Abstract
The diarrheagenic pathogen enteropathogenic Escherichia coli (EPEC) limits the death of infected enterocytes early in infection. A number of bacterial molecules and host signaling pathways contribute to the enhanced survival of EPEC-infected host cells. EspZ, a type III secreted effector protein that is unique to EPEC and related “attaching and effacing” (A/E) pathogens, plays a role in limiting host cell death, but the precise host signaling pathways responsible for this phenotype are not known. We hypothesized that EspZ contributes to the survival of infected intestinal epithelial cells by interfering with apoptosis. Consistent with previous studies, scanning electron microscopy analysis of intestinal epithelial cells infected with an EPEC espZ mutant (ΔespZ) showed increased levels of apoptotic and necrotic cells compared to cells infected with the isogenic parent strain. Correspondingly, higher levels of cytosolic cytochrome c and increased activation of caspases 9, 7, and 3 were observed for ΔespZ strain-infected cells compared to wild-type (WT) EPEC-infected cells. Finally, espZ-transfected epithelial cells were significantly protected from staurosporine-induced, but not tumor necrosis factor alpha (TNF-α)/cycloheximide-induced, apoptosis. Thus, EspZ contributes to epithelial cell survival by mechanisms that include the inhibition of the intrinsic apoptotic pathway. The enhanced survival of infected enterocytes by molecules such as EspZ likely plays a key role in optimal colonization by A/E pathogens.
INTRODUCTION
The Gram-negative pathogen enteropathogenic Escherichia coli (EPEC) causes diarrhea and contributes significantly to childhood morbidity and mortality in developing countries (18). EPEC, along with enterohemorrhagic E. coli (EHEC) and the murine pathogen Citrobacter rodentium, belongs to a group of extracellular attaching and effacing (A/E) pathogens that adhere to mature enterocytes and efface the brush border microvilli (27). The EPEC type III secretion system (T3SS), a critical virulence determinant, serves as a conduit for the transport of specific bacterial proteins directly into the host cytosol. These “effector” proteins contribute to disease by specifically altering host cell structures and signaling systems. While the signaling and structural alterations mediated by many secreted effector molecules have been elucidated, their contribution to pathogenesis remains to be established (8).
Despite altering a number of structural and functional components of host epithelial cells, EPEC infection causes only a modest degree of host cell death (5, 31). Epithelial cells killed as a result of EPEC infection display elements of both necrosis and apoptosis (5). The effector protein EspF contributes to host cell death by localizing to mitochondria and activating the intrinsic apoptotic pathway (6). This involves mitochondrial depolarization, the release of cytochrome c into the cytosol, and the subsequent activation of the cysteine protease caspase 9; caspase 9 is an “initiator” caspase that cleaves and activates “executioner” caspases such as caspases 3 and 7, which ultimately trigger apoptosis (6, 26, 28, 29).
Interestingly, EPEC also limits host cell death by activating prosurvival signaling pathways such as the epidermal growth factor receptor (EGFR)/phosphatidylinositol 3-kinase (PI3K)/Akt pathway (5, 31). The premature death of host epithelial cells early in infection is likely detrimental to colonization by an extracellular pathogen such as EPEC. Thus, EPEC appears to dynamically regulate the survival of infected epithelial cells. While the inhibition of apoptosis by bacteria is an emerging theme in the pathogenesis of intracellular pathogens (10), relatively little is known about the role of host cell survival in the colonization and virulence of extracellular pathogens like EPEC and EHEC.
Two recent studies implicated a role for the EPEC-secreted effector EspZ in the protection of infected host cells (33, 34). EPEC EspZ is a 98-amino-acid protein predicted to contain two transmembrane domains with an intervening 8- to 10-amino-acid loop. EspZ is conserved in all members of the A/E group but is not present in any of the other T3SS-containing organisms. Within the A/E pathogens, EspZ is hypervariable, with homologs of the group displaying 60 to 81% identity with the EPEC protein. EspZ does not contain any conserved motifs and does not display similarity to any other known protein. A nonpolar ΔespZ mutant does not exhibit any impairment in type III secretion, the formation of A/E lesions, bacterial attachment, or the ability to disrupt host epithelial tight junction barriers (15).
Based on studies with nonintestinal cell lines, two mechanisms of EspZ-mediated host cell survival have been proposed. EHEC EspZ (strain EDL933) (60% identical and 78% similar to EPEC EspZ) interacts with CD98, a transmembrane glycoprotein, and promotes HeLa cell survival by inducing focal adhesion kinase (FAK) phosphorylation (34). In a more recent study, EHEC EspZ was also shown to localize to the mitochondria of transfected HeLa cells, where it interacted with translocase of inner mitochondrial membrane 17b (TIM17b) and helped maintain the mitochondrial membrane potential (33). Furthermore, infection-induced death was exacerbated in HeLa cells depleted of TIM17b. It is not known, however, if EspZ-TIM17b interactions in mitochondria inhibit the downstream intrinsic apoptotic pathway in infected host epithelial cells. We hypothesized that EspZ contravenes the proapoptotic signaling of effectors like EspF in EPEC-infected intestinal epithelial cells (33).
We extended these studies using intestinal epithelial cell lines and explored the downstream pathways that contribute to the EspZ-mediated protection of infected host cells. Specifically, the protective effects of EspZ in the Caco-2 BBE (C2BBE) and T84 intestinal epithelial cell lines were verified. The role of CD98/FAK signaling in EspZ-dependent protective effects was evaluated by using a pharmacological inhibitor of FAK phosphorylation in C2BBE and T84 cells as well as HeLa cells. The effects of EPEC, the ΔespZ strain, and a cis-complemented strain (single-copy espZ with its native promoter) on the intrinsic apoptotic pathway in intestinal epithelial cells were compared. Finally, espZ-transfected epithelial cells were evaluated for their ability to resist chemically induced apoptosis. Our results show that EspZ protects infected epithelial cells, in part, by inhibiting the intrinsic apoptotic pathway and that the prosurvival signaling mechanisms likely have some cell line-specific attributes.
(Portions of this work were presented at the 2009 Digestive Diseases Week, Chicago, IL.)
MATERIALS AND METHODS
Cell lines.
The human intestinal epithelial cell lines C2BBE (30) and T84 (ATCC CCL-248) were used; several studies were also performed with HeLa (ATCC CCL-2) cells. C2BBE cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 25 mM glucose, 10% fetal bovine serum (FBS), and 20 mM HEPES at 37°C in the presence of 5% CO2. Cells employed in these studies were used between passages 25 and 45 and were used for experiments at days 7 to 10 postplating. T84 and HeLa cells were treated as described above, except that T84 cells were grown in DMEM–F-12 medium (supplemented with 5% FBS, 0.12% sodium bicarbonate, and 0.356% HEPES). HeLa cells were grown in 5.56 mM glucose–DMEM (Invitrogen Corporation, Grand Island, NY) supplemented with 10% FBS and 0.12% sodium bicarbonate.
To generate stable transfectants expressing green fluorescent protein (GFP) alone or EspZ and GFP (HeLa-vector and HeLa-espZ cells respectively), HeLa cells were stably transfected with the pCMV-EGFP vector (Clontech Laboratories, Inc., Mountain View, CA) or with pCMV::espZ (described below). C2BBE cells were trypsinized and resuspended in Opti-MEM I reduced serum medium (Invitrogen Life Technologies, Grand Island, NY). C2BBE cells (1 × 106) were electroporated with 30 μg of plasmid DNA (260 V, 850 μF, and 720 Ω) (Gene Pulser X Cell; Bio-Rad, Hercules, CA). Transfected cells were repeatedly sorted for GFP expression by using a BD FACS Aria III cell sorter (Becton Dickinson, San Jose, CA), until >90% of the cells were consistently expressing GFP.
Growth of bacteria and infection of cell lines.
C2BBE culture medium was changed to serum-free DMEM 14 h prior to bacterial infection. The bacterial strains used in this study included enteropathogenic Escherichia coli O127:H6 strain E2348/69 (EPEC) and the nonpolar espZ deletion derivative MK41 (ΔespZ) (15). The single-copy cis-complemented strain (cis-espZ) was constructed via Tn7 transposition (described below) (23). For infections, bacterial cultures grown overnight (in Luria-Bertani broth) were subcultured at a 1:20 dilution in serum-free DMEM and grown to the mid-log growth phase (optical density at 600 nm [OD600] of 0.4) at 37°C. Bacteria were added to the apical surface of C2BBE cells, corresponding to an initial multiplicity of infection (MOI) of 100. Following incubation at 37°C in a 5% CO2 water-jacketed incubator for 1 h, unattached bacteria were washed away, replaced with fresh medium, and incubated for additional periods. The times indicated in the figures are from the point of the initial addition of bacteria.
Plasmid and strain constructions.
pCMV::espZ, used to create stable HeLa-espZ transfectants, was generated in two steps: espZ from rabbit EPEC (REPEC) strain E22 (O103:H2; a kind gift from Edgar Boedeker) was amplified with primers CCATGGAAGCAGCAAATTTAAGCCC and GGCATATTTCATCGCTAATCCG and cloned into the vector pTrcHis2 TOPO (Invitrogen Corporation, Grand Island, NY), in frame with sequences for the Myc and 6×His tags, to generate pXZ1. The sequences corresponding to espZ and the epitope tags were amplified from this construct by using primers AACTCGAGATGGAAGCAGCAAATTTAAGC and CCTCTAGATCAATGATGATGATGATGATGATGGTCGAC, and the fragment was cloned into the XhoI-XbaI sites in the pCMV-EGFP vector to generate pCMV::espZ. The cis-espZ strain was constructed by complementing the ΔespZ strain with a single copy of espZ, with its native promoter, inserted downstream of glmS via mini-Tn7 transposition. Plasmid pJSW101, used for creating the cis-espZ strain, was generated by PCR amplifying EPEC espZ (with primers TCATCTGCAGGCTCTGAAGTA and AGGCAATAGCATAATGCATCC) and cloning the corresponding fragment into pGEMT Easy (Promega, Madison, WI), adding flanking NotI sites to the PCR product. The NotI fragment was then ligated into the NotI site of pGRG36 (23). This vector was used to insert a single copy of espZ onto the chromosome, just outside the glmS gene. The inserted fragment included the native espZ (LEE2) promoter and was designed to approximate wild-type (WT) regulation of espZ. Where appropriate, plasmids and strains were confirmed by PCR and sequence analyses of the relevant regions.
Scanning electron microscopy (SEM) of infected C2BBE cells.
Polarized C2BBE cells were grown on collagen-coated (collagen type I; BD Biosciences) 0.33-cm2 Transwell inserts (Corning, Inc., Corning, NY) for 7 to 10 days until they achieved trans-epithelial electrical resistance values of at least 300 Ω (EVOM2; World Precision Instruments, Sarasota, FL). At 4 h postinfection, Transwell inserts were transferred into a fresh tray containing phosphate-buffered saline (PBS), and the apical medium was replaced with 200 μl of PBS. The cells/filters were then fixed and dried as previously described (17). Samples were critical-point dried, mounted onto glass slides, and sputter coated with gold-palladium. Images were obtained by using a Hitachi S-4800 type II field emission scanning electron microscope (Hitachi High-Technologies Canada, Inc., Toronto, Canada).
Ethidium homodimer cell death assays and immunofluorescence microscopy.
Epithelial cell death was monitored by using the Live/Dead viability/cytotoxicity kit (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer's instructions. Only dead cells, which were stained red, are shown in the figures. Fluorescent (dead) cells were counted from a minimum of nine separate fields from three independent experiments and are reported as a percentage of the total number of cells in the corresponding field. Images were captured by using a Nikon Opti-Phot microscope and a Spot-RT digital imaging system.
Propidium iodide uptake assay.
C2BBE or transfected HeLa cells were cultured in 96-well white-walled microplates. To quantitate the relative number of dead cells, propidium iodide (PI; Molecular Probes) was added to mock- and bacterium/chemical-treated cells to a final concentration of 2 μg/ml. After 30 min, the PI uptake of cells was measured by using a fluorescence microplate reader (Synergy HT; BioTek Instruments, Winooski, VT). To estimate maximum PI uptake, cells in a set of control wells were killed/permeabilized with 70% methanol for 30 min prior to the addition of PI.
Caspase activity assay.
C2BBE cells were grown in 96-well white-walled microplates. After bacterial infection, spent medium was carefully removed, and 50 μl of Caspase-Glo 3/7 reagent (Promega Corporation, Madison, WI) was added to each well. Active caspases 3 and 7 cleave the substrate DEVD-aminoluciferin to release aminoluciferin; the subsequent conversion of aminoluciferin by luciferase results in a luminescent signal proportional to caspase 3/7 activity. Luminescence readings were obtained by using a Synergy HT plate reader.
Cytochrome c release assay.
C2BBE cells infected for 4 h with WT EPEC or the ΔespZ or cis-espZ strain were assayed for cytochrome c release into the cytosol. Mitochondrial and cytosolic fractions were extracted by using the ApoAlert cell fractionation kit (Clontech Laboratories, Inc., Mountain View, CA). Twenty-five micrograms of each cytosolic and membrane fraction was separated on a 4-to-20% gradient SDS-PAGE gel. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane and immunoblotted for cytochrome c (Clontech). To verify the integrity of each fraction, the samples were also immunoblotted for the mitochondrial marker Cox4 (Clontech).
Stimulation of intrinsic and extrinsic apoptosis.
Transfected epithelial cells expressing EspZ (HeLa-espZ cells) or the corresponding vector-only controls (HeLa-vector cells) were seeded onto a 96-well white-walled clear-bottom tray (Costar 3610; Corning, Inc., Corning, NY) and allowed to grow to confluence. Cells were treated with 0.2 μM or 2 μM staurosporine (STS) to stimulate intrinsic apoptosis, 150 ng/ml human tumor necrosis factor alpha (TNF-α) and 10 μg/ml cycloheximide (CHX) to stimulate extrinsic apoptosis, or 0.1% dimethyl sulfoxide (DMSO) as a vehicle control (3, 13, 19). Replicate wells were treated with 100% methanol for 30 min to set a baseline for total cells per well for each strain. Samples were also incubated with 2 μM PI. To determine mean background fluorescence, replicate wells were cultured without PI. We then performed a Live/Dead assay as described above. The PI uptake of cells was corrected by subtracting the mean background value. Differences in cell numbers between the two cell lines were normalized by multiplying the corrected PI fluorescence by the ratio of the methanol-treated wells. The data displayed are the average increases in the relative fluorescence of each treatment over time. Student's t test was performed to determine the significance of the observed differences.
RESULTS
EspZ suppresses host intestinal epithelial cell death.
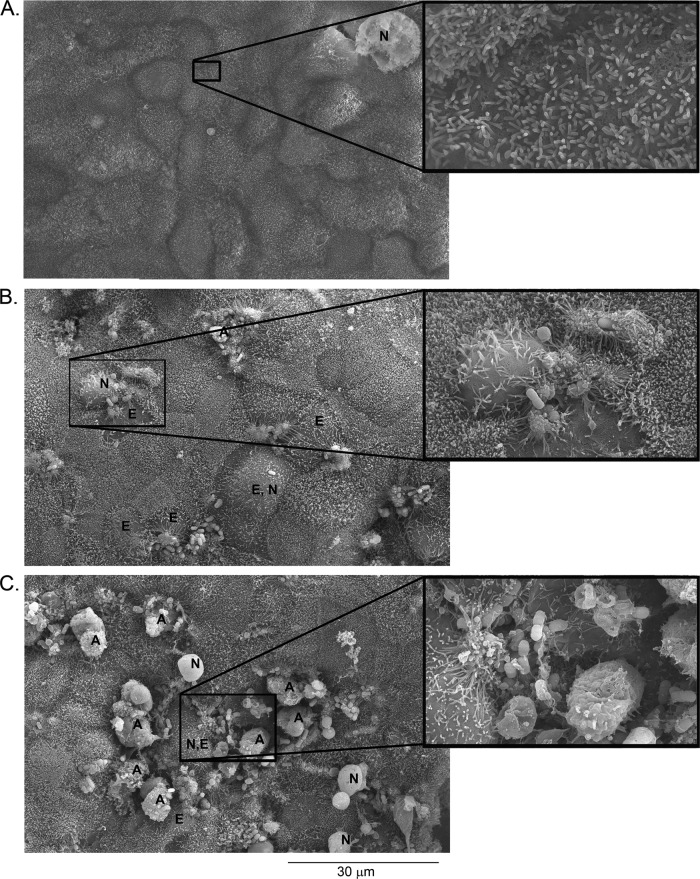
C2BBE monolayers infected with a nonpolar ΔespZ mutant, but not WT EPEC, detached from the plastic substratum at between 4 and 6 h postinfection. Microscopic examination revealed greater cell rounding in ΔespZ mutant-infected C2BBE monolayers than in WT EPEC-infected monolayers (see Fig. S1 in the supplemental material). To visualize the corresponding morphological alterations, WT- and ΔespZ strain-infected polarized C2BBE cells were subjected to scanning electron microscopy (SEM) (Fig. 1). Both strains colonized epithelial cells, formed signature actin pedestals, and caused microvillus effacement. The SEM images of infected C2BBE cells revealed the swelling of some cells, which is suggestive of necrotic death; late-stage necrosis manifesting as swollen, rounded cells with a relatively smoother cell surface was also evident. In addition, apoptotic cells with characteristic membrane blebbing and cell splitting were observed. SEM images of ΔespZ strain-infected cells revealed larger numbers of dying cells than of cells infected with WT EPEC (Fig. 1).
Fig 1.
SEM images of ΔespZ mutant-infected cells reveal larger numbers of dying cells. C2BBE cells were mock treated (A), infected with EPEC (B), or infected with the ΔespZ mutant (C) for 4 h. Selected cells are labeled as follows: A, apoptotic cells; E, effaced cells; N, necrotic cells. Images shown are representative of those collected from cells infected on three separate days. Insets show selected regions at a higher magnification.
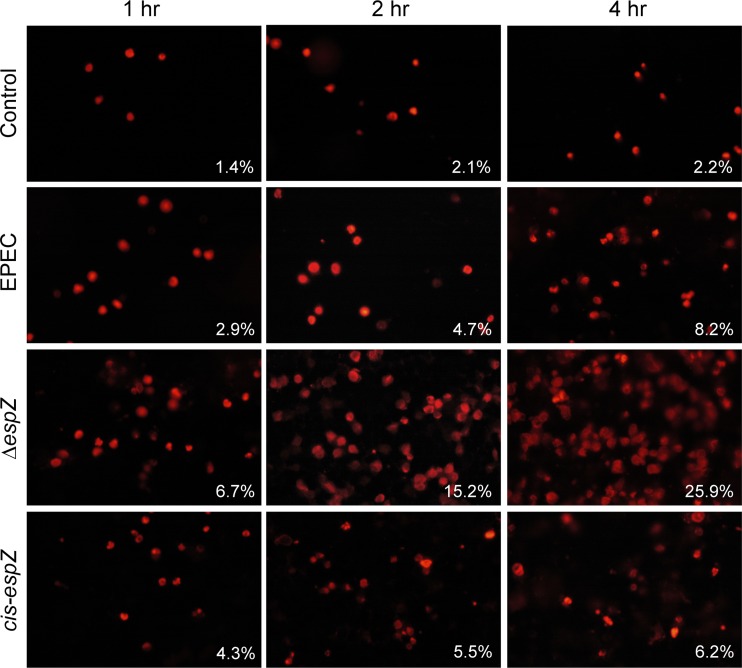
To confirm the protective effect of EspZ on intestinal epithelial cells and to determine if EspZ influences the kinetics of EPEC-induced cell death, the time course of cell death was evaluated in C2BBE monolayers infected with WT EPEC and the ΔespZ and cis-espZ strains by using an ethidium homodimer uptake assay (Fig. 2). WT EPEC caused a progressive increase in the rate of epithelial cell death, reaching 8.2% by 4 h postinfection. Host cell death was evident earlier, and was more pronounced, in monolayers infected with the ΔespZ mutant, with 15.2% and 25.9% dead cells by 2 and 4 h postinfection, respectively. Infection with the cis-espZ strain significantly reduced the numbers of dead cells to 5.5% and 6.2% at 2 and 4 h, respectively (P value of <0.001 for the cis-espZ strain versus the ΔespZ mutant). Similar results were observed for experiments using a plate reader to monitor the uptake of propidium iodide by infected C2BBE and HeLa cells (see Fig. S2A and S2B in the supplemental material). Thus, EspZ protects against infection-induced death in host intestinal epithelial cells as well as in nonintestinal epithelial cell lines such as HeLa and MDCK cells (34).
Fig 2.
EspZ decreases the rate of death of infected enterocytes. C2BBE cells grown on glass coverslips were treated with medium alone or infected with EPEC, the ΔespZ mutant, or the single-copy complemented cis-espZ strain for 1, 2, and 4 h and then stained with an ethidium homodimer 15 min prior to imaging. Representative data from three independent experiments are shown. Percentages represent averages from 9 fields.
FAK activation is not required for EspZ-mediated survival of enterocytes.
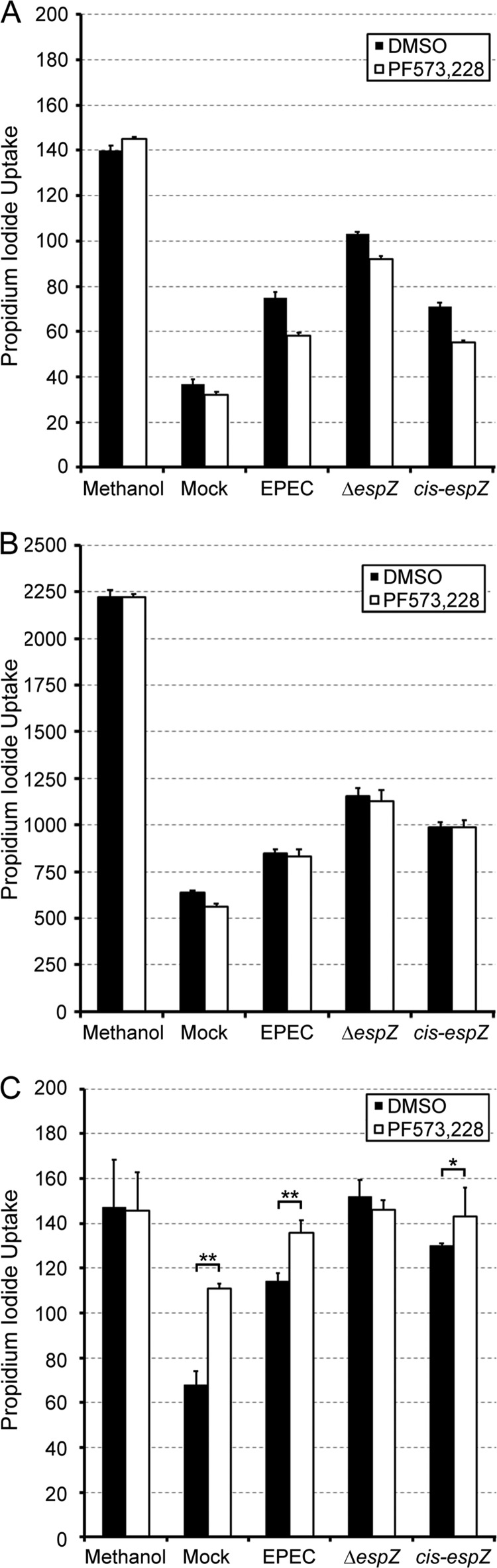
Based on studies with HeLa cells ectopically expressing EHEC EspZ, it was hypothesized that the interaction of EspZ with the glycoprotein CD98 promotes cell survival via FAK phosphorylation (34). We therefore evaluated the role of FAK activation in EspZ-mediated survival in HeLa cells as well as in the human intestinal epithelial cell line C2BBE. Western blot analysis of extracts from EPEC-infected HeLa and C2BBE cells revealed time-dependent FAK phosphorylation in both cell lines (see Fig. S3 in the supplemental material). Interestingly, the kinetics of FAK phosphorylation differed between the two cell lines. In HeLa cells, FAK phosphorylation was evident by 30 min postinfection and was sustained for up to 120 min. In C2BBE cells, however, phosphorylation was evident by 15 min postinfection but displayed a biphasic activation profile, with dephosphorylation being evident from 60 to 105 min. Phospho-FAK (pFAK) was again detectable at 120 min in these cells. Next, we explored whether EPEC-induced FAK phosphorylation contributed to the survival of HeLa and C2BBE cells and another intestinal epithelial cell line, T84. Propidium iodide uptake assays revealed increased host cell death following WT EPEC infection in all three cell lines; the three cell lines also displayed a greater degree of cell death following infection with the ΔespZ mutant (than that displayed by WT-infected cells), and this was reversed in infections with the cis-espZ strain (Fig. 3). FAK inhibition with PF-573228 significantly increased death rates in mock-infected as well as WT-infected HeLa cells but not in ΔespZ mutant-infected cells (Fig. 3C). Curiously, however, PF-573228 had no effect on the EPEC-induced death of intestinal epithelial cells (Fig. 3A and B). These results confirm a role for FAK phosphorylation in the EspZ-dependent protection of infected HeLa cells. While FAK phosphorylation is evident in EPEC-infected C2BBE cells, data from the inhibitor studies suggest that this is likely not relevant for EspZ-dependent survival signaling in these cells.
Fig 3.
FAK phosphorylation is not involved in espZ-dependent intestinal epithelial cell survival. Mean propidium iodide uptake values (six technical replicates) are shown for T84 (A), C2BBE (B), and HeLa (C) cells infected with WT EPEC or the ΔespZ or cis-espZ strain in the presence of the FAK inhibitor PF-573228 (50 μM) or DMSO as a vehicle control. After 4 h, cells were treated with propidium iodide (2 μg/ml), and fluorescence was measured. To obtain a measurement of maximum fluorescence (100% death), control samples were treated with methanol prior to the addition of PI. *, P < 0.05; **, P < 0.01 (determined by Student's t test). Each experiment was repeated at least 2 times (n = 3 for panel A, n = 2 for panel B, and n = 3 for panel C).
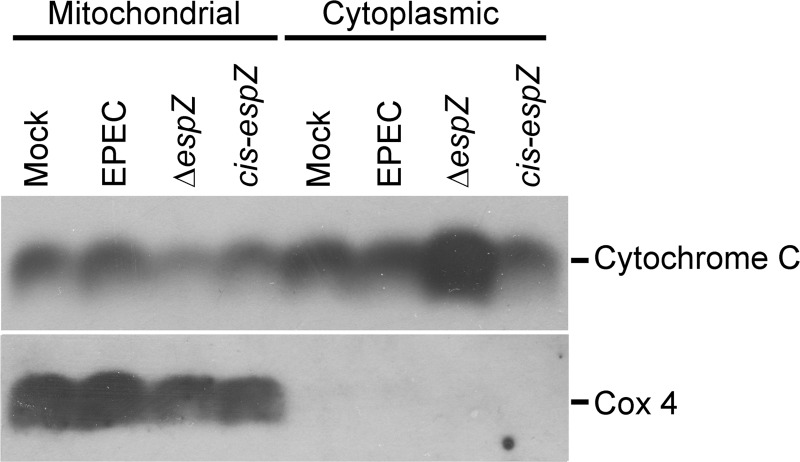
EspZ inhibits the release of mitochondrial cytochrome c.
The secreted effector EspF localizes to the mitochondria of infected epithelial cells and triggers mitochondrial depolarization, cytochrome c release, and the consequent activation of the intrinsic apoptotic pathway (26, 29). EspZ was shown previously to interact with the mitochondrial protein TIM17b (33) and to prevent mitochondrial depolarization. To determine if EPEC EspZ consequently inhibits the downstream intrinsic apoptotic pathway, mitochondrial and cytosolic fractions were extracted from C2BBE cells infected with WT EPEC or the ΔespZ or cis-espZ strain, and the samples were immunoblotted for cytochrome c. ΔespZ mutant infection resulted in increased cytochrome c release into the cytosol, and this was reversed in epithelial cells infected with the cis-espZ strain, the complemented mutant (Fig. 4). The mitochondrial protein Cox4 was detected only in mitochondrial fractions, confirming the integrity of the fractionated samples and ruling out cross-contamination. These data suggest that the EspZ-mediated maintenance of mitochondrial potential is accompanied by the retention of cytochrome c in the mitochondria (33).
Fig 4.
EspZ inhibits cytochrome c release in EPEC-infected cells. C2BBE cells were treated with medium alone or infected for 4 h with WT EPEC or the ΔespZ or cis-espZ strain. Host cell cytosolic and mitochondrial fractions were separated and analyzed for the abundance of cytochrome c by Western blotting. Blots were also probed for Cox4 as a control marker for the mitochondrial fractions. The blot shown is representative of three independent experiments.
EspZ inhibits activation of caspases 9, 7, and 3.
In the intrinsic apoptotic pathway, the release of mitochondrial cytochrome c induces the activation of the cytosolic cysteine protease caspase 9 (28). Caspase 9 is an initiator caspase with N-terminal adaptor domains that permit autocleavage and the consequent activation of downstream caspases. To explore the possibility that EspZ protects epithelial cells by limiting caspase 9 activation, extracts of C2BBE cells infected with WT EPEC and the ΔespZ mutant were immunoblotted for caspase 9. Caspase 9 cleavage was evident in C2BBE cells infected with EPEC but not in control uninfected monolayers (see Fig. S4A in the supplemental material). ΔespZ mutant-infected cells, however, had higher levels of cleaved caspase 9 (and also a corresponding decrease in the level of full-length procaspase 9) than cells infected with WT EPEC. This response is not cell line specific, since similar observations were made with EPEC-infected HeLa cells (data not shown). Caspase 7, an “executioner” caspase that lacks N-terminal adaptor domains, is cleaved/activated by initiator caspases. To confirm the effect of EspZ on the apoptotic pathway, caspase 7 cleavage was also monitored by immunoblot analysis. As with caspase 9, EPEC infection of C2BBE cells for 4 h resulted in caspase 7 cleavage (see Fig. S4B in the supplemental material). Again, ΔespZ mutant infection increased the levels of cleaved caspase 7 (with a corresponding decrease in the levels of procaspase 7).
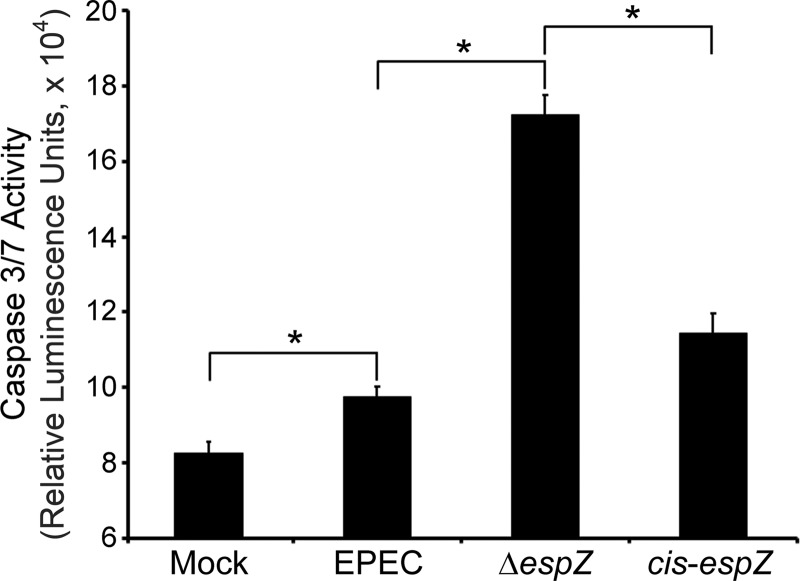
To correlate cytochrome c release and caspase cleavage with caspase activation (4), infected intestinal epithelial cells were assayed for caspase activity. After 4 h of infection, cells infected with WT EPEC had higher levels of caspase 3/7 activity than did uninfected control monolayers (Fig. 5). ΔespZ mutant infection resulted in increased caspase 3/7 activity compared to that with WT EPEC infection. Caspase 3/7 activity levels of cells infected with the cis-espZ strain were restored to WT EPEC infection levels. This further confirms that EspZ prevents the activation of the intrinsic apoptotic pathway in EPEC-infected intestinal epithelial cells. Collectively, these data suggest that EspZ inhibits the intrinsic apoptotic pathway upstream of cytochrome c release in intestinal epithelial cells and HeLa cells.
Fig 5.
EspZ decreases caspase 3/7 activities of infected cells. C2BBE cells were treated with medium alone or were infected for 4 h with WT EPEC or the ΔespZ or cis-espZ strain and then monitored for caspase 3/7 activity by using a Promega Caspase-Glo 3/7 kit. Mean relative luminescence units (n = 6) are reflective of caspase 3 and 7 activities. The asterisk represents a P value of <0.01, determined by Student's t test.
EspZ inhibits intrinsic apoptosis.
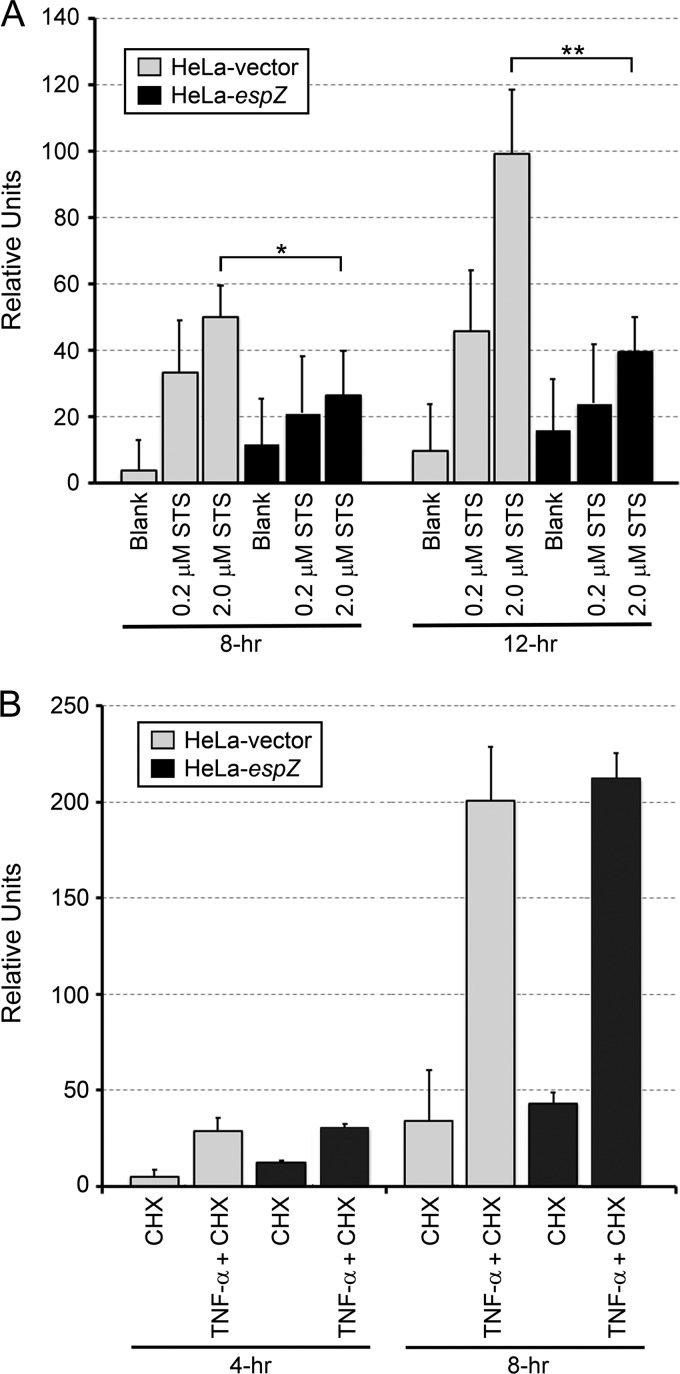
Since EPEC, and EspZ, appears to influence both apoptotic and necrotic death, an infection-independent system was devised to confirm the role of the EspZ-mediated inhibition of intrinsic apoptosis. For this, stably transfected epithelial cell lines expressing EspZ were evaluated for their abilities to withstand chemically induced apoptosis. The HeLa-espZ and HeLa-vector cell lines were treated with staurosporine (STS), a nonspecific protein kinase inhibitor that induces intrinsic apoptosis (3, 13, 14), or TNF-α and cycloheximide (CHX), which induces extrinsic apoptosis via the activation of caspase 8 (19). Cell death was monitored by PI uptake assays. STS induced a dose- and time-dependent increase in the death rate of HeLa-vector cells, while HeLa-espZ cells were significantly protected from death at 8 h and 12 h after treatment with 2 μM STS (Fig. 6A). The combination of TNF-α and CHX also induced the death of epithelial cells; in contrast to STS-induced death, however, HeLa-espZ and control HeLa-vector cells showed similar levels of dead cells (Fig. 6B). Thus, cells transfected with EspZ are resistant to staurosporine-induced, but not to TNF-α/CHX-induced, cell death. Collectively, our data demonstrate that EPEC EspZ contributes to the survival of infected host epithelial cells by inhibiting the intrinsic apoptotic pathway.
Fig 6.
EspZ inhibits staurosporine-induced apoptosis. HeLa-vector and HeLa-espZ stable transfectants were stimulated with STS, an intrinsic apoptotic pathway inducer (A), or 150 ng/ml TNF-α and 10 μg/ml CHX, which, in combination, induce the extrinsic apoptotic pathway (B). Death was then monitored via PI uptake for the next 4, 8, or 12 h. Average changes in the fluorescence of 4 wells at the given time points (relative units) are shown. Data are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (determined by Student's t test).
DISCUSSION
Attaching and effacing pathogens such as EPEC and REPEC actively modulate the survival of host intestinal epithelial cells in vitro and in vivo (12, 36). EPEC effector molecules secreted into host cells reconfigure the structural and functional elements of these cells (37), including the apparent overt activation of apoptotic signaling (1, 5, 20–22, 24). The secreted effector EspF localizes to the mitochondria, stimulates cytochrome c release, and induces apoptosis (16, 26, 28). Consistent with data from previous reports, our studies on intestinal epithelial cells suggest that EPEC-induced cell death exhibits features of both apoptosis and necrosis (7). The concomitant activation of prosurvival signaling pathways in host cells suggests that these extracellular pathogens dynamically modulate the fate of infected cells (36). Recent studies showed that EspZ, an effector molecule secreted into host cells early in infection, promotes the survival of infected host cells and is essential for pathogenesis (33, 34).
EspZ, a 98- to 100-amino-acid protein, is a highly variant protein encoded on the locus of enterocyte effacement (LEE) of the A/E class of pathogens (11). It does not display significant sequence similarity to any known protein (15). EspZ is secreted into infected epithelial cells early in infection via the LEE-encoded T3SS (25) and is essential for the virulence of Citrobacter rodentium (an A/E pathogen of mice) (9). Although EspZ may localize to the pedestal-like structures induced by EPEC on HeLa cells, it is likely not involved in pedestal formation (15). EHEC EspZ (60% identity and 77% similarity to EPEC EspZ), ectopically expressed in HeLa cells, localized to the cell membrane and mitochondria (15, 33, 34). For these cells, the interaction of EspZ with the membrane glycoprotein CD98, and the subsequent phosphorylation of FAK, was proposed previously to be a mechanism for the protection of host cells (34). Consistent with this hypothesis, we observed that FAK is phosphorylated in EPEC-infected HeLa and C2BBE cells and that the pharmacological inhibition of FAK phosphorylation augments the death of EPEC-infected, but not ΔespZ mutant-infected, HeLa cells. FAK also seems to play a general role in HeLa cell survival, since a FAK inhibitor caused increased rates of death of uninfected HeLa cells. Curiously, however, this mechanism appears to be cell line specific, since FAK inhibition had no effect on the EspZ-dependent survival of the intestinal epithelial cell lines, even though FAK phosphorylation was observed in infected C2BBE cells.
In another report, EspZ was shown to localize to mitochondria and interact with TIM17b, preventing the rapid decrease in the host mitochondrial membrane potential in EPEC-infected HeLa cells (33). Consistent with this finding, and extending the results to downstream signaling events, our studies show that EspZ inhibits (mitochondrial) cytochrome c release into the cytosol as well as the downstream activation of caspases 9, 7, and 3, thus limiting intrinsic apoptosis in infected intestinal epithelial cells. It has been proposed that mitochondrion-localized EspZ may interfere directly with the functioning of proapoptotic effector molecules, such as EspF. However, since transfected EspZ blocks staurosporine-induced apoptosis, it is more likely that the prosurvival role of this molecule results from the engagement of specific host signaling pathways (such as TIM17b-dependent signaling) rather than direct interference with the functioning of other (proapoptotic) effector molecules.
The protective role of EspZ extends beyond its ability to inhibit apoptotic signaling (33); apoptosis accounts marginally for the increased rate of death of ΔespZ mutant-infected epithelial cells, since a pancaspase inhibitor (Q-VD-OPh hydrate; Sigma, St. Louis, MO) had only a modest protective effect on these cells (data not shown). This finding suggests that the signaling pathway(s) triggered by EspZ has a broad protective effect against both apoptotic, such as that induced by EspF, and necrotic death. Finally, EspZ failed to protect epithelial cells from TNF-α/CHX-mediated extrinsic apoptosis. Since EspZ is secreted into host cells early in infection, preceded only by Tir (25), it is tempting to speculate that this molecule serves the function of preserving host cells from the onslaught of subsequent infection/colonization processes, including the effects of other secreted molecules.
The protection of infected host cells from death is an emerging theme in bacterial pathogenesis, especially in the context of extracellular pathogens like EPEC (36). In addition to EspZ, A/E pathogens express several other proteins that have protective effects on infected cells. EPEC and related pathogens harbor homologs of the Shigella-secreted effector OspE, which interacts with host integrin-linked kinase, enhances β1-integrin surface localization, maintains focal adhesion, and prevents cell detachment (35). The EPEC-secreted effector molecules NleH1 and NleH2 inhibit death by engaging antiapoptotic Bax inhibitor 1 in infected epithelial cells (37). Another secreted effector, NleD, cleaves and inactivates Jun N-terminal protein kinase (JNK), thereby blocking downstream proapoptotic signaling (2). Bacterial flagellin, and likely other molecules, promotes the activation of the prosurvival transcription factor NF-κB, and the pharmacological inhibition of NF-κB increases the rate of death of infected epithelial cells (data not shown). EPEC also stimulates the prosurvival EGFR/PI3K/Akt and protein kinase C (PKC) pathways, and these signals have been shown to contribute to the survival of host cells (5, 7, 31, 32). On the other hand, the expression of proapoptotic effector molecules by A/E pathogens, and the overt death of infected epithelial cells observed during later stages of infection, suggests that these bacteria dynamically modulate host cell survival.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge research support from the following organizations: the National Institutes of Health (V.K.V.; grant no. NIAID1R01AI081742), the U.S. Department of Veterans Affairs (G.V.; grant no. BX1I01BX001183), and the U.S. Department of Agriculture (CSREES Hatch Program) (G.V. and V.K.V.; grant no. ARZT-570410-A-02-139 and ARZT-570410-A-02-140, respectively).
We thank Al Agellon for assistance with electron microscopy and Paula Campbell and Junesse Farley of the AZCC/ARL Division of Biotechnology Cytometry Core Facility, which is supported by a grant from the National Cancer Institute (grant no. CCSG-CA 023074). We thank James Kaper for providing strain MK41, Edgar Boedeker for strain E22, and Nancy Craig for Tn7-targeting plasmid pGRG36.
We confirm that we do not have any conflict of interest.
Footnotes
Published ahead of print 20 August 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Abul-Milh M, Wu Y, Lau B, Lingwood CA, Barnett Foster D. 2001. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect. Immun. 69:7356–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baruch K, et al. 2011. Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. EMBO J. 30:221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chae HJ, et al. 2000. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol. Res. 42:373–381 [DOI] [PubMed] [Google Scholar]
- 4. Chua BT, Guo K, Li P. 2000. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J. Biol. Chem. 275:5131–5135 [DOI] [PubMed] [Google Scholar]
- 5. Crane JK, Majumdar S, Pickhardt DF., III 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crane JK, McNamara BP, Donnenberg MS. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197–211 [DOI] [PubMed] [Google Scholar]
- 7. Crane JK, Oh JS. 1997. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect. Immun. 65:3277–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean P, Kenny B. 2009. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr. Opin. Microbiol. 12:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng W, et al. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U. S. A. 101:3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faherty CS, Maurelli AT. 2008. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 16:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilmour MW, et al. 2006. Use of the espZ gene encoded in the locus of enterocyte effacement for molecular typing of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 44:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heczko U, Carthy CM, O'Brien BA, Finlay BB. 2001. Decreased apoptosis in the ileum and ileal Peyer's patches: a feature after infection with rabbit enteropathogenic Escherichia coli O103. Infect. Immun. 69:4580–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inada H, et al. 2001. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J. Cell Biol. 155:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabir J, Lobo M, Zachary I. 2002. Staurosporine induces endothelial cell apoptosis via focal adhesion kinase dephosphorylation and focal adhesion disassembly independent of focal adhesion kinase proteolysis. Biochem. J. 367:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanack KJ, Crawford JA, Tatsuno I, Karmali MA, Kaper JB. 2005. SepZ/EspZ is secreted and translocated into HeLa cells by the enteropathogenic Escherichia coli type III secretion system. Infect. Immun. 73:4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenny B, Jepson M. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579–590 [DOI] [PubMed] [Google Scholar]
- 17. Knutton S. 1995. Electron microscopical methods in adhesion. Methods Enzymol. 253:145–158 [DOI] [PubMed] [Google Scholar]
- 18. Kosek M, Bern C, Guerrant RL. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 81:197–204 [PMC free article] [PubMed] [Google Scholar]
- 19. Lin Y, Devin A, Rodriguez Y, Liu ZG. 1999. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13:2514–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malish HR, et al. 2003. Potential role of the EPEC translocated intimin receptor (Tir) in host apoptotic events. Apoptosis 8:179–190 [DOI] [PubMed] [Google Scholar]
- 21. Malladi V, Puthenedam M, Williams PH, Balakrishnan A. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce iNOS by activation of NF-kappaB and MAP kinases. Inflammation 28:345–353 [DOI] [PubMed] [Google Scholar]
- 22. Malladi V, Shankar B, Williams PH, Balakrishnan A. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of Caco-2 cells through activation of PKCalpha. Microbes Infect. 6:38–50 [DOI] [PubMed] [Google Scholar]
- 23. McKenzie GJ, Craig NL. 2006. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6:39 doi:10.1186/1471-2180-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melo AR, et al. 2005. Expression of the virulence factor, BfpA, by enteropathogenic Escherichia coli is essential for apoptosis signalling but not for NF-kappaB activation in host cells. Scand. J. Immunol. 61:511–519 [DOI] [PubMed] [Google Scholar]
- 25. Mills E, Baruch K, Charpentier X, Kobi S, Rosenshine I. 2008. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3:104–113 [DOI] [PubMed] [Google Scholar]
- 26. Nagai T, Abe A, Sasakawa C. 2005. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for the bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280:2998–3011 [DOI] [PubMed] [Google Scholar]
- 27. Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nougayrede JP, Donnenberg MS. 2004. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6:1097–1111 [DOI] [PubMed] [Google Scholar]
- 29. Nougayrede JP, Foster GH, Donnenberg MS. 2007. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell. Microbiol. 9:680–693 [DOI] [PubMed] [Google Scholar]
- 30. Peterson MD, Mooseker MS. 1992. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J. Cell Sci. 102:581–600 [DOI] [PubMed] [Google Scholar]
- 31. Roxas JL, Koutsouris A, Viswanathan VK. 2007. Enteropathogenic Escherichia coli-induced epidermal growth factor receptor activation contributes to physiological alterations in intestinal epithelial cells. Infect. Immun. 75:2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sason H, et al. 2009. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol. Biol. Cell 20:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shames SR, Croxen MA, Deng W, Finlay BB. 2011. The type III system-secreted effector EspZ localizes to host mitochondria and interacts with the translocase of inner mitochondrial membrane (TIM) 17b. Infect. Immun. 79:4784–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shames SR, et al. 2010. The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signalling. Cell. Microbiol. 12:1322–1339 [DOI] [PubMed] [Google Scholar]
- 35. Tegtmeyer N, Backert S. 2009. Bacterial type III effectors inhibit cell lifting by targeting integrin-linked kinase. Cell Host Microbe 5:514–516 [DOI] [PubMed] [Google Scholar]
- 36. Vossenkamper A, Macdonald TT, Marches O. 2011. Always one step ahead: how pathogenic bacteria use the type III secretion system to manipulate the intestinal mucosal immune system. J. Inflamm. 8:11 doi:10.1186/1476-9255-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong AR, et al. 2011. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol. Microbiol. 80:1420–1438 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.