of antioxidant genes, causing decomposition of the reactive oxygen species (ROS) induced by drought stress (24). For this reason, the level of H2O2 in alfalfa leaves subjected to drought stress was tested by DAB staining. Our results showed that less H2O2 accumulated in leaves of alfalfa plants inoculated with the engineered strains than in those of controls (Fig. 5A). The transcription of ROS-scavenging enzymes was analyzed by RT-PCR. Consistently, the transcripts of SOD, CAT, sAPX, thylAPX, DHAR, MDHAR, GR, and GPX were all higher in leaves of alfalfa plants inoculated with LMG202 after 4 days of drought treatment than in the control strain (Fig. 5B). These results suggested that the inoculation of the engineered S. meliloti strain probably promoted decomposition of ROS by increasing the expression of antioxidant enzymes.
Fig 5.
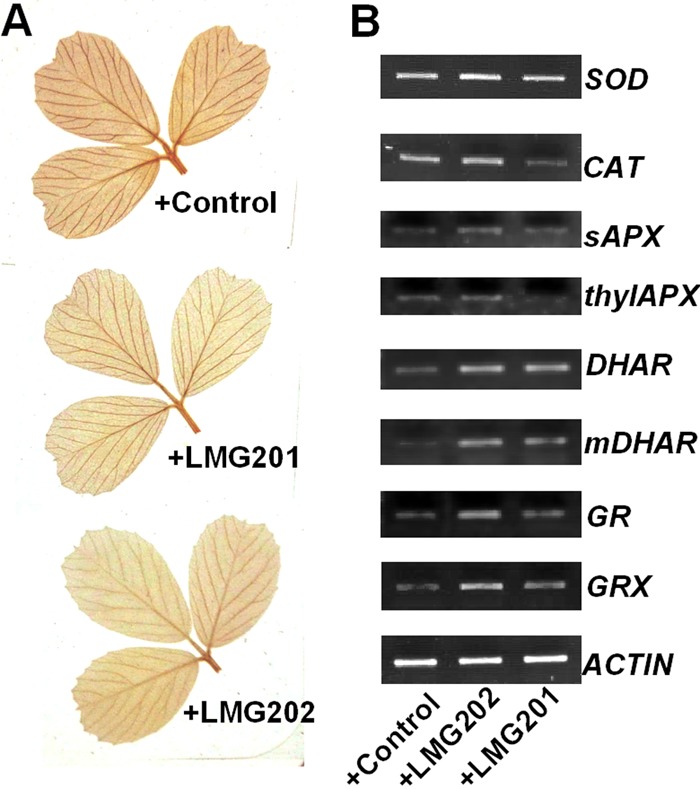
Levels of ROS were detected in alfalfa leaves after inoculation with engineered S. meliloti strains. (A) H2O2 accumulation in alfalfa leaves as detected by DAB staining. (B) Expression of ROS-scavenging enzyme genes in leaves of alfalfa plants subjected to 4 days of severe drought. Data from one representative experiment are shown. The experiment was repeated at least three times.
DISCUSSION
It has been reported that engineered rhizobia expressing foreign indole-3-acetic acid (IAA) biosynthetic genes can cause host plants to form fewer, larger nodules and so improve their tolerance to salt and low-phosphate stresses (3–6). However, there has not yet been a description of the effects of engineered cytokinin-producing rhizobia on the tolerance of host plants to abiotic stresses. In this study, we described that engineered Sinorhizobium strains synthesized more CK and improved host tolerance to severe drought stress during the period analyzed.
The production of zeatins from Bradyrhizobium japonicum was first determined 4 decades ago (22). Here, the wild-type S. meliloti was also found to synthesize zeatins (Fig. 1C). These zeatins are probably derived from tRNA molecules, as indicated by the fact that no gene homologous to ipt was found in the genomes of symbiotic Rhizobium species (25).
Cytokinin is required for the formation of leguminous nodules (18, 21, 25). The slight decrease in the number of alfalfa nodules induced by engineered strains (Fig. 4B) may be due to the modification of the cytokinin-auxin balance (5, 23). It has been reported that overproduction of IAA by Rhizobium can reduce the number of Medicago nodules (6). And CRE1-dependent inhibition of PINs changes the polar transportation of auxins (CRE1 is a sensor kinase of CK, and PINs are the transporters of auxin [12, 23, 28]).
The lack of any significant difference in nitrogen fixation capacity and growth of alfalfa plants during the period analyzed suggests that larger nodules fix nitrogen more efficiently (Fig. 2A, 3C and D, and 4C). The use of our engineered S. meliloti strains might not affect the yield of alfalfa plants under the conditions tested. Longer-term studies must be performed to confirm this.
The high nitrogenase activity of alfalfa nodules hosting engineered strains under severe drought conditions (Fig. 4C) could be attributed to the delay of leaf senescence. By maintaining leaves, photosynthate may thus be provided to nodules. It is possible that the synthesized zeatins are transported from nodules to leaves, which may increase the level of this phytohormone in leaves.
The increase in zeatin content from plants inoculated with engineered strains was determined by both immunological and chromatographic methods and may play a key role in alfalfa tolerance to severe drought stress by increasing the expression of scavenging genes and fostering the decomposition of ROS (Fig. 5). These data are consistent with those from a study of transgenic plants expressing an ipt gene (24). The increased concentration of zeatins in alfalfa leaves could also be attributed to synthesis by colonized engineered strains, considering that Chi et al. reported that S. meliloti lives freely in the plant roots, stems, and leaves (7).
In summary, the engineered Sinorhizobium strain carrying different ipt constructs secreted more CKs. They did not affect the ability of alfalfa nodules to fix nitrogen, but they did improve host tolerance to severe drought stress during the period analyzed. This engineered Rhizobium strain has shown potential for development as a new biotechnological approach to improving the tolerance of host legumes to abiotic stresses.
ACKNOWLEDGMENTS
We thank Sharon Long for providing the pTZS plasmid, Haiying Xue for preparing the plant materials, and Yi-Ning Liu for Q-TOF LC-MS analysis.
This work was supported by the National Key Program for Basic Research (2011CB100702 and 2010CB126501), Natural Science Foundation of China (31070218), Natural Science Foundation of Shanghai (09ZR1436500), and Knowledge Innovation Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (2009KIP206) (all to L.L.).
Footnotes
Published ahead of print 7 September 2012
REFERENCES
- 1. Akiyoshi DE, Klee H, Amasino RM, Nester EW, Gordon PM. 1984. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 81:5994–5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barry GF, Rogers SG, Fraley RT, Brand L. 1984. Identification of a cloned cytokinin biosynthetic gene. Proc. Natl. Acad. Sci. U. S. A. 81:4776–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianco C, Defez R. 2009. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 60:3097–3107 [DOI] [PubMed] [Google Scholar]
- 4. Bianco C, Defez R. 2010. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 76:4626–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bianco C, Imperlini E, Defez R. 2009. Legumes like more IAA. Plant Signal. Behav. 4:763–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camerini S, et al. 2008. Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch. Microbiol. 190:67–77 [DOI] [PubMed] [Google Scholar]
- 7. Chi F, et al. 2005. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 71:7271–7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper JB, Long SR. 1994. Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell 6:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galibert F, et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672 [DOI] [PubMed] [Google Scholar]
- 10. Goicoechea N, Merino S, Sánchez-Díaz M. 2005. Arbuscular mycorrhizal fungi can contribute to maintain antioxidant and carbon metabolism in nodules of Anthyllis cytisoides L. subjected to drought. J. Plant Physiol. 162:27–35 [DOI] [PubMed] [Google Scholar]
- 11. Goodner B, et al. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323–2328 [DOI] [PubMed] [Google Scholar]
- 12. Grunewald W, et al. 2009. Manipulation of auxin transport in plant roots during rhizobium symbiosis and nematode parasitism. Plant Cell 21:2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huynh LN, Van Toai T, Streeter J, Banowetz G. 2005. Regulation of flooding tolerance of SAG12:ipt Arabidopsis plants by cytokinin. J. Exp. Bot. 56:1397–1407 [DOI] [PubMed] [Google Scholar]
- 14. Kamada-Nobusada T, Sakakibara H. 2009. Molecular basis for cytokinin biosynthesis. Phytochemistry 70:444–449 [DOI] [PubMed] [Google Scholar]
- 15. Khan SR, Gaines J, Roop RM, II, Farrand SK. 2008. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 74:5053–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kudo T, Kiba T, Sakakibara H. 2010. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 52:53–60 [DOI] [PubMed] [Google Scholar]
- 17. Leigh JA, Signer ER, Walker GC. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. U. S. A. 82:6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo L, Xu J, Li XL. A new approach improving plant quality. 2010 Chinese patent no. 2010101330890. [Google Scholar]
- 19. Ma QH. 2008. Genetic engineering of cytokinins and their application to agriculture. Crit. Rev. Biotechnol. 28:213–232 [DOI] [PubMed] [Google Scholar]
- 20. Murray JD, et al. 2007. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315:101–104 [DOI] [PubMed] [Google Scholar]
- 21. Pan X, Welti R, Wang X. 2010. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 5:986–992 [DOI] [PubMed] [Google Scholar]
- 22. Phillips DA, Torrey JG. 1972. Studies on cytokinin production by Rhizobium. Plant Physiol. 49:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plet J, et al. 2011. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 65:622–633 [DOI] [PubMed] [Google Scholar]
- 24. Rivero RM, et al. 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. U. S. A. 104:19631–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakakibara H. 2006. Cytokinins: activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 57:431–449 [DOI] [PubMed] [Google Scholar]
- 26. Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11:1187–1194 [Google Scholar]
- 27. Tirichine L, et al. 2007. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315:104–107 [DOI] [PubMed] [Google Scholar]
- 28. van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. 2006. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 140:1494–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, et al. 2010. GGDEF and EAL proteins play different roles in the control of Sinorhizobium meliloti growth, motility, exopolysaccharide production, and competitive nodulation on host alfalfa. Acta Biochim. Biophys. Sin. (Shanghai) 42:410–417 [DOI] [PubMed] [Google Scholar]
- 30. Yao SY, et al. 2004. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 186:6042–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Van Toai T, Huynh L, Preiszner J. 2000. Development of flooding-tolerant Arabidopsis thaliana by autoregulated cytokinin production. Mol. Breeding 6:135–144 [Google Scholar]


