Abstract
Isolation of Clostridium mutants based on gene replacement via allelic exchange remains a major limitation for this important genus. Use of a heterologous counterselection marker can facilitate the identification of the generally rare allelic exchange events. We report on the development of an inducible counterselection marker and describe its utility and broad potential in quickly and efficiently generating markerless DNA deletions and integrations at any genomic locus without the need for auxotrophic mutants or the use of the mobile group II introns. This system is based on a codon-optimized mazF toxin gene from Escherichia coli under the control of a lactose-inducible promoter from Clostridium perfringens. This system is potentially applicable to almost all members of the genus Clostridium due to their similarly low genomic GC content and comparable codon usage. We isolated all allelic-exchange-based gene deletions (ca_p0167, sigF, and sigK) or disruptions (ca_p0157 and sigF) we attempted and integrated a 3.6-kb heterologous DNA sequence (made up of a Clostridium ljungdahlii 2.1-kb formate dehydrogenase [fdh] gene plus a FLP recombination target [FRT]-flanked thiamphenicol resistance marker) into the Clostridium acetobutylicum chromosome. Furthermore, we report on the development of a plasmid system with inducible segregational instability, thus enabling efficient deployment of the FLP-FRT system to generate markerless deletion or integration mutants. This enabled expeditious deletion of the thiamphenicol resistance marker from the fdh integrant strain as well as the sigK deletion strain. More generally, our system can potentially be applied to other organisms with underdeveloped genetic tools.
INTRODUCTION
The genus Clostridium is composed of Gram-positive, spore-forming bacteria that belong to the phylum Firmicutes. Members of this genus are obligate anaerobes and contain both medically and commercially important species. Clostridium botulinum, C. difficile, C. perfringens, and C. tetanii are medically important human pathogens (5). Clostridium sporogenes and C. novyi were shown to have the potential for use as anticancer drug delivery systems that specifically target hypoxic and necrotic areas of tumors (30). Other species of the Clostridium genus are industrially important for producing commodity chemicals and biofuels from a variety of carbon sources. These species include Clostridium species that produce solvents, such as C. acetobutylicum, C. pasteurianum, and C. beijerinckii, the acetogens that grow on CO2/CO/H2, including C. ljungdahlii and C. carboxidivorans (48), and the cellulolytic clostridia, including C. thermocellum, C. cellulolyticum, and C. phytofermentans among others (48). Industrial and medical applications of these organisms are hindered by the limited availability of effective genetic tools (6, 17, 38, 45, 48), and the inherent difficulty of genetically modifying these organisms has been a roadblock in the development of desirable strains. Gene deletions (knockouts [KOs]) and chromosomal integrations (knockins [KIs]) for both fundamental investigations and practical applications remain a difficult, slow, and inefficient task (6, 17, 38, 45, 48).
Until recently, most of the relatively few homologous recombination mutants isolated in Clostridium spp. have been single-crossover mutants brought about by a Campbell-like integration of the entire plasmid backbone (3, 13, 21, 35, 49, 55). To date, only a few mutants with gene replacement via allelic exchange have been isolated (1, 8, 22, 59).
To bypass the difficulty of isolating allelic exchange mutants in Clostridium, three main methods are currently employed for gene inactivation and DNA chromosomal integration. The first method is based on the mobile group II intron from the ltrB gene of Lactococcus lactis (commercially known as the TargeTron technology [Sigma-Aldrich]) (17, 57, 60). These are large “retrohoming” ribozymes capable of self-splicing from pre-mRNA and can be designed to target a specific locus in the host genome, thus disrupting the gene of interest (17, 45). This method can be effective but is not without limitations (25, 39). One possible drawback is that target sequences smaller than 400 bp may not contain optimal target sites for the mobile group II intron recognition sequence as discussed in the TargeTron Gene Knockout System User Guide (45a). Moreover, since the mutants generated by the mobile group II intron system are disruption mutants and not true deletion mutants, some variation in phenotype may be observed depending on the intron targeting sequence, and hence its insertion into the gene of interest. This phenomenon, where different mutant phenotypes are observed has been reported when attempting to inactivate ca_c0437 in Clostridium acetobutylicum (47). Additionally, integration efficiency drastically decreases when attempting to deliver foreign “cargo” DNA larger than 1 kb (24, 39), and thus, this method cannot be effectively used to integrate DNA into the chromosome.
A second method relies upon the creation of auxotrophic mutants, and specifically auxotrophic mutants for uracil (16, 50). The pyrE, pyrF, and upp genes are targeted for initial disruption leading to an auxotrophic mutant that requires uracil-supplemented medium for growth. In addition, these mutants exhibit resistance to 5-fluoroorotic acid (5-FOA) or 5-fluorouracil (5-FU), both toxic antimetabolites (4). Subsequently, these mutants serve as a genetic background for isolating further mutations in target gene(s) by utilizing a counterselection method. A plasmid expressing the pyrF gene, for example, and containing an antibiotic resistance marker flanked by two regions of homology to the target gene is transformed into the host cells. To isolate double-crossover mutants, cells are plated onto media containing 5-FOA for negative selection. Mutants that have undergone the recombination event survive, while those that still maintain the plasmid, and hence express the pyrF gene, perish. This method has been used to inactivate the pta gene in Clostridium thermocellum (50). A similar approach was utilized to isolate unmarked chromosomal mutants in C. perfringens, albeit the initial mutant created (called the HN13 strain with a disrupted galKT operon) was not an auxotroph but rather was unable to metabolize galactose (32). The HN13 strain was used as genetic background to isolate unmarked chromosomal mutants. GalK is responsible for phosphorylating galactose, which in HN13 is not further metabolized, causing toxicity to the cells. Thus, the integration vector expresses galK to enable negative selection or counterselection once cells are plated on galactose-supplemented media. This approach was utilized to disrupt the virRS operon as well as six other genes in C. perfringens HN13 (32).
A recently reported method for integrating DNA into the chromosome is based upon coupling the expression of a promoterless antibiotic resistance marker (16) to that of the constitutively expressed thiolase (thl) promoter (14, 53) to screen for integrants. Integration mutants that have undergone the desired recombination event to fuse the silent antibiotic resistance marker immediately downstream of the thl gene (thus leading to the expression of the antibiotic marker) can be easily selected on solid media supplemented with the appropriate antibiotic (16). Current data (53) on the strength of clostridial promoters suggest that there is a limited number of constitutively expressed promoters that are suitable for this promoter “hijacking” strategy (53). This constitutes a limitation as to where the integration event can be targeted to in the chromosome.
Most recently, Cartman et al. described the use of the codA gene that codes for cytosine deaminase from Escherichia coli as a counterselection marker in Clostridium difficile for isolating mutant strains with allelic exchange mutations in the tcdC gene (8). CodA converts cytosine to uracil. However, its nonstringent substrate specificity allows it to act upon the pyrimidine analog 5-fluorocytosine (5-FC) and convert it to 5-FU, a toxic antimetabolite. The upp gene, which codes for uracil phosphoribosyltransferase, is responsible for conferring toxicity in the presence of 5-FU. Thus, a precondition for the successful use of codA as a heterologous counterselection marker is the presence of the upp gene in the host organism and the absence of the codA gene. Clostridium genome sequences indicate that several Clostridium organisms contain codA homologues. These species include C. perfringens, C. botulinum, C. lentocellum, and C. ljungdahlii.
Here we report on the development of a method based on counterselection that overcomes the limitations discussed above in order to enable gene replacement KOs as well as KIs in Clostridium. Two components are necessary for our method: an inducible promoter and a toxic gene based on a general mechanism that would be applicable to Clostridium and many, if not all, other organisms. A recent study identified a lactose-inducible promoter and its divergent regulator (bgaR; CPE0770) in C. perfringens strain 13. For a toxic gene, we chose the Escherichia coli gene mazF, an mRNA interferase, which exhibits a general mechanism for toxicity and which has been successfully used as a counterselection marker in Bacillus subtilis (58). The MazE-MazF complex is part of the toxin-antitoxin system in E. coli (Fig. 1). The toxin, MazF, is stable, while the antitoxin MazE is labile (56). The mazF gene was synthetically constructed for optimized translation in C. acetobutylicum. Once expressed and translated, MazF cleaves mRNA at ACA sequences, therefore arresting cell growth (Fig. 1) (56). We show that the combination of the lactose-inducible promoter with this mazF gene leads to the development of an effective counterselection method for generating both KOs and KIs in C. acetobutylicum.
Fig 1.
Mode of action of the MazF mRNA interferase. MazF is part of the toxin-antitoxin system of E. coli coded by the mazEF operon. Under normal conditions, the antitoxin MazE binds to and inhibits the MazF protein. Once the cells are subjected to stress, the MazE protein is degraded by cellular proteases, and the MazF protein is relieved from inhibition. MazF then targets mRNA at ACA sequences, thus leading to growth arrest, followed by cell death. P, promoter.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Relevant characteristics of bacterial strains and plasmids used in this work are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristic(s)a | Sourceb or reference |
|---|---|---|
| E. coli strains | ||
| ER2275 | hsdR mcrA recA1 endA1 | NEB |
| Top10 | hsdR mcrA recA1 endA1 | LT |
| DB3.1 | ccdB survival cells | LT |
| Turbo | lacIq endA1 | NEB |
| C. acetobutylicum strains | ||
| ATCC 824 | Wild-type strain | ATCC |
| M5 | ATCC 824; pSOL1 negative | ATCC |
| 824 ΔsigK | Mostly deleted sigK; Thr | This study |
| 824 ΔsigK_um | Unmarked sigK deletion | This study |
| 824 Δca_p0167 | Mostly deleted ca_p0167; Thr | This study |
| 824 sigF::Thr | Disrupted sigF; Thr | This study |
| 824 ca_p0157::Thr | Disrupted ca_p0157; Thr | This study |
| 824 ΔsigF | Mostly deleted sigF; Thr | This study |
| M5 sigK::fdh | fdh integrated in the sigK locus; Thr | This study |
| M5 sigK::fdh_um | Unmarked fdh integrated in the sigK locus | This study |
| Plasmids | ||
| pAN3 | Kmr; Φ3T I gene | This study |
| pKORECU | Thr MLSr; recU | 49 |
| pCR8/GW/TOPO TA | Spr; topoisomerized | LT |
| pCR8/sigF/ThR | pCR8/GW/sigF::ThR | 21 |
| pKORD_mazF | Thr MLSr; ccdB recU repL; ori; bgaR and PbgaL upstream of mazF; DEST cassette | This study |
| pKOD_mazF | Thr MLSr; ccdB repL; ori; bgaR and PbgaL upstream of mazF; DEST cassette | This study |
| pAH2 | Thr; bgaR and PbgaL upstream of cpe-gusA | 15 |
| pKRAH1 | Thr; bgaR and PbgaL upstream of the MCS | 15 |
| pKRAH1_mazF | Thr; bgaR and PbgaL upstream of mazF | This study |
| p94FLP_asRepL | Emr; flippase; ptb promoter; bgaR and PbgaL upstream of asRNA to the repL origin | This study |
| pCR8/cap0157/ThR | pCR8/GW/ca_p0157::Thr | This study |
| pCR8/cap0167/ThR | pCR8/GW/ThR-FRT flanked by ca_p0167 ∼1,000-bp regions of homology upstream and downstream | This study |
| pCR8/sigK/ThR | pCR8/GW/ThR-FRT flanked by sigK ∼1,000-bp regions of homology upstream and downstream | This study |
| pCR8/sigF2/ThR | pCR8/GW/ThR-FRT flanked by sigF ∼1,000-bp regions of homology upstream and downstream | This study |
| pKOSIGF_mazF | Thr-FRT; MLSr; recU repL; ori; bgaR and PbgaL upstream of mazF; sigF::Thr | This study |
| pKOCAP0157_mazF | Thr MLSr; recU repL; ori; bgaR and PbgaL upstream of mazF; ca_p0157::Thr | This study |
| pKOSIGK_mazF | Thr-FRT; MLSr; recU repL; ori; bgaR and PbgaL upstream of mazF; Thr-FRT flanked by sigK ∼1,000-bp regions of homology upstream and downstream | This study |
| pKOCAP0167_mazF | Thr-FRT; MLSr; recU repL; ori; bgaR and PbgaL upstream of mazF; Thr-FRT flanked by ca_p0167 ∼1,000-bp regions of homology upstream and downstream | This study |
| pKISIGK::FDH_mazF | Thr-FRT; MLSr; recU repL; ori; bgaR and PbgaL upstream of mazF; Thr and fdh flanked by sigK ∼1,000 bp regions of homology upstream and downstream | This study |
| pKOSIGF2_mazF | Thr-FRT; MLSr; repL; ori; bgaR and PbgaL upstream of mazF; Thr-FRT flanked by sigF ∼1,000-bp regions of homology upstream and downstream | This study |
| pKOSIGK2_mazF | Thr-FRT; MLSr; repL; ori; bgaR and PbgaL upstream of mazF; Thr-FRT flanked by sigK ∼1,000-bp regions of homology upstream and downstream | This study |
| p94MCS | Ampr MLSr; ptb promoter; MCS | This study |
| p94FDH | Ampr MLSr; ptb promoter; fdh | This study |
hsdR, host specific restriction deficient; mcrA, methylcytosine specific restriction deleted; recA1, homologous recombination deleted; endA1, endonuclease deleted; lacIq, lac repressor; ptb, phosphotransbutyrylase; DEST cassette, Invitrogen Destination cassette for Gateway cloning system; Spr, spectinomycin resistance; cpe-gusA, β-glucuronidase reporter; Cmr, chloramphenicol resistance; Thr, thiamphenicol resistance; Kmr, kanamycin resistance; Thr-FRT, FRT-flanked thiamphenicol resistance; Φ3T I, B. subtilis phage Φ3T I methyltransferase gene; MLSr, macrolide-lincosamide-streptogramin B resistance; mazF, codon-optimized E. coli MazF toxin gene; bgaR, beta-galactosidase regulator; PbgaL, promoter of the beta-galactosidase gene; MCS, multiple cloning site; fdh, C. ljungdahlii formate dehydrogenase gene; recU, B. subtilis resolvase gene; repL, pIM13 gram-positive origin of replication; ori, ColE1 origin of replication; Ampr, ampicillin resistance.
NEB, New England Biolabs, Ipswich, MA; LT, Life Technologies, Grand Island, NY; ATCC, American Type Culture Collection, Manassas, VA.
Codon optimization of the mazF gene.
The mazF gene was synthetically constructed (DNA 2.0, Menlo Park, CA) for optimized codon usage in Clostridium acetobutylicum (see Table S1 in the supplemental material). Codons that had a usage frequency of less than 5 per 1,000 were exchanged for those that exhibited a higher frequency. Additionally, the lacI operator sequence was placed upstream of the optimized mazF gene to enable the cloning of mazF-containing vectors in E. coli strains that express lacI or lacIq.
Construction of plasmids.
All primers are listed in Table S2 in the supplemental material. The pKOSIGK_mazF plasmid was constructed by PCR amplifying the two large regions of homology flanking the sigK gene utilizing splicing by overhang extension (SOE) PCR (19). The generated amplicon (∼2 kb) was then cloned into the pCR8/GW/TOPOTA (Life Technologies [LT], Grand Island, NY), linearized with ScaI (New England BioLabs [NEB], Ipswich, MA), and finally ligated to the FLP recombination target (FRT)-flanked thiamphenicol resistance (Thr-FRT) gene. The resulting plasmid (pCR8/sigK/ThR) was then recombined with either the pKORD_mazF or the pKOD_mazF (no recU) destination plasmids by utilizing the Gateway LR Clonase (LT) reaction, yielding plasmids pKOSIGK_mazF and pKOSIGK2_mazF, respectively.
The pKOCAP0167_mazF plasmid was constructed by PCR amplifying the complete region of homology, including the ca_p0167 open reading frame (ORF), and the resulting amplicon was cloned into pCR8/GW/TOPOTA (LT). This plasmid was then doubly digested with SspI/BsgI (NEB), resulting in the removal of 285 bp from the ca_p0167 ORF, blunt ended with the large (Klenow) fragment of DNA polymerase I (NEB), and finally ligated to the Thr-FRT gene. The resulting plasmid (pCR8/cap0167/ThR) was shuffled into the pKORD_mazF destination plasmid using the Gateway LR Clonase (LT) reaction to yield plasmid pKOCAP0167_mazF.
The sigF disruption plasmid was constructed (21) by amplifying a 391-bp internal fragment and cloning it into pCR8/GW/TOPOTA (LT). The resulting plasmid was then ScaI linearized (NEB) (ScaI has a single digestion site in the middle of the amplified sigF region), ligated to the Thr marker to yield plasmid pCR8/sigF/ThR, which was then recombined with the pKORD_mazF destination plasmid to generate pKOSIGF_mazF.
The ca_p0157 disruption plasmid was constructed by amplifying a 498-bp internal fragment and cloning it into pCR8/GW/TOPOTA (LT). The resulting plasmid was HindIII (NEB) linearized (HindIII has a single cut site approximately in the middle in the ca_p0157 fragment), blunt ended with the large (Klenow) fragment of DNA polymerase I (NEB), and ligated to the Thr gene, yielding plasmid pCR8/cap0157/ThR. This plasmid was then shuffled into the pKORD_mazF destination plasmid as described above, yielding plasmid pKOCAP0157_mazF.
The pKISIGK::FDH_mazF was constructed as follows. First, the formate dehydrogenase (fdh) gene (CLJU_c08930) from C. ljungdahlii (RefSeq [RS] database accession no. NC_014328) was PCR amplified from C. ljungdahlii genomic DNA (gDNA) using primers incorporating the NcoI and SacII digestion sites and then cloned into the NcoI/SacII (NEB) doubly digested p94MCS plasmid, yielding plasmid p94FDH. In this construct, the fdh gene is flanked upstream by a strong clostridial promoter (Pptb) and downstream by the adc gene's rho-independent terminator (46, 53). Next, the full-length fragment, including the promoter and terminator, was PCR amplified from p94FDH and cloned into the NotI (NEB)-linearized and blunt-ended pKOSIGK_mazF plasmid, yielding plasmid pKISIGK::FDH_mazF.
The p94FLP_asRepL plasmid was constructed by treating the p94FLP plasmid with SbfI (NEB) to linearize it and subsequently clone the bgaR-asRepL fragment, yielding plasmid p94FLP_asRepL. The lactose-inducible promoter and its divergent regulator (bgaR-PbgaL) were utilized and fused immediately upstream of a 100-bp antisense RNA (asRNA) that targets the first 100 bp of the repL transcript coding for the replication protein (RepL), creating plasmid p94FLP_asRepL. The asRNA is designed to be a reverse and complement RNA strand to that of the repL transcript. Once transcribed, the asRNA binds to the transcript and inhibits its translation.
The pKOSIGF2_mazF plasmid designed to generate a sigF deletion mutant was constructed by PCR amplifying the two large regions of homology flanking the sigF gene using SOE PCR. The resulting PCR product (∼ 2 kb) was cloned into the pCR8/GW/TOPOTA (LT), linearized with ScaI (NEB), and ligated to the Thr-FRT gene. The resulting plasmid (pCR8/sigF2/ThR) was then recombined with the pKOD_mazF destination plasmid that is lacking the recU gene by utilizing the Gateway LR Clonase (LT) reaction, yielding plasmid pKOSIGF2_mazF.
Culture conditions.
E. coli strains were grown aerobically at 37°C and 220 rpm in liquid LB medium or solid LB with agar (1.5%) supplemented with ampicillin (Amp) (50 μg/ml) or chloramphenicol (Cm) (35 μg/ml) as needed. Single Clostridium acetobutylicum colonies grown anaerobically on solid 2× YTG medium (16 g/liter Bacto tryptone, 10 g/liter yeast extract, 4 g/liter NaCl, and 5 g/liter glucose; pH 5.8) for at least 5 days were transferred to 10 ml of liquid clostridial growth medium [CGM; 0.75 g/liter K2HPO4, 0.75 g/liter KH2PO4, 0.7 g/liter MgSO4·7H2O, 0.017 g/liter MnSO4·5H2O, 0.01 g/liter FeSO4·7H2O, 2 g/liter (NH4)2SO4, 1 g/liter NaCl, 2 g/liter asparagine, 0.004 g/liter p-aminobenzoic acid, 5 g/liter yeast extract, 4.08 g/liter CH3COONa·3H2O, and 80 g/liter glucose] and heat shocked at 80°C for 10 min. Recombinant strains were grown as described above in media supplemented with erythromycin (Em) (40 μg/ml for solid media and 100 μg/ml for liquid media) or thiamphenicol (Th) (5 μg/ml) as necessary. Where needed, 40 mM β-lactose (Sigma-Aldrich, St. Louis, MO) was added to the culture media to induce the expression from the lactose-inducible promoter.
Reporter enzyme assay.
C. acetobutylicum cultures were grown to an A600 of 1.0 in liquid 2× YTG (pH 5.2) supplemented with 5 μg/ml of Th where needed. Lactose was then added to the respective culture to a final concentration of 0 mM, 1 mM, or 10 mM, and the cultures were incubated for 30 min at 37°C in an anaerobic chamber. The cells were then collected by centrifugation at 5,000 × g for 10 min at 4°C, and the pellets were stored at −20°C until used. The β-glucuronidase enzyme assay was performed as described in reference 15. All experiments were done with at least two biological replicates.
Plasmid transformation.
Newly constructed plasmids were transformed into E. coli Top10 (LT) cells and subsequently isolated and confirmed using restriction digests. A plasmid(s) destined to be electrotransformed into C. acetobutylicum was first transformed into electrocompetent E. coli ER2275(pAN3) cells for DNA methylation. pAN3-bearing cells are a derivative of the ER2275(pAN1) cells except that they have kanamycin resistance (28). The vectors were then isolated and confirmed from their respective E. coli ER2275(pAN3) strains and subsequently transformed into C. acetobutylicum ATCC 824 (824) or M5 by electroporation in an anaerobic chamber as described previously (29) except that 5 to 10 μg of plasmid DNA was used to transform the cells.
Isolation and enrichment of gene replacement mutants via allelic exchange.
Upon transforming the appropriate plasmid(s) in C. acetobutylicum and following 4 h of outgrowth in liquid 2× YTG, cells were collected by centrifugation at 5,000 × g for 10 min and resuspended in 500 μl of 2× YTG. One hundred microliters of the cell suspension was spread onto solid 2× YTG medium supplemented with 5 μg/ml Th and 40 mM lactose (Th-lactose plates) (4 plates) and Th-only plates (1 plate). The plates were incubated for 48 to 72 h in an anaerobic chamber at 37°C or until colonies were observed. Putative mutants appearing on the Th-lactose plates were isolated and screened by PCR to confirm the presence of the desired allelic exchange event.
If no colonies were seen on the Th-and-lactose plates, colonies from the Th-only plate were grown in liquid CGM, and a series of subcultivations were undertaken to enrich for desired gene replacement mutants using an approach similar to the one described in reference 49, except that lactose-containing plates were utilized for the final selection plate (see Fig. S1 in the supplemental material). Transformants were vegetatively transferred every 24 h for 5 days on 2× YTG solid medium supplemented with Th at 5 μg/ml. After 5 days, the cells were again vegetatively transferred for an additional 5 days under no antibiotic selection to facilitate plasmid curing. After 5 days of curing, the cells were plated on Th-lactose plates and allowed to grow for 24 h, and colonies were isolated and confirmed. Optionally, these plates were replica plated (21, 49) onto plates supplemented with Em at 40 μg/ml (erythromycin resistance [Emr] is encoded on the vector backbone) and allowed to grow for 24 h. The plates were then visually compared to the Th-lactose plates. Ideally, areas of growth on Th-lactose plates should show no growth on plates containing Em, an indication of chromosomal integration via allelic exchange. Areas on the Th-lactose plates that showed no growth on the Em-supplemented plates were restreaked on fresh plates to isolate single colonies for screening. Alternatively, a liquid transfer protocol may be undertaken (see Fig. S3 in the supplemental material). One colony from the Th-only plate was used to inoculate 10 ml liquid CGM supplemented with 5 μg/ml Th and allowed to grow overnight at 37°C. Subsequently, a 5% inoculum was transferred to a fresh 10-ml CGM tube supplemented with 5 μg/ml Th and allowed to grow for 8 to 12 h. This transfer process was repeated a total of 5 times. Finally, 500 μl from the final transfer was used to inoculate 10 ml of CGM supplemented with 5 μg/ml Th and 40 mM β-lactose and allowed to grow for 6 h. Serial dilutions were plated onto solid 2× YTG medium supplemented with 5 μg/ml Th and 40 mM β-lactose. Single colonies were then isolated and screened to confirm the presence of the desired allelic exchange event.
RNA isolation and semiquantitative reverse transcription-PCR (sq-RT-PCR).
RNA isolation, cDNA generation, and semiquantitative real-time PCR were carried out as previously described except that 2 μg of total RNA was reverse transcribed (3). Primers were designed to amplify an ∼700-bp fragment from the reverse-transcribed fdh transcript. All amplification reactions were carried out using Phusion high-fidelity DNA polymerase (NEB).
Analytical techniques.
Cell density was measured at 600 nm using a Beckman Coulter DU 730 spectrophotometer.
Confirmation of chromosomal or megaplasmid insertions by PCR and sequencing.
To confirm allelic exchange chromosomal integration mutants, gDNA was isolated from cells using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) following the manufacturer's protocol. Appropriate primer combinations (see Table S2 in the supplemental material) were used to confirm the putative integrants. Amplification reactions were carried out using Phusion high-fidelity DNA polymerase (NEB). Mutants were also confirmed by Sanger sequencing.
Nucleotide sequence accession numbers.
The nucleotide sequences for pKOD_mazF and p94FLP_asRepL have been deposited in the GenBank database and assigned accession numbers JX644442 and JX644443, respectively.
RESULTS
Development of an inducible counterselection marker.
A lactose-inducible promoter that was developed for use in Clostridium perfringens strain 13 (15) was tested for functionality in C. acetobutylicum. Plasmid pAH2 (15) with the inducible promoter placed upstream of the reporter gene, the β-glucuronidase gene (Fig. 2A), was introduced into C. acetobutylicum, giving rise to strain 824(pAH2), and its response to induction with lactose was tested. Previous work (15) reported that lactose concentrations above 10 mM did not significantly increase the level of expression with this promoter. The results showed that the promoter did indeed respond to induction with lactose in C. acetobutylicum. After 30 min of induction, the reporter's specific activity increased by 10- and 15-fold upon the addition of 1 mM and 10 mM lactose, respectively (Fig. 2B). Baseline levels of the reporter's activity (i.e., without the addition of lactose) were close to wild-type (WT) levels. Without lactose induction, the reporter's specific activity in strain 824(pAH2) was 0.6 ± 0.2 U/mg compared to 0.2 ± 0.01 U/mg for the WT strain. These results suggest slight promoter leakiness, but we hypothesized that it would not be sufficient to induce the expression of the mazF gene (described below) to high enough levels that would promote cell death.
Fig 2.
bgaR-PbgaL promoter reporter activity in response to 30 min of induction by lactose and toxicity of MazF in C. acetobutylicum. (A) Schematic diagram showing the arrangement of the lactose-inducible promoter (PbgaL) and divergent regulator (BgaR) upstream of the β-glucuronidase (gusA) gene. (B) Specific activity of the reporter in response to lactose addition. The average activities (n = 2) were 0.6 U/mg, 8.0 U/mg, and 13.0 U/mg when 0 mM, 1 mM, and 10 mM lactose were added, respectively. (C) Schematic diagram of the pKRAH1_mazF expression plasmid, whereby the codon-optimized mazF gene was placed under the control of the lactose-inducible promoter and cloned into C. acetobutylicum. Ori+, Gram-positive origin of replication; Ori−, Gram-negative origin of replication. (D) Plates showing the effects of mazF expression in C. acetobutylicum. Cells harboring the pKRAH1_mazF plasmid were grown in the absence of lactose (−lactose) and in the presence of lactose (+lactose). Without lactose in the medium, cells grow abundantly on the plate, but no growth is observed when lactose is added to the medium, and thus, mazF is expressed. One of two biological replicates is shown.
The synthetic mazF gene was cloned downstream of the lactose-inducible promoter and its divergent regulator (bgaR-PbgaL) on plasmid pKRAH1 (15). This generated plasmid pKRAH1_mazF (Fig. 2C). The plasmid was electrotransformed into C. acetobutylicum. Once it was confirmed that the strain carried the plasmid, colonies were grown in liquid media supplemented with 5 μg/ml of Th for 24 h. Subsequently, equal volumes of culture were plated onto solid 2× YTG medium supplemented with 5 μg/ml of Th with and without lactose. After 24 h of anaerobic incubation at 37°C, plates were visually inspected for growth. As anticipated, plates containing lactose did not have any colonies, while those lacking lactose showed an abundance of growth (Fig. 2D). These data suggested that the toxicity of MazF might enable us to utilize it for counterselection to screen for cells that have targeted allelic exchange events.
Utilizing mazF as a counterselectable marker to isolate sigK and ca_p0167 gene deletions via allelic exchange using large regions of homology.
To demonstrate the application and utility of the developed inducible counterselection marker, the sporulation-specific sigma factor K (σK) and the gene encoding CA_P0167, a putative sigma factor of unknown function, carried on the C. acetobutylicum pSOL1 megaplasmid were targeted for inactivation (9). The KO plasmids were designed to delete the majority of the ORF of each gene. Plasmid pKOSIGK_mazF included a Thr-FRT that is further flanked by two large regions of homology of ∼1,000 bp both upstream and downstream of the sigK gene (with only 12 bp of the 5′ sigK ORF and 29 bp of the 3′ end included in the upstream and downstream regions of homology, respectively) (Fig. 3A). This design was to ensure that no DNA regulatory elements of adjacent genes would be affected. Consequently, the resulting sigK mutant would have 664 bp of the 705 bp of the sigK gene deleted. Similarly, plasmid pKOCAP0167_mazF was designed with two regions of homology for the ca_p0167 gene that ensured the removal of 285 bp of the 555-bp coding sequence (Fig. 3B). Allelic exchange mutants were isolated by utilizing the solid replica plating protocol described in Materials and Methods. Putative mutants appearing on the Th-lactose plates were isolated and screened by PCR to confirm the presence of the desired gene replacement event using primers listed in Table S2 in the supplemental material. Of the 6 colonies screened for the sigK mutant, 3 contained the targeted gene replacement via allelic exchange to delete a significant portion of the sigK ORF as confirmed by PCR (Fig. 3C) and Sanger sequencing. Similarly, of the 10 colonies screened for the ca_p0167 mutant, 8 contained the desired allelic exchange event to delete 285 bp of the ca_p0167 ORF as confirmed by PCR (Fig. 3D), as well as by sequencing.
Fig 3.
Gene replacement via allelic exchange at the sigK and ca_p0167 loci. (A) Selection of unmarked sigK double-crossover deletion mutants. The boxed regions of the ca_c1688 and pilT genes show the approximate regions of homology (∼1 kb each) incorporated in the knockout (KO) vector. After the cells were placed on plates containing thiamphenicol (Th) and lactose to negatively select against cells bearing the plasmid, putative deletion mutants were selected and screened by PCR with the indicated primers. (B) Isolation of ca_p0167 deletion mutants. The boxed areas of the adc and amyA genes indicate the approximate locations of the two regions of homology (∼1 kb each). Colonies growing on plates containing Th plus lactose were isolated, and mutants were confirmed with the indicated primers. (C) PCR confirmation of double-crossover sigK deletion mutants in panel A with primers that anneal to the chromosome as indicated. Three of the six colonies isolated and screened contained the desired double-crossover recombination. One of the three colonies is shown. Additionally, the Thr marker was excised by the FLP recombinase as described in the text, thus creating an unmarked sigK deletion (ΔsigK_um). Lane M, 2-log DNA ladder (0.1 to 10 kb) (NEB). (D) PCR confirmation of deletion mutants in panel B with the indicated primers. Out of 10 colonies screened, 8 contained the expected double-crossover integration. One of the eight colonies is shown.
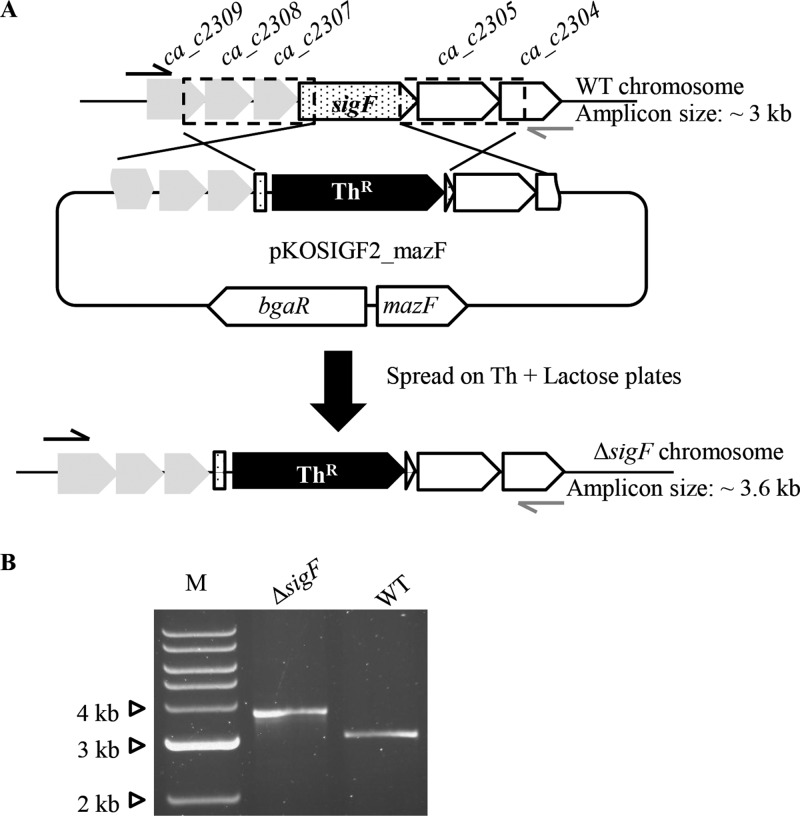
Isolation of sigF and ca_p0157 disruption mutants with small (<300-bp) internal regions of homology.
To demonstrate the strength of our method, gene disruption mutants of the sporulation-specific sigma factor F (σF) and the predicted megaplasmid sigma factor CA_P0157 were generated by utilizing the Thr marker flanked by two internal regions of homology of <300 bp (see Fig. S2A in the supplemental material). Previous work in attempting to isolate mutant strains with allelic exchange disruption mutations in the sigF gene yielded only mutants that had undergone a Campbell-like single-crossover event that resulted in the entire plasmid backbone rolling into the targeted region of the chromosome (21). As described above, the replica plating protocol was employed to enrich for mutants that have undergone the allelic exchange recombination with the target gene. All 5 putative mutants isolated contained the desired recombination event (see Fig. S2B in the supplemental material). Similarly, we aimed to isolate mutants with allelic exchange disruption mutations in the ca_p0157 gene. The pKOCAP0157_mazF plasmid was methylated and electrotransformed into wild-type C. acetobutylicum, and the replica plating protocol was performed as described above. All 5 colonies screened had undergone the desired allelic exchange event (Fig. S2B). It was previously reported that the rate of recombination decreases significantly when the regions of homology are less than 400 bp in various unicellular organisms (33, 37) and in mammalian cells (43). Notwithstanding this, the selection protocol described herein is capable of identifying mutant strains with rare allelic exchange mutations generated by small regions of homology (<300 bp) with the target gene(s).
Unmarked genomic integration of the 2.1-kb formate dehydrogenase gene from C. ljungdahlii.
To further demonstrate the utility of our method, integration of the 2.1-kb formate dehydrogenase (fdh) gene (CLJU_c08930) from C. ljungdahlii was undertaken. The expected sizes of the wild-type and integrant loci are shown in Fig. 4A. The fdh KI plasmid was methylated and electrotransformed into the asporogenous C. acetobutylicum M5 strain that has lost the pSOL1 megaplasmid (9, 31). Due to the relatively large size of the pKISIGK::FDH_mazF plasmid (∼12.3 kb) and in order to increase the transformation efficiency (28, 34), the plasmid was incubated with competent cells on ice for 20 min before electrotransformation. Following 4 h of outgrowth in liquid 2× YTG, cells were plated onto solid media containing 5 μg/ml Th and 40 mM β-lactose with the aim of isolating putative integrants immediately after plasmid transformation. Of the estimated 5 × 103 transformants, we were immediately able to isolate three transformants (i.e., 0.06% of the total estimated 5 × 103 transformants) that had undergone the desired allelic exchange event, namely, to integrate the ∼3.6-kb “cargo” DNA (2.1-kb fdh and a 1.5-kb Thr-FRT marker) as confirmed by PCR (Fig. 4B) and sequencing. To test active expression of the newly integrated fdh gene, sq-RT-PCR was carried out as described in Materials and Methods. Two biological replicates from each strain (M5 sigK::fdh and M5 control strain) were used for RNA isolation and cDNA generation. Both M5 sigK::fdh biological replicates showed the expected size band, while none of the control strains did (Fig. 4C), thus confirming expression of the integrated fdh gene.
Fig 4.
Isolation of unmarked chromosomal integration of the fdh gene in the sigK locus. RH1 and RH2 indicate the first and second regions of homology, respectively. (A) Schematic diagram showing the WT, marked, and unmarked fdh integration sites in the C. acetobutylicum M5 sigK locus and the expected amplicon sizes with the indicated primers. (B) PCR screening and confirmation of integration mutants with the primers indicated in panel A. Three integrants were isolated immediately following transformation of the pKOSIGK::FDH_mazF plasmid on plates containing Th and lactose, and data for one integrant are shown. The unmarked (sigK::fdh_um) strain and WT strain are also shown. Lanes: MW, λ HindIII-digested ladder (NEB); M, 2-log DNA ladder (0.1 to 10 kb) (NEB). (C) sq-RT-PCR confirming expression of the integrated fdh gene in the sigK locus of strain M5. Primers were designed to amplify 700 bp in the middle of the fdh transcript. Two biological replicates (replicates A and B) are shown for both the integration strain as well as the control M5 strain. Lane M, 2-log DNA ladder (0.1 to 10 kb) (NEB). (D) Effectiveness of asRNA-based induction of segregational plasmid instability shown by the loss of the plasmid expressing FLP recombinase after induction with lactose to express the asRNA against the repL origin of replication. After six serial transfers in liquid CGM, with and without lactose, equal volumes were plated onto solid media supplemented with Em (encoded on the plasmid backbone). Following incubation, the plates were visually inspected for growth. As anticipated, the culture that was induced with lactose did not show any growth, while the uninduced culture showed growth.
Easy marker removal: expression of the FLP recombinase from a plasmid with inducible segregational instability.
Due to the limited number of antibiotic resistance markers (18) that are suitable for use in C. acetobutylicum, we set out to “flip out” the Thr marker from the C. acetobutylicum M5 sigK::fdh integration strain and the 824 ΔsigK strain via expression of FLP recombinase (44, 61). Anecdotal evidence suggested that once excision of the FRT-flanked marker is confirmed, stimulating plasmid loss is laborious and time-consuming due to the relatively good stability of the repL Gram-positive replicon (27). There are no known temperature-sensitive replicons in Clostridium. There are few established replication origins suitable for use on plasmids to be propagated in Clostridium (18). However, these origins are segregationally very stable with an estimated retention frequency of >90% after 60 generations without any selective pressure (23, 27). Hence, we set out to develop a means to easily and readily cure the FLP recombinase expression plasmid once it was confirmed that the antibiotic marker had been excised. Once again, the C. perfringens lactose-inducible promoter and its divergent regulator (bgaR-PbgaL) were utilized and fused immediately upstream of a 100-bp antisense RNA (asRNA) that targets the first 100 bp of the repL transcript coding for the replication protein (RepL), creating plasmid p94FLP_asRepL. This plasmid was transformed into the C. acetobutylicum M5 sigK::fdh and 824 ΔsigK strains, and the following procedure was used to stimulate FLP activity. First, a single colony was grown overnight in 10 ml of CGM supplemented with 100 μg/ml of Em. One milliliter of this culture was used to inoculate a new 10-ml tube of CGM which was incubated in an anaerobic chamber for 6 h at 30°C to ensure optimal FLP activity (7). Serial dilutions were plated onto solid 2× YTG medium containing 40 μg/ml Em and incubated at 37°C until colonies were observed. Subsequently, a replica of the plate was made on 2× YTG plates supplemented with 5 μg/ml Th. Colonies that did not exhibit growth on the Th-supplemented plate were screened to confirm excision of the Thr gene by PCR. For the fdh integration mutant, 3 of the 14 colonies screened had the Thr gene excised, while all 14 colonies screened for the sigK deletion mutant had lost the Thr gene. The resulting unmarked sigK deletion mutant was named 824 ΔsigK_um (um stands for unmarked) (Fig. 3B), and the unmarked integration mutant was renamed M5 sigK::fdh_um (Fig. 4B). To stimulate the loss of the p94FLP_asRepL plasmid, a single colony was grown overnight in liquid CGM. A 5% inoculum from the overnight culture was used to inoculate two fresh 10-ml CGM tubes simultaneously (with and without lactose) and allowed to grow for 8 to 12 h. Lactose was added to induce the expression of the asRNA fragment to stimulate plasmid loss. This process was repeated a total of six times. One hundred microliters was then plated onto solid 2× YTG plates with Em (Emr gene contained on the plasmid backbone) and allowed to grow for 48 h to confirm loss of the plasmid. Following incubation, the plates were visually inspected for growth. The culture that was not induced with lactose showed growth (indicating the presence of the plasmid), while the culture that was induced with lactose did not (indicating plasmid loss) (Fig. 4D). We conclude that the strategy of inducing segregational instability by inducing an asRNA against the repL origin of replication is sound and effective.
Isolation of sigF and sigK deletion mutants with a KO plasmid lacking the resolvase (recU) gene.
Previous work in our group aimed to enhance the homologous recombination machinery by expressing the B. subtilis resolvase gene (recU) (49). Resolvases are a class of proteins responsible for Holliday junction resolution during homologous recombination. However, these attempts yielded mutants that were only single-crossover integrants, similar to what others have reported without the expression of recU (12, 13, 46, 55). Thus far, all our KO/KI plasmids were designed to express the recU gene. To test whether recU was dispensable in our counterselection strategy, sigF and sigK deletion plasmids were designed without including the recU gene. The sigF deletion mutant was designed to remove 521 bp of the 759-bp sigF ORF (Fig. 5A). Upon transforming C. acetobutylicum cells with the pKOSIGF2_mazF plasmid as described above, 14 colonies were immediately isolated after transformation and outgrowth from the Th-lactose plates. PCR confirmation revealed that 8 of those colonies had undergone the desired gene replacement via allelic exchange to generate an in-frame deletion of the sigF gene (Fig. 5B). No colonies were observed on the Th-lactose plates upon transformation of the cells with plasmid pKOSIGK2_mazF, which was designed to generate a sigK in-frame deletion mutant as described above, albeit without the expression of recU. Thus, the sigK deletion mutant was generated by utilizing a liquid transfer protocol described in Materials and Methods (see Fig. S3 in the supplemental material). In conclusion, the sigF mutant was immediately isolated without using the extended replica plating protocol described above in the absence of recU. Likewise, the sigK deletion mutant was isolated without the expression of recU on the vector backbone.
Fig 5.
Gene replacement via homologous recombination at the sigF locus without recU expression. (A) Schematic diagram showing the KO vector design and isolation of sigF deletion mutants. The boxed areas (outlined by a broken line) indicate the approximate locations of the two regions of homology (∼1 kb each). (B) Colonies appearing on plates containing Th and lactose immediately after transformation were isolated, and mutants were confirmed with the primers indicated in panel A. Of the 14 colonies screened, 8 showed the correct PCR product sizes indicating a double crossover as shown in panel A. Data for one colony are shown. Lane M, 2-log DNA ladder (0.1 to 10 kb) (NEB).
DISCUSSION
The lack of a generally applicable counterselection marker for use in Clostridium, without the need to rely on auxotrophic strains, has been described as a major limitation for efficiently and readily isolating allelic exchange mutants (16). Unlike yeast cells or other naturally competent prokaryotes like B. subtilis, which can be transformed with linear DNA or suicide plasmids, respectively (11, 20), isolating rare homologous recombination mutants is a major obstacle due to difficulty in identifying desirable gene replacement mutants via allelic exchange, low transformation efficiency in clostridia, and the poor recombination systems of most Clostridium organisms (13, 18, 26, 48). However, these deficiencies can be overcome if a suitable counterselection marker is placed on the vector backbone to select against cells bearing the plasmid, enabling the isolation of gene replacement mutants (16). Nonetheless, identifying suitable counterselection markers and optimizing the conditions for their use is a challenging and laborious task (41).
Here, we report on the development of an easy-to-implement counterselection method based on an inducible mazF gene for isolating rare allelic exchange chromosomal insertions and deletions in C. acetobutylicum. The work flow and the estimated effort involved in designing and isolating a desired mutant are shown in Fig. S4 in the supplemental material. Our method does not resort to generating auxotrophic strains, and it does not limit the isolation of integrants in certain genomic loci. Furthermore, the method developed here can be utilized to study the conditions that can potentially enhance native homologous recombination in the host organism (e.g., culture age, temperature, etc.). We anticipate that this new method will prove invaluable for easily and efficiently creating mutant strains for studying gene function or for integrating DNA sequences that confer desirable traits in Clostridium spp.
Unlike the group II intron system, where target sequences smaller than 400 bp may not contain optimal group II intron target sites, we show here that we can target specific DNA sequences that are smaller than 300 bp and are able to readily isolate homologous recombination mutants.
Due to the good stability of the repL origin of replication (27) and in order to swiftly cure the cells of the plasmid bearing the FLP recombinase gene, an inducible segregational instability element was utilized. Antisense RNA (asRNA) against a target transcript(s) has been successfully utilized to downregulate the expression of a number of genes in Clostridium (10, 40, 42, 51, 52). The asRNA is a single-stranded RNA molecule that is complementary to the target gene's synthesized mRNA molecule. Therefore, upon transcription of the asRNA, it binds to the target mRNA molecule and inhibits its translation. To exploit this feature for curing the plasmid, a lactose-inducible asRNA fragment against the repL transcript was designed and implemented to promote inducible segregational instability of the replicon. Consequently, this system enables a fast turnaround time if the desired mutant is required for further genetic manipulations.
Although our work focused on C. acetobutylicum, our method could be adapted to most, if not all, Clostridium organisms and other organisms with underdeveloped genetic tools. The method described herein may prove invaluable for clostridial geneticists aiming to generate chromosomal fusion proteins (54) or for creating whole-genome KO libraries in Clostridium spp., enabling a functional genomics approach in studying these important and poorly understood organisms (2).
Supplementary Material
ACKNOWLEDGMENTS
We thank Bryan Tracy (Elcriton Inc., DE) for providing plasmid p94FLP and for insightful discussions and suggestions. We also thank Stephen Melville (Virginia Tech) for generously donating plasmids pAH2 and pKRAH1.
This work was supported by U.S. Department of Energy grant DE-SC0007092. Financial support for M. A. Al-Hinai was provided by the Government of Oman.
The University of Delaware has filed a patent application covering the work described in this article. The application names M. A. Al-Hinai and E. T. Papoutsakis as inventors.
Footnotes
Published ahead of print 14 September 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Awad MM, Bryant AE, Stevens DL, Rood JI. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15: 191–202 [DOI] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bi CH, Jones SW, Hess DR, Tracy BP, Papoutsakis ET. 2011. SpoIIE is necessary for asymmetric division, sporulation, and expression of σF, σE, and σG but does not control solvent production in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 193: 5130–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boeke JD, Trueheart J, Natsoulis G, Fink GR. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175 [DOI] [PubMed] [Google Scholar]
- 5. Bruggemann H, et al. 2003. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc. Natl. Acad. Sci. U. S. A. 100: 1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brüggemann H, Gottschalk G. 2009. Clostridia: molecular biology in the post-genomic era. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 7. Buchholz F, Ringrose L, Angrand PO, Rossi F, Stewart AF. 1996. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res. 24: 4256–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. 2012. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl. Environ. Microbiol. 78: 4683–4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cornillot E, Nair RV, Papoutsakis ET, Soucaille P. 1997. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J. Bacteriol. 179: 5442–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai RP, Papoutsakis ET. 1999. Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl. Environ. Microbiol. 65: 936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gietz D, St Jean A, Woods RA, Schiestl RH. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425 doi:10.1093/nar/20.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green EM, Bennett GN. 1996. Inactivation of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. Appl. Biochem. Biotechnol. 57-88: 213–221 [DOI] [PubMed] [Google Scholar]
- 13. Green EM, et al. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142: 2079–2086 [DOI] [PubMed] [Google Scholar]
- 14. Harris LM, Welker NE, Papoutsakis ET. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 184: 3586–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartman AH, Liu H, Melville SB. 2011. Construction and characterization of a lactose-inducible promoter system for controlled gene expression in Clostridium perfringens. Appl. Environ. Microbiol. 77: 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heap JT, et al. 2012. Integration of DNA into bacterial chromosomes from plasmids without a counter-selection marker. Nucleic Acids Res. 40: e59 doi:10.1093/nar/gkr1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70: 452–464 [DOI] [PubMed] [Google Scholar]
- 18. Heap JT, Pennington OJ, Cartman ST, Minton NP. 2009. A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 78: 79–85 [DOI] [PubMed] [Google Scholar]
- 19. Higuchi R, Krummel B, Saiki RK. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16: 7351–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Itaya M, Tanaka T. 1990. Gene-directed mutagenesis on the chromosome of Bacillus subtilis 168. Mol. Gen. Genet. 223: 268–272 [DOI] [PubMed] [Google Scholar]
- 21. Jones SW, Tracy BP, Gaida SM, Papoutsakis ET. 2011. Inactivation of σF in Clostridium acetobutylicum ATCC 824 blocks sporulation prior to asymmetric division and abolishes σE and σG protein expression but does not block solvent formation. J. Bacteriol. 193: 2429–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennedy CL, et al. 2005. The alpha-toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 57: 1357–1366 [DOI] [PubMed] [Google Scholar]
- 23. Khan SA. 1997. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 61: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein JR, et al. 2004. A conjugation-based system for genetic analysis of group II intron splicing in Lactococcus lactis. J. Bacteriol. 186: 1991–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein JR, Dunny GM. 2002. Bacterial group II introns and their association with mobile genetic elements. Front. Biosci. 7: d1843–d1856 [DOI] [PubMed] [Google Scholar]
- 26. Lee SY, Mermelstein LD, Bennett GN, Papoutsakis ET. 1992. Vector construction, transformation, and gene amplification in Clostridium acetobutylicum ATCC 824. Ann. N. Y. Acad. Sci. 665: 39–51 [DOI] [PubMed] [Google Scholar]
- 27. Lee SY, Mermelstein LD, Papoutsakis ET. 1993. Determination of plasmid copy number and stability in Clostridium acetobutylicum ATCC 824. FEMS Microbiol. Lett. 108: 319–323 [DOI] [PubMed] [Google Scholar]
- 28. Mermelstein LD, Papoutsakis ET. 1993. In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi 3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59: 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mermelstein LD, Welker NE, Bennett GN, Papoutsakis ET. 1992. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology (NY) 10: 190–195 [DOI] [PubMed] [Google Scholar]
- 30. Minton NP, et al. 1995. Chemotherapeutic tumor targeting using clostridial spores. FEMS Microbiol. Rev. 17: 357–364 [DOI] [PubMed] [Google Scholar]
- 31. Nair RV, Papoutsakis ET. 1994. Expression of plasmid-encoded Aad in Clostridium acetobutylicum M5 restores vigorous butanol production. J. Bacteriol. 176: 5843–5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nariya H, Miyata S, Suzuki M, Tamai E, Okabe A. 2011. Development and application of a method for counterselectable in-frame deletion in Clostridium perfringens. Appl. Environ. Microbiol. 77: 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson RT, Pryor BA, Lodge JK. 2003. Sequence length required for homologous recombination in Cryptococcus neoformans. Fungal Genet. Biol. 38: 1–9 [DOI] [PubMed] [Google Scholar]
- 34. Nickoloff JA. 1995. Electroporation protocols for microorganisms. Humana Press, Totowa, NJ [Google Scholar]
- 35. O'Connor JR, et al. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61: 1335–1351 [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37. Papadopoulou B, Dumas C. 1997. Parameters controlling the rate of gene targeting frequency in the protozoan parasite Leishmania. Nucleic Acids Res. 25: 4278–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papoutsakis ET. 2008. Engineering solventogenic clostridia. Curr. Opin. Biotechnol. 19: 420–429 [DOI] [PubMed] [Google Scholar]
- 39. Plante I, Cousineau B. 2006. Restriction for gene insertion within the Lactococcus lactis Ll.LtrB group II intron. RNA 12: 1980–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raju D, Setlow P, Sarker MR. 2007. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl. Environ. Microbiol. 73: 2048–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reyrat JM, Pelicic V, Gicquel B, Rappuoli R. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66: 4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roberts AP, et al. 2003. Development of an integrative vector for the expression of antisense RNA in Clostridium difficile. J. Microbiol. Methods 55: 617–624 [DOI] [PubMed] [Google Scholar]
- 43. Rubnitz J, Subramani S. 1984. The minimum amount of homology required for homologous recombination in mammalian cells. Mol. Cell. Biol. 4: 2253–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlake T, Bode J. 1994. Use of mutated Flp recognition target (Frt) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry 33: 12746–12751 [DOI] [PubMed] [Google Scholar]
- 45. Shao L, et al. 2007. Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res. 17: 963–965 [DOI] [PubMed] [Google Scholar]
- 45a. Sigma-Aldrich 2008. TargeTron gene knockout system user guide. Sigma-Aldrich, St. Louis, Mo [Google Scholar]
- 46. Sillers R, Chow A, Tracy B, Papoutsakis ET. 2008. Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab. Eng. 10: 321–332 [DOI] [PubMed] [Google Scholar]
- 47. Steiner E, et al. 2011. Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol. Microbiol. 80: 641–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tracy BP, Jones SW, Fast AG, Indurthi DC, Papoutsakis ET. 2012. Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr. Opin. Biotechnol. 23: 364–381 [DOI] [PubMed] [Google Scholar]
- 49. Tracy BP, Jones SW, Papoutsakis ET. 2011. Inactivation of σE and σG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J. Bacteriol. 193: 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tripathi SA, et al. 2010. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl. Environ. Microbiol. 76: 6591–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tummala SB, Junne SG, Papoutsakis ET. 2003. Antisense RNA downregulation of coenzyme A transferase combined with alcohol-aldehyde dehydrogenase overexpression leads to predominantly alcohologenic Clostridium acetobutylicum fermentations. J. Bacteriol. 185: 3644–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tummala SB, Welker NE, Papoutsakis ET. 2003. Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J. Bacteriol. 185: 1923–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tummala SB, Welker NE, Papoutsakis ET. 1999. Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 65: 3793–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Webb CD, et al. 1998. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol. 28: 883–892 [DOI] [PubMed] [Google Scholar]
- 55. Wilkinson SR, Young M. 1994. Targeted integration of genes into the Clostridium acetobutylicum chromosome. Microbiology 140: 89–95 [Google Scholar]
- 56. Yamaguchi Y, Inouye M. 2009. mRNA interferases, sequence-specific endoribonucleases from the toxin-antitoxin systems. Prog. Mol. Biol. Transl. Sci. 85: 467–500 [DOI] [PubMed] [Google Scholar]
- 57. Yao J, et al. 2006. Use of targetrons to disrupt essential and nonessential genes in Staphylococcus aureus reveals temperature sensitivity of Ll.LtrB group II intron splicing. RNA 12: 1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang XZ, Yan X, Cui ZL, Hong Q, Li SP. 2006. mazF, a novel counter-selectable marker for unmarked chromosomal manipulation in Bacillus subtilis. Nucleic Acids Res. 34: e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang YL, Yu MR, Yang ST. 2012. Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnol. Prog. 28: 52–59 [DOI] [PubMed] [Google Scholar]
- 60. Zhong J, Karberg M, Lambowitz AM. 2003. Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Res. 31: 1656–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu XD, Sadowski PD. 1995. Cleavage-dependent ligation by the Flp recombinase—characterization of a mutant Flp protein with an alteration in a catalytic amino acid. J. Biol. Chem. 270: 23044–23054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.