Abstract
It has been proposed that intracellular pathogens may interfere with expression or function of proteins that mediate vesicular traffic in order to survive inside cells. Brucella melitensis is an intracellular pathogen that evades phagosome-lysosome fusion, surviving in the so-called Brucella-containing vacuoles (BCV). Vesicle-associated membrane protein 3 (VAMP3) is a v-SNARE protein that promotes the exocytosis of the proinflammatory cytokine TNF at the phagocytic cup when docking to its cognate t-SNARE proteins syntaxin-4 and SNAP-23 at the plasma membrane. We determined the expression level of VAMP3 in J774.1 murine macrophages stimulated with B. melitensis lipopolysaccharide (LPS) and detected a transitory increase of VAMP3 mRNA expression at 30 min. A similar result was obtained when cells were incubated in the presence of LPS from Salmonella enterica serovar Minnesota (SeM). This increase of VAMP3 mRNA was also observed on infected cells with B. melitensis even after one hour. In contrast, infection with Salmonella enterica serovar Enteritidis (SeE) did not cause such increase, suggesting that membrane components other than LPS modulate VAMP3 expression differently. To determine the effect of VAMP3 inhibition on macrophages infection, the expression of VAMP3 in J774.A1 cells was silenced and then infected with wild-type B. melitensis. Although a slight decrease in the rate of recovery of surviving bacteria was observed between 12 h and 36 h post-infection with B. melitensis, this was not significant indicating that VAMP3 is not involved in Brucella survival.
Keywords: Brucella, intracellular, pathogen, phagocytosis, macropinocytosis, SNARE, siRNA
Introduction
Brucellosis is an important zoonotic bacterial disease with a high incidence in developing countries. This infection is caused by multiple species of Brucella spp, although B. melitensis is the species most frequently associated with human brucellosis. More than 500,000 new cases per year are reported worldwide.1 The organism is a Gram-negative bacterium that causes infertility, abortion, feverish and septicemia in natural artiodactyls hosts (e.g., sheep, goats and cattle) and undulant fever, debilitation and disability in humans.2 All species of Brucella are facultative intracellular pathogens that possess the ability to survive and multiply in professional and non-professional phagocytes3 and their classification is mainly based on differences in pathogenicity and host preference.2,4 These intracellular pathogens have or produce a set of factors, including lipopolysaccharides, virulence regulatory proteins and phosphatidylcholine, which are essential for invasion of host cells, whereas others are crucial to avoid elimination by the host.3 Strains pathogenic for humans (e.g., B. abortus, B. suis and B. melitensis) possess a smooth LPS that has also been associated to their infection capabilities. The LPS O-chain protects the bacteria from cellular cationic peptides, oxygen metabolites and complement-mediated lysis and it is a key factor for Brucella survival and replication within the host.5 It is known that Brucella spp possess a non-classical LPS that, in the case of B. abortus lipid A, has a diaminoglucose backbone and the acyl groups are longer and linked to the core by simple amide bonds. It is proposed that this non-classical structure confers B. abortus LPS particular characteristics as a virulence factor. On the other hand, the O-chain and lipid A are also implicated in entry and in the early stages of B. abortus trafficking. The rough strains of Brucella spp (which do not possess the O-chain) do not enter the cells by lipid rafts but by a different mechanism and once inside rapidly fuse with lysosomes and get destroyed. By contrast, smooth B. suis entry is lipid-raft dependent and impairs phagosome-lysosome fusion during the first few hours of infection (for review, see ref. 3).
Characterization of pathogen entry pathways is essential for a clear understanding of infectious diseases, because individual pathogens have developed a range of strategies to modulate the host’s normal macropinocytic pathways to invade the host cells, evade the host immune system and promote survival by manipulating the lipid and protein composition of the encapsulating macropinosome.6 Brucella invades phagocytic and nonphagocytic cells through lipid rafts.4,7 Once inside the cell Brucella is surrounded by a membrane-bound compartment to form the Brucella-containing vacuoles (BCV).8,9 In these vacuoles it is able to escape the endocytic pathway to reach the endoplasmic reticulum (ER), where the bacteria are protected from host-defense mechanisms to ensure their replication and persistence within the host.10-12
One characteristic of phagocytes is their ability to ingest large numbers of particles or microbes quickly, suggesting that these cells have the capacity to rapidly replenish their plasma membrane. Using chimeric constructs of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein VAMP3 (vesicle-associated membrane protein 3) tagged with green fluorescent protein (GFP), it was demonstrated that endosomes bearing VAMP3 first fuse with the plasma membrane where phagocytosis is being initiated and are subsequently internalized.13 Focal exocytosis of VAMP3-containing vesicles, presumably originating from recycling endosomes, was shown to occur in the vicinity of nascent phagosomes, and insertion of these membranes was suggested to account for the growth of pseudopods.13 In fact, membrane traffic in activated macrophages has been linked for two critical events: proinflammatory cytokine secretion and phagocytosis of pathogens. In this way, it has been possible to associate the tumor necrosis factor (TNFα) secretion to VAMP3 incorporation at the site of phagocytic cup formation. Fusion of secretory vesicles at the cup simultaneously allows rapid release of TNFα and expansion of the membrane for phagocytosis.14 In addition to VAMP3, syntaxin 4, syntaxin 6, Vti1b and SNAP-23 are upregulated during LPS-mediated activation to accommodate the increased trafficking during TNF secretion and provides excess of plasma membrane for microorganism engulfment at the lipid rafts.15,16
Because vesicular traffic is controlled by the regulation of vesicular traffic proteins expression,17 early internalization events must be decisive for the fate of endocytic vacuoles. In the current study we have shown that VAMP3 mRNA is upregulated by live B. melitensis and also by LPS isolated from this microorganism. However, when the expression of VAMP3 mRNA was silenced in J774.A1 cells no significant differences in B. melitensis survival were observed compared with control cells.
Results
It has been reported that during the early stages of phagocytosis, Syntaxin 4 is recruited to the phagocytic cup in a cholesterol-dependent manner, thus allowing insertion of VAMP3-positive recycling endosome membrane that is required for efficient ingestion of a pathogen.16 Based on this notion, we wanted to investigate whether B. melitensis LPS or whole bacteria upregulate VAMP3 mRNA levels in macrophages to ensure VAMP3 availability during infection.
Extraction of B. melitensis LPS (Bm-LPS) yielded 4.5 mg/ml with < 0.5% residual protein, which is comparable with previously reported results for the extraction of LPS from Brucella spp.18 As shown in Figure 1, J774.A1 cells treated with 200 ng/ml of Bm-LPS showed a 4.5-fold transient increase of VAMP3 mRNA levels at 30 min post-treatment, followed by a return to control levels, as measured by qRT-PCR. In the case of S. enterica serovar Minnesota LPS (Sigma Aldrich), we detected a similar transient increase of VAMP3 mRNA levels, but in this case control levels were not restored at this time point. In both conditions the increment observed was transitory even though LPS was present in the milieu along the experiment. Significant differences were detected (p ≤ 0.05) in both cases.
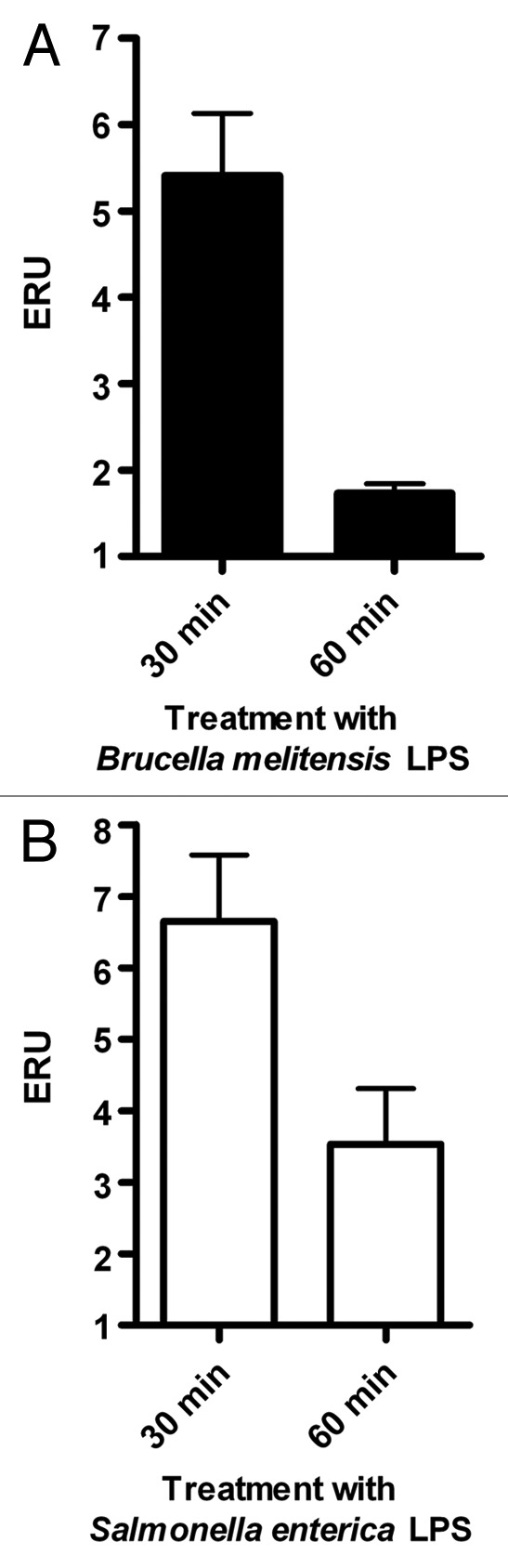
Figure 1. LPS treatment causes a transient increase in VAMP3 mRNA expression. Expression of VAMP3 mRNA was analyzed in J774.A1 cells treated with either S. enterica serotype Minnesota LPS (SeM) or B. melitensis LPS. J774.1 cell were incubated for 30 or 60 min at 37°C in the presence of 200 ng/ml of SeM LPS or B. melitensis LPS. VAMP3 mRNA was quantified as described in Materials and Methods. Values are expressed in expression relative units (ERU). Bars represent the fold increase compared with its respective control cells at the same time points, by the Pfaffl equation. The results are representative of three independent experiments conducted in triplicate.
Next, we determined the levels of VAMP3 expression in response to B. melitensis and SeE infection. Upon infection of J774.A1 cells with B. melitensis, VAMP3 mRNA expression was also temporarily upregulated as with purified LPS. Here the increase reached a peak by 30 min (2.1 times over the control) and remained at this level even after 60 min. Values show significant differences as compared with that of control cells non-treated (p ≤ 0.05) (Fig. 2A). In contrast, the expression of VAMP3 in cells infected with SeE was nearly constant during the course of the experiment (Fig. 2B), but below that seen in non-infected control cells, no significant differences (p ≤ 0.05) were detected.
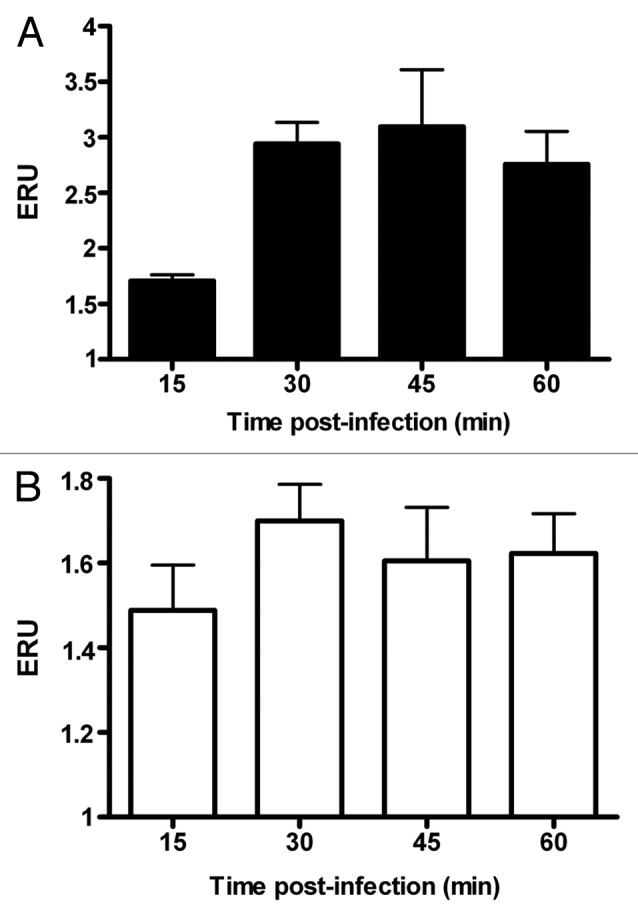
Figure 2. Infection by B. melitensis increases VAMP3 expression in J774.A1 macrophtages. J774.A1 cells were infected with B. melitensis (solid bars) or S. enterica serotype Enteritidis (SeE) (open bars) at a MOI of 50:1, at 37°C. After 15, 30, 45 and 60 min cultures were washed and cells harvested for RNA purification. VAMP3 mRNA was quantified as described in Materials and Methods. Values are expressed in expression relative units (ERU). Bars represent the fold increase compared with respective control cells at the same time points, by the Pfaffl equation. These results are representative of three independent experiments conducted in triplicate.
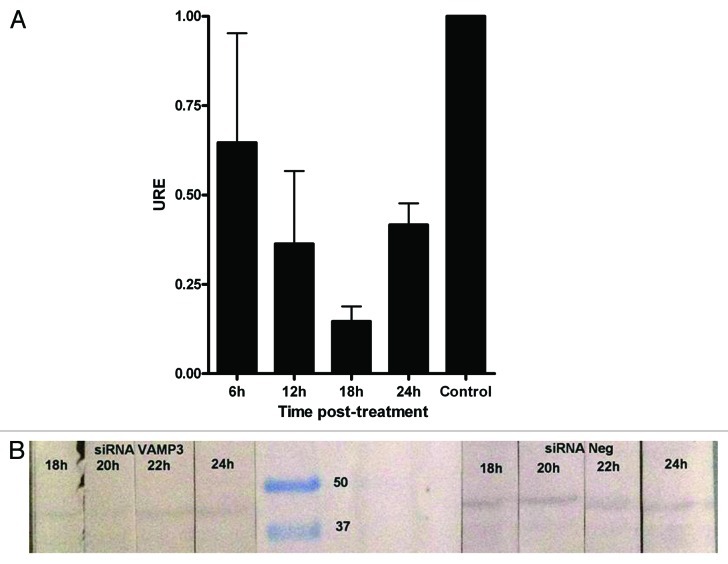
Lastly, we infected J774.A1 cells silenced with VAMP3 siRNA (100 nM/1 × 106 cells) with B. melitensis and measured bacterial survival by determining the CFU at different time points up to 36 h. Using this approach, we found that the maximum decrease for the expression of VAMP3 was between 18 and 20 h (less than 15%, Fig. 3) and a slight decrease in bacterial replication compared with control cells after 12, 24 and 36 h, with about 10% less bacteria recovered from cells that had been silenced for VAMP3 mRNA expression (Fig. 4). However, this reduction was not statistically significant with the exception of 12 h time point. Time zero represents total CFU counts (6.8 × 106 CFU/ml = 100%) after 1 h of phagocytosis and previous to gentamicin treatment.
Figure 3. Expression of VAMP3 in J774.A1 cells treated with siRNA VAMP3. (A) qRT-PCR was performed at 6 h intervals to determine the time lapse in which VAMP3 is inhibited at maximum. Control corresponds to siRNA negative in western blotting. (B) Western blotting assay for VAMP3 of J774.A1 cells treated with siRNA VAMP3, samples were monitored each 2 h in order to detect maximum inhibition more precisely.
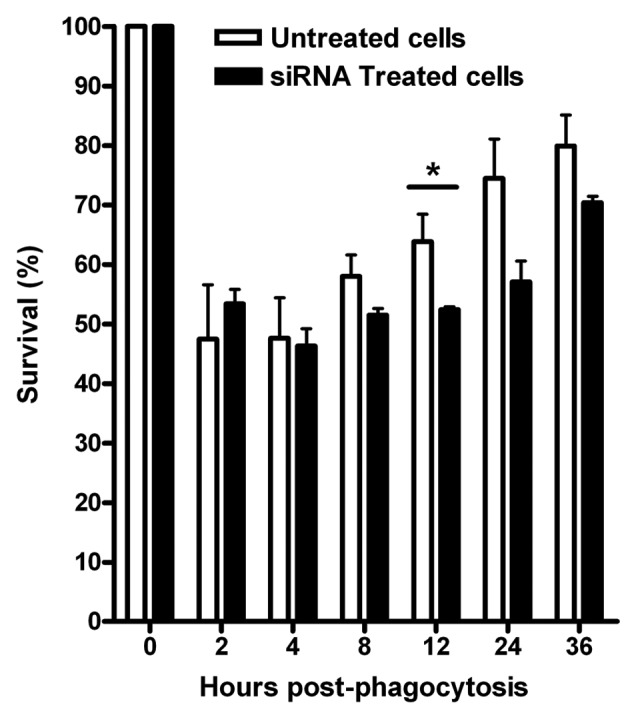
Figure 4. siRNA silencing of VAMP3 expression does not affect B. melitensis replication and survival in J774.A1 macrophages. J774.A1 cells transfected with three different siRNAs designed to silence mouse VAMP3 were infected with B. melitensis at a MOI of 50:1. The CFU counts, expressed as survival %, were determined at different intervals during a period of 36 h. Viable intracellular bacteria were determined by the gentamicin protection assay. Time zero represents total CFU counts previous to gentamicin treatment. The graph shows the mean of CFU counts from three independent experiments. *At this time point, a significant difference (p ≤ 0.05) was determined.
Discussion
Here we report that endocytosis of Brucella or Salmonella LPS in macrophages produced a transitory increment in VAMP3 mRNA levels that return to normal levels at later time points. This transitory increment was also seen in J774 cells infected with Brucella, but not with Salmonella. However, no significant differences were observed in Brucella survival when macrophages were silenced with VAMP3 siRNAs. These results further indicate that VAMP3 could be linked to the membrane expansion during macropinocytosis and suggest that external stimuli such as LPS or infection by Brucella trigger an increase in VAMP3 expression probably to make the protein readily available during this process. However, changes in VAMP3 levels post infection do not seem to be of particular relevance for Brucella survival and thus for the biogenesis of the BCVs.
Different observations suggest that the soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) protein VAMP3, an integral component of the recycling endosomes, plays an important role in cytokine secretion and in phagocytosis, possibly participating in localized fusion events at the site of phagosome formation and thus driving pseudopodial extension.13,19 Supporting this idea Coppolino and colleagues used a GFP-tagged chimera of VAMP3 that they found to accumulate at sites of Salmonella and Candida albicans invasion.16,19 Moreover, microinjection of tetanus or botulinum toxins (which cleave and degrade VAMP2 and VAMP3) reduced phagocytosis of a variety of particles by ~66% in J774 cells.20,21
However, studies performed with S. enterica serovar Typhimurium indicate that VAMP3 has no direct role in its phagocytosis. For example, recruitment of GFP-VAMP3 at sites of Salmonella invasion was blocked by tetanus toxin; nevertheless, it did not inhibit invasion.19 Furthermore, in dominant-negative NSF cells, cellular invasion by S. Typhimurium or the associated membrane remodeling were not affected, but fusion of Salmonella-containing vacuoles with endomembranes was significantly impaired.19 Along these lines, we observed a reduction in VAMP3 mRNA levels when S. Enteritidis was internalized by J744 cells. The different effect on VAMP3 expression observed between Salmonella LPS-treated and infected J774 cells, suggest that membrane components other than LPS also play a role in this process.
Contradictory results have been published with respect to the role of the non-canonical structure and composition of the Brucella LPS in the modulation of TNF secretion,20 a fact that could be linked to the modulation of the expression of vesicular trafficking proteins or to other intracellular factors. These findings suggest that fluctuations in the expression of VAMP3 very early during phagocytosis possibly influence the movement of secretory vesicles to the plasma membranes.21,22 Murray et al.15 showed that the maximum expression of VAMP3 was detected at 2 h after stimulation of RAW264.7 murine macrophages using LPS from S. enterica serovar Minnesota.
The possibility that Brucella modifies the expression and/or function of proteins that promote intracellular trafficking during phagocytosis has been previously proposed.20,23 It was demonstrated that LPS is a bacterial constituent capable of modulating the secretion of proinflammatory cytokines mediated by the v-SNARE VAMP3, which in turn is located primarily in recycling endosomes.24,25 This protein must engage itself to the t-SNARE proteins syntaxin-4 and SNAP23 in the plasma membrane thus promoting the fusion of membranes and secretion. It seems reasonable to suppose that if lipid rafts were preferred sites for Brucella entry into macrophages, then proteins associated with these macrodomains would be affected at the beginning of phagocytosis. In this stage, it is likely that bacteria have to resolve the fate of the phagosomes.23 In contrast to this notion, we did not find significant differences in the ability of Brucella to survive and replicate in macrophages upon VAMP3 mRNA silencing. However, we cannot rule out the possibility that other SNARE proteins such as the SNAREs syntaxin-3, Ti-VAMP and VAMP8,26 could play a role during Brucella invasion, whether or not their expression is modified in response to infection.
Materials and Methods
Bacterial strains and LPS extraction
The smooth, virulent Brucella melitensis strain BM133, a reference Mexican strain,27 was used both for infections and for the extraction of LPS. Additionally, a wild-type strain of Salmonella enterica serovar Enteritidis (SeE) from our departmental collection and commercially available LPS from S. enterica serovar Minnesota (Sigma Aldrich) were also used. B. melitensis LPS was extracted from a 1 L culture as previously described18 and quantified by the 2-keto-3-deoxyoctonate (KDO) method.28 The protein concentration in the LPS extract was determined by the Bradford method.29
Cell culture and infections
Murine J774.A1 macrophage cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal calf serum (FCS) (Gibco), 2 mM l-glutamine and 1 mM sodium pyruvate and cultured at 37°C in a 5% CO2 atmosphere. Cells were plated in 6-well plates (Nunc) (1 × 106 cells per well) 24 h before stimulation with LPS. Briefly, the cells were grown until 90–100% of confluence and stimulated with 200 ng/ml of B. melitensis LPS (Bm-LPS) or SeM LPS (Se-LPS) for 30 and 60 min. Then total protein was extracted from each well using RIPA buffer30 [150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0, 1 mM PMSF and Complete Protease Inhibitor (Roche)]. For infections, J774.A1 cells were cultured as previously indicated and infected at a MOI of 50:1 for 15, 30, 45 or 60 min at 37°C with B. melitensis BM133 grown in BBL Medium (BD Diagnostics) or SeE grown in LB broth. These experiments were performed three times, each in triplicate.
Gentamicin protection assay (GPA)
J774.A1 cells were plated in 6-well plates at 1 × 106 per well and cultured as indicated, to attain 90 to 100% confluence. Cell monolayers were washed twice with PBS before infection. B. melitensis BM133 grown in BBL Medium up to an OD600nm of 1.0 (approximately 3 × 109 bacteria/mL) was diluted to set the infection at a MOI of 50:1. The plates were centrifuged at 500 rpm for 2 min at 4°C to synchronize phagocytosis and then incubated at 37°C in a 5% CO2 atmosphere for 1 h. Time cero was set at this point. Then the cells were washed and further incubated for 2 h in DMEM containing 100 μg/ml of gentamicin to kill adherent bacteria, but not those located within the cells. After this time the cells were washed again and the medium replaced once more but now containing 15 μg/ml of gentamicin for the rest of the experiment. Control cells were also incubated with DMEM supplemented with gentamicin at the same concentration with no effect on cells. The monolayer was washed once with PBS at different intervals up to 36 h and lysed with 1% sterile Triton X-100. Appropriate dilutions were plated on Brucella agar (BBL Medium, BD Diagnostics) to determine the number of viable intracellular bacteria by bacterial colony counting.
Real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed with the Two-Step qRT-PCR kit (Invitrogen) according to the manufacturer’s instructions using total RNA extracted from infected or LPS-treated cells with Trizol (Invitrogen), according to company’s protocol. Primers were designed from the sequence of VAMP3 mRNA reported in the Gen Bank (accession number NM_009498). The sequences of the primers were VAMP3-1 (forward) 5′-AGAACCTGCCGTGTTATCGAGCTT-3′ and VAMP3-2 (reverse) 5′-ACACAAGTCCTCTTTCCCAGTCCA-3′; these primers generated a 195-bp product. Control primers for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene were modified from those reported by Fan et al.31 The sequences obtained were GP-1 (forward) 5′-GCTCATGACCACAGTCCATGCC-3′ and GP-2 (reverse) 5′-GCACATTGGGGGTAGGAACACA-3′; these primers generated a 201-bp product. The following PCR conditions were used: 50°C for 2 min; 95°C for 10 min; 35 cycles of 95°C for 15 sec (denaturation), 60°C for 60 sec (annealing) and 72°C for 90 sec (elongation); and a final extension at 72°C for 10 min. All reactions were performed using a Light Cycler 480 (Roche) and the results were analyzed with the Pfaffl equation, which indicates changes in the expression of target genes.32 Values are expressed in expression relative units (ERU) that indicate the number of times that the expression changed with respect to the control. GAPDH levels were unchanged and used to normalize VAMP3 expression (data not shown).
Small interfering RNA (siRNA) treatment
J774.A1 cells plated on 6-well plates were transfected with three different siRNAs designed to silence mouse VAMP3 expression (Silencer Validated siRNA ID#186988-90, Ambion) using siIMPORTER (UPSTATE-Millipore) according to the manufacturer’s instructions. qRT-PCR experiments were performed at different times upon transfection to establish the earliest time point when the VAMP3 mRNA was at its lower level with respect to untreated cells. Those experiments were conducted at 6 h intervals, but for western blotting intervals were reduced for 2 h, in order to identify the time lapse more precisely. Hence, J774.A1 cells were cultured for 18 to 20 h prior to infection with B. melitensis. Subsequently, bacterial colony counting was performed in a GPA assay as previously described.
Statistics
Differences in data from qPCR from LPS treatment and infections were analyzed by Student's t-test.
Acknowledgments
This work was supported by PAPIIT IN-222907 and PAPIIT IN-212610, UNAM. The authors acknowledge Daniel Martínez for his helpful discussions, Raymundo Iturbe for his technical support, Ma. Teresa Ramírez for her support in the translation of this paper and Mrs. Francisca Muños for her administrative support.
Glossary
Abbreviations:
- VAMP3
vesicle-associated membrane protein 3 (cellubrevin)
- SNARE
soluble NSF attachment protein receptor
- LPS
lipopolysaccharide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/21251
References
- 1.World Health Organization. Brucellosis in humans and animals: WHO guidance. Geneva, 2005. [Google Scholar]
- 2.Adams LG, Schutta CL. Natural resistance against brucellosis: A review. Open Vet Sci J. 2010;4:61–71. [Google Scholar]
- 3.Carvalho Neta AV, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. Pathogenesis of bovine brucellosis. Vet J. 2010;184:146–55. doi: 10.1016/j.tvjl.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Xavier MN, Paixão TA, den Hartigh AB, Tsolis RM, Santos RL. Pathogenesis of Brucella spp. Open Vet Sci J. 2010;4:109–18. [Google Scholar]
- 5.Cardoso PG, Macedo GC, Azevedo V, Oliveira SC. Brucella spp noncanonical LPS: structure, biosynthesis, and interaction with host immune system. Microb Cell Fact. 2006;5:13–24. doi: 10.1186/1475-2859-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–71. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Watarai M, Makino S, Shirahata T. Membrane sorting during swimming internalization of Brucella is required for phagosome trafficking decisions. Microb Pathog. 2002;33:225–37. doi: 10.1006/mpat.2002.0531. [DOI] [PubMed] [Google Scholar]
- 8.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–56. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roop RM, 2nd, Gaines JM, Anderson ES, Caswell CC, Martin DW. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med Microbiol Immunol. 2009;198:221–38. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorvel JP, Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol. 2002;90:281–97. doi: 10.1016/S0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 11.Roop RM, 2nd, Bellaire BH, Valderas MW, Cardelli JA. Adaptation of the Brucellae to their intracellular niche. Mol Microbiol. 2004;52:621–30. doi: 10.1111/j.1365-2958.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 12.Celli J. Surviving inside a macrophage: the many ways of Brucella. Res Microbiol. 2006;157:93–8. doi: 10.1016/j.resmic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–29. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 15.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–5. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 16.Kay JG, Murray RZ, Pagan JK, Stow JL. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J Biol Chem. 2006;281:11949–54. doi: 10.1074/jbc.M600857200. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 18.Kreutzer DL, Buller CS, Robertson DC. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979;23:811–8. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppolino MG, Kong C, Mohtashami M, Schreiber AD, Brumell JH, Finlay BB, et al. Requirement for N-ethylmaleimide-sensitive factor activity at different stages of bacterial invasion and phagocytosis. J Biol Chem. 2001;276:4772–80. doi: 10.1074/jbc.M007792200. [DOI] [PubMed] [Google Scholar]
- 20.Watarai M, Makino S, Fujii Y, Okamoto K, Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4:341–55. doi: 10.1046/j.1462-5822.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 21.Allen LAH, Yang C, Pessin JE. Rate and extent of phagocytosis in macrophages lacking vamp3. J Leukoc Biol. 2002;72:217–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Niedergang F, Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol. 2004;16:422–8. doi: 10.1016/j.ceb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Mañes S, del Real G, Martínez-A C. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–68. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 24.van Ijzendoorn SCD. Recycling endosomes. J Cell Sci. 2006;119:1679–81. doi: 10.1242/jcs.02948. [DOI] [PubMed] [Google Scholar]
- 25.Hu K, Rickman C, Carroll J, Davletov B. A common mechanism for the regulation of vesicular SNAREs on phospholipid membranes. Biochem J. 2004;377:781–5. doi: 10.1042/BJ20031164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pocard T, Le Bivic A, Galli T, Zurzolo Ch. Distinct v-SNAREs regulate direct and indirect apical delivery in polarized epithelial cells. J Cell Sci. 2007;120:3309–20. doi: 10.1242/jcs.007948. [DOI] [PubMed] [Google Scholar]
- 27.Herńndez-Castro R, Verdugo-Rodríguez A, Puente JL, Súrez-Güemes F. The BMEI0216 gene of Brucella melitensis is required for internalization in HeLa cells. Microb Pathog. 2008;44:28–33. doi: 10.1016/j.micpath.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Karkhanis YD, Zeltner JY, Jackson JJ, Carlo DJ. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 29.Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-P. [DOI] [PubMed] [Google Scholar]
- 30.Rao N, Miyake S, Reddi AL, Douillard P, Ghosh AK, Dodge IL, et al. Negative regulation of Lck by Cbl ubiquitin ligase. Proc Natl Acad Sci U S A. 2002;99:3794–9. doi: 10.1073/pnas.062055999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Q, Ding J, Zhang J, Guan N, Deng J. Effect of the knockdown of podocin mRNA on nephrin and alpha-actinin in mouse podocyte. Exp Biol Med (Maywood) 2004;229:964–70. doi: 10.1177/153537020422900914. [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]