Abstract
The pmr gene is predicted to encode a Ca2+-ATPase in the secretory pathway. We examined two strains of Neurospora crassa that lacked PMR: the Δpmr strain, in which pmr was completely deleted, and pmrRIP, in which the gene was extensively mutated. Both strains had identical, complex phenotypes. Compared to the wild type, these strains required high concentrations of calcium or manganese for optimal growth and had highly branched, slow-growing hyphae. They conidiated poorly, and the shape and size of the conidia were abnormal. Calcium accumulated in the Δpmr strains to only 20% of the wild-type level. High concentrations of MnCl2 (1 to 5 mM) in growth medium partially suppressed the morphological defects but did not alter the defect in calcium accumulation. The Δpmr Δnca-2 double mutant (nca-2 encodes a Ca2+-ATPase in the plasma membrane) accumulated 8-fold more calcium than the wild type, and the morphology of the hyphae was more similar to that of wild-type hyphae. Previous experiments failed to show a function for nca-1, which encodes a SERCA-type Ca2+-ATPase in the endoplasmic reticulum (B. J. Bowman, S. Abreu, E. Margolles-Clark, M. Draskovic, and E. J. Bowman, Eukaryot. Cell 10:654-661, 2011). The pmrRIP Δnca-1 double mutant accumulated small amounts of calcium, like the Δpmr strain, but exhibited even more extreme morphological defects. Thus, PMR can apparently replace NCA-1 in the endoplasmic reticulum, but NCA-1 cannot replace PMR. The morphological defects in the Δpmr strain are likely caused, in part, by insufficient concentrations of calcium and manganese in the Golgi compartment; however, PMR is also needed to accumulate normal levels of calcium in the whole cell.
INTRODUCTION
Calcium is an important signaling molecule in all cells, essential for growth yet toxic if cytosolic concentrations increase above micromolar concentrations. The “filamentous” morphology of the nonyeast fungi has been postulated to be controlled by intracellular calcium gradients (25, 28, 40, 46). However, the transporters that regulate calcium homeostasis in filamentous fungi have not been investigated to nearly the extent that they have been in some yeasts.
Saccharomyces cerevisiae has been the primary model organism for the study of proteins that move calcium across biological membranes (9, 11, 54). In S. cerevisiae, three proteins have been shown to be particularly important. Pmc1p, a Ca2+-ATPase, and Vcx1p, a Ca2+/H+ exchange protein, reside in the vacuolar membrane. They transport calcium from the cytosol into the vacuole, thus sequestering more than 95% of the cellular calcium (12, 15, 17, 45). Δpmc1 strains accumulate only 20% of the calcium seen in the wild type. Δvcx strains accumulate calcium to the same levels observed for the wild type, but Δpmc1 Δvcx1 double mutant strains accumulate only 10% of the wild-type levels and do not grow in media with elevated levels of calcium (12). The SPCA-type Ca2+-ATPase Pmr1p is seen primarily in the Golgi compartment. It delivers calcium and manganese to organelles in the secretory pathway (2, 16, 41, 48, 55, 56). In addition, S. cerevisiae has calcium channels in the vacuolar membrane (Yvc1p) (14, 19, 27, 42) and in the plasma membrane (the Cch1p/Mid1p complex) (19, 27, 42).
Sequencing of the genomes of many filamentous fungi has revealed larger numbers of calcium transporters in these organisms (3, 26, 58). In addition to homologs of VCX1 and PMC1, Neurospora crassa and Aspergillus species, for example, have genes encoding 2 to 5 PMCA-type transporters (the family to which yeast PMC1 belongs). Unlike yeasts, the filamentous fungi also have the SERCA-type Ca2+-ATPase found in the endoplasmic reticulum (ER) of plants and animal cells (6, 31). The PMR Ca2+-ATPase has been investigated in Aspergillus niger and Aspergillus fumigatus. The deletion of this enzyme in A. fumigatus was reported previously to reduce radial growth by 60% and to cause defects in the synthesis of the cell wall (43). In contrast, the loss of PMR in A. niger did not cause a significant change in the growth rate or hyphal morphology (3, 57). In both species of Aspergillus, the PMR deletion strains grew poorly in media with low concentrations of calcium.
Using N. crassa as our model organism, we have investigated the cellular distribution and function of calcium transporters (4, 5). The cax gene encodes the homolog of VCX1. As in S. cerevisiae, this Ca2+/H+ exchanger is important for sequestering calcium in vacuoles. The nca-2 and nca-3 genes encode PMCA-type Ca2+-ATPases. Different from S. cerevisiae but similar to animals cells, they appear to function primarily in the plasma membrane, pumping calcium out of the cell. The deletion of nca-2 in N. crassa causes a 4- to 10-fold elevation of the cell calcium concentration. The nca-1 gene encodes a SERCA-type Ca2+-ATPase, which, when tagged with green fluorescent protein (GFP), is observed in the nuclear envelope and ER, again as in animal cells. The Δnca-1 strains of N. crassa were indistinguishable from the wild-type strain. Indeed, we were surprised that deletions of the genes cax, nca-1, nca-2, and nca-3 or double and triple combinations of these deletions failed to cause significant changes in cell morphology: the sizes and branching patterns of hyphae were much like those of the wild type.
In contrast to the results seen with other calcium transporters, preliminary experiments with pmr mutant strains of N. crassa showed that cells lacking this SPCA-type Ca2+-ATPase were dramatically affected both in hyphal morphology and in the ability to grow in standard media (1). Experiments with Aspergillus species showed that the deletion of the pmr gene causes defects in cell wall synthesis and slower growth, especially in media with lower concentrations of calcium (43, 51, 57). In S. cerevisiae, strains lacking Pmr1p accumulate higher levels of calcium and manganese and are defective in secretion and glycosylation (2, 24, 29, 30, 48). Changes in the size or shape of haploid cells have not been reported for S. cerevisiae; however, diploid Δpmr cells fail to sporulate and appear abnormal (48). In Schizosaccharomyces pombe, the typical cylindrical shape of cells became more rounded when the pmr gene was deleted (35). In yeasts, there is evidence that Pmr1p may function in the ER as well as the Golgi compartment (52). N. crassa has a SERCA-type Ca2+-ATPase, encoded by nca-1, localized to the ER (5). In this report, we constructed a pmr nca-1 double mutant. The phenotype of this strain suggests that both the SERCA-type Ca2+-ATPase and the SPCA-type Ca2+-ATPase are needed for the normal growth of N. crassa and probably other filamentous fungi. The major goals of this paper are to determine the role of the PMR transporter in N. crassa and to ask if PMR and NCA-1 have important roles in calcium homeostasis and in morphological development.
MATERIALS AND METHODS
Deletion strains.
Except for the pmrRIP strain described below, all mutant strains were generated by the Neurospora Genome Project (7) and were obtained from the Fungal Genetics Stock Center (39). In these strains, the protein-encoding region was replaced by the hph+ gene, which confers resistance to the drug hygromycin. The procedure used to generate deletion strains made use of multinucleated conidia, and viable hygromycin-resistant homokaryons were not obtained for a small percentage of the strains. For these deletions, the transformed conidia were saved as heterokaryons. The Neurospora Genome Project produced heterokaryon strain FGSC 11616, in which pmr (locus NCU03292.2) was replaced with hph+. We streaked conidia from FGSC 11616 and observed a small number of colonies that were slow growing and morphologically abnormal (described more fully in Results). They had the same phenotype as that of the pmrRIP strain described below. When crossed to wild-type strain 74A, the slow-growing, abnormal colonies represented approximately 50% of the progeny. Analysis by PCR showed that the pmr gene had been replaced by hph+. We selected one of these with the pmr::hph+ A genotype and named it the Δpmr strain. The other deletion strains used, Δcax, Δnca-1, Δnca-2, and Δnca-3, were described previously (4).
Isolation of strains with two mutations.
The Δpmr strain was fertile as either a male or a female parent. It was mated with the Δcax, Δnca-2, or Δnca-3 strain, and progeny were isolated by using standard genetic procedures for N. crassa (13). The progeny were analyzed by PCR to identify double mutants and to verify that no endogenous copy of either gene was present.
Multiple attempts to isolate the Δpmr Δnca-1 double mutant strain were unsuccessful because pmr is closely linked to nca-1, separated by only 57 kb (approximately 1 map unit), and because the double mutant had a low level of viability. Therefore, we developed a method by which we could enrich for the presence of pmr-null Δnca-1 progeny. First, we generated a pmr-null strain with the RIPing (repeat-induced point mutations) procedure (49). A 3,211-bp fragment of DNA containing the pmr gene was amplified by PCR using primers 5′-ACGATGTTCTTCCCTTCTACC-3′ (forward) and 5′-CCGTACTTCCACCATTTTCTC-3′ (reverse). The pmr gene was inserted into pBM61 (38). The resulting plasmid was used to transform the his-3 strain (FGSC 6103) of N. crassa, producing a strain that had both endogenous pmr+ and a second copy of pmr+ targeted to the his-3 locus. The transformed strain was crossed with wild-type strain 74A. Among the progeny from this cross were isolates that had an abnormal hyphal morphology and failed to conidiate, a phenotype that we now know is indistinguishable from the phenotype of Δpmr strains. An isolate with abnormal morphology was crossed again to wild-type strain 74A. We selected progeny that were his-3+ and had the abnormal-morphology phenotype. The pmr gene from one such isolate, named pmrRIP, was amplified by PCR and sequenced. The gene had 120 nucleotide changes, resulting in 80 amino acid changes, with stop codons introduced at amino acids 490 and 856 (1,025 amino acids total).
We next crossed the Δnca-1 strain obtained from the Neurospora Genome Project to the his-3 strain (his-3 lies 10 map units from pmr), generating the his-3 Δnca-1 strain, which is a histidine auxotroph that is resistant to hygromycin. The his-3 Δnca-1 strain was crossed with the pmrRIP strain, and ascospores were germinated on agar plates with Vogel's minimal medium containing 2% sucrose and hygromycin (400 μg/ml). The order of the genes is his-3, pmr, and nca-1. The only progeny that could grow were his+ and hygromycin-resistant progeny, produced by recombination between the his-3 and Δnca-1 genes. Ten percent of the recombinants were predicted to be pmrRIP Δnca-1 recombinants. More than 99% of the viable progeny had a normal morphology, as expected for his-3+ Δnca-1 strains. A few of the viable progeny grew very slowly with an abnormal morphology (described in Results). Analysis by PCR (for the Δnca-1 mutation) and sequencing (for the pmrRIP mutation) showed that these progeny were pmrRIP Δnca-1 strains.
Generation and analysis of strains with GFP- and red fluorescent protein (RFP)-tagged proteins.
We identified N. crassa homologs of the VPS52 (NCU05273) and VRG4 (NCU06198) genes of S. cerevisiae. Vps52p is a component of the Golgi-associated retrograde protein complex, required for the recycling of proteins from endosomes to the late Golgi compartment (8). Vrg4p is a GDP-mannose transporter (44). Primers 5′-cacatctagaCACCATGGCCAACAAGAGAAAC-3′ (forward) and 5′-ggttggatccAAGCATTTGCCGCATCCCTG-3′ (reverse) were used for pvrg-4-GFP (the region in uppercase letters is the sequence in the gene, and the region in lowercase letters contains the restriction site used for construction of the plasmid). The primers used to construct pvps-52-GFP, pRFP-vps-52, ppmr-GFP, and pRFP-pmr were described previously (5). The product of the NCU06777 gene served as a marker of tubular vacuolar membranes. We previously reported that this gene encodes the homolog of VAM3 from S. cerevisiae (5). As pointed out by an anonymous reviewer, this is almost certainly incorrect. N. crassa and other filamentous fungi lack VAM3, and NCU06777 appears to encode the homolog of PEP12, which has a similar sequence (22). Pep12p is a multifunctional syntaxin involved in trafficking to the prevacuolar compartment in S. cerevisiae (20); a high level of expression of PEP12 can suppress defects in VAM3 mutants (21). We have shown that the N. crassa protein encoded by NCU06777 colocalizes with the vacuolar H+-ATPase (VMA-1 and VMA-5) and the vacuolar calcium/H+ transporter (CAX) (5). The construction of plasmids, the transformation of N. crassa strain 74A (FGSC 987), and analysis by confocal microscopy were carried out as described previously (5).
Analysis of growth and morphology.
The growth yields of wild-type and mutant strains were measured by using Vogel's medium with 2% sucrose (13) in which CaCl2 and MnCl2 had been omitted. CaCl2 and MnCl2 were added as indicated in the figure legends. Six milliliters of medium was put into 20-ml vials; each vial was then inoculated with 100,000 conidia, and conidia were grown at 30°C for 2 days. Mycelia were collected by filtration, rinsed with water, and dried. All growth experiments were done at least twice, with triplicate samples in each experiment.
To observe growth on solid medium (2% agar), we used Vogel's medium containing normal concentrations of MnCl2 (0.3 μM) and CaCl2 (0.68 mM) and 2% sucrose. Some plates were supplemented with 3 mM MnCl2 or 10 mM CaCl2, as indicated. Plates were inoculated with a 3-mm plug of mycelium from another plate and incubated at 30°C for the times indicated. The morphology of hyphae at the edge of the colonies was photographed after 24 h. In other experiments. we used the same medium and growth conditions but spread conidia onto the agar plates at a low density. Colonies growing from single conidia were photographed after 24 h of growth.
To obtain conidia from wild-type strain 74A and the Δpmr strain, we inoculated agar plates with Vogel's medium (2% agar and 2% sucrose) in the center of the plates and grew the conidia for 3 days at 30°C and for an additional 7 to 10 days on the benchtop at room temperature. As described in Results, the Δpmr strain produced conidia only after 10 days postinoculation and only in a narrow region around the rim of the plate. Strain 74A produced abundant conidia starting 3 to 4 days after inoculation. Conidia were suspended in water and photographed to measure the conidial diameter. For the rod-shaped conidia produced by the Δpmr strain, the average of the width and length was used in place of the diameter.
Measurement of calcium uptake.
Strains were grown in 20-ml vials containing 6 ml of Vogel's medium with 2% sucrose and 0.3 μCi 45Ca. Vials were inoculated with conidia (300,000 conidia per ml) from 74A and the Δpmr, Δpmr Δcax, and Δpmr Δnca-3 strains. For the aconidial pmrRIP Δnca-1 and Δpmr Δnca-2 strains, a 200-μl aliquot of mycelium from a liquid culture (approximately 0.1 mg [dry weight]) was used as an inoculum. Experiments with the Δpmr strain showed that the amounts of calcium accumulated per mg dry weight were the same in cultures inoculated with conidia or mycelia. The strains were grown for 2 to 6 days until they reached a dry weight of approximately 2 mg/ml, which is in the mid-log phase. Mycelia were collected by filtration and rinsed four times with 6-ml aliquots of Vogel's medium supplemented with 20 mM CaCl2. The dry weight was determined, and the uptake of 45Ca was measured with a liquid scintillation counter. The experiments were performed at least twice with triplicates for each strain.
RESULTS
Intracellular location of PMR.
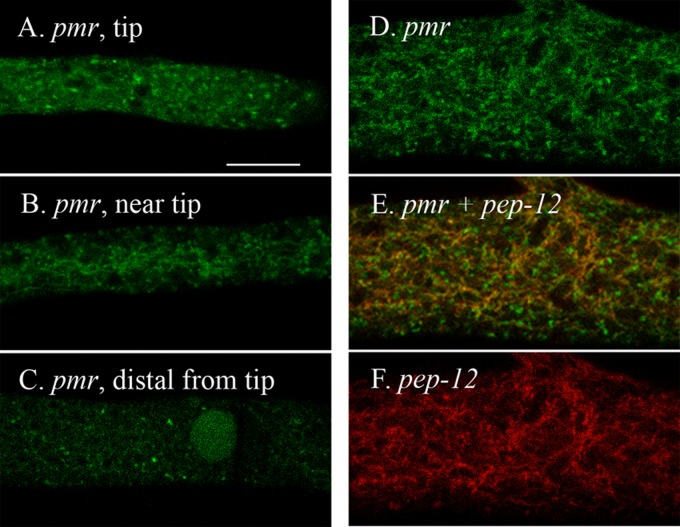
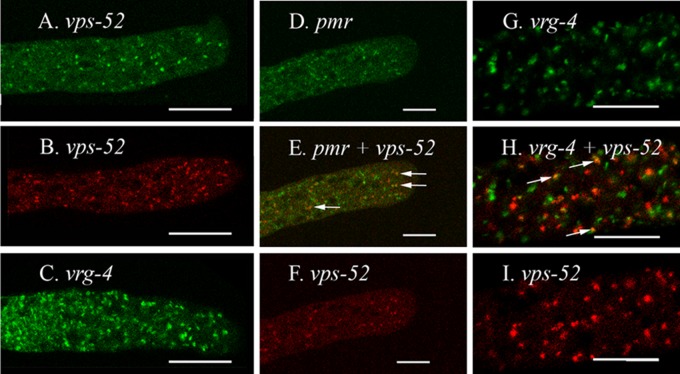
To visualize the location of PMR, we constructed recombinant genes to produce proteins with GFP fused to the C terminus or RFP fused to the N terminus. These recombinant genes were introduced into wild-type strain 74A and the Δpmr strain. With the RFP-PMR protein, the morphology and growth defects observed for the Δpmr strain were unchanged, while the PMR-GFP protein restored nearly normal growth and morphology (data not shown). We observed PMR-GFP in irregularly shaped particles roughly 0.5 to 1.0 μm in diameter. These particles occurred in all regions of the hypha (Fig. 1A to C) but were most abundant near the tip (Fig. 1A). In the region where the tubular vacuolar network was observed (50 to 200 mm behind the tips), we also saw PMR-GFP located in thin tubules (Fig. 1B). The tubules were part of the tubular vacuolar network because they colabeled with RFP–PEP-12 (discussed in Materials and Methods) (Fig. 1D to F). The small green particles in this region did not overlap RFP–PEP-12. To see if the particles visualized with PMR-GFP were components of the Golgi compartment, we tagged two putative Golgi-localized proteins with GFP and RFP. The proteins which we used were encoded by N. crassa homologs of the S. cerevisiae genes VPS52 and VRG4. We observed VPS-52–GFP and RFP–VPS-52 in small particles similar in size to those seen with PMR-GFP (Fig. 2A and B). In heterokaryons made by fusing VPS-52–GFP and RFP–VPS-52, we saw the two tagged proteins in the same compartments (data not shown). VRG-4–GFP gave the strongest signal of any of the Golgi markers, appearing in organelles of similar sizes but in a greater abundance than PMR-GFP, VPS-52–GFP, or RFP–VPS-52 (Fig. 2C). In a heterokaryon expressing both PMR-GFP and RFP–VPS-52, a small proportion of the tagged particles had both GFP and RFP (Fig. 2D to F). Similarly, a heterokaryon expressing both VRG-4–GFP and RFP–VPS-52 had a small proportion of particles with both GFP and RFP (Fig. 2G to I). Despite several attempts, we did not succeed in making a red-tagged version of PMR or VRG-4 that we could use to see if PMR and VRG-4 colocalized. The localization data are discussed further below.
Fig 1.
Intracellular location of PMR. The scale bar in panel A is 10 μm. All panels are shown at the same magnification. (A to C) Cells have been transformed with pmr+::sgfp. The regions shown are the hyphal tip (A) and the regions 150 μm from the tip (B) and 2 mm from the tip (C). (D to F) Shown is the region 150 μm from the tip in a heterokaryon formed by coinoculating strains transformed with pmr+::sgfp and rfp::pep-12+. Panels D and F were merged in panel E.
Fig 2.
Intracellular location of the PMR, VPS-52, and VRG-4 proteins. The scale bars are 10 μm in panels A to F and 5 μm in panels G to I. (A to C) Cells were transformed with vps-52+::sgfp (A), rfp::vps-52+ (B), and vrg-4+::sgfp (C). (D to F) Shown is a region at the hyphal tip in a heterokaryon formed by the coinoculation of strains transformed with pmr+::sgfp (D) and rfp::vps-52+ (F). (Panels D and F were merged in panel E. (G to I) Shown is a heterokaryon formed by coinoculating strains transformed with vrg-4+::sgfp (G) and rfp::vps-52+ (I). Panels G and I were merged in panel H. Arrows indicate particles with both GFP and RFP. The region shown is approximately 100 μm from the hyphal tip.
Effect of calcium and manganese on the growth of strains lacking PMR.
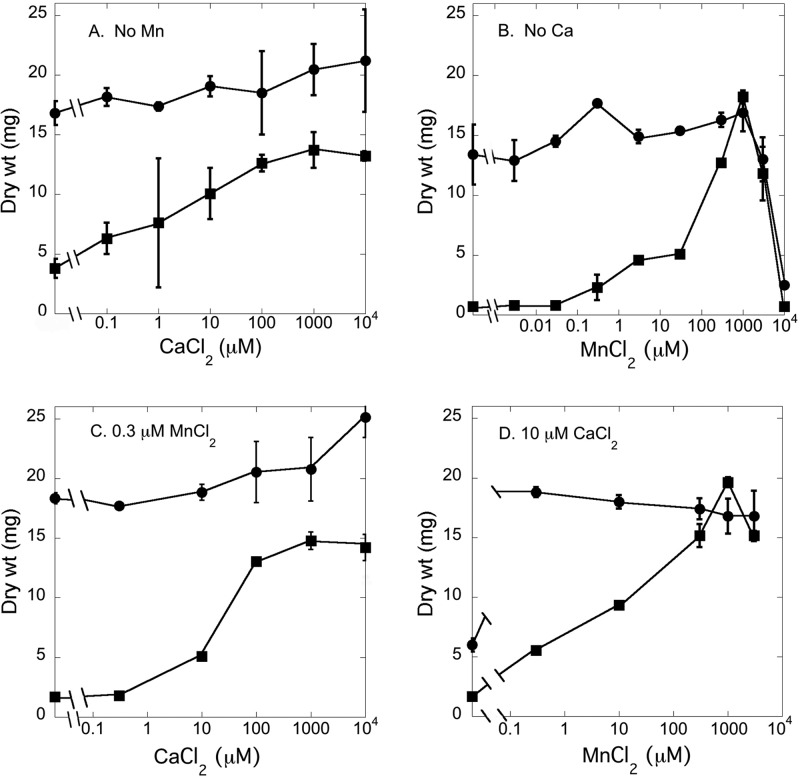
Previous reports have shown that the PMR protein has a role in the transport of both calcium and manganese in fungi (2, 24, 30, 36, 48, 57). We compared the growths of the Δpmr strain and wild-type strain 74A in Vogel's minimal medium, which is widely used for N. crassa. This medium contains 8.9 mM citrate, a weak chelator of divalent cations. In preliminary experiments, we found that the omission of citrate allowed the Δpmr strain to grow in a mass nearly as well as the wild type, presumably using trace levels of divalent cations. In the experiments shown in Fig. 3, the medium contained citrate. The growth of the wild-type strain was barely affected by the omission of both calcium and manganese from Vogel's medium, while the Δpmr strain grew poorly in this medium (Fig. 3A and B). The addition of calcium up to 1 mM increased the growth yield for the Δpmr strain, although maximal growth was still less than that for the wild type. The addition of manganese at a concentration of 1 mM produced the same growth yield for the Δpmr strain as that for the wild type. Higher concentrations of manganese were toxic to both the Δpmr and wild-type strains.
Fig 3.
Growth of wild-type and Δpmr strains with various concentrations of calcium and manganese. Strains were grown in Vogel's medium with the indicated concentrations of CaCl2 and MnCl2, as described in Materials and Methods. Circles represent wild-type strain 74A, and squares represent the Δpmr strain.
Similar results were obtained if we omitted only one of the divalent cations. The growth of the Δpmr strain approached that of the wild type in medium with 0.1 to 10.0 mM calcium (Fig. 3C). At manganese concentrations of 0.3 to 3.0 mM, the growth of the Δpmr strain was equivalent to that of the wild type (Fig. 3D). Note that the wild type grew poorly in medium with 10 μM calcium and no added manganese (Fig. 3D), perhaps indicating that calcium can block the uptake of trace amounts of manganese. The addition of just 0.3 μM manganese was sufficient for the maximal growth of the wild type. These results show that PMR plays an important role in providing the calcium and manganese needed for growth.
Morphological defects in the Δpmr strain.
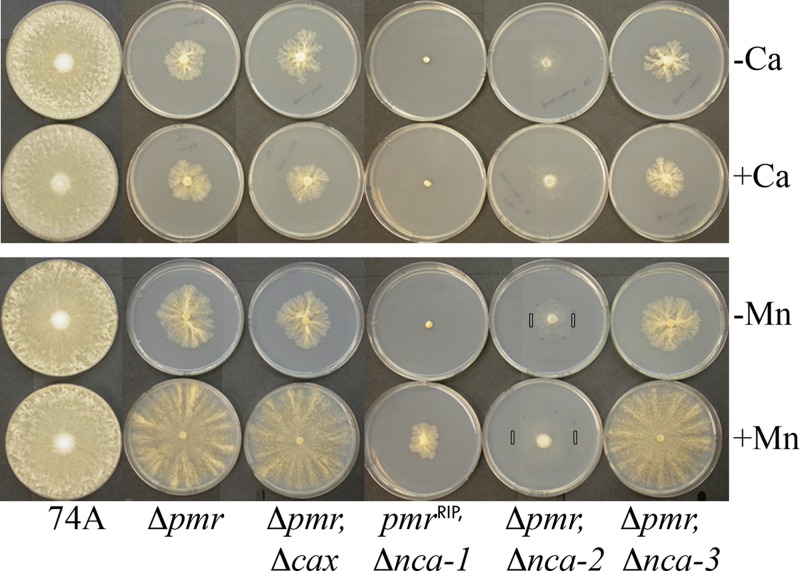
When grown on agar plates with Vogel's minimal medium, the Δpmr strain exhibited an unusual colony morphology. The linear growth rate was much lower, 0.23 mm/h, than the growth rate for the wild type, 4.6 mm/h. The edge of the colony was highly branched, the production of aerial hyphae was suppressed, and the general appearance of the mycelial mat was different (Fig. 4). The addition of a high concentration of calcium (10 mM) did not affect the growth rate or morphology. The addition of manganese (1 mM) stimulated the radial growth of the Δpmr strain 4-fold and partially corrected the morphological defects. Higher concentrations of calcium or manganese had no additional effects or were toxic.
Fig 4.
Growth of wild-type and mutant strains on agar plates. Strains were grown for 2 days at 30°C, as described in Materials and Methods. As indicated, either 1 mM MnCl2 or 10 mM CaCl2 was added to some plates. The black bars on the plates with the Δpmr Δnca-2 strain show the position of the edge of the colony, which is barely visible in the photograph.
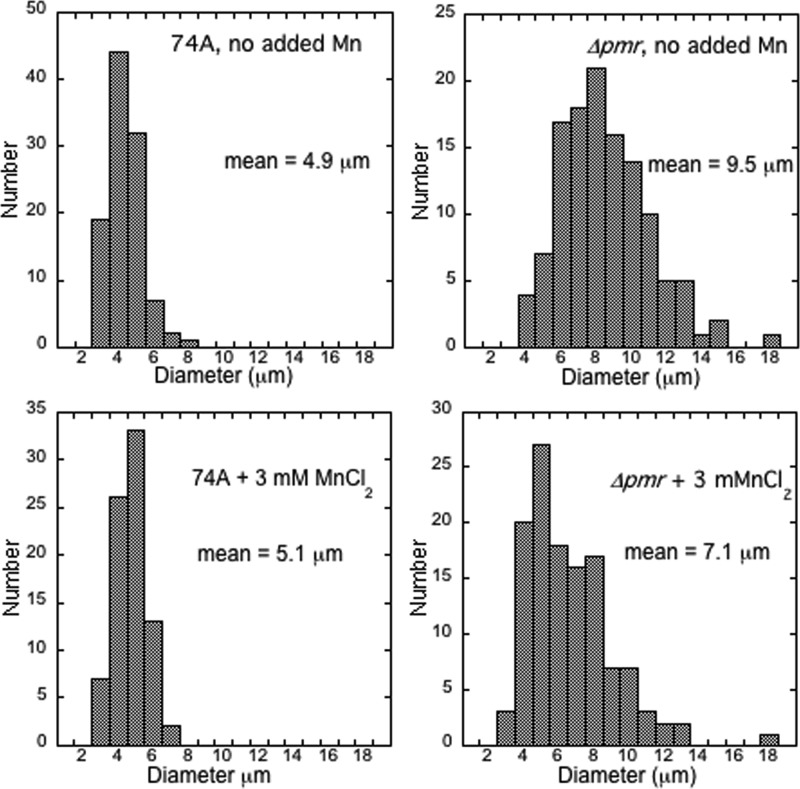
The Δpmr strain did not produce conidia (asexual spores) when cultured in ways that result in abundant conidial production from the wild type, for example, by growth in agar slants or in Erlenmeyer flasks. The only reproducible way that we found to produce conidia was to grow Δpmr cells on agar plates for at least 10 days. Conidia formed in the narrow space where the plate contacted the lid (see data for the Δpmr strain in Fig. 8). The conidia that were produced were highly variable in size and shape. The conidia from the wild type had an average diameter of 4.9 μm, while the average diameter for conidia of the Δpmr strain was nearly twice as large, 9.5 μm (Fig. 5, top). The addition of MnCl2 to the medium did not significantly change the size of conidia compared to the size of the wild-type conidia, but it did affect the size of conidia of the Δpmr strain, resulting in the production of conidia that were a bit more like those of the wild type (Fig. 5, bottom). The shape of conidia is shown in Fig. 6. For the Δpmr strain, conidia were often highly vacuolated or shaped like rods. The addition of manganese only partially reduced the proportion of abnormal conidia.
Fig 8.
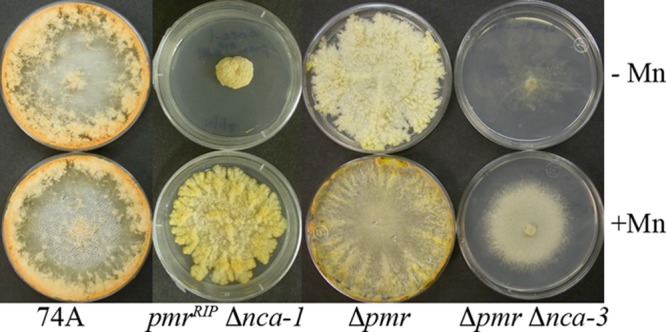
Wild-type and mutant strains grown on agar plates for 10 days. Strains were inoculated with 3-mm plugs of mycelia grown on Vogel's medium, with and without added MnCl2. Strains were grown for 3 days at 30°C, followed by 7 days at room temperature.
Fig 5.
Sizes of conidia from wild-type and Δpmr strains. Conidia were harvested from cultures grown on agar plates containing Vogel's medium with no supplements (top) or with 3 mM MnCl2 (bottom).
Fig 6.
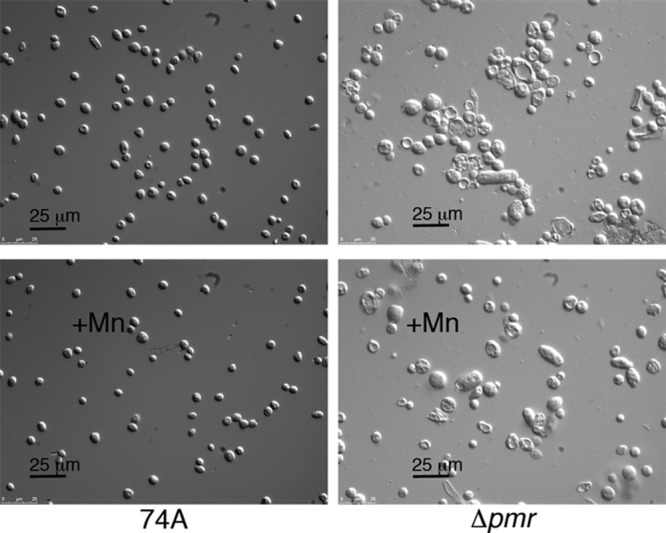
Effect of high concentrations of MnCl2 on the size and shape of conidia. The conidia measured in Fig. 5 are shown.
A microscopic examination of the leading edge of colonies showed that Δpmr hyphae were more highly branched than those of the wild type and were less likely to develop the pattern seen in the wild type, a few large hyphae with subsidiary branches (Fig. 7, top). To obtain quantitative data, we took time-lapse photographs during 8 h of growth at room temperature, observing hyphae at the leading edge of the colony. The wild-type strain formed a branch at an average time interval of 4.9 min, and the average distance between branches was 0.35 mm. The Δpmr strain formed a branch at an average time interval of 10.8 min, and the average distance between branches was 0.07 mm. The differences were even more pronounced when early growth from a single conidium was observed (Fig. 7, bottom). The wild type produced long hyphae with few branches, often as much as 1 mm apart. Many hyphae grew into the agar (out of the focal plane in Fig. 7). Hyphae from the Δpmr strain grew almost exclusively on the agar surface, with frequent branches. The addition of manganese did not significantly affect the wild type. For the Δpmr strain, hyphal growth with a high manganese concentration was still largely on the surface, but the branching pattern was more similar to that of the wild type.
Fig 7.
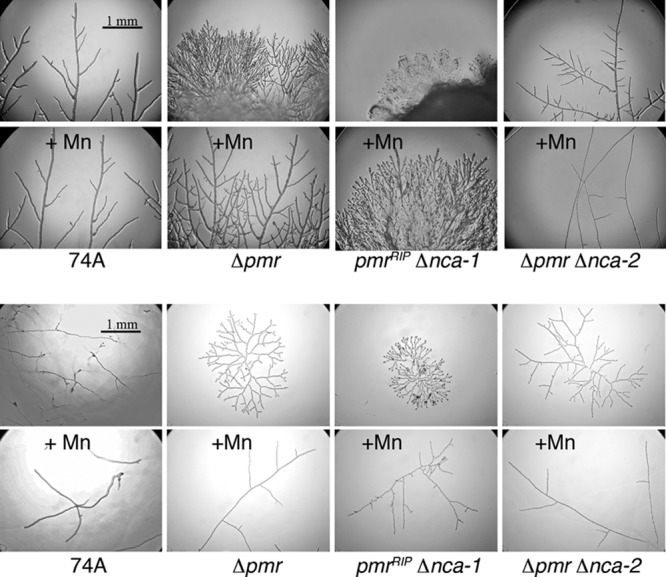
Hyphal morphology of wild-type and mutant strains. The top eight photographs show the edges of colonies inoculated with 3-mm plugs of mycelia and grown for 24 h at 30°C. The bottom eight photographs show colonies growing from single conidia after 24 h at 30°C. All photographs were taken at the same magnification. The strains were grown on Vogel's medium, with and without added MnCl2, as described in Materials and Methods.
Phenotypes of double mutant strains.
We previously observed that strains lacking two calcium transporters can reveal unpredicted phenotypes. For example, the growth of the Δcax strain is indistinguishable from that of the wild type in media with 0.01 to 400 mM CaCl2. However, the growth of the Δcax Δnca-2 strain is highly sensitive to calcium, much more so than strains lacking only NCA-2 (4). We constructed double mutant strains with the Δpmr mutation and either the Δcax, Δnca-1, Δnca-2, or Δnca-3 mutation (Fig. 4). The radial growth rates and morphologies of the Δpmr Δcax and Δpmr Δnca-3 strains were indistinguishable from those of the single Δpmr strain. The pmrRIP Δnca-1 and Δpmr Δnca-2 strains had severe, but different, defects in growth and morphology.
NCA-1 is a SERCA-type Ca2+-ATPase in the ER. The Δnca-1 strain of N. crassa has no observable defect in growth, morphology, or calcium uptake, either by itself or in combination with the Δnca-2, Δnca-3, or Δcax mutation (4). The pmrRIP Δnca-1 double mutant exhibited extreme defects in growth and morphology. Conidiation was never observed. On agar plates containing Vogel's minimal medium, the strain barely grew in 3 days, forming a tiny ball of mycelia (Fig. 4). After 2 weeks, the ball of mycelia enlarged but never spread across the plate. The addition of manganese moderately stimulated radial growth but not to the extent observed for the Δpmr strain (Fig. 8). Hyphae in the pmrRIP Δnca-1 colony remained tubular, but tiny, and even more highly branched than in the Δpmr strain (Fig. 7). A high frequency of split hyphal tips was observed. As with the Δpmr strain, the addition of manganese, but not calcium, partially suppressed the morphological defects (Fig. 4 and 8). The results show that NCA-1 is indeed functional; however, the effects of the deletion of the gene encoding nca-1 were visible only in cells lacking a functional PMR transporter.
In a previous report, we suggested that NCA-2 resides in the plasma membrane in N. crassa, pumping calcium out of the cell (4). The Δpmr Δnca-2 strain grew more slowly than either of the single mutants. On agar plates, the mycelial mat was much less dense, and conidiation was never observed. However, the morphology of individual hyphae was surprisingly more like that of the wild type (Fig. 7), suggesting that the deletion of nca-2 partially suppresses the morphological defects of the Δpmr mutation. The hyphal morphology of the Δpmr Δnca-2 strain was even more like that of the wild type if manganese was added (Fig. 7, bottom).
Uptake of calcium by the Δpmr and double mutant strains.
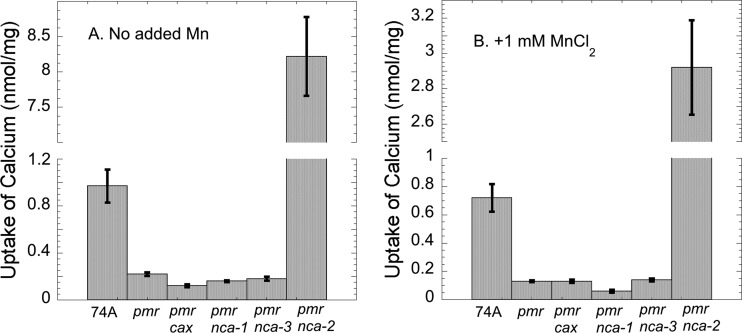
We wanted to determine if the deletion of pmr affected the amount of calcium in cells. Wild-type strain 74A and mutant strains were grown in standing liquid cultures to approximately the mid-log phase. The amount of calcium in the Δpmr strain was only 20% of that in the wild-type strain. The pmrRIP Δnca-1, Δpmr Δnca-3, and Δpmr Δcax double mutants had similarly low levels of calcium (Fig. 9A). We reported previously that the Δnca-2 strain accumulates 4- to 10-fold more calcium than the wild type (4). The Δpmr Δnca-2 double mutant accumulated 8-fold more calcium than the wild type (Fig. 9A), showing that the loss of nca-2 is epistatic to the loss of pmr. The accumulation of calcium was also measured in medium with 1 mM MnCl2 (Fig. 9B). The results were qualitatively the same, showing that high levels of manganese did not suppress the defect in calcium accumulation in Δpmr strains.
Fig 9.
Uptake of calcium by wild-type and mutant strains. The strains were grown at 30°C in liquid Vogel's medium for 2 to 6 days. For panel B, the medium was supplemented with 1 mM MnCl2. The accumulation of 45Ca was determined as described in Materials and Methods.
DISCUSSION
The data from this and two previous reports (4, 5) provide a reasonably comprehensive view of the role of calcium transport proteins in N. crassa (Fig. 10). The locations and functions of these transporters in this filamentous fungus make it more similar to animal cells than to yeasts. Like the yeasts, N. crassa has a calcium/H+ antiporter, encoded by the cax gene, in the vacuolar membrane. Like animal cells, the PMCA-type Ca2+-ATPase, NCA-2, appears to function in the plasma membrane, pumping calcium out of the cell. N. crassa and other filamentous fungi in fact have multiple homologs of the PMCA-type Ca2+-ATPase (3, 18, 58). The function of these other PMCA homologs has not yet been discovered, although we have shown that NCA-3 from N. crassa is located in the plasma membrane. Yeasts and animal cells have an SPCA-type Ca2+-ATPase (PMR) in the secretory pathway. In S. cerevisiae, Pmr1p was proposed previously to do double duty, functioning in the ER and the Golgi compartment (52), whereas animal cells and filamentous fungi have a SERCA-type Ca2+-ATPase in the ER.
Fig 10.
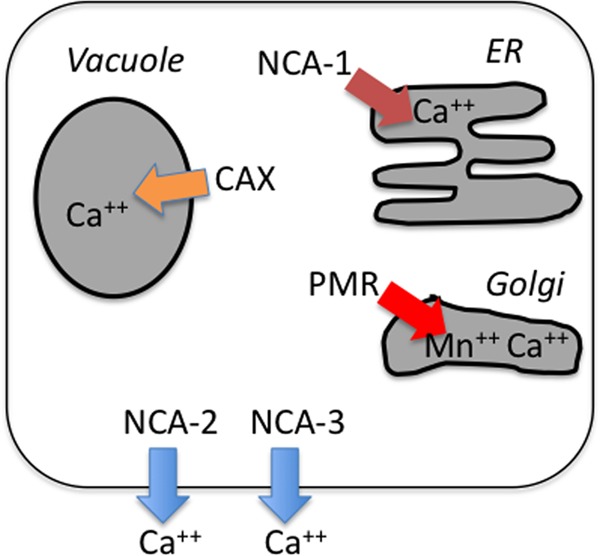
Model of the function of calcium transporters in N. crassa. Note that NCA-2 and NCA-3, when tagged with GFP or RFP, are observed in both the plasma membrane and the vacuole. However, the data do not indicate that they are functional in the vacuolar membrane (4).
Because strains lacking the SERCA-type Ca2+-ATPase (Δnca-1) had no observable defects, we lacked evidence that this enzyme had an important function in N. crassa (4). Our results support the idea that in the absence of a Ca2+-ATPase in the ER, e.g., as in the Δnca-1 strain, the PMR transporter can function in the ER in N. crassa. The amount of PMR in the ER may be quite small, because we could not detect PMR-GFP in the ER, even in a Δnca-1 background (data not shown). The deletion of both nca-1 and pmr gives rise to a strain with defects far more severe than the deletion of only pmr. The double mutant barely grows on normal media, although it is weakly stimulated by high levels of manganese. The morphological phenotype is unusual, with the mycelia forming a spherical colony. Individual hyphae are still tubular but highly branched. Thus, our analysis of the pmrRIP Δnca-1 strain strongly indicates that NCA-1 does have a functional role in N. crassa.
To see the location of PMR in N. crassa, we tagged the protein with GFP. It was predominately localized in 0.5- to 1.0-μm particles, most abundant near the hyphal tip. In other organisms, the SPCA family of Ca2+-ATPases has been observed in the Golgi compartment (41, 55). The N. crassa homologs of Vps52p and Vrg4p, proteins that have been observed in the Golgi compartment in S. cerevisiae and other organisms (8, 32, 44), appeared in particles similar in size and distribution to the particles visualized with PMR-GFP.
However, the observation of N. crassa hyphae expressing both PMR-GFP and RFP–VPS-52 indicated that these proteins were not all in the same compartment. In merged images, 5 to 15% of the particles contained both proteins, but most of the tagged particles appeared as different, spatially separated compartments. Other investigators reported observations of different Golgi proteins in different compartments of the Golgi compartment (33). Thus, our data are consistent with a Golgi localization for PMR in N. crassa. The particles that we saw may be early Golgi cisternae, some of which were transitioning to late Golgi cisternae. However, other explanations are also possible. VPS-52 was also shown previously to interact with endosomes (8, 47, 50). PMR could also be localized to vesicles that travel between the ER and the Golgi compartment. Determining the exact location of PMR, and, indeed, of other secretory pathway proteins in N. crassa, will be a challenging project.
The complex phenotype of the Δpmr strain indicates that PMR has multiple important functions. Consistent with data from previous reports, PMR appears to transport both calcium and manganese (2, 24, 30, 36, 48, 57). The wild-type strain, but not the Δpmr strain, can grow at nearly normal rates in Vogel's medium in which calcium and manganese have been omitted. PMR is thus required to either scavenge trace amounts of calcium and manganese from the medium or maintain sufficient levels of calcium and manganese in an intracellular compartment. Interestingly, an increase of the concentration of only one of these divalent cations is sufficient to stimulate the growth rate; i.e., calcium can substitute for manganese and vice versa. However, this is not true for the defects in hyphal morphology. A high concentration of manganese in the medium, but not calcium, partially suppressed the aberrant hyphal branching pattern and the production of atypically large, misshapen conidia. The most likely explanation is that enzymes involved in glycosylation require manganese as a cofactor and that PMR is required to pump manganese into the Golgi compartments where these enzymes reside (2, 16, 53). Support for this hypothesis comes from the recent report that the deletion of OCH1, a 1,6-mannosyltransferase, causes morphological defects in N. crassa that are very similar to what we observed for the Δpmr strain (34).
Given the observations that more than 95% of cell calcium is stored in vacuoles (4, 15, 23), we were surprised to find that the Δpmr strain accumulates 80% less calcium than the wild type. In S. cerevisiae, Δpmr1 strains accumulate more calcium than the wild type, supporting the hypothesis that Pmr1p has a role in calcium efflux in this organism, presumably by pumping calcium into secretory vesicles which then fuse with the plasma membrane (10, 24, 29, 37). The loss of PMR in N. crassa may disrupt Golgi function and thereby disrupt the synthesis of compartments in the cell that normally accumulate calcium. We must also consider the possibility that a loss of function, such as an accumulation of normal levels of calcium, may simply be an indirect consequence of the poor growth of the Δpmr strain.
Do the phenotypes of Δpmr and pmrRIP Δnca-1 strains provide evidence for or against the hypothesis that calcium plays a key role in polarized growth and/or branching in filamentous fungi? The lack of morphological defects in Δnca-1, Δnca-2, Δnca-3, or Δcax strains led us to conclude that these calcium transporters were not essential for a calcium signal involved in polarized growth. In contrast, the Δpmr and pmrRIP Δnca-1 strains have severe morphological defects that are only partially suppressed by added manganese. The hyphae are still tubular, with new growth occurring at the tip, albeit at a greatly reduced rate, but the frequency and pattern of branching are highly dependent on PMR and also on NCA-1 in the absence of PMR. Also noteworthy is the phenotype of the Δpmr Δnca-2 strain, which has a more nearly normal hyphal morphology. The deletion of NCA-2 causes excess calcium to accumulate in N. crassa (4), a phenomenon also observed for the Δpmr Δnca-2 double mutant strain. Thus, there is a correlation: the Δpmr strain has small amounts of calcium and exhibits hyperbranching, while the Δpmr Δnca-2 strain has elevated concentrations of calcium and suppressed hyperbranching. However, these experiments do not rule out the possibility that the morphological defects are a secondary effect. Insufficient calcium may disrupt secretory pathway functions that in turn cause defects in polarized growth and branching.
ACKNOWLEDGMENTS
Most strains used in this report were generated by the Neurospora Genome Project and were obtained from the Fungal Genetics Stock Center. We thank Christopher Chavez for contributions to early work relating to this project and Marija Draskovic for technical assistance.
This study was supported by Public Health Service grant GM058903 from the Institute of General Medicine to B.J.B.
Footnotes
Published ahead of print 14 September 2012
REFERENCES
- 1. Abreu S. 2003. The roles of the organellar calcium transport proteins nca-2, nca-3, cax and pmr1 in cell morphology and growth. M.S. thesis University of California—Santa Cruz, Santa Cruz, CA [Google Scholar]
- 2. Antebi A, Fink GR. 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3:633–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bencina M, Bagar T, Lah L, Krasevec N. 2009. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet. Biol. 46(Suppl 1):S93–S104 doi:10.1016/j.fgb.2008.07.019 [DOI] [PubMed] [Google Scholar]
- 4. Bowman BJ, Abreu S, Margolles-Clark E, Draskovic M, Bowman EJ. 2011. Role of four calcium transport proteins, encoded by nca-1, nca-2, nca-3, and cax, in maintaining intracellular calcium levels in Neurospora crassa. Eukaryot. Cell 10:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowman BJ, Draskovic M, Freitag M, Bowman EJ. 2009. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa. Eukaryot. Cell 8:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carafoli E. 2002. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. U. S. A. 99:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colot HV, et al. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103:10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conibear E, Stevens TH. 2000. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol. Biol. Cell 11:305–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui J, Kaandorp JA, Sloot PM, Lloyd CM, Filatov MV. 2009. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res. 9:1137–1147 [DOI] [PubMed] [Google Scholar]
- 10. Culotta VC, Yang M, Hall MD. 2005. Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot. Cell 4:1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham KW, Fink GR. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham KW, Fink GR. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis RH. 2000. Neurospora. Oxford University Press, New York, NY [Google Scholar]
- 14. Denis V, Cyert MS. 2002. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 156:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn T, Gable K, Beeler T. 1994. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269:7273–7278 [PubMed] [Google Scholar]
- 16. Durr G, et al. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9:1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eilam Y, Lavi H, Grossowicz N. 1985. Cytoplasmic Ca2+ homeostasis maintained by a vacuolar Ca2+ transport-system in the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 131:623–629 [DOI] [PubMed] [Google Scholar]
- 18. Findon H, et al. 2010. Analysis of a novel calcium auxotrophy in Aspergillus nidulans. Fungal Genet. Biol. 47:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer M, et al. 1997. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 419:259–262 [DOI] [PubMed] [Google Scholar]
- 20. Gerrard SR, Mecklem AB, Stevens TH. 2000. The yeast endosomal t-SNARE, Pep12p, functions in the absence of its transmembrane domain. Traffic 1:45–55 [DOI] [PubMed] [Google Scholar]
- 21. Gotte M, Gallwitz D. 1997. High expression of the yeast syntaxin-related Vam3 protein suppresses the protein transport defects of a pep12 null mutant. FEBS Lett. 411:48–52 [DOI] [PubMed] [Google Scholar]
- 22. Gupta GD, Heath IB. 2002. Predicting the distribution, conservation, and functions of SNAREs and related proteins in fungi. Fungal Genet. Biol. 36:1–21 [DOI] [PubMed] [Google Scholar]
- 23. Halachmi D, Eilam Y. 1989. Cytosolic and vacuolar Ca2+ concentrations in yeast cells measured with the Ca2+-sensitive fluorescence dye indo-1. FEBS Lett. 256:55–61 [DOI] [PubMed] [Google Scholar]
- 24. Halachmi D, Eilam Y. 1996. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett. 392:194–200 [DOI] [PubMed] [Google Scholar]
- 25. Harold FM. 2002. Force and compliance: rethinking morphogenesis in walled cells. Fungal Genet. Biol. 37:271–282 [DOI] [PubMed] [Google Scholar]
- 26. Harris SD, et al. 2009. Morphology and development in Aspergillus nidulans: a complex puzzle. Fungal Genet. Biol. 46(Suppl 1):S82–S92 doi:10.1016/j.fgb.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 27. Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14:8259–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson SL, Heath IB. 1993. Roles of calcium ions in hyphal tip growth. Microbiol. Rev. 57:367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kellermayer R, Aiello DP, Miseta A, Bedwell DM. 2003. Extracellular Ca2+ sensing contributes to excess Ca2+ accumulation and vacuolar fragmentation in a pmr1Δ mutant of S. cerevisiae. J. Cell Sci. 116:1637–1646 [DOI] [PubMed] [Google Scholar]
- 30. Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang F, Cunningham KW, Harper JF, Sze H. 1997. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 94:8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lobstein E, et al. 2004. The putative Arabidopsis homolog of yeast Vps52p is required for pollen tube elongation, localizes to Golgi, and might be involved in vesicle trafficking. Plant Physiol. 135:1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Losev E, et al. 2006. Golgi maturation visualized in living yeast. Nature 441:1002–1006 [DOI] [PubMed] [Google Scholar]
- 34. Maddi A, Free SJ. 2010. α-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa. Eukaryot. Cell 9:1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maeda T, et al. 2004. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: the importance of Mn2+ homeostasis. Genes Cells 9:71–82 [DOI] [PubMed] [Google Scholar]
- 36. Mandal D, Woolf TB, Rao R. 2000. Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 275:23933–23938 [DOI] [PubMed] [Google Scholar]
- 37. Marchi V, Sorin A, Wei Y, Rao R. 1999. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 454:181–186 [DOI] [PubMed] [Google Scholar]
- 38. Margolin BS, Freitag M, Selker EU. 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 47:34–36 [Google Scholar]
- 39. McCluskey K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52:245–262 [DOI] [PubMed] [Google Scholar]
- 40. McGillviray AM, Gow NAR. 1987. The transhyphal electrical current of Neurospora crassa is carried principally by protons. J. Gen. Microbiol. 133:2875–2881 [Google Scholar]
- 41. Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F. 2007. Calcium in the Golgi apparatus. Cell Calcium 41:405–416 [DOI] [PubMed] [Google Scholar]
- 42. Paidhungat M, Garrett S. 1997. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 17:6339–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinchai N, et al. 2010. The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot. Cell 9:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poster JB, Dean N. 1996. The yeast VRG4 gene is required for normal Golgi functions and defines a new family of related genes. J. Biol. Chem. 271:3837–3845 [DOI] [PubMed] [Google Scholar]
- 45. Pozos TC, Sekler I, Cyert MS. 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16:3730–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess IB. 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256:104–114 [DOI] [PubMed] [Google Scholar]
- 47. Reggiori F, Wang CW, Stromhaug PE, Shintani T, Klionsky DJ. 2003. Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion. J. Biol. Chem. 278:5009–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rudolph HK, et al. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133–145 [DOI] [PubMed] [Google Scholar]
- 49. Selker EU, Garrett PW. 1988. DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc. Natl. Acad. Sci. U. S. A. 85:6870–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siniossoglou S, Pelham HRB. 2002. Vps51p links the VFT complex to the SNARE Tlg1p. J. Biol. Chem. 277:48318–48324 [DOI] [PubMed] [Google Scholar]
- 51. Soriani FM, et al. 2005. A PMR1-like calcium ATPase of Aspergillus fumigatus: cloning, identification and functional expression in S. cerevisiae. Yeast 22:813–824 [DOI] [PubMed] [Google Scholar]
- 52. Strayle J, Pozzan T, Rudolph HK. 1999. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 18:4733–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ton VK, Mandal D, Vahadji C, Rao R. 2002. Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277:6422–6427 [DOI] [PubMed] [Google Scholar]
- 54. Ton VK, Rao R. 2004. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am. J. Physiol. Cell Physiol. 287:C580–589 [DOI] [PubMed] [Google Scholar]
- 55. Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J. 2011. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol. 3:pii=a004184. doi:10.1101/cshperspect.a004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei Y, et al. 2000. Phenotypic screening of mutations in PMR1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem. 275:23927–23932 [DOI] [PubMed] [Google Scholar]
- 57. Yang J, et al. 2001. Cloning of the Aspergillus niger pmrA gene, a homologue of yeast PMR1, and characterization of a pmrA null mutant. FEMS Microbiol. Lett. 199:97–102 [DOI] [PubMed] [Google Scholar]
- 58. Zelter A, Bencina M, Bowman BJ, Yarden O, Read ND. 2004. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 41:827–841 [DOI] [PubMed] [Google Scholar]