Abstract
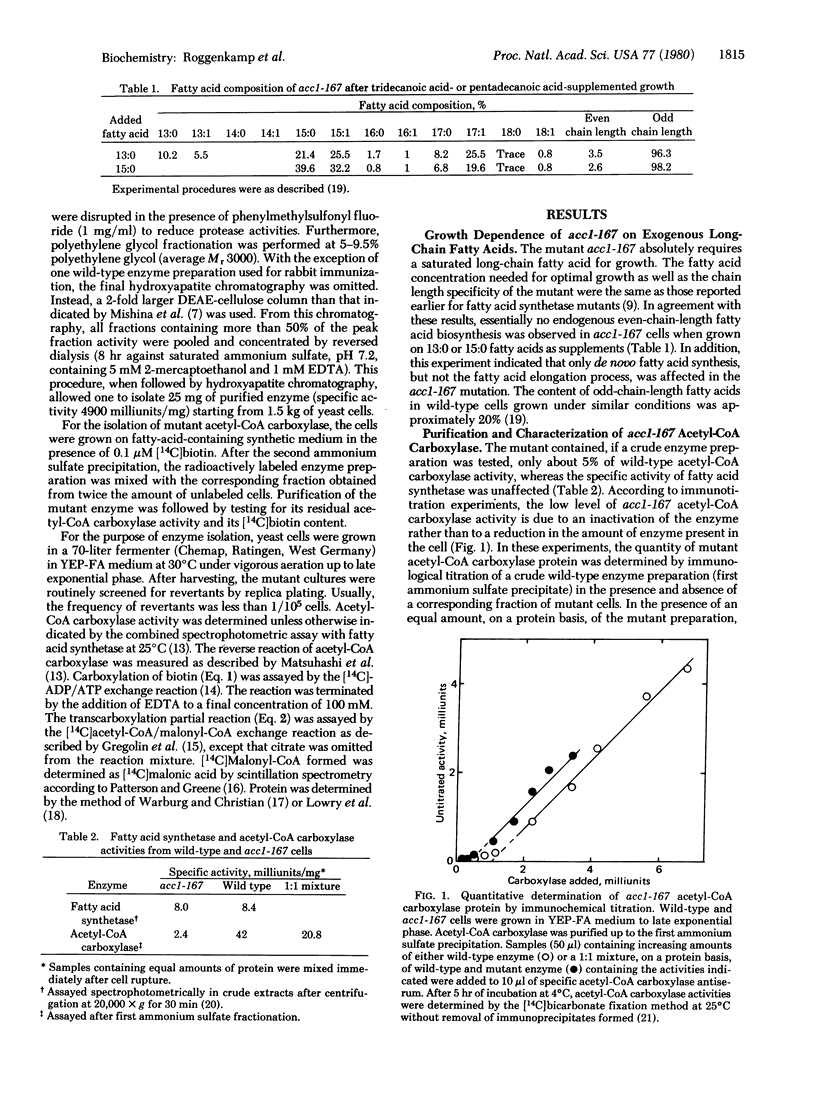
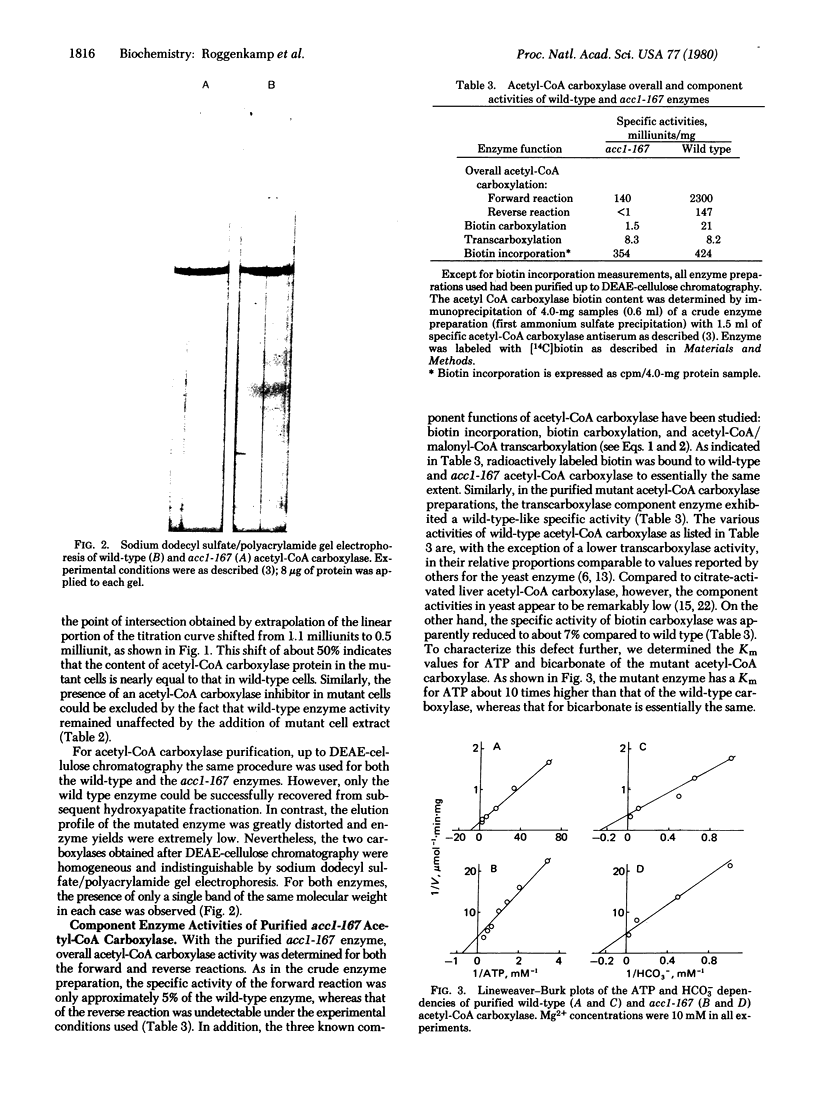
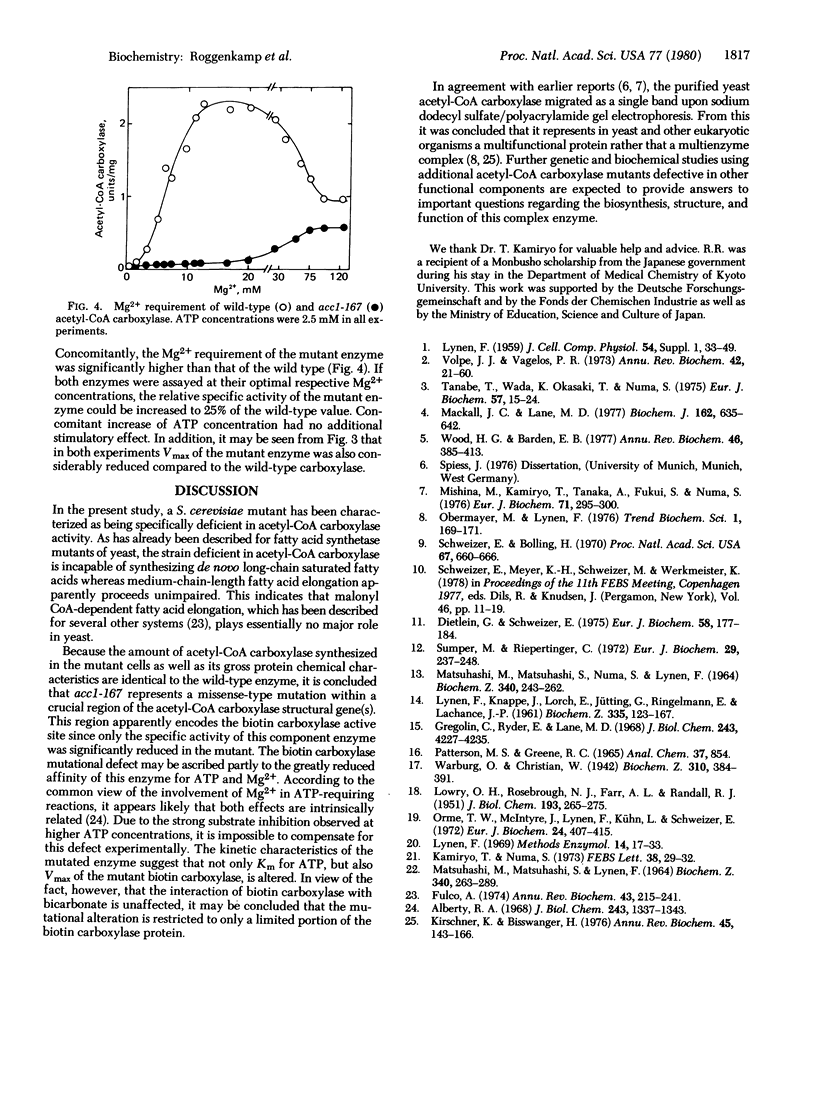
The isolation and biochemical properties of a Saccharomyces cerevisiae mutant (acc1-167) defective in acetyl-CoA carboxylase [acetyl-CoA:carbon-dioxide ligase (ADP forming), EC 6.4.1.2] activity are described. The mutant is deficient in de novo biosynthesis of long-chain fatty acids and specifically requires a saturated fatty acid of chain length 14-16 C atoms for growth. Fatty acid synthetase levels were normal, but the acetyl-CoA carboxylase specific activity of the purified enzyme was reduced to approximately 5% compared to wild-type yeast. Upon sodium dodecyl sulfate/polyacrylamide gel electrophoresis the purified mutant enzyme migrated as a single band and was essentially indistinguishable from the wild-type enzyme. The study of acetyl-CoA carboxylase partial activities revealed that the biotin incorporation capacity and the transcarboxylase partial activity were unaffected whereas the biotin carboxylase component enzyme exhibited less than 10% of wild-type specific activity. This biotin carboxylase mutational deficiency could be ascribed to a more than 90% reduction of Vmax and to a comparable increase in the Km value for ATP, which was accompanied by an increased requirement for Mg2+. It is concluded that acc1-167 contains a structural gene mutation in the biotin carboxylase domain of acetyl-CoA carboxylase.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Dietlein G., Schweizer E. Control of fatty-acid synthetase biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1975 Oct 1;58(1):177–184. doi: 10.1111/j.1432-1033.1975.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. Metabolic alterations of fatty acids. Annu Rev Biochem. 1974;43(0):215–241. doi: 10.1146/annurev.bi.43.070174.001243. [DOI] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Lane M. D. Liver acetyl coenzyme A carboxylase. I. Isolation and cat- alytic properties. J Biol Chem. 1968 Aug 25;243(16):4227–4235. [PubMed] [Google Scholar]
- Kamiryo T., Numa S. Reduction of the acetyl coenzyme A carboxylase content of Saccharomyces cerevisiae by exogenous fatty acids. FEBS Lett. 1973 Dec 15;38(1):29–32. doi: 10.1016/0014-5793(73)80505-8. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNEN F., KNAPPE J., LORCH E., JUETTING G., RINGELMANN E., LACHANCE J. P. [On the biochemical function of biotin. II. Purification and mode of action of beta-methyl-crotonyl-carboxylase]. Biochem Z. 1961;335:123–167. [PubMed] [Google Scholar]
- LYNEN F. Participation of acyl--CoA in carbon chain biosynthesis. J Cell Comp Physiol. 1959 Dec;54:33–49. doi: 10.1002/jcp.1030540406. [DOI] [PubMed] [Google Scholar]
- MATSUHASHI M., MATSUHASHI S., LYNEN F. ZUR BIOSYNTHESE DER FETTSAEUREN. V. DIE ACETYL-COA CARBOXYLASE AUS RATTENLEBER UND IHRE AKTIVIERUNG DURCH CITRONENSAEURE. Biochem Z. 1964 Aug 11;340:263–289. [PubMed] [Google Scholar]
- MATSUHASHI M., MATSUHASHI S., NUMA S., LYNEN F. ZUR BIOSYNTHESE DER FETTSAEUREN. IV. ACETYL-COA CARBOXYLASE AUS HEFE. Biochem Z. 1964 Aug 11;340:243–262. [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem J. 1977 Mar 15;162(3):635–642. doi: 10.1042/bj1620635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tanaka A., Fukui S., Numa S. Acetyl-coenzyme-A carboxylase of Candida Lipolytica. 1. Purification and properties of the enzyme. Eur J Biochem. 1976 Dec;71(1):295–300. doi: 10.1111/j.1432-1033.1976.tb11115.x. [DOI] [PubMed] [Google Scholar]
- Orme T. W., McIntyre J., Lynen F., Kühn L., Schweizer E. Fatty-acid elongation in a mutant of Saccharomyces cerevisiae deficient in fatty-acid synthetase. Eur J Biochem. 1972 Jan 21;24(3):407–415. doi: 10.1111/j.1432-1033.1972.tb19700.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Schweizer E., Bolling H. A Saccharomyces cerevisiae mutant defective in saturated fatty acid biosynthesis. Proc Natl Acad Sci U S A. 1970 Oct;67(2):660–666. doi: 10.1073/pnas.67.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M., Riepertinger C. Structural relationship of biotin-containing enzymes. Acetyl-CoA carboxylase and pyruvate carboxylase from yeast. Eur J Biochem. 1972 Sep 18;29(2):237–248. doi: 10.1111/j.1432-1033.1972.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Wada K., Okazaki T., Numa S. Acetyl-coenzyme-A carboxylase from rat liver. Subunit structure and proteolytic modification. Eur J Biochem. 1975 Sep 1;57(1):15–24. doi: 10.1111/j.1432-1033.1975.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Saturated fatty acid biosynthesis and its regulation. Annu Rev Biochem. 1973;42:21–60. doi: 10.1146/annurev.bi.42.070173.000321. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]




