Abstract
Adhesion of bacteria to mucosal surfaces and epithelial cells is one of the key features for the selection of probiotics. In this study, we assessed the adhesion property of Lactococcus lactis subsp. lactis BGKP1 based on its strong autoaggregation phenotype and the presence of the mucin binding protein (MbpL). Genes involved in aggregation (aggL) and possible interaction with mucin (mbpL), present on the same plasmid pKP1, were previously separately cloned in the plasmid pAZIL. In vivo and in vitro experiments revealed potentially different physiological roles of these two proteins in the process of adherence to the intestine during the passage of the strain through the gastrointestinal tract. We correlated the in vitro and in vivo aggregation of the BGKP1-20 carrying plasmid with aggL to binding to the colonic mucus through nonspecific hydrophobic interactions. The expression of AggL on the bacterial cell surface significantly increased the hydrophobicity of the strain. On the other hand, the presence of AggL in the strain reduced its ability to adhere to the ileum. Moreover, MbpL protein showed an affinity to bind gastric type mucin proteins such as MUC5AC. This protein did not contribute to the binding of the strain to the ileal or colonic part of the intestine. Different potential functions of lactococcal AggL and MbpL proteins in the process of adhesion to the gastrointestinal tract are proposed.
INTRODUCTION
Lactococci are commonly used in the dairy industry, although, traditionally, they are not considered to be natural inhabitants of the human gastrointestinal tract (GIT) (48). This is the main reason why the probiotic activity of Lactococcus strains has been poorly analyzed in the past. Nevertheless, several studies indicated the presence of Lactococcus strains in the flora of the human or animal GIT (18, 19, 24, 44). Therefore, it would be quite beneficial for the food industry, especially for the dairy industry, to develop new probiotic lactococcal strains. One of the desirable properties of bacteria, recommended as the selection criteria for probiotic strain, is host-specific adherence.
Adhesion is a complex bacterial trait that appears to be a multistep process in which both nonspecific and specific ligand receptor mechanisms play important roles. Cell surface proteins of lactic acid bacteria (LAB) have been shown to mediate adhesion to intestinal mucosa, but the mechanisms of adherence to the epithelial surface involve both protein-specific binding and hydrophobic interactions (22, 40).
It has been previously demonstrated that autoaggregation is strongly related to adhesion (10). Autoaggregation of probiotic strains appears to be necessary for adhesion to intestinal epithelial cells (3, 10, 38). Also, physicochemical characteristics of the bacterial cell surface, such as hydrophobicity, may influence autoaggregation and the adhesion of bacteria to different surfaces (10, 36, 54). Previous analyses revealed that the surfaces of autoaggregating LAB strains were highly hydrophobic, whereas the surfaces of nonaggregating strains were hydrophilic (34). Accordingly, hydrophobicity and autoaggregation abilities seem to be important for adhesion (10).
Numerous studies on the physicochemical properties of microbial cell surfaces have shown that the presence of (glyco)proteinaceous material at the cell surface results in higher hydrophobicity, whereas hydrophilic surfaces are associated with the presence of polysaccharides (2, 50). One of the mechanisms for bacterial adherence also involves the binding of microbial cell surface molecules to the protective mucus layer covering the epithelial cells of the GIT of the host (51).
Gut mucus presents the first physical barrier to host-bacterium interaction and plays an important role in the adhesion of microorganisms to host surfaces (51). The presence of mucus is particularly relevant in the colon, where mucus is thickest and microorganisms are most abundant. This protective layer consists of complex mixture of large, highly glycosylated proteins (mucins) and glycolipids that cover the epithelial cells of the intestine and presents the attachment site for the bacteria colonizing the intestine (9). Adherence to the intestinal mucus has been demonstrated for many strains belonging to LAB that promote health in humans and animals (39, 45). In most cases, the adhesion has been shown to be mediated by proteins such as mucin binding proteins (MucBP) that are most abundant in lactobacilli inhabiting the GIT, indicating their potential function in host-microbe interactions (7, 8, 40). Nevertheless, other surface proteins, such as S-layer protein and mannose-specific adhesin protein, have been implicated in contributing to the adhesive properties of lactobacilli (37, 52).
Several studies investigated the aggregation phenomenon of lactococci and associated it with a sex factor and lactose plasmid cointegration event or duplication of the cell wall-spanning (CWS) domain of PrtP proteinase (13, 14). In addition, CluA surface protein was shown to be the only sex factor component required for the aggregation in Lactococcus lactis subsp. cremoris MG1363 (46).
In our previous study, a novel lactococcal aggregation protein AggL from autoaggregating Lactococcus lactis subsp. lactis BGKP1 was identified (25). The gene for AggL is located on a newly characterized plasmid, pKP1, and was shown to be sufficient for cell aggregation in homologous and heterologous lactococci and enterococci (25). Along with the aggL gene, this plasmid contains a mucin binding protein-like gene (mbpL) coding for a potential mucin binding protein that could be involved in the adhesion to mucosa.
The primary structure of AggL is characterized by its modular architecture and a number of repeat regions that share high mutual identity (98 to 100%), among which collagen binding protein B domain is the most abundant. The importance of repeated units in the aggregation of lactococci was previously shown for a CWS region of PrtP (13). Moreover, repeated units could possibly serve as a support for the region involved in adherence, thus facilitating not only aggregation but also bacterial adherence to collagen. The presence of the MucBP-like domain in the primary structure of the MbpL protein is an indication of an interaction with GIT mucosa being its most likely function.
In this context, the aim of the present study was to evaluate the potential roles of AggL and MbpL proteins in the adhesion and autoaggregation of BGKP1. We used previously constructed plasmid derivatives carrying aggL and mbpL genes for in vitro, ex vivo, and in vivo experiments in order to provide evidence of AggL and MbpL in processes important for GIT colonization.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The AggL and MbpL proteins were expressed in L. lactis subsp. lactis BGKP1-20, a nonaggregating strain of wild-type BGKP1, as previously described (25). The strains used in this study are listed in Table 1. Bacteria were grown at 30°C in M17 medium (Oxoid, Basingstoke, United Kingdom) supplemented with 0.5% glucose (GM17). For the selection erythromycin (Serva, Heidelberg, Germany) was added into the medium at a final concentration of 15 μg/ml.
Table 1.
Strains tested in the present study
| Strain | Origin | Phenotype | Designation used in the present study | Reference |
|---|---|---|---|---|
| L. lactis subsp. lactis BGKP1-20 | Spontaneous derivative of L. lactis subsp. lactis BGKP1 | Nonaggregating | BGKP1-20 | 25 |
| L. lactis subsp. lactis BGKP1-20/pAZIL | Derivative of BGKP1-20 carrying pAZIL vector | Nonaggregating | BGKP1-20/pAZIL | 25 |
| L. lactis subsp. lactis BGKP1-20/pAZIL-KPPvScI | Derivative of BGKP1-20 carrying aggL cloned in pAZIL | Aggregating | BGKP1-20/pAZIL-aggL | 25 |
| L. lactis subsp. lactis BGKP1-20/pAZIL-KPE6 | Derivative of BGKP1-20 carrying mbpL cloned in pAZIL | Nonaggregating | BGKP1-20/pAZIL-mbpL | 25 |
In vitro adhesion to HT29-MTX cells.
HT29-MTX cells were kindly supplied by T. Lesuffleur (27). This cell line is considered to be representative of surface or villus-type cells presenting mucus-producing goblet cells (16). The culture and maintenance of the cell lines, as well as the adhesion test, were carried out according to the method of Sánchez et al. (42). Cells were propagated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and with a mixture of antibiotics (50 μg of penicillin/ml, 50 μg of streptomycin/ml, 50 μg of gentamicin/ml). Media and reagents were purchased from PAA, Pasching, Austria. Intestinal cells were seeded in 24-well plates and cultivated until a confluent differentiated state was reached. Overnight cultures of bacteria were diluted as follows. Initial dilutions (1:10) were prepared in phosphate-buffered saline (PBS), subsequent dilutions (1:10) were prepared in DMEM, and final dilutions (1:10) were prepared in DMEM. The wells of cell culture plate that contained HT29-MTX cells were washed three times in PBS. In the next step, 500 μl of bacterial suspension in DMEM was added into wells with the monolayer. An additional 100 μl of bacterial suspension was used for the preparation of dilutions in saline and subsequent colony enumeration that represents the bacterial number available for interaction with HT29-MTX cells. After 1 h of incubation at 37°C and 5% CO2 in a HERACell 150 incubator (Thermo Electron, Osterode, Germany), DMEM with unbound bacteria was aspirated, and the wells were washed three times in PBS. Afterward, 500 μl of 0.25% trypsin (PAA) in PBS was added to dissociate the cell monolayer and detach adhered bacterial cells. After 15 min of incubation at 37°C and 5% CO2, trypsinized well content was used for the preparation of dilutions in saline for counting bacterial cells that had adhered to HT29-MTX monolayer. Aliquots (10 μl) of dilutions prepared before and after interaction with HT29-MTX cells were plated on GM17 erythromycin (15 μg/ml) agar plates. The adhesion test was performed in triplicate for each BGKP1-20 strain.
Ex vivo adhesion test.
Experiments involving animals were approved by the ethical committee of the Faculty of Pharmacy, University of Belgrade, and were conducted in accordance with institutional regulations on animal experimentation. The test was done according to the method of Muñoz-Provencio et al. (32). Three female Wistar rats weighing 200 ± 10 g were sacrificed by increasing the concentration of CO2. Pieces of ileum and colon from each rat were removed and opened longitudinally, and the luminal contents were carefully removed. Segments were washed gently with PBS. Fragments weighing ∼100 mg were positioned along their upper luminal sides on tissue culture inserts (500-μm-pore-size bottom mesh) that were placed inside 15-mm-diameter wells (Netwell culture systems; Costar, Cambridge, MA). Bacterial overnight cultures were diluted 1:100 in RPMI 1640 (Roswell Park Memorial Institute) containing HEPES and l-glutamine (PAA) supplemented with 10% FBS. RPMI 1640 containing prepared bacterial suspensions was added into wells with tissue fragments. For each BGKP1-20 strain, three tissue sections originating from different rats were used for the adhesion test. After 4 h of incubation at 37°C in a 5% CO2 atmosphere in a HERACell 150 incubator (Thermo Electron), the tissues were placed in sterile tubes with 10 ml of PBS for a washing step. Each tube was slowly inverted for 90 s, and the tissue was transferred to a manual glass homogenizer containing 1 ml of saline. In order to determine the number of bacterial cells (before and after adhesion), dilutions of incubation medium (RPMI 1640), wash medium, and homogenization buffers were prepared. Aliquots (10 μl) of dilutions were plated on GM17 erythromycin (15 μg/ml)-agar plates. The plates were incubated at 30°C for 48 h aerobically, and each colony number was determined.
Affinity for hydrophobic solvent.
The partition of studied strains between water and apolar solvent was tested according to the method of Samot et al. (41), with slight modifications. Briefly, cells from overnight cultures were washed in potassium phosphate buffer (10 mM, pH 7) and resuspended in the same buffer to an OD600 of 0.5 ± 0.05. Hexadecane (150 μl) was added to 3 ml of prepared bacterial suspension. The mixture was vortexed twice for 30 s with 30-s intermissions between vortexing. Because of the rapid aggregation of the BGKP1-20/pAZIL-aggL strain, the lower phase was taken 30 s after vortexing. The OD600s of the lower phase and the bacterial suspension before mixing with hexadecane were measured and compared. The experiment was performed in triplicate with independent bacterial overnight cultures. According to the method of Ocaña et al. (35), values that define hydrophobic characteristics are divided into three categories: high (71 to 100%), medium (36 to 70%), and low (0 to 35%) hydrophobicity.
In vitro adhesion to porcine gastric mucin.
The binding of bacterial cells to mucin was tested according to the method of Muñoz-Provencio et al. (32), with modifications. The wells of Maxisorb plates (Nunc, Roskilde, Denmark) were coated at 4°C for 24 h with 200 μl of porcine stomach mucin, type II (Sigma, Germany), and resuspended in carbonate buffer (pH 9.6; 30 mg/ml). The same volume of carbonate buffer was added to control wells. Coated and control wells were washed three times with PBS, and then PBS with 1% Tween 20 was added to saturate the uncoated binding places. After 1 h of incubation at room temperature, the wells were washed once more with PBS and, subsequently, 200 μl of bacterial suspension in PBS was added (the suspension was adjusted to an OD600 of 1). Each BGKP1-20 strain was tested in quadruplicate in both coated and control wells. After overnight incubation at 4°C, the wells were washed three times with PBS containing 0.05% Tween 20 to remove nonadhered cells. Fixation of bound bacterial cells was performed by drying the plate in oven at to 65°C for 45 min. Subsequently, 200 μl of crystal violet (Serva, Heidelberg, Germany) in a final concentration of 1 mg/ml was added to the wells. After 45 min, the unbound stain was removed by six washes with PBS. Citrate buffer (50 mM; pH 4) was added to dissolve the stain bound to the bacterial cells. After 1 h of incubation at room temperature, the absorbance was measured at 620 nm by using a Labsystems Multiskan RC plate reader (Life Sciences International, Hampshire, United Kingdom).
In vivo adhesion test.
Female Wistar rats weighing 200 ± 10 g were used in the study. The rats were housed in cages (three per cage) in a ventilated room with 12-h light/dark cycles and free access to water and food. Bedding was changed daily. The animals were divided in four groups with three rats per group. Three groups were fed with different bacterial strains (Table 1) resuspended in skimmed milk, and a control group received skimmed milk only. For the treatment of rats with bacteria, overnight bacterial cultures were washed with saline, and ∼109 bacterial cells were resuspended in 1 ml of 11% reconstituted skimmed milk (Subotica, Serbia). The rats were treated orally with either 1 ml of bacterial suspension or skimmed milk, using a gastric feeding tube (stainless steel, 18-gauge diameter, 76 mm in length; Instech Solomon, Plymouth Meeting, PA). After 2 weeks of daily treatment, the rats were sacrificed by exposure to increasing CO2 concentrations. Ileum and colon sections were cut longitudinally, the luminal content was carefully removed, and the tissue was gently washed with saline until the content particles were removed. Approximately 100 mg of the ileal and colonic content and the same weight of tissue were sampled in triplicate and resuspended in 1 ml of saline. The content was vortexed vigorously until reaching homogeneity. Tissue was homogenized with a manual glass homogenizer. Aliquots of content and tissue homogenates were taken for direct inoculation (10 μl) on appropriate selective (antibiotic) plates and for serial dilutions in saline. For samples of intestinal and colon content, 10−1 and 10−2 dilutions were prepared. For tissue samples, undiluted homogenate and the first dilution were taken for the inoculation on selective agar plates. After 48 h of aerobic growth at 30°C, the colony number was determined.
Statistical analysis.
Statistical analyses of obtained data were performed using online available software for two-tailed Mann-Whitney U test (http://elegans.som.vcu.edu/∼leon/stats/utest.html). The strength (correlation coefficient [r]) and significance (two-tailed probability [P]) of correlation between expression phenotypes and tested properties were calculated using the Spearman rank-order correlation test (http://vassarstats.net/corr_rank.html).
RESULTS
Adhesion to HT29-MTX cells.
The adhesion of strains to HT29-MTX intestinal cells was calculated as the ratio of bacterial cells (in CFU) in trypsin-treated HT29-MTX bacterial suspensions to bacterial cells (in CFU) diluted in DMEM before being loaded into cell culture wells. The CFU were determined by counting colonies on selective GM17 agar plates after 48 h of aerobic growth. The results are presented graphically as the percent bacterial adhesion (Fig. 1). Among the tested strains, BGKP1-20/pAZIL-aggL showed the lowest binding affinity to HT29-MTX cells (P < 0.05), with an aggregating phenotype correlating negatively (r = −0.8729, P < 0.05) with the adhesion ability. The BGKP1-20/pAZIL-mbpL strain exhibited significantly higher binding to HT29-MTX cells than did strain BGKP1-20/pAZIL (P < 0.05). MbpL expression shows statistically significant correlation (r = 0.7638, P < 0.05) with the adhesion to HT29-MTX cell line.
Fig 1.
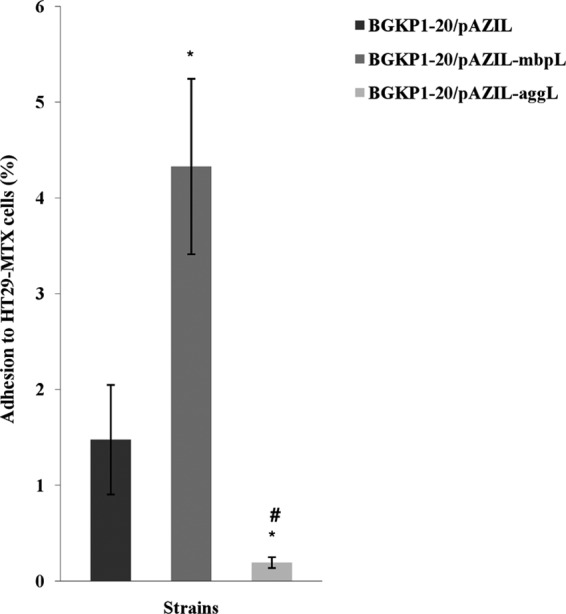
Percent adhesion of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to cultured HT29-MTX cells. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL.
Ex vivo adhesion test.
The number of colonies (CFU) obtained after 48 h of growth on GM17 selective plates was taken as a measure of live bacterial cells present in the tested fractions: (i) tissue homogenate, (ii) incubation medium (RPMI 1640), and (iii) wash medium (saline). The level of adhesion to mucosal segments was calculated as follows: CFU in tissue homogenate/(CFU in tissue homogenate + RPMI + wash medium). The results of the ex vivo test are presented as the mean percent adhesion (Fig. 2). A statistically significant (P < 0.05) lower level of adhesion to ileal tissues of strains expressing aggregation factor AggL than for strains expressing mucin binding protein MbpL or strains carrying plasmid pAZIL was observed. An AggL-expressing phenotype has shown negative correlation (r = −0.869, P < 0.05) with ileal binding affinity. In contrast, testing with colonic fragments showed better adhesion of aggregating strain pAZIL-aggL compared to the other two strains, with a statistically significant difference (P < 0.05). Aggregation ability correlated positively (r = 0.7725, P < 0.05) with adhesion to colonic tissue. The strain harboring the pAZIL-mbpL construct showed significantly less capacity (P < 0.05) to adhere to the intestinal and colonic mucosa compared to strain BGKP1-20/pAZIL. Statistical analysis revealed a negative correlation between MbpL expression and ileal (r = −0.869, P < 0.05) and colonic (r = −0.7725, P < 0.05) tissue binding.
Fig 2.
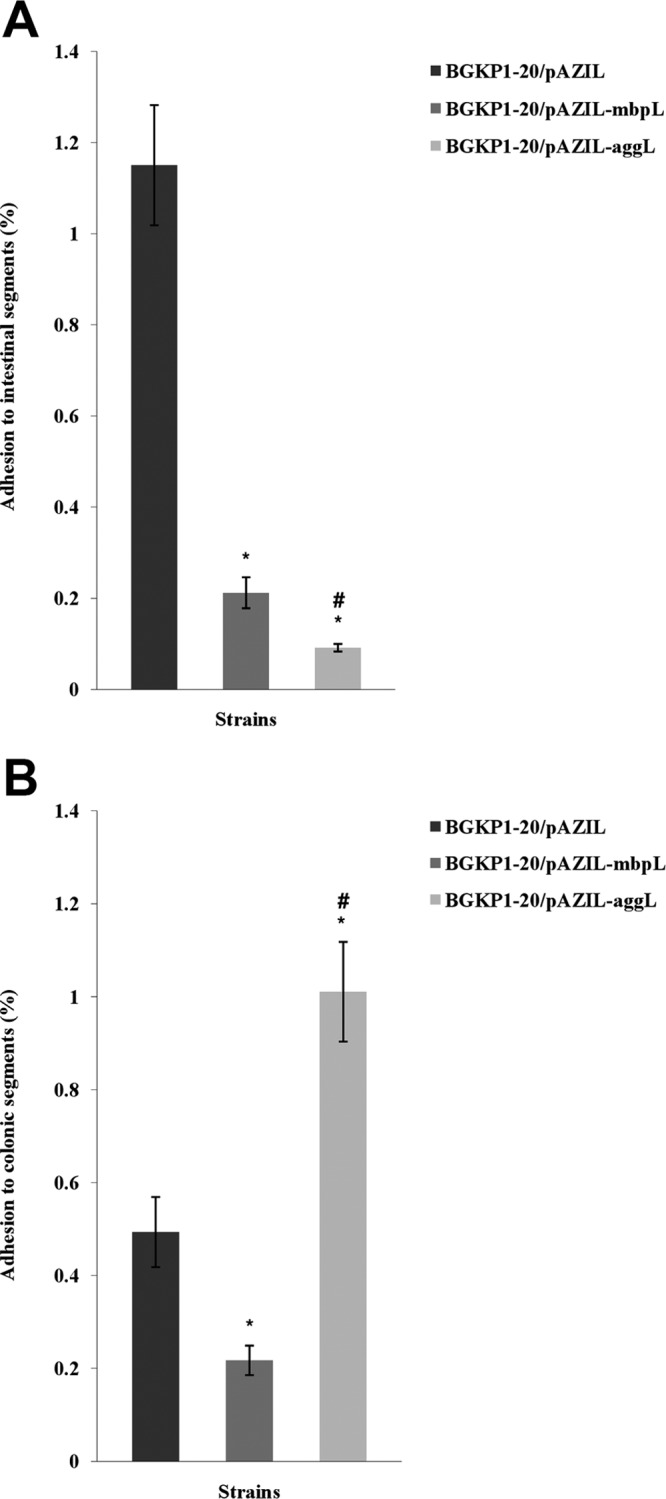
(A) Percent adhesion of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to resected segments of rat ileal tissue. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL. (B) Percent adhesion of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to resected segments of rat colonic tissue. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL.
Hydrophobicity test.
The affinity of tested derivatives to hexadecane in the mix of hexadecane and buffer phases was calculated according to the following formula: (ODa − ODb)/ODa, where ODa is the OD600 of the bacterial suspension in buffer, and ODb is the OD600 of the lower phase after the bacterial suspension and hexadecane were mixed.
The results are presented graphically and are expressed as the mean percentages of affinity to hexadecane (Fig. 3). Strain BGKP1-20/pAZIL showed significantly less (P < 0.05) affinity to hexadecane than both the BGKP1-20/pAZIL-mbpL and BGKP1-20/pAZIL-aggL strains. Among the three tested strains, BGKP1-20/pAZIL-aggL displayed the highest affinity for hexadecane, with a statistically significant difference (P < 0.05). The expression of both MbpL and AggL proteins significantly correlated (r = 0.9333) with the affinity to hexadecane. Furthermore, the absolute values that define their hydrophobic characteristics were 3% for BGKP1-20/pAZIL, 15.7% for BGKP1-20/pAZIL-mbpL, and 48.3% for BGKP1-20/pAZIL-aggL.
Fig 3.
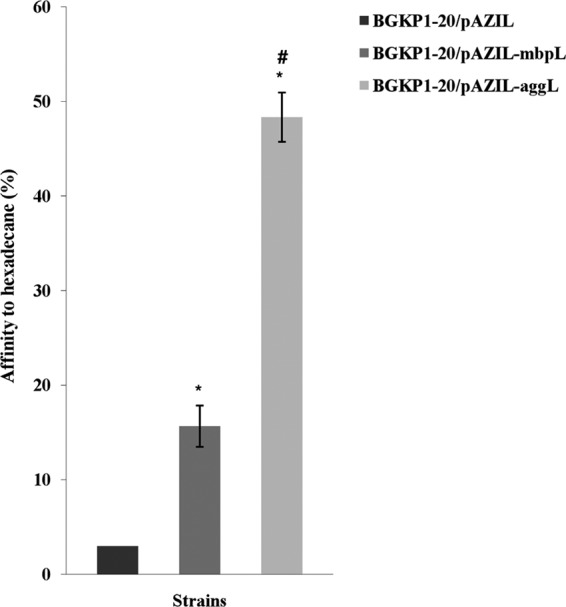
Affinities of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to hexadecane. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL.
In vitro adhesion to porcine stomach mucin.
The results for the in vitro adhesion of BGKP1-20 strains to mucin and to control wells are presented graphically (Fig. 4), where the absorbance values are shown on the y axis. Although the strain expressing an aggregation phenotype exhibited maximal binding capacity to Maxisorb plate plastics (P < 0.05), with significant positive correlation (r = 0.8729), its affinity to mucin-coated wells was similar to the adhesion exhibited by the strain with pAZIL. The correlation in the latter case was not statistically significant. BGKP1-20/pAZIL-mbpL displayed the highest affinity to mucin-coated wells, with a statistically significant difference compared to both BGKP1-20/pAZIL and BGKP1-20/pAZIL-aggL (P < 0.05). MbpL expression correlated positively (r = 0.93, P < 0.05) with binding to mucin-coated wells but has not shown a statistically significant correlation with binding to uncoated wells. Accordingly, the binding of BGKP1-20/pAZIL-mbpL to control wells did not differ from the binding of strain with pAZIL.
Fig 4.
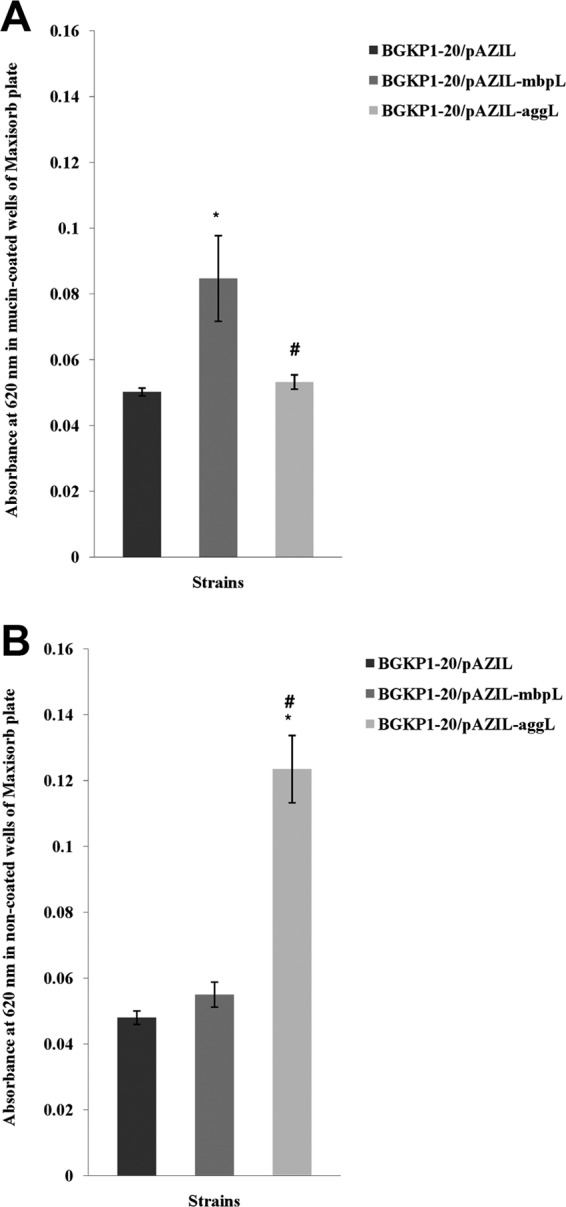
(A) Adhesion levels of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to mucin-coated wells of cell culture plate. The error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL. (B) Adhesion levels of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to empty wells of a Maxisorb culture plate. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL.
In vivo adhesion test.
The number of colonies (i.e., the CFU) visible on selective GM17 plates after 48 h was taken as a measure of the bacterial cell number in luminal content and tissue homogenates. No visible colonies on plates corresponding to the luminal content and the tissues of control rats were detected. Adhesion was calculated as a ratio of the cell number in the tissue homogenate to the cell count in the tissue homogenate plus the cell count in the luminal content: adhesive ability = CFU in tissue/(CFU in tissue + CFU in luminal content).
Results are presented graphically as means of the percent adhesion values (Fig. 5). Strain BGKP1-20/pAZIL-aggL had a higher adhesive ability (P < 0.05) to rat colonic mucosa than did the BGKP1-20/pAZIL and BGKP1-20/pAZIL-mbpL strains. In contrast, strains BGKP1-20/pAZIL and BGKP1-20/pAZIL-mbpL adhered to the intestinal mucosa significantly more (P < 0.05) than did BGKP1-20/pAZIL-aggL. Although a positive correlation between an aggregating phenotype and adhesion to colonic mucosa was obtained (r = 0.8197, P < 0.05), the same phenotype correlated negatively (r = −0.87, P < 0.05) with ileal mucosal binding affinity. There was no difference in the adhesive ability to ileal and colonic mucosa between strains BGKP1-20/pAZIL and BGKP1-20/pAZIL-mbpL. As expected, no significant correlation between MbpL expression and binding to mucosal tissue was detected.
Fig 5.
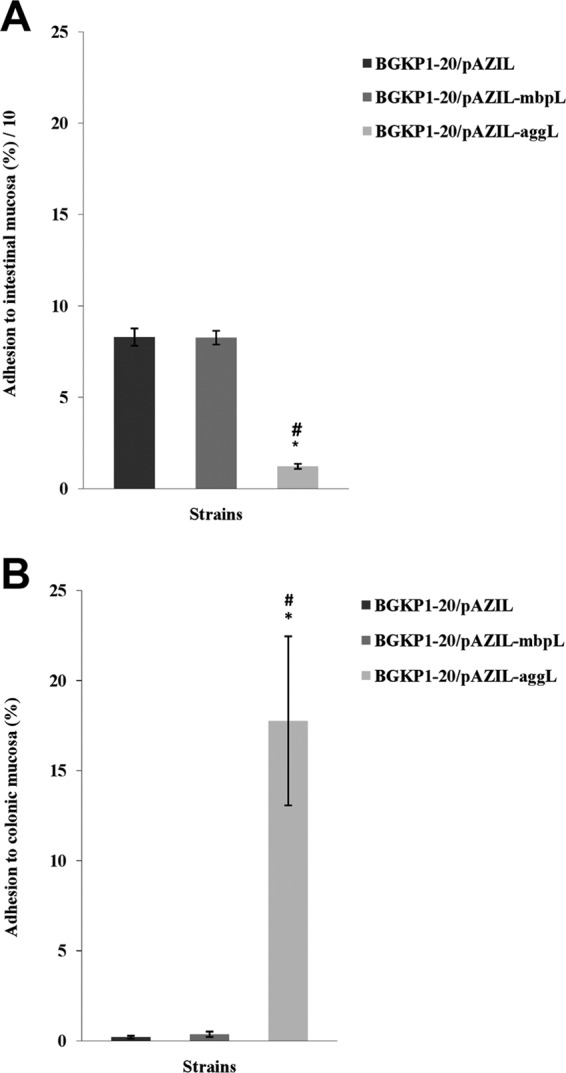
(A) Percent adhesion of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to rat intestinal mucosa. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL. (B) Percent adhesion of L. lactis subsp. lactis BGKP1-20/pAZIL, BGKP1-20/pAZIL-mbpL, and BGKP1-20/pAZIL-aggL strains to rat colonic mucosa. Error bars represent the standard errors. *, statistically significant difference with regard to BGKP1-20/pAZIL; #, statistically significant difference with regard to BGKP1-20/pAZIL-mbpL.
DISCUSSION
Adhesion to intestinal mucosa is considered one of the main features that accounts for the beneficial health effects of specific LAB. Adhesion is a complex process, and it has been correlated with different factors, including the presence of mucin binding proteins and autoaggregation (5, 23, 33). The aggregation ability of probiotic bacteria can potentially inhibit the adherence of pathogenic bacteria to the intestinal mucosa (43). The aggregation phenomenon is often related to adhesion to intestinal epithelial cells in vitro (10, 26). Different factors responsible for the adhesion to mucous components have been identified and their role in this process has been confirmed (1, 29, 30). Moreover, mucous binding proteins have been revealed as one of the effector molecules involved in both mechanisms: the adherence of lactobacilli to the host and cell aggregation (30).
The GIT is covered by mucous gel secreted by epithelial surfaces, and this secretion varies in composition in different gut regions. The ileum and colon are the two most important regions of the GIT in the process of microbial colonization (21). In the present study, we have shown that the presence of mbpL gene on plasmid pAZIL in Lactococcus lactis subsp. lactis BGKP1-20 enabled significantly better adhesion of the strain to HT29-MTX intestinal cells compared to strain BGKP1-20/pAZIL. Similar results were obtained for Bifidobacterium bifidum A8, where Tal protein (transaldolase) was shown to have a role of the surface mucin binding protein, enabling this strain to adhere to HT29 cells (16).
Interestingly, the presence of the aggL gene and aggregation phenotype reduced the ability of BGKP1-20/pAZIL-aggL strain to adhere to this type of intestinal cells. On the other hand, Gonzales-Rodriguez et al. (16) showed that Tal protein, unlike AggL in our study, contributed to both adherence to epithelial cells and to strong autoaggregation phenotype of B. bifidum A8.
Moreover, we can speculate that lactococcal MbpL aids the adhesion to MUC3 and MUC5AC, since these two mucins are the forms predominantly produced by HT29-MTX cells (17). These mucins are normally produced in the stomach and lungs (6, 27, 47). Taking into account our results, it can be inferred that additional factors such as direct interactions between bacteria and HT-29 cell's surface might also be involved in binding to HT29-MTX monolayer (47).
On the other hand, MUC2, MUC3, MUC5AC, and MUC6 are the mucins that are present along the GIT. Moreover, the predominant mucin forms produced in the ileum and a healthy colon are MUC2 and MUC3 (31).
The results obtained here revealed the potential of a strain producing MbpL to bind to the commercially available pig gastric mucin, which is homologous to human MUC5AC on cDNA level (49). It should be also noted that mucins from different species are generally similar, since they mostly differ in their glycosylation pathways. Nevertheless, experiments did not demonstrate the ability of AggL to contribute to adhesion of the strain to this protein. It was previously shown that the aggregation-promoting factor Apf from Lactobacillus gasseri 4B2 served as an adhesion factor that participated in the interaction with the host's mucous layer and intestinal epithelial cells (15). Nevertheless, this protein was not involved in the aggregation of the strain.
Both ex vivo and in vivo experiments showed that expressed AggL protein significantly increased adhesion of the strain to colonic tissue. It appears that aggregation phenotype helps this strain to target colon, since this effect was not observed in the ileum. These results are in accordance with the findings of Voltan et al. (53), who demonstrated that in vivo the aggregation phenotype is required for L. crispatus persistence in the mouse colon. Nevertheless, MbpL protein did not contribute to the adhesion of the strain to both parts of the GIT. The mucous gel present in ileum is not continuous because it is produced in the base of the villi and moved to the surface; thus, there are epithelial regions in distal intestine that are even mucous free (21). In addition, the results indicate that adhesion of bacteria to ileal mucosa reflects binding to both mucous components and cell surface as well (20). Furthermore, mucous gel present in ileum has no stratification such as the one described for colon. It is also known that the mucous layer in colon is thicker compared to the ileum (21), and previous studies using contact angle measurements have demonstrated the difference in hydrophobicity between intestinal and colonic mucosa (28).
Moreover, there is a degree of correlation between hydrophobicity and adhesion to the hydrophobic mucosal surface. When the hydrophobicity of all strains was measured, the strain expressing AggL showed the highest hydrophobicity and, according to Ocaña et al. (35), the strain was classified as “medium hydrophobic.” The strain carrying MbpL and the construct-free plasmid strain were classified as “low hydrophobic” strains, although MbpL carrying strain exhibited higher hydrophobicity. With the hydrophobicity of mucosal surfaces tested in the study, it is likely that the adhesion of the strain expressing AggL is due to nonspecific hydrophobic interactions. This is in agreement with previously published data (12, 26, 28, 54). The hydrophobicity of intestinal surfaces arises from surfactant lipids coating the mucous gel (4). It was hypothesized that lipids secreted in ileum move distally and become established on the surface of colonic mucus gel (11). The surfactant lipids might be responsible for initial nonspecific hydrophobic interactions of bacteria with mucous gel. In light of the already-established differences in the hydrophobicities of the ileum and colon, the different adhesion potentials of the strains to rat mucosa described here could be explained by the different surface properties of tested strains.
Conclusions.
L. lactis subsp. lactis BGKP1 expresses two types of adhesion proteins, AggL and MbpL. In this study, we demonstrate the function of aggregation factor AggL present in the strain BGKP1 in the process of adhesion to GIT mucous tissues. Aggregation of this strain is involved in adhesion to the colonic tissue, a feature that can be used as a targeted action to treat different conditions related to the colon. On the other hand, we demonstrated the potential of strain expressing mucin binding protein MbpL to bind gastric type mucin proteins. This may give an advantage to a strain that expresses this protein for application in other organ tissues such as the stomach, where MUC5AC is predominantly expressed. Due to the different adhesion specificities of the proteins, AggL and MbpL, BGKP1 bacterial cells could colonize different parts of GIT. Further analyses will focus on the screening of lactococci and other LAB for the presence of aggL and mbpL genes.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education and Science, Republic of Serbia (grant 173019).
We thank Myra Macpherson Poznanović, official native English editor of Archives of Biological Sciences.
Footnotes
Published ahead of print 7 September 2012
REFERENCES
- 1. Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. 2006. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology 152: 273– 280 [DOI] [PubMed] [Google Scholar]
- 2. Boonaert CJP, Rouxhet PG. 2000. Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties. Appl. Environ. Microbiol. 66: 2548– 2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boris S, Suárez JE, Barbés C. 1997. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J. Appl. Microbiol. 83: 413– 420 [DOI] [PubMed] [Google Scholar]
- 4. Bourlioux P, Koletzko B, Guarner F, Braesco V. 2003. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine.” Am. J. Clin. Nutr. 78: 675– 683 [DOI] [PubMed] [Google Scholar]
- 5. Buck BL, Altermann E, Svingerud T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71: 8344– 8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callaghan Rose MC, Voynow JA. 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86: 245– 278 [DOI] [PubMed] [Google Scholar]
- 7. Coconnier MH, Klaenhammer TR, Kerneis S, Bernet MF, Servin AL. 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 58: 2034– 2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conway PL, Kjelleberg S. 1989. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J. Gen. Microbiol. 135: 1175– 1186 [DOI] [PubMed] [Google Scholar]
- 9. Dekker J, Rossen JW, Buller HA, Einerhand AW. 2002. The MUC family: an obituary. Trends Biochem. Sci. 27: 126– 131 [DOI] [PubMed] [Google Scholar]
- 10. Del Re B, Sgorbati B, Miglioli M, Palenzona D. 2000. Adhesion, autoaggregation, and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 31: 438– 442 [DOI] [PubMed] [Google Scholar]
- 11. Ehehalt R, Braun A, Karner M, Füllekrug J, Stremmel W. 2010. Phosphatidylcholine as a constituent in the colonic mucosal barrier: physiological and clinical relevance. Biochim. Biophys. Acta 1801: 983– 993 [DOI] [PubMed] [Google Scholar]
- 12. Ehrmann MA, Kurzak P, Bauer J, Vogel RF. 2002. Characterization of lactobacilli toward their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 92: 966– 975 [DOI] [PubMed] [Google Scholar]
- 13. Gajic O, et al. 2003. Novel mechanism of bacteriocin secretion and immunity carried out by lactococcal multidrug resistance proteins. J. Biol. Chem. 278: 34291– 34298 [DOI] [PubMed] [Google Scholar]
- 14. Gasson MJ, Swindell S, Maeda S, Dodd HM. 1992. Molecular rearrangement of lactose plasmid DNA associated with high-frequency transfer and cell aggregation in Lactococcus lactis 712. Mol. Microbiol. 6: 3213– 3223 [DOI] [PubMed] [Google Scholar]
- 15. Goh YJ, Klaenhammer TR. 2010. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 76: 5005– 5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzàles-Rodrìguez I, et al. 2012. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl. Environ. Microbiol. 78: 3992– 3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gouyer V, et al. 2001. Specific secretion of gel-forming mucins and TFF peptides in HT-29 cells of mucin-secreting phenotype. BBA-Mol. Cell Res. 1539: 71– 84 [DOI] [PubMed] [Google Scholar]
- 18. Grahn E, Holm SE, Lilja H, Sellgren K. 1994. Interference of a Lactococcus lactis strain on the human gut flora and its capacity to pass the stomach and intestine. Scand. J. Nutr. 38: 2– 4 [Google Scholar]
- 19. Gruzza M, Duval-Iflah Y, Ducluzeau R. 1992. Colonization of the digestive tract of germ-free mice by genetically engineered strains of Lactococcus lactis: study of recombinant DNA stability. Microb. Releases 1: 165– 171 [PubMed] [Google Scholar]
- 20. Jensena SR, Finka LN, Nielsenb OH, Brynskovb J, Brixa S. 2011. Ex vivo intestinal adhesion of Escherichia coli LF82 in Crohn's disease. Microb. Pathog. 51: 426– 431 [DOI] [PubMed] [Google Scholar]
- 21. Johansson MEV, Larsson JMH, Hansson GC. 2011. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. U. S. A. 108: 4659– 4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirjavainen PV, Ouwehand AC, Isolauri E, Salminen SJ. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167: 185– 189 [DOI] [PubMed] [Google Scholar]
- 23. Kleerebezem M, et al. 2010. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 34: 199– 230 [DOI] [PubMed] [Google Scholar]
- 24. Klijn N, Weerkamp AH, De Vos WM. 1995. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 61: 2771– 2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kojic M, et al. 2011. Cloning and expression of a novel lactococcal aggregation factor from Lactococcus lactis subsp. lactis BGKP1. BMC Microbiol. 11: 265 doi:10.1186/1471-2180-11-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kos B, et al. 2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 94:981– 987 [DOI] [PubMed] [Google Scholar]
- 27. Lesuffleur T, et al. 1993. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 106: 771– 783 [DOI] [PubMed] [Google Scholar]
- 28. Lichtenberger LM. 1995. The hydrophobic properties of gastrointestinal mucus. Annu. Rev. Physiol. 57: 565– 583 [DOI] [PubMed] [Google Scholar]
- 29. Macías-Rodríguez ME, Zagorec M, Ascencio F, Vázquez-Juárez R, Rojas M. 2009. Lactobacillus fermentum BCS87 expresses mucus- and mucin-binding proteins on the cell surface. J. Appl. Microbiol. 107: 1866– 1874 [DOI] [PubMed] [Google Scholar]
- 30. Mackenzie DA, et al. 2010. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156: 3368– 3378 [DOI] [PubMed] [Google Scholar]
- 31. McGuckin AM, Linden KS, Sutton P, Florin HT. 2011. Mucin dynamics and enteric pathogens. Nat. Rev. 9: 265– 278 [DOI] [PubMed] [Google Scholar]
- 32. Muñoz-Provencio D, et al. 2009. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 191: 153– 161 [DOI] [PubMed] [Google Scholar]
- 33. Nikolic M, Jovcic B, Kojic M, Topisirovic L. 2010. Surface properties of Lactobacillus and Leuconostoc isolates from homemade cheeses showing auto-aggregation ability. Eur. Food Res. Technol. 231: 925– 931 [Google Scholar]
- 34. Nikolic M, et al. 2008. Phenotypic and genotypic characterization of the microflora in the autochthonous homemade semi-hard goat's milk cheese. Int. J. Food Microbiol. 122: 162– 170 [DOI] [PubMed] [Google Scholar]
- 35. Ocaña VS, Bru E, Ruiz Holgado AAP, Nader-Macias ME. 1999. Surface characteristics of lactobacilli isolated from human vagina. J. Gen. Appl. Microbiol. 45: 203– 212 [DOI] [PubMed] [Google Scholar]
- 36. Pérez PF, Minnaard J, Disalvo E, De Antoni G. 1998. Surface properties of bifidobacterial strains of human origin. Appl. Environ. Microbiol. 6: 21– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pretzer G, et al. 2005. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187: 6128– 6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reid G, McGroarty JA, Angotti R, Cook RL. 1988. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can. J. Microbiol. 34: 344– 351 [DOI] [PubMed] [Google Scholar]
- 39. Reid G, et al. 2003. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 37: 105– 118 [DOI] [PubMed] [Google Scholar]
- 40. Roos S, Jonsson H. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148: 433– 442 [DOI] [PubMed] [Google Scholar]
- 41. Samot J, Lebreton J, Badet C. 2011. Adherence capacities of oral lactobacilli for potential probiotic purposes. Anaerobe 17: 69– 72 [DOI] [PubMed] [Google Scholar]
- 42. Sánchez B, Fernández-GarcíA M, Margolles A, De los Reyes-Gavilán CG, Ruas-Madiedo P. 2010. Technological and probiotic selection criteria of a bile-adapted Bifidobacterium animalis subsp. lactis strain. Int. Dairy J. 20: 800– 805 [Google Scholar]
- 43. Schachtsiek M, Hammes WP, Hertel C. 2004. Characterization of Lactobacillus coryniformis DSM 20001T surface protein Cpf mediating coaggregation with and aggregation among pathogens. Appl. Environ. Microbiol. 70: 7078– 7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlundt J, Saadbye P, Lohmann B, Jacobsen BL, Nielsen EM. 1994. Conjugal transfer of plasmid DNA between Lactococcus lactis strains and distribution of transconjugants in the digestive tract of gnotobiotic rats. Microb. Ecol. Health Dis. 7: 59– 69 [Google Scholar]
- 45. Servin AL. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28: 405– 440 [DOI] [PubMed] [Google Scholar]
- 46. Stentz R, Gasson M, Shearman C. 2006. The Tra domain of the lactococcal CluA surface protein is a unique domain that contributes to sex factor DNA transfer. J. Bacteriol. 188: 2106– 2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tassell M, Miller M. 2011. Lactobacillus adhesion to mucus. Nutrients 3: 613– 636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teuber M, Geis A, Neve H. 1992. The genus Lactococcus, p 1482–1501 In Balows A, et al. (ed), The prokaryotes, 2nd ed, vol 2 Springer Verlag, New York, NY [Google Scholar]
- 49. Turner B, Bansil R, Afdhal NH. 2007. Expression of cysteine-rich C-terminal domains of pig gastric mucin in Pichia pastoris. FASEB J. 21: 928.4 [Google Scholar]
- 50. Van der Mei HC, Van de Belt-Gritter B, Pouwels PH, Martinez B, Busscher HJ. 2003. Cell surface hydrophobicity is conveyed by S-layer proteins a study in recombinant lactobacilli. Colloid Surface B 28:127– 134 [Google Scholar]
- 51. Velez MP, De Keersmaecker SCJ, Vanderleyden J. 2007. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 276: 140– 148 [DOI] [PubMed] [Google Scholar]
- 52. Viljanen MJ, Palv A. 2007. Isolation of surface (S) layer protein carrying Lactobacillus species from porcine intestine and faeces and characterization of their adhesion properties to different host tissues. Vet. Microbiol. 124: 264– 273 [DOI] [PubMed] [Google Scholar]
- 53. Voltan S, et al. 2007. Aggregating phenotype in Lactobacillus crispatus determines intestinal colonization and TLR2 and TLR4 modulation in murine colonic mucosa. Clin. Vaccine Immunol. 14: 1138– 1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wadstrom T, et al. 1987. Surface properties of lactobacilli isolated from the small intestine of pigs. J. Appl. Bacteriol. 62: 513– 520 [DOI] [PubMed] [Google Scholar]


