Abstract
Salmonellosis is one of the most common causes of food-borne disease in the United States. Increasing antimicrobial resistance and corresponding increases in virulence present serious challenges. Currently, empirical therapy for invasive Salmonella enterica infection includes either ceftriaxone or ciprofloxacin (E. L. Hohmann, Clin. Infect. Dis. 32:263–269, 2001). The blaCMY-2 gene confers resistance to ceftriaxone, the antimicrobial of choice for pediatric patients with invasive Salmonella enterica infections, making these infections especially dangerous (J. M. Whichard et al., Emerg. Infect. Dis. 11:1464–1466, 2005). We hypothesized that blaCMY-2-positive Salmonella enterica would exhibit increased MICs to multiple antimicrobial agents and increased resistance gene expression following exposure to ceftriaxone using a protocol that simulated a patient treatment in vitro. Seven Salmonella enterica strains survived a simulated patient treatment in vitro and, following treatment, exhibited a significantly increased ceftriaxone MIC. Not only would these isolates be less responsive to further ceftriaxone treatment, but because the blaCMY-2 genes are commonly located on large, multidrug-resistant plasmids, increased expression of the blaCMY-2 gene may be associated with increased expression of other drug resistance genes located on the plasmid (N. D. Hanson and C. C. Sanders, Curr. Pharm. Des. 5:881–894, 1999). The results of this study demonstrate that a simulated patient treatment with ceftriaxone can alter the expression of antimicrobial resistance genes, including blaCMY-2 and floR in S. enterica serovar Typhimurium and S. enterica serovar Newport. Additionally, we have shown increased MICs following a simulated patient treatment with ceftriaxone for tetracycline, amikacin, ceftriaxone, and cefepime, all of which have resistance genes commonly located on CMY-2 plasmids. The increases in resistance observed are significant and may have a negative impact on both public health and antimicrobial resistance of Salmonella enterica.
INTRODUCTION
Salmonellosis is one of the most common causes of food-borne disease in the United States (3). An estimated 1.2 million people in the United States are infected with Salmonella enterica annually (16). Each year, Salmonella infections in the United States result in an estimated 160,000 physician visits, 15,000 hospitalizations, and 400 deaths (13, 21). Among the major food-borne pathogens, Salmonella enterica is the leading cause of hospitalization and death (16). Ninety-five percent of Salmonella enterica cases in the United States result from ingestion of contaminated, incompletely cooked foods, such as poultry, beef, eggs, milk, vegetables, and fruit (20, 21, 24).
Salmonella enterica is an important food-borne pathogen which is distributed worldwide. Antimicrobials are not indicated for the majority of Salmonella enterica infections, which typically result in a self-limited gastroenteritis illness. However, antimicrobial therapy is indicated, and may be life-saving, for the management of invasive infection (17). Increasing virulence and corresponding increases in antimicrobial resistance present serious challenges to human medicine, veterinary medicine, and public health (7, 9). As the number of effective treatment choices for invasive Salmonella enterica infections continues to decline, careful utilization of antimicrobial agents is necessary. Currently, empirical therapy for invasive Salmonella enterica infection includes either ceftriaxone or ciprofloxacin (11). The blaCMY-2 gene encodes an AmpC β-lactamase, which confers reduced susceptibility or resistance to extended-spectrum cephalosporins, such as ceftriaxone (23). Ciprofloxacin is contraindicated in pediatric patients, making invasive infections in this population especially concerning (22).
Invasive bloodstream infection and hospitalization as a result of Salmonella enterica infection is associated with antimicrobial resistance. In patients with Salmonella enterica serovar Typhimurium infection, the association between resistance, invasive infection, and hospitalization is particularly strong (7, 9, 20). Therefore, not only does multiple-drug-resistant Salmonella enterica contribute to increased morbidity and mortality, it also contributes to increased health care costs. Isolates utilized in this study include Salmonella Typhimurium and Salmonella enterica serovar Newport strains, all of which have a plasmid containing the blaCMY-2 and floR genes. While blaCMY-2 encodes a β-lactamase that confers resistance to β-lactam antimicrobials, the floR gene encodes a phenicol-specific efflux pump which confers resistance to phenicol antimicrobials, such as chloramphenicol (2). The blaCMY-2 AmpC β-lactamase enzymes found in these strains are divergent from traditional extended-spectrum β-lactamases in that blaCMY-2 AmpC β-lactamase enzymes are not inhibited by clavulanic acid.
A series of Salmonella Typhimurium and Salmonella Newport strains were subjected to a simulated patient treatment regimen with ceftriaxone in vitro. We hypothesized that blaCMY-2-positive Salmonella enterica would exhibit increased MICs to multiple antimicrobial agents and increased resistance gene expression following exposure to ceftriaxone using a protocol that simulated patient treatment. Here, we show a statistically significant increase in MICs to ceftriaxone, cefepime, amikacin, and tetracycline following treatment. Additionally, the expression of both blaCMY-2 and floR was influenced by exposure to ceftriaxone.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Four Salmonella enterica serovar Typhimurium strains and three Salmonella enterica serovar Newport strains were utilized in this study. Strains utilized were obtained from a laboratory stock of CMY-2-positive Salmonella strains which were screened for the ability to survive a simulated patient treatment with ceftriaxone. The strains had been previously obtained from human stool, porcine liver, bovine liver, and bovine intestine. Those designated pretreatment isolates were the strains in their original state. Isolates labeled posttreatment were pretreatment isolates which had been exposed to ceftriaxone using a protocol that simulated patient treatment with ceftriaxone. Following the simulated patient treatment, these isolates were designated posttreatment isolates. Isolates designated posttreatment with ceftriaxone pressure were posttreatment isolates grown with 8 μg/ml ceftriaxone prior to MIC and gene expression assays. Isolates retained their designated strain numbers with the addition of the treatment designation throughout the experiment. All isolates were stored at −80°C until further use.
Replication of patient treatment in vitro.
The pharmacokinetics of a 2-g ceftriaxone adult dose was simulated in vitro using the following protocol. Bacterial cells were grown on Mueller-Hinton agar (MH; Becton, Dickinson, and Company, Sparks, MD) at 37°C for 18 h. A colony from each plate was used to inoculate 10 ml MH broth (BD) in a 14-ml Falcon tube (BD) which was grown at 37°C for 2 h on an orbital shaker (New Brunswick Scientific, Edison, NJ) at 100 rpm. The simulated patient treatments with ceftriaxone (Sigma-Aldrich, St. Louis, MO) were all performed with 50 ml culture in 150-ml flasks while shaking at 100 rpm and 37°C. Briefly, the treatment concentrations, all with ceftriaxone (Sigma-Aldrich), were as follows: 257 μg/ml for 45 min, 192 μg/ml for 1 h, 154 μg/ml for 2 h, and 60 μg/ml for 18 h. The concentrations used were based off the mean plasma concentration of ceftriaxone for an adult receiving a 2-g intravenous dose (14).
Antimicrobial susceptibility testing.
MICs were determined by broth microdilution as described by the Clinical and Laboratory Standards Institute (CLSI) standard protocol (4, 5). The following antimicrobials were used: ampicillin (AMP; Fisher Scientific, Fair Lawn, NJ), cephalothin (CEF; Sigma-Aldrich, St. Louis, MO), ceftriaxone (CRO; Sigma-Aldrich), cefepime (FEP; USP, Rockville, MD), gentamicin (GEN; Fisher Scientific), amikacin (AMK; Fisher Scientific), tetracycline (TET; MP Biomedicals, Solon, OH), chloramphenicol (CHL; MP Biomedicals), ciprofloxacin (CIP; LKT Laboratories, St. Paul, MN), and piperacillin-tazobactam (TZP; MP Biomedicals/Chem-Impex International, Wood Dale, IL). Escherichia coli ATCC 25922 was used as a quality control organism in all antimicrobial susceptibility assays. All assays were run at least twice in duplicate.
RNA isolation and quantitative reverse transcriptase PCR (qRT-PCR).
Total RNA was isolated from liquid culture with an optical density at 600 nm (OD600) of 0.4 for all Salmonella Typhimurium and Salmonella Newport isolates using the Promega SV total RNA isolation system (Promega Corporation, Madison, WI) according to the manufacturer's instructions. An additional DNase treatment was performed using RQ1 RNase-free DNase (Promega Corporation). The concentration and quality of the RNA in each sample were determined by measuring the absorbance at 230, 260, and 280 nm. Reverse transcription reactions were performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's recommendations.
Relative expression levels of blaCMY-2 and floR were determined by performing qRT-PCR on DNA obtained following the reverse transcription reactions. Amplification was performed with consensus primers (Table 1) for blaCMY-2, floR, and recA genes as previously described (19). The qPCR amplifications were performed by combining 10 μl of 10-ng/μl cDNA with a 15 μl mixture of SYBR green Supermix (Bio-Rad, Hercules, CA) and 10 nM primers. The fluorescence signal was detected by a Mini-Opticon real-time PCR instrument (Bio-Rad). The recA gene was used as an internal control for all copy number determinations (19). Data shown are the means from three replicates. DNA fold change of the blaCMY-2 and floR genes was determined using the Pfaffl method (15).
Table 1.
Primers used for qRT-PCR
Statistical analysis.
Data are presented as means and standard deviations. Statistical comparisons of MIC data were made using the Kruskal-Wallis test with Dunn's post hoc test where appropriate (8). Gene expression data were statistically analyzed using the independent Student t test (8). A P value of less than 0.05 was considered significant.
RESULTS
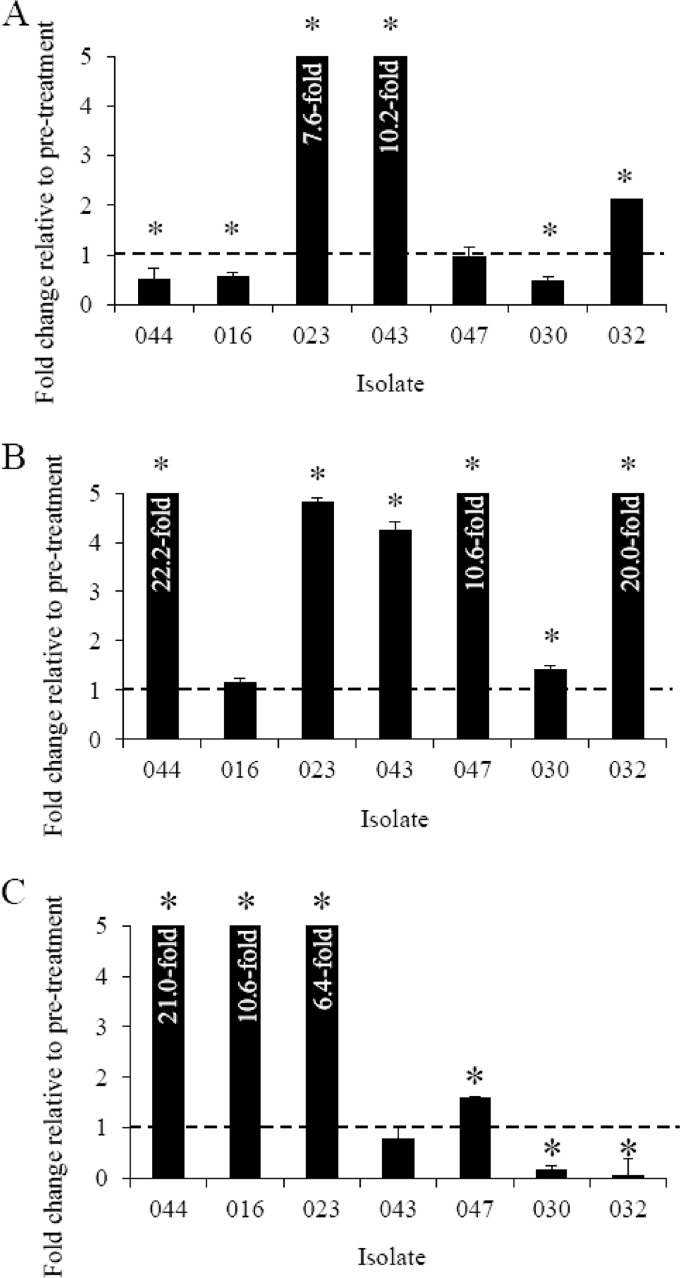
To determine if a simulated patient treatment had an effect on blaCMY-2 gene expression and resistance to ceftriaxone, qRT-PCR and MIC assays were performed. The data in Fig. 1 show the relative change in blaCMY-2 and floR gene expression following exposure to ceftriaxone using a protocol that simulated patient treatment in vitro. Presence of the extended-spectrum β-lactamase gene blaCMY-2 was detected in all isolates. Salmonella Typhimurium strains 043 and 032 and Salmonella Newport strain 023 demonstrated a significant increase (2.1- to 10.2-fold) in blaCMY-2 gene expression following a simulated patient treatment with ceftriaxone (Fig. 1A). However, Salmonella Newport 044 and 016 and Salmonella Typhimurium 030 showed a significant decrease (0.5- to 0.6-fold) in blaCMY-2 expression, while Salmonella Typhimurium 047 exhibited no change (1.0-fold) in posttreatment isolates (Fig. 1A). MICs increased significantly in posttreatment isolates (155 μg/ml) relative to those of pretreatment isolates (52 μg/ml) (Table 2).
Fig 1.
Posttreatment blaCMY-2 (A), posttreatment-with-ceftriaxone-pressure blaCMY-2 (B), and floR (C) gene expression (fold change) among S. enterica serovar Newport 044, 016, and 023 and S. enterica serovar Typhimurium 043, 047, 030, and 032 by qRT-PCR, relative to the expression of their respective pretreatment isolates. Expression of blaCMY-2 and floR was normalized to recA for each sample. Gene expression data were statistically analyzed using independent Student t tests. Bars show the means of three replicates. Dashed lines show a fold change of 1, error bars indicate the standard deviation, and asterisks show significant differences (P < 0.05).
Table 2.
Average MICs for Salmonella Typhimurium and Salmonella Newport isolates used in this study
| Breakpoint or isolate group | MIC (μg/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CEF | CRO | FEP | AMK | GEN | TET | CHL | CIP | TZP | |
| Breakpoint | 32 | 32 | 4 | 32 | 64 | 16 | 16 | 32 | 4 | 128 |
| Isolate group | ||||||||||
| Pretreatment | 1,836 (418) | ≥2,000 (0) | 52 (5) | 91 (33) | 13 (4) | 6 (3) | 79 (19) | 150 (40) | 0.3 (0) | 400 (0) |
| Posttreatment | ≥2,000 (0) | ≥2,000 (0) | 155* (84) | 128* (0) | 18* (5) | 6 (2) | 107* (23) | 157 (43) | 0.3 (0) | 386 (36) |
| Posttreatment with CRO pressure | ND | ND | 162* (86) | 69 (28) | ND | 6 (2) | 112* (35) | 186 (46) | ND | 73* (139) |
Values represent means (standard deviations) from 7 strains utilized in this study. AMP, ampicillin; CEF, cephalothin; CRO, ceftriaxone; FEP, cefepime; AMK, amikacin; GEN, gentamicin; TET, tetracycline; CHL, chloramphenicol; CIP, ciprofloxacin; TZP, piperacillin-tazobactam. Statistical comparisons of MIC data were made using the Kruskal-Wallis test with Dunn's post hoc test where appropriate. *, P < 0.05; ND, not determined.
Examination of the effect of continued ceftriaxone pressure on gene expression and resistance to ceftriaxone and cefepime was assessed by qRT-PCR and MICs. Salmonella Newport 044 and 023 and Salmonella Typhimurium 043, 047, 030, and 032 posttreatment isolates all displayed statistically significant increases (1.4- to 22.2-fold) in blaCMY-2 expression when grown in 8 μg/ml ceftriaxone (Fig. 1B). Expression of blaCMY-2 increased in Salmonella Newport 016 when grown in ceftriaxone; however, the increase was not significant (1.2-fold) (Fig. 1B). The increase in blaCMY-2 expression correlated well with a significant increase in MIC in posttreatment isolates with ceftriaxone pressure (162 μg/ml) relative to that in pretreatment isolates (52 μg/ml) (Table 2). The MICs to another extended-spectrum cephalosporin, cefepime, also showed a statistically significant increase in posttreatment isolates (128 μg/ml) relative to that in pretreatment isolates (91 μg/ml). Interestingly, MICs to cefepime decreased in posttreatment isolates with ceftriaxone pressure relative to that in pretreatment isolates; however, the decrease was not statistically significant (Table 2). Further investigation into the decrease in cefepime MIC is necessary in order to determine the mechanism behind the decreased MIC observed.
The effect of a simulated patient treatment with ceftriaxone on chloramphenicol resistance and floR expression was determined via MIC and qRT-PCR assays. The expression of floR in the posttreatment isolates with ceftriaxone pressure increased significantly (1.6- to 21.0-fold) in Salmonella Newport 044, 016, and 023 and Salmonella Typhimurium 047 (Fig. 1C). Decreased expression (0.1- to 0.2-fold) was seen in Salmonella Typhimurium 030 and 032, while no significant change (0.8-fold) was observed in Salmonella Typhimurium 043 (Fig. 1C). Chloramphenicol MICs increased in the posttreatment isolates with ceftriaxone pressure (186 μg/ml) relative to that in the pretreatment isolates (150 μg/ml); however, the increase was not statistically significant.
Following a simulated patient treatment with ceftriaxone, significant increases in MICs were observed for ceftriaxone, cefepime, amikacin, and tetracycline (Table 2). It is important to note that the increase in MICs observed was not due to differences in growth rate between the pretreatment and posttreatment isolates (data not shown). There was no statistically significant increase in MIC for ampicillin, cephalothin, gentamicin, ciprofloxacin, or piperacillin-tazobactam (Table 2). Tetracycline MICs exhibited a pattern similar to that for ceftriaxone, with statistically significant increases in posttreatment isolates (107 μg/ml) and posttreatment isolates with ceftriaxone pressure (112 μg/ml) relative to the MICs in pretreatment isolates (79 μg/ml) (Table 2).
DISCUSSION
We have shown that a simulated patient treatment with ceftriaxone altered blaCMY-2 and floR expression as well as the phenotypic resistance of multiple isolates. Relative blaCMY-2 expression increased significantly in six of seven posttreatment isolates with ceftriaxone pressure, with one isolate showing an increase that was not statistically significant (Fig. 1B). There was no difference in growth between the pretreatment and posttreatment isolates, as determined by growth curve assays, which indicated that the phenotypic change in resistance was not due to an alteration in growth parameters. Interestingly, of the posttreatment isolates grown without ceftriaxone pressure, just three of the seven isolates exhibited a significant increase in blaCMY-2 gene expression (Fig. 1A). Compared to blaCMY-2 expression in the presence of ceftriaxone, these data suggest that increases in blaCMY-2 expression are short-lived and require the presence of ceftriaxone.
The increase in blaCMY-2 expression observed in the posttreatment isolates with ceftriaxone pressure correlated with the MIC data. The MICs to cefepime also increased following treatment with ceftriaxone (Table 2), a result which indicated that a common mechanism likely conferred resistance to both of these cephalosporins. Extended-spectrum cephalosporin resistance, mediated by blaCMY genes, is the major mechanism of cephalosporin resistance among Salmonella enterica strains (1, 6). Resistance to β-lactam antimicrobials, such as ceftriaxone, is linearly correlated with the lactamase level over a large range, and resistance to β-lactams can be achieved by increasing enzyme levels (22). Thus, with increased blaCMY-2 expression, increased resistance would be expected. Our data indicate that therapeutic concentrations of ceftriaxone can induce increased blaCMY-2 expression. This finding builds upon previous studies with Enterobacteriaceae which have shown inducible AmpC expression in response to β-lactam antimicrobials (10, 12).
In a recent study, Salmonella Typhimurium lineages carrying a blaTEM-1 β-lactamase gene, which is similar to the blaCMY-2 gene in function, were allowed to develop resistance to progressively increased concentrations of cephalothin and cefaclor (19). As the lineages developed resistance to higher antimicrobial concentrations, amplification of the blaTEM-1 gene was found to be the primary and most common resistance mechanism (19). Though this study does not present evidence of gene amplification as a resistance mechanism, increases in blaCMY-2 expression as a result of exposure to cephalosporins highlight an additional mechanism leading to increased resistance as a result of cephalosporin exposure. The data in the present study complement this work while employing extended-spectrum cephalosporins. The study on blaTEM-1-mediated resistance utilized slowly increasing concentrations of antimicrobials not commonly used to treat invasive Salmonella enterica infections; however, this study enlisted one of the drugs of choice for treatment of invasive infections. Additionally, the isolates were exposed to ceftriaxone in a manner which simulated patient infection and treatment. The simulated patient treatment with ceftriaxone closely mimics the mean plasma concentrations seen in both adult and pediatric patients (14, 18). Thus, this study contributes to the growing body of knowledge of Salmonella enterica drug resistance and response to antimicrobial treatment.
Expression of floR was determined via qRT-PCR for pretreatment isolates and posttreatment isolates with ceftriaxone pressure. Statistically significant increases in expression of floR were observed in four of seven posttreatment isolates under ceftriaxone pressure, significant decreases in expression were observed in two posttreatment isolates under ceftriaxone pressure, and one isolate exhibited no significant change in floR expression (Fig. 1C). The MICs to chloramphenicol did increase in posttreatment isolates grown under ceftriaxone pressure relative to those in pretreatment isolates; however, the increase was not statistically significant (Table 2). Additional study is necessary to better understand the effect of ceftriaxone on floR gene expression and phenotypic resistance to chloramphenicol. Further, unlike the blaCMY-2 gene product, an extended-spectrum β-lactamase enzyme in which resistance is directly proportional to the amount of enzyme present, the floR gene product is a multidrug efflux pump (2). Thus, floR regulation is more sensitive and small changes in expression can result in increased phenotypic resistance. Unlike with blaCMY-2, there is not a linear relationship between floR expression and phenotypic resistance (2).
It is important to note that the system utilized in this study does have some inherent limitations. Performing the simulated patient treatment in vitro did allow duplication of the pharmacokinetics of ceftriaxone but did not incorporate any type of host immune response. The host immune response is an important factor in any infectious process, especially for intracellular pathogens such as Salmonella enterica. Employing 7 strains, the data presented here characterize a response in a small sample of Salmonella enterica strains. A larger sample would need to be studied before any generalizations could be made. This study focused on the interaction of Salmonella enterica with ceftriaxone and its effect on phenotypic characteristics as well as expression of the blaCMY-2 and floR genes; however, moving this study into a model with a host immune response is necessary to better characterize the response in vivo.
Though this initial study was performed in vitro with a small number of Salmonella enterica isolates, the data describe an important phenomenon. We intend to expand the study to include a larger number of Salmonella enterica isolates which also contain the blaCMY-2 plasmid. Additionally, shifting the simulated patient infection and treatment to an animal model will provide necessary data for evaluating the effects of ceftriaxone treatment when combined with a functioning immune system. Since Salmonella enterica is capable of host cell invasion and intracellular proliferation, an animal model is absolutely necessary to fully evaluate our initial findings. Performing gene expression arrays and, subsequently, qRT-PCR on additional resistance genes would give a more complete picture of the impact of a simulated patient treatment with ceftriaxone on Salmonella enterica. Finally, sequencing of the blaCMY-2 plasmids and construction of plasmid maps would shed additional light on potential regulatory mechanisms involved in this phenomenon.
The fact that seven Salmonella enterica isolates were able to survive a simulated patient treatment and then exhibit an increased ceftriaxone MIC is an important observation. Not only would these isolates be less responsive to further ceftriaxone treatment, but because the blaCMY-2 genes are commonly located on large, multidrug-resistant plasmids, increased expression of the blaCMY-2 gene may also be associated with increased expression of other drug resistance genes located on the plasmid (10). The results of this study demonstrate that a simulated patient treatment with ceftriaxone can alter the expression of antimicrobial resistance genes, including blaCMY-2 and floR in Salmonella Typhimurium and Salmonella Newport. Here, we show increased MICs following a simulated patient treatment with ceftriaxone for tetracycline, amikacin, ceftriaxone, and cefepime, all of which have resistance genes commonly located on CMY-2 plasmids. The increases in resistance observed are significant and may have a negative impact on both public health and antimicrobial resistance of Salmonella enterica.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Marie Nguyen.
This work was supported by the Iowa Osteopathic Education and Research Foundation and the Mercy Foundation.
Footnotes
Published ahead of print 7 September 2012
REFERENCES
- 1. Biedenbach DJ, Toleman M, Walsh TR, Jones RN. 2006. Analysis of Salmonella spp. with resistance to extended-spectrum cephalosporins and fluoroquinolones isolated in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn. Microbiol. Infect. Dis. 54:13–21 [DOI] [PubMed] [Google Scholar]
- 2. Braibant M, Chevalier J, Chaslus-Dancla E, Pages JM, Cloeckaert A. 2005. Structural and functional study of the phenicol-specific efflux pump FloR belonging to the major facilitator superfamily. Antimicrob. Agents Chemother. 49:2965–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333–337 [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5.Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. CLSI M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Dunne EF, et al. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase. JAMA 284:3151–3156 [DOI] [PubMed] [Google Scholar]
- 7. Fluit AC. 2005. Towards more virulent and antibiotic-resistant Salmonella. FEMS Immunol. Med. Microbiol. 43:1–11. [DOI] [PubMed] [Google Scholar]
- 8.GraphPad Software 2003. GraphPad InStat (version 3.06). GraphPad Software, San Diego, CA [Google Scholar]
- 9. Guerra B, Junker E, Miko A, Helmuth R, Mendoza MC. 2004. Characterization and localization of drug resistance determinants in multidrug-resistant, integron-carrying Salmonella enterica serotype Typhimurium strains. Microb. Drug Resist. 10:83–91 [DOI] [PubMed] [Google Scholar]
- 10. Hanson ND, Sanders CC. 1999. Regulation of inducible AmpC β-lactamase expression among Enterobacteriaceae. Curr. Pharm. Des. 5:881–894 [PubMed] [Google Scholar]
- 11. Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263–269 [DOI] [PubMed] [Google Scholar]
- 12. Jacobs C, Frère JM, Normark S. 1997. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell 88:823–832 [DOI] [PubMed] [Google Scholar]
- 13. Molbak K. 2005. Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens. Clin. Infect. Dis. 41:1613–1620 [DOI] [PubMed] [Google Scholar]
- 14. Patel IH, et al. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob. Agents Chemother. 20:634–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 doi:10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sjölund-Karlsson M, et al. 2010. Salmonella isolates with decreased susceptibility to extended-spectrum cephalosporins in the United States. Foodborne Pathog. Dis. 7:1503–1509 [DOI] [PubMed] [Google Scholar]
- 18. Steele RW, et al. 1983. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob. Agents Chemother. 23:191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun S, Berg OG, Roth JR, Andersson DI. 2009. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella Typhimurium. Genetics 182:1183–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varma JK, et al. 2006. Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002-2003. J. Infect. Dis. 194:222–230 [DOI] [PubMed] [Google Scholar]
- 21. Voetsch AC, et al. 2004. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin. Infect. Dis. 38(Suppl 3):S127–S134 [DOI] [PubMed] [Google Scholar]
- 22. Whichard JM, et al. 2007. Human Salmonella and concurrent decreased susceptibility to quinolones and extended-spectrum cephalosporins. Emerg. Infect. Dis. 13:1681–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whichard JM, et al. 2005. β-Lactam resistance and Enterobacteriaceae, United States. Emerg. Infect. Dis. 11:1464–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winokur PL, et al. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]