Abstract
Heat shock protein 90 (Hsp90) is a eukaryotic molecular chaperone. Its involvement in the resistance of Candida albicans to azole and echinocandin antifungals is well established. However, little is known about Hsp90's function in the filamentous fungal pathogen Aspergillus fumigatus. We investigated the role of Hsp90 in A. fumigatus by genetic repression and examined its cellular localization under various stress conditions. Failure to generate a deletion strain of hsp90 suggested that it is essential. Genetic repression of Hsp90 was achieved by an inducible nitrogen-dependent promoter (pniiA-Hsp90) and led to decreased spore viability, decreased hyphal growth, and severe defects in germination and conidiation concomitant with the downregulation of the conidiation-specific transcription factors brlA, wetA, and abaA. Hsp90 repression potentiated the effect of cell wall inhibitors affecting the β-glucan structure of the cell wall (caspofungin, Congo red) and of the calcineurin inhibitor FK506, supporting a role in regulating cell wall integrity pathways. Moreover, compromising Hsp90 abolished the paradoxical effect of caspofungin. Pharmacological inhibition of Hsp90 by geldanamycin and its derivatives (17-AAG and 17-DMAG) resulted in similar effects. C-terminal green fluorescent protein (GFP) tagging of Hsp90 revealed mainly cytosolic distribution under standard growth conditions. However, treatment with caspofungin resulted in Hsp90 accumulation at the cell wall and at sites of septum formation, further highlighting its role in cell wall stress compensatory mechanisms. Targeting Hsp90 with fungal-specific inhibitors to cripple stress response compensatory pathways represents an attractive new antifungal strategy.
INTRODUCTION
The heat shock protein 90 (Hsp90) is a molecular chaperone present in all eukaryotes (39). In fungi, Hsp90 has been mainly studied in yeasts (3). Hsp90 of Saccharomyces cerevisiae was found to be at the center of an extended network of key signaling proteins or transcription factors involving more than 10% of the entire proteome (41). Moreover, Hsp90 has been demonstrated to play an important role in the emergence of resistance to azole and echinocandin antifungal drugs in Candida albicans (5–9, 19, 28, 31).
The recent application of Hsp90 inhibitors as anti-cancer therapy (38, 39) has raised considerable interest in this protein as a target for new antifungal therapies. Geldanamycin (a benzoquinone ansamycin antibiotic binding to the ATP-binding pocket of Hsp90) and its derivatives 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) have demonstrated a potentiating in vitro effect when combined with azoles or echinocandins against C. albicans (8, 9, 28, 31). However, due to their toxicity, their use in vivo could be assessed only in an invertebrate host model (the wax moth, Galleria mellonella) (9). While deletion of hsp90 was never achieved in yeasts, genetic repression was reported for both S. cerevisiae and C. albicans and resulted in increased susceptibility to azoles and echinocandins in vitro and in a murine model of invasive candidiasis (8, 9, 31). Compromising Hsp90 in C. albicans also revealed important functions of this chaperone in morphogenesis and virulence, such as a regulatory role in the temperature-dependent transition from yeasts to filamentous growth (30). Filaments resulting from Hsp90 repression mimicked those induced by cell cycle arrest and were associated with cells of two-lobed morphology exhibiting defects in cytokinesis, which further highlights a role in cell division and cell cycle progression (29).
Little is known about the role of Hsp90 in molds such as Aspergillus fumigatus, one of the most important human pathogenic fungi. Hsp90 inhibitors increased the effect of caspofungin in vitro and in an invertebrate model of invasive aspergillosis (9, 28). However, genetic repression of Hsp90 has never been achieved in A. fumigatus or other molds, thus preventing further molecular characterization of its actual role in growth, virulence, and drug resistance. Here, we investigated the role of Hsp90 in A. fumigatus via genetic and pharmacologic repression of Hsp90 and analyzed its subcellular localization by GFP tagging. Our results suggest an important role of Hsp90 in A. fumigatus conidiation and in cell wall stress-compensatory mechanisms.
(This work was presented in part [P132] at the 5th Advances Against Aspergillosis meeting in Istanbul, Turkey, 26 to 28 January 2012.)
MATERIALS AND METHODS
Strains, media, and culture conditions.
The A. fumigatus akuBKU80 strain, which is auxotrophic for uracil/uridine and possesses an increased rate of homologous recombination (11), was used as the transformation recipient as well as the control strain. A. fumigatus wild-type strain (AF293) DNA was used for molecular cloning. Cultures were grown at 37°C on glucose minimal medium (GMM) supplemented with 5 mM uracil and 5 mM uridine as previously described (32), unless otherwise specified. For the transformants harboring the nitrogen-inducible pniiA promoter, modified GMM containing various sources of nitrogen were used to achieve repression of the promoter (14, 22): (i) ammonium minimal medium (AMM, with 20 mM ammonium tartrate [C4H12N2O6] as the sole nitrogen source), (ii) GMM supplemented with ammonium (GMM+Am, where 20 mM ammonium tartrate was added to GMM, thus containing a concomitant source of nitrate sodium [NaNO3] to support growth).
Escherichia coli DH5α competent cells (New England BioLabs, Ipswich, MA) were used for cloning and grown in Luria-Bertani broth (Fisher Scientific, Pittsburgh, PA) at 37°C with the addition of carbenicillin. Transformations in A. fumigatus were performed as previously described (27, 32).
A. fumigatus hsp90 gene deletion.
Genetic deletion of hsp90 was attempted by replacing the 2.2-kb hsp90 gene (Afu5g04170; www.aspergillusgenome.org) with the 3.1-kb pyrG gene from Aspergillus parasiticus as previously described (32). Approximately 1-kb upstream and downstream flanking sequences of hsp90 were amplified from AF293 genomic DNA and cloned into plasmid pJW24 (32) to flank pyrG at SalI/EcoRI and BamHI/NotI sites, respectively. The pyrG gene was used to complement the uracil auxotrophy of both the A. fumigatus akuBKU80 and AF293.1 strains (26).
Construction of the Hsp90 inducible strain (pniiA-Hsp90).
In order to modulate expression of hsp90, the promoter of the gene was replaced with an inducible promoter (pniiA) as previously described (14, 27). This nitrogen-dependent promoter is induced by the presence of nitrate in the absence of ammonium, while repression is achieved by ammonium whether nitrate is present or not (14, 22). The modified plasmid pBluescript II SK(-) containing the pniiA sequence from AF293 and the hygromycin B resistance cassette (pBSK-pniiA) was used (27). A 1-kb sequence of the 5′ flanking region of hsp90 (located 0.6 kb upstream of hsp90, thus excluding the putative site of the promoter) was amplified and cloned at the KpnI/ApaI sites into pBSK-pniiA (left arm). The entire 2.2-kb sequence of hsp90 was cloned at the PstI/NotI sites (right arm). A sequence including the left arm, the pniiA promoter, the hygromycin B resistance cassette, and approximately 1 kb from the start codon of hsp90 that was sequenced for the absence of mutation, was all amplified from this construct and transformed into the akuBKU80 A. fumigatus strain. Transformants were selected by resistance to hygromycin B. Integration of the construct was confirmed by PCR and Southern analysis using the digoxigenin PCR labeling system (Roche Applied Science, Indianapolis, IN).
Construction of the Hsp90-EGFP strain.
Localization of Hsp90 was achieved by fusion to the enhanced green fluorescent protein (EGFP). The 1-kb 3′-terminal sequence of hsp90 (excluding the stop codon) was amplified and cloned at the KpnI/BamHI sites in the N terminus of egfp in plasmid pUCGH (20) (left arm). An approximately 1.5-kb sequence of the 3′ flanking region of hsp90 was cloned at the HindIII site (right arm). The resulting plasmid was linearized by KpnI and then transformed into akuBKU80. Transformants were selected by resistance to hygromycin B and verified via PCR and fluorescence microscopy. For fluorescence microscopy, the Hsp90-EGFP strain was grown in 5 ml GMM broth on coverslips at 37°C for 16 to 20 h before visualization using an Axioskop 2 Plus microscope (Zeiss) equipped with AxioVision 4.6 imaging software as previously described (18). Calcofluor white and propidium iodide were used for costaining of the cell wall and the nuclei, respectively, as previously described (18).
Radial growth and conidiation.
Conidia (104 each from the akuBKU80 and pniiA-Hsp90 strains) were inoculated on GMM, AMM, or GMM+Am agar and incubated at 37°C. Colony diameters were measured every 24 h for 5 days. Conidia were harvested after 5 days of incubation and counted with a hemocytometer. All experiments were performed in triplicate. The mean and standard deviation of the colony diameter and conidial concentration (total number of conidia per mm2 of colony) were calculated. For spore viability assays, 100 conidia of each strain grown under repression conditions were spread on GMM agar and incubated at 37°C until the appearance of colonies. The number of growing colonies was assessed for each strain in triplicate. For germination analysis, 104 conidia of each strain were inoculated in GMM or AMM broth and incubated at 37°C. Conidia were counted, and germination was expressed as the percent germlings present from 100 analyzed conidia at each time point, determined in triplicate.
Quantification of β-1,3-glucan content.
The β-1,3-glucan content of the cell wall was measured by the aniline blue assay as previously described (13). Fluorescence measurement was performed using an Flx800 fluorescence microplate reader (BioTek Instruments Inc., Winooski, VT) at 360-nm (±40) excitation and 460-nm (±40) emission wavelengths. Curdlan (Sigma, St. Louis, MO) dilutions were used to generate a standard curve. Values are mean relative fluorescent units (RFU) per nmol curdlan per milligram of mycelial tissue and standard deviations for triplicates.
Gene expression by real-time reverse transcription-PCR (RT-PCR).
To assess Hsp90 repression in the pniiA-Hsp90 strain, hsp90 expression was measured under repression conditions (AMM) and compared to the expression level in the akuBKU80 control strain. In order to investigate the link between Hsp90 and conidiation, the expression of the brlA, abaA, and wetA genes, known to be involved in conidial formation (10, 35), was assessed. Expression of hsp90 was also measured in the wild-type (AF293) under basal and various stress conditions to assess the role of Hsp90 in various stress response mechanisms. Each strain was grown at a concentration of 106 conidia/ml for 48 h at 37°C. RNA extraction, cDNA synthesis, and real-time PCR assay were performed as previously described (12). The 2−ΔΔCt analytic method (21) normalized to beta-tubulin was used to calculate expression changes. Results are the means (± standard deviations) from three triplicate assays.
Determination of Hsp90 inhibitors' antifungal activity.
The in vitro antifungal activity of Hsp90 inhibitors was assessed against the wild-type AF293 and akuBKU80 strains. The Hsp90 inhibitors geldanamycin (Sigma, St. Louis, MO), 17-(allylamino)-17-demethoxygeldanamycin (17-AAG; Selleck Chemicals, Houston, TX), and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; Selleck Chemicals, Houston, TX) were used alone or in a checkerboard combination with other antifungal agents (caspofungin, nikkomycin Z, voriconazole, amphotericin B, and FK506). Conidia (104) were inoculated on GMM agar containing a defined dose of each drug. Growth was visualized after 5 days of incubation at 37°C. For liquid medium assays, each strain was tested according to Clinical and Laboratory Standards Institute (CLSI) standards (23). The minimal effective concentration (MEC) was defined as the lowest concentration of the drug that produced significantly abnormal growth (13). Antifungal checkerboard interactions were assessed by the fractional inhibitory concentration index (FICI), with synergy defined as an FICI of ≤0.5 (25).
Statistical analyses.
Unpaired Student's t test was used for comparison of two separate sets of independent samples. A P value of <0.05 was considered statistically significant.
RESULTS
Hsp90 is likely an essential gene in A. fumigatus.
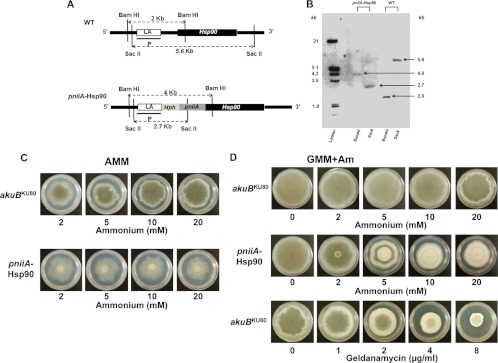
As a first step toward investigating the function of Hsp90 in A. fumigatus, we attempted to delete the gene but were unsuccessful. Results from three transformation attempts in two different recipient strains, coupled with the previous reports in yeasts (2) and in the filamentous fungus Neurospora crassa (4), led us to conclude that hsp90 is an essential gene in A. fumigatus. We therefore generated the pniiA-Hsp90 strain in which hsp90 was under the control of an inducible nitrogen-dependent promoter (pniiA) (Fig. 1A). Proper integration of the construct was verified by PCR and Southern analysis using two different restriction enzyme digestions (Fig. 1A and B). This strain exhibited an important aerial growth defect and conidiation defect on AMM agar plates when ammonium was used as the sole nitrogen source for maximal genetic repression (Fig. 1C and 2A). This effect was present at ammonium concentrations as low as 2 mM (Fig. 1C). When grown in liquid AMM (with ammonium concentrations ranging from 2 to 20 mM), the pniiA-Hsp90 strain displayed a near complete lack of hyphal growth. RT-PCR analyses performed from this small amount of tissue confirmed an 8.42-fold decrease (standard deviation [SD], ±1.02; P < 0.0001) in hsp90 expression compared to expression in the akuBKU80 control strain.
Fig 1.
Genetic construction of the pniiA-Hsp90 strain and phenotypes under Hsp90 repression. (A) Schematic representation of the genomic locus of hsp90 in the wild-type (WT) and pniiA-Hsp90 strains. Correct integration of the left arm (LA, consisting of a 1-kb sequence located downstream of the putative hsp90 encoding gene) and the right arm (1-kb 5′-end sequence of hsp90) was confirmed by Southern analysis using the left arm sequence as the probe (P) and two different enzyme digestions (BamHI and SacII). Hph, hygromycin B resistance cassette; pniiA, nitrogen-dependent inducible promoter. (B) Southern analysis of the pniiA-Hsp90 strain. Digestion with BamHI revealed 2-kb and a 4-kb fragments in the wild-type and pniiA-Hsp90 strains, respectively. Digestion with SacII shows a 5.6-kb fragment (wild type) and a 2.7-kb fragment (pniiA-Hsp90). (C and D) Morphological changes of the pniiA-Hsp90 strain grown under different levels of ammonium for Hsp90 genetic repression compared to the akuBKU80 control strain. (C) AMM (where ammonium is the unique source of nitrogen); (D) GMM+Am (ammonium with a concomitant and constant source of nitrate), which is comparable to the pharmacologic inhibition of Hsp90 with geldanamycin (concentrations ranging from 0 to 8 μg/ml) in the akuBKU80. Pictures were taken after 5 days of growth at 37°C.
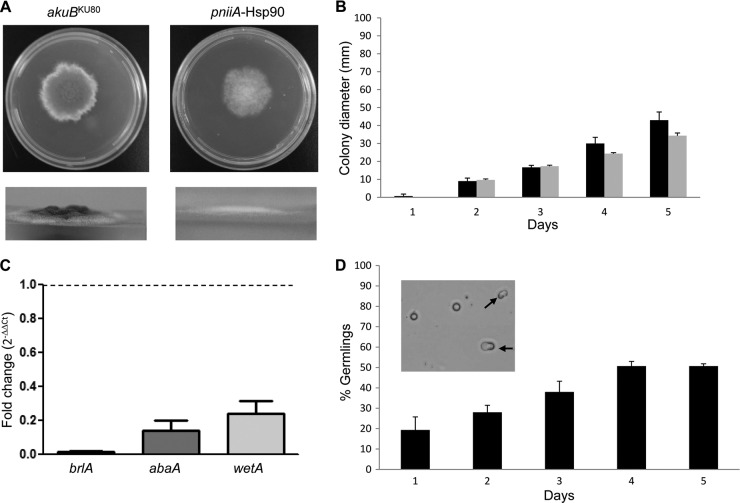
Fig 2.
Hyphal growth, germination, and conidiation defects of the pniiA-Hsp90 strain under Hsp90 repression. (A) Culture morphology of the control akuBKU80 and the pniiA-Hsp90 strains on AMM (20 mM ammonium as the sole nitrogen source) showing both radial growth (top) and aerial growth (bottom). Pictures were taken after 5 days of growth at 37°C. (B) Hyphal growth (colony diameter, in mm) of the control akuBKU80 strain (black bars) and the pniiA-Hsp90 strain (gray bars) over 5 days on AMM (20 mM ammonium). Results are means ± SD for triplicates. (C) Quantification of the transcription of the conidiation-specific genes brlA (black bar), abaA (dark gray bar), and wetA (light gray bar) via real-time reverse transcription-PCR in the pniiA-Hsp90 strain under repression conditions (20 mM ammonium). Results are mean fold change (2−ΔΔCT) ± SD compared to the wild-type (dotted line). (D) Germination rate (expressed as the mean percentage of germlings observed in triplicates ± SD) of the pniiA-Hsp90 strain (black bars) over time on AMM (20 mM ammonium). Emergence of germ tubes (black arrows) was achieved in only 50% of conidia after 4 days, while hyphal elongation was absent. The picture was taken after 4 days of growth at 37°C.
Due to this severe growth defect in the presence of ammonium as the sole nitrogen source, we used GMM supplemented with ammonium (GMM+Am) in order to provide a concomitant source of nitrate to support growth. Although this did not improve the growth defect in liquid medium, aerial hyphal growth was improved, and phenotypes that matched with pharmacological repression of Hsp90 by geldanamycin were obtained on agar plates (Fig. 1D). This growth medium (GMM+Am) was used to test the phenotypic effect of Hsp90 repression under various stress conditions.
Genetic repression of Hsp90 resulted in decreased hyphal growth and a severe conidiation defect.
Radial growth of the pniiA-Hsp90 strain and the akuBKU80 strain (control) were similar in the absence of ammonium (Fig. 1D). However, the pniiA-Hsp90 strain under repression (AMM with 20 mM ammonium) exhibited a 20% relative radial growth deficit compared to the control strain (34.3 cm ± 1.5 versus 43 ± 4.6 at 5 days, respectively; P = 0.04) (Fig. 2A and B) and a complete lack of aerial hyphal growth (Fig. 2A). When grown on AMM, the pniiA-Hsp90 strain was unable to produce conidia despite prolonged incubation. After growth for 5 days on GMM+Am (with 20 mM ammonium), the pniiA-Hsp90 strain showed a similar radial growth defect (25% reduction compared to the control strain) (Fig. 1D). However, aerial hyphal growth and a few conidia were observed (1,861 ± 568 conidia/mm2 versus 1.41 × 106 ± 0.18 × 106 in the control strain; P < 0.001). These few conidia lacked green pigmentation. Additionally, pniiA-Hsp90 under repression did not grow on rich nutrient media, such as yeast extract peptone dextrose and Sabouraud's dextrose agar.
Light microscopy of the pniiA-Hsp90 strain under repression showed both hyphae and conidia that appeared similar to those of the control strain. Normal hyphal and conidial structures were subsequently confirmed by scanning electron microscopy (data not shown).
Because of the severe conidiation defect under Hsp90 repression, we quantified the expression of brlA, abaA, and wetA, three genes encoding transcription factors that are known to control asexual sporulation in Aspergillus species (10, 35). Compared with the akuBKU80 strain, pniiA-Hsp90 under repression exhibited a significant decrease in the expression of brlA (70.2- ± 5.5-fold decrease; P = 0.0004), abaA (7.8- ± 2.6-fold decrease; P = 0.008), and wetA (4.5- ± 1.4-fold decrease; P = 0.003) (Fig. 2C).
Quantification of germination in liquid AMM revealed both a delay and a defect in germination. While the akuBKU80 control strain achieved 100% germination after approximately 9 h, germination of pniiA-Hsp90 reached 50% after 4 days and did not exceed this level despite prolonged incubation (Fig. 2D). Although initial germ tub emergence could be observed, we did not see any hyphal elongation. Results of the spore viability assay showed a 28% decrease in spore viability of the pniiA-Hsp90 strain compared to that of the control strain under repression (89 ± 13.9 versus 122.7 ± 0.6 growing colonies, respectively; P = 0.014).
Pharmacological inhibition of Hsp90 mimics but does not equal Hsp90 genetic repression.
In order to compare the effect of the currently available Hsp90 inhibitors with the Hsp90 repressive growth phenotypes, we first determined the minimal effective concentration (MEC) of the Hsp90 inhibitor geldanamycin to be 4 μg/ml, while that of its analogs 17-AAG and 17-DMAG was 8- to 10-fold higher. At these concentrations, light microscopy revealed a similar morphology to that resulting from genetic repression with apparently normal hyphae. On GMM agar plates, exposure to Hsp90 inhibitors resulted in similar morphological alterations observed in the pniiA-Hsp90 strain under repression, demonstrating a substantial, albeit somewhat less severe, conidiation defect and a moderate reduction of radial growth (Fig. 1D). Current Hsp90 inhibitors thus revealed only a limited efficacy against A. fumigatus, indicating a requirement for high doses.
Hsp90 repression led to increased sensitivity to caspofungin and abolition of the paradoxical effect.
We then investigated the impact of Hsp90 repression under various stress conditions. Due to the severe growth deficit of the pniiA-Hsp90 strain under basal growth conditions on AMM, we used GMM+Am (GMM supplemented with 20 mM ammonium).
As the cell wall inhibitor caspofungin has been shown to increase the effect of Hsp90 inhibitors against C. albicans (31) and A. fumigatus (9), we investigated its effect on the pniiA-Hsp90 strain under repression. While radial growth was decreased approximately 20% in the untreated pniiA-Hsp90, the addition of caspofungin resulted in a considerably increased inhibition of growth compared to the control strain (Fig. 3B). Hsp90 genetic repression combined with caspofungin (≥0.25 μg/ml) yielded very small colonies that did not exhibit any radial hyphal growth despite prolonged incubation time. Moreover, the decreased efficacy of caspofungin at higher concentrations (≥2 μg/ml), known as the “paradoxical effect” (40), was abolished in the pniiA-Hsp90 strain under repression, while it was present in the control strain (Fig. 3B).
Fig 3.
Effect of Hsp90 repression in cell wall stress response. (A) Transcriptional profile of hsp90 in the A. fumigatus wild-type strain (AF293) under various stress conditions: heat stress (black bar, 23 h growth at 37°C followed by heat shock 55°C for 1 h), caspofungin treatment (dark gray bar, 24 h growth in the presence of 1 μg/ml caspofungin) and FK506 treatment (light gray bar, 24 h growth in the presence of 20 ng/ml FK506). Results are presented as the mean fold change (2−ΔΔCT) for triplicates ± SD compared to the wild-type values under basal growth conditions (dotted line; 37°C, no treatment). (B) Genetic and pharmacologic inhibition of Hsp90 increases the effect of caspofungin and abolishes the paradoxical effect of caspofungin. Growth of akuBKU80 (control), pniiA-Hsp90 under genetic Hsp90 repression conditions (GMM+Am with 20 mM ammonium) and AF293 (wild type) under pharmacologic Hsp90 inhibition (geldanamycin [GdA], 8 μg/ml) with increasing caspofungin concentrations (0.125 to 4 μg/ml). Pictures were taken after 5 days of growth at 37°C. (C) Comparative growth of the akuBKU80 and pniiA-Hsp90 strains under various stress conditions on GMM+Am (20 mM ammonium). The pniiA-Hsp90 strain is unable to grow under heat stress (55°C) and in the presence of Congo red (binding to the cell wall). The effect of FK506 (calcineurin inhibitor) is increased by concomitant Hsp90 repression. Pictures were taken after 5 days of growth. (D) The cell wall stabilizer sorbitol (at a concentration of 1 M) remediates the conidiation defect of the pniiA-Hsp90 strain under repression conditions (GMM+Am with 20 mM ammonium), as shown by the greenish appearance of the colonies. This effect was also observed in the presence of caspofungin or FK506. Pictures were taken after 5 days of growth at 37°C.
As observed with the genetic repression of Hsp90, we confirmed that Hsp90 inhibitors strongly potentiate the effect of caspofungin (Fig. 3B). The loss of paradoxical response to caspofungin at concentrations ≥2 μg/ml was similarly observed after pharmacological inhibition of Hsp90 (Fig. 3B). However, in checkerboard dilutions, the MEC of both drugs in combination did not reach the criteria for synergism (FICI = 1.5).
An additive effect of Hsp90 repression was also observed in the presence of the calcineurin inhibitor FK506 (Fig. 3C), yet to a lesser extent than the combination of Hsp90 repression with caspofungin. No additive effect was observed with the combination of Hsp90 genetic or pharmacologic repression and nikkomycin Z, voriconazole, or amphotericin B.
Hsp90 is involved in maintenance of cell wall integrity.
We assessed the effect of Hsp90 repression along with other physical or chemical stress conditions. As Hsp90 is a heat stress protein, we first tested the effect of heat (55°C), which resulted in a complete lack of growth (Fig. 3C). Compared to the akuBKU80 strain, pniiA-Hsp90 under repression was more sensitive to the cell wall inhibitor Congo red, including a complete lack of growth at a concentration as low as 10 μg/ml (Fig. 3C). However, in the presence of calcofluor white (preferential binding to chitin) or calcium chloride (that does not directly affect the cell wall), the hyphal growth of pniiA-Hsp90 under repression was altered in a similar proportion as the control strain.
To further investigate a possible link between Hsp90 and the β-glucan synthesis pathway, we measured the total β-1,3-glucan content of the cell wall. While β-1,3-glucan content of pniiA-Hsp90 and akuBKU80 was found to be similar in the absence of ammonium, there was a 19% decrease in pniiA-Hsp90 under repression compared to the control strain (3.69 ± 0.18 versus 4.56 ± 0.25 RFU/nmol curdlan/mg; P = 0.008).
Because repression of Hsp90 had an inhibitory effect on hyphal growth and conidiation indicative of cell wall stress, we examined if sorbitol, an osmotic cell wall stabilizer, could remediate these defects. Although sorbitol (1 M) did not restore the hyphal growth defect of the pniiA-Hsp90 strain under repression, it remediated the conidiation defect, as noted by the greenish colony appearance (Fig. 3D). The pniiA-Hsp90 strain under repression was also able to partially conidiate when grown in glucose enriched GMM (5% glucose) in the absence of sorbitol.
To further support the role of Hsp90 in response to heat stress, as well as caspofungin and FK506 stress, we investigated the gene expression profile of hsp90 in the wild-type strain (AF293). As expected, transcription of hsp90 was found to substantially increase (83.1 ± 37.1-fold increase; P = 0.0002) in response to heat stress. However, caspofungin induced a significant decrease of hsp90 expression (1.74 ± 0.01-fold decrease; P = 0.01). A similar effect, albeit not statistically significant, was observed with the calcineurin inhibitor FK506 (1.54 ± 0.39-fold decrease; P = 0.08) (Fig. 3A).
Subcellular localization patterns of Hsp90 indicate its multifunctional role under normal growth and cell wall stress conditions.
To examine the localization pattern of Hsp90 in hyphae both under normal growth and stress-induced conditions, a strain expressing Hsp90-EGFP fusion protein was generated by tagging egfp to the C terminus of hsp90 at its native locus (Fig. 4A). Under standard growth conditions, Hsp90 was found distributed widely in the cytosol (Fig. 4B and C), and this localization was not affected by treatment with nikkomycin Z, FK506, or Hsp90 inhibitors (geldanamycin, 17-AAG, and 17-DMAG) (data not shown). Hsp90 was also localized to punctate structures (Fig. 4B and C) and in the nucleus in some hyphae, although it did not appear to be related to any specific growth condition. With various concentrations of caspofungin treatment (0.125 to 1 μg/ml), Hsp90 accumulated at points of hyphal damage along the cell wall and at the septa (Fig. 4D, E, and F). Several points of intrahyphal hyphae were also decorated by the concentration of Hsp90 close to regions of newly forming hyphal tips (Fig. 4G). The localization of Hsp90 at the sites of cell wall regeneration was confirmed by costaining with calcofluor white (Fig. 4H to O). These observations suggest a role for Hsp90 in a cell wall repair mechanism.
Fig 4.
Localization of Hsp90-EGFP under standard growth conditions (untreated) and under caspofungin exposure. (A) Schematic representation of the genomic locus of hsp90 in the wild-type (WT) and the Hsp90-EGFP strains. The enhanced green fluorescent protein gene (egfp) and the hygromycin B resistance cassette (Hph) were linked to the 3′-end of the hsp90 gene by homologous recombination of the left arm (LA, consisting of the 1-kb terminal sequence of hsp90) and the right arm (RA, a 1.5-kb sequence downstream of hsp90). Correct integration of the genetic construct was confirmed by PCR analysis showing a 2.3-kb band in the Hsp90-EGFP strain and no amplification in the control strain. (B and C) Localization of Hsp90-EGFP under standard growth conditions (untreated). Hsp90 is widely and homogenously distributed within the cytosol with the presence of some dotted (or punctate) structures (white arrows). (D and E) In the presence of low caspofungin concentration (0.125 μg/ml), Hsp90 moves from the cytosol to the cell wall and the tips of hyphae or to the sites of septum formation (white arrows). (F and G) At a higher caspofungin concentration (0.25 μg/ml), while more hyphal lysis is observed (dotted black arrows), Hsp90 further accumulates at the sites of hyphal regeneration, such as sites of septum formation (white arrows) (F) and tips of intrahyphal hyphae (arrowheads) (G). (H to O) Costaining with calcofluor white (blue) confirms the localization of EGFP-Hsp90 (green) in the cell wall and septa (white arrows). Panels I, K, M, and O show overlay pictures. Pictures were taken by fluorescence microscopy after 17 h growth on coverslips in liquid GMM at 37°C. Bar, 10 μm.
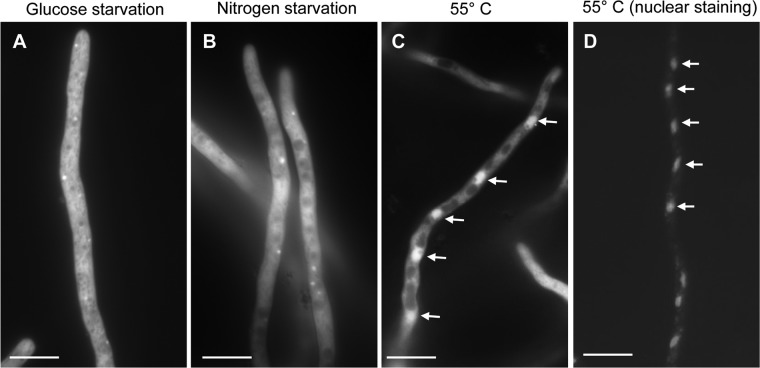
Localization of Hsp90 was also assessed after various other stress conditions. Glucose starvation and nitrogen deprivation both resulted in the formation of multiple dots within the cytosol (Fig. 5A and B). A shift from the cytosol to the nucleus was observed after heat shock (Fig. 5C) and confirmed by costaining with propidium iodide (Fig. 5D), which suggests a role of Hsp90 in transcriptional regulation during heat stress response. In agreement with these results, we have identified a predicted nuclear localization sequence (NLS) in A. fumigatus Hsp90 at its N terminus (248-KKKKKKTKTVK-258) by the NLStradamus program (http://www.moseslab.csb.utoronto.ca/NLStradamus/) (24).
Fig 5.
Localization of Hsp90-EGFP under various stress conditions. The Hsp90-EGFP strain was grown for 19 h on coverslips in liquid GMM at 37°C and then exposed to the stress condition (heat shock [55°C] or glucose or nitrogen starvation) for 1 h, before observation by fluorescence microscopy. Bar, 10 μm. (A) Glucose starvation. Hsp90 remains widely distributed in the cytosol, while more dotted structures are observed (compared to the standard growth conditions). (B) Nitrogen starvation. Many dotted structures are observed, similarly to glucose starvation. (C) Heat shock at 55°C. Hsp90 moves from the cytosol to the nuclei (white arrows). (D) Costaining with propidium iodide confirms the nuclear localization of EGFP-Hsp90 on the overlay picture.
DISCUSSION
In this study, we investigated the role of Hsp90 in Aspergillus fumigatus. Failure to delete hsp90 suggested its essentiality for fungal survival, in concordance with previous attempts reported for Neurospora crassa (4) and S. cerevisiae (2). While strains with low Hsp90 expression levels have been constructed in yeasts (8, 9), we achieved for the first time the genetic repression of Hsp90 in a filamentous fungus. Hsp90 repression resulted in major disturbances in A. fumigatus conidiation, germination, and hyphal growth. The 8- to 9-fold decrease of Hsp90 expression measured from the very few conidia that were able to produce hyphae should be interpreted cautiously, as the actual level of Hsp90 repression in the majority of conidia that were not viable or unable to grow could not be assessed. The important decrease in spore viability and the severe aerial growth defect observed under strict Hsp90 repression conditions (i.e., ammonium as the sole nitrogen source) confirmed the essential role of Hsp90 but prevented further characterization of the phenotypic consequences of Hsp90 and its role in stress responses. Although the use of repressible promoters may be helpful in characterizing the essential role of a defined gene (14), we conclude that their utility in studying the function of a gene can be limited. We finally achieved a model of genetic repression using ammonium with a concomitant nitrate source (GMM+Am), which resulted in an effect similar to the pharmacologic inhibition by geldanamycin and an acceptable level of growth to pursue our aim of better defining Hsp90 function.
While the growth defect was increased in the presence of drugs or environmental agents stressing the fungal cell wall through inhibition of its β-1,3-glucan component, those affecting its chitin component or the cell membrane did not show any additional impact. Hsp90 repression in combination with the β-1,3-glucan synthase inhibitor caspofungin caused complete growth inhibition at concentrations corresponding to the caspofungin therapeutic range in humans and also abolished the paradoxical effect of caspofungin (i.e., decreased efficacy at increasing concentrations). The β-1,3-glucan concentration of the cell wall was found to be slightly decreased following Hsp90 repression. These observations suggest a role for Hsp90 in A. fumigatus growth and in the maintenance of cell wall integrity, possibly linked with the β-glucan synthesis axis. Hsp90 localization showing a shift of Hsp90 from the cytosol to the cell wall and the sites of hyphal regeneration during caspofungin exposure supported this hypothesis. The decreased expression of Hsp90 under caspofungin exposure in the wild-type strain also supports a role of Hsp90 in response to the stress induced by this drug, although it could result from an indirect effect.
As a molecular chaperone, Hsp90 controls a myriad of proteins and signaling pathways, either by physical interactions or by regulation of their expression (39, 41). In other fungi, one of the most important client proteins of Hsp90 is calcineurin (16). The role of Hsp90 in azole and echinocandin resistance of C. albicans was shown to be mediated by the calcineurin pathway (7, 8, 31). Our finding that the effect of the calcineurin inhibitor FK506 was increased after Hsp90 repression supports this link in A. fumigatus. Comparison of Hsp90 genetic repression and calcineurin deletion (in the ΔcnaA strain from our previous work) (12, 13, 32, 33) shows some similarities supporting this link, such as the lower cell wall β-1,3-glucan content, the increased susceptibility to caspofungin, and the abolition of the caspofungin paradoxical effect. However, compromising Hsp90 had no further impact on the action of the chitin synthase inhibitor nikkomycin Z, while calcineurin inhibition resulted in increased sensitivity (33). Our recent work also found both calcineurin subunits localized at the hyphal septum and tip under basal growth conditions and this localization was not altered in the presence of cell wall stress agents (17, 18). Hsp90 was visualized at the septum, but this localization was specifically observed in response to the stress induced by caspofungin and not under basal conditions or with concomitant calcineurin inhibition by FK506. Taken together, these data suggest that mechanisms involved in the stress response against caspofungin and the maintenance of the cell wall integrity are complex and may be under the control of Hsp90 in concert with the calcineurin pathway.
We did not find any previous report of a role for Hsp90 in conidium formation in Aspergillus species. Deletion of the heat shock transcription factor hsf2 has been associated with an altered expression profile of hsp90 and an aconidial phenotype in N. crassa (37). In S. cerevisiae, compromising Hsp90 function also resulted in defective spore formation (36). Nutrient limitation triggers asexual sporulation and conidium formation in Aspergillus spp. (1). The fact that pniiA-Hsp90 had an aerial growth defect and did not grow on enriched media might suggest a role for Hsp90 in environmental sensing. This conidiation defect could be partially reversed under various growth conditions, such as an osmotically stabilized environment (sorbitol) or an increase in glucose content.
Our finding that Hsp90 moved from the cytosol to the nucleus during heat stress and the presence of a predicted nuclear localization sequence (NLS) in Hsp90 suggests that it might have a role in transcriptional regulation. While Hsp90 repression was associated with a significant decrease of the expression of the conidiation-specific genes brlA, abaA, and wetA, we cannot determine if this is the result of a direct transcriptional regulatory effect of Hsp90 or of an indirect effect. The brlA gene is the first key transcription factor to be activated in the conidiation process, which then activates abaA during the middle stage of conidiation and wetA at the later stage of spore wall synthesis (35). These genes are also involved in the synthesis of the conidial wall pigment (35), which was absent under Hsp90 repression.
As previously suggested (5, 8, 9), there are significant differences in the chaperoning network of Hsp90 and/or the Hsp90-calcineurin interaction between yeasts and molds, possibly resulting in different mechanisms of resistance to various classes of antifungal agents. Neither calcineurin nor Hsp90 repression enhanced the effect of voriconazole in A. fumigatus (34). This contrasts with the positive interaction reported for both calcineurin and Hsp90 with azoles in C. albicans (8, 9). As previously described for C. albicans (29, 30), compromising Hsp90 in A. fumigatus resulted in important defects in morphogenesis. Although a full comparison is not possible between these two evolutionarily distant fungi, we also found substantial defects in A. fumigatus morphogenesis, including the processes of conidiation, germination, and hyphal elongation. Fluorescent labeling of Hsp90 was performed in S. cerevisiae and revealed the same cytosolic and nuclear localization under basal conditions (15, 36). A shift from the cytosol to the nucleus was seen in quiescent cells, but not following heat stress or any other stress conditions (36). We did not find any previous report of a shift of Hsp90 to the sites of stress and regeneration induced by an antifungal drug.
In conclusion, this first report of Hsp90 genetic compromise in A. fumigatus revealed various important phenotypic consequences, confirming the crucial role of this heat stress protein in multiple essential functions for fungal survival and stress adaptation. The mechanisms by which Hsp90 controls these functions remain to be fully investigated. Our next objectives are to further define the network and interactions of Hsp90 via phosphoproteomic analyses and to better characterize the role of Hsp90 in stress responses by targeting the promoter of hsp90 via different approaches such as substitution by other repressible or constitutive promoters and serial truncations of the promoter. Hsp90 also represents an attractive antifungal target against invasive aspergillosis. However, the currently available Hsp90 inhibitors had limited efficacy and displayed a fungicidal effect in combination with caspofungin only at high concentrations (i.e., beyond the maximal tolerated dose in humans) (38). As geldanamycin and its derivatives are not specific to fungal Hsp90, their low toxic threshold combined with their high MEC against both C. albicans and A. fumigatus limit their use for the treatment of fungal diseases. New drugs specifically targeting fungal Hsp90 are warranted.
ACKNOWLEDGMENTS
F.L. was supported by the Swiss National Science Foundation (grant PBLAP3-134303). J.R.F. was supported by the Molecular Mycology and Pathogenesis Training Program grant at Duke University (5T32-AI052080). W.J.S. is supported by NIH/NIAID (grant 1R56AI077648-01A2).
We are grateful to Michelle Gignac from the Duke University Shared Materials Instrumentation Facility for technical assistance with scanning electron microscopy.
Footnotes
Published ahead of print 20 July 2012
REFERENCES
- 1. Adams TH, Wieser JK, Yu JH. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. 1989. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 9:3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnie JP, Carter TL, Hodgetts SJ, Matthews RC. 2006. Fungal heat-shock proteins in human disease. FEMS Microbiol. Rev. 30:53–88 [DOI] [PubMed] [Google Scholar]
- 4. Colot HV, et al. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103:10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowen LE. 2008. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 6:187–198 [DOI] [PubMed] [Google Scholar]
- 6. Cowen LE. 2009. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 5:e1000471 doi:10.1371/journal.ppat.1000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. 2006. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 5:2184–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowen LE, Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189 [DOI] [PubMed] [Google Scholar]
- 9. Cowen LE, et al. 2009. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. U. S. A. 106:2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coyle CM, Kenaley SC, Rittenour WR, Panaccione DG. 2007. Association of ergot alkaloids with conidiation in Aspergillus fumigatus. Mycologia 99:804–811 [DOI] [PubMed] [Google Scholar]
- 11. da Silva Ferreira ME, et al. 2006. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fortwendel JR, et al. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 54:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fortwendel JR, et al. 2009. Differential effects of inhibiting chitin and 1,3-β-d-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu W, et al. 2007. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3:e24 doi:10.1371/journal.ppat.0030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huh WK, et al. 2003. Global analysis of protein localization in budding yeast. Nature 425:686–691 [DOI] [PubMed] [Google Scholar]
- 16. Imai J, Yahara I. 2000. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol. Cell. Biol. 20:9262–9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juvvadi PR, et al. 2008. Calcineurin localizes to the hyphal septum in Aspergillus fumigatus: implications for septum formation and conidiophore development. Eukaryot. Cell 7:1606–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juvvadi PR, et al. 2011. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 82:1235–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaFayette SL, et al. 2010. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 6:e1001069 doi:10.1371/journal.ppat.1001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langfelder K, Philippe B, Jahn B, Latge JP, Brakhage AA. 2001. Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect. Immun. 69:6411–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 22. Muro-Pastor MI, Gonzalez R, Strauss J, Narendja F, Scazzocchio C. 1999. The GATA factor AreA is essential for chromatin remodelling in a eukaryotic bidirectional promoter. EMBO J. 18:1584–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Committee for Clinical Laboratory Standards 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard–second edition. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 24. Nguyen Ba AN, Pogoutse A, Provart N, Moses AM. 2009. NLStradamus: a simple hidden Markov model for nuclear localization signal prediction. BMC Bioinformatics 10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 26. Osherov N, Kontoyiannis DP, Romans A, May GS. 2001. Resistance to itraconazole in Aspergillus nidulans and Aspergillus fumigatus is conferred by extra copies of the A. nidulans P-450 14alpha-demethylase gene, pdmA. J. Antimicrob. Chemother. 48:75–81 [DOI] [PubMed] [Google Scholar]
- 27. Pinchai N, et al. 2009. Aspergillus fumigatus calcipressin CbpA is involved in hyphal growth and calcium homeostasis. Eukaryot. Cell 8:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robbins N, et al. 2011. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 7:e1002257 doi:10.1371/journal.ppat.1002257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Senn H, Shapiro RS, Cowen LE. 2012. Cdc28 provides a molecular link between Hsp90, morphogenesis, and cell cycle progression in Candida albicans. Mol. Biol. Cell 23:268–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shapiro RS, et al. 2009. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 19:621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh SD, et al. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 5:e1000532 doi:10.1371/journal.ppat.1000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinbach WJ, et al. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinbach WJ, et al. 2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:2979–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinbach WJ, et al. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao L, Yu JH. 2011. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 157:313–326 [DOI] [PubMed] [Google Scholar]
- 36. Tapia H, Morano KA. 2010. Hsp90 nuclear accumulation in quiescence is linked to chaperone function and spore development in yeast. Mol. Biol. Cell 21:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson S, Croft NJ, Sotiriou A, Piggins HD, Crosthwaite SK. 2008. Neurospora crassa heat shock factor 1 Is an essential gene; a second heat shock factor-like gene, hsf2, is required for asexual spore formation. Eukaryot. Cell 7:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trepel J, Mollapour M, Giaccone G, Neckers L. 2010. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whitesell L, Lindquist SL. 2005. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5:761–772 [DOI] [PubMed] [Google Scholar]
- 40. Wiederhold NP. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr. Opin. Infect. Dis. 20:574–578 [DOI] [PubMed] [Google Scholar]
- 41. Zhao R, et al. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120:715–727 [DOI] [PubMed] [Google Scholar]