Abstract
The M1 serotype of Streptococcus pyogenes plays an important role in streptococcal toxic shock syndrome. Simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, has been shown to inhibit streptococcal M1 protein-induced acute lung damage, although downstream mechanisms remain elusive. Protein isoprenylation, such as farnesylation and geranylgeranylation, has been suggested to regulate anti-inflammatory effects exerted by statins. Here, we examined the effect of a farnesyltransferase inhibitor (FTI-277) on M1 protein-triggered lung inflammation. Male C57BL/6 mice were treated with FTI-277 prior to M1 protein challenge. Bronchoalveolar fluid and lung tissue were harvested for quantification of neutrophil recruitment, edema, and CXC chemokine formation. Flow cytometry was used to determine Mac-1 expression on neutrophils. The gene expression of CXC chemokines was determined in alveolar macrophages by using quantitative reverse transcription (RT)-PCR. We found that the administration of FTI-277 markedly decreased M1 protein-induced accumulation of neutrophils, edema formation, and tissue damage in the lung. Notably, inhibition of farnesyltransferase abolished M1 protein-evoked production of CXC chemokines in the lung and gene expression of CXC chemokines in alveolar macrophages. Moreover, FTI-277 completely inhibited chemokine-induced neutrophil migration in vitro. However, farnesyltransferase inhibition had no effect on M1 protein-induced expression of Mac-1 on neutrophils. Our findings suggest that farnesyltransferase is a potent regulator of CXC chemokine formation in alveolar macrophages and that inhibition of farnesyltransferase not only reduces neutrophil recruitment but also attenuates acute lung injury provoked by streptococcal M1 protein. We conclude that farnesyltransferase activity is a potential target in order to attenuate acute lung damage in streptococcal infections.
INTRODUCTION
Streptococcal toxic shock syndrome (STSS) is a fatal condition associated with acute lung injury (2, 5, 24). Due to the limited understanding of the underlying pathophysiology, management of patients with STSS poses a major challenge to clinicians and is largely limited to antibiotics and supportive therapy. It is known that the M1 serotype of Streptococcus pyogenes is most frequently associated with fatal STSS (14, 25). M1 protein is a potent stimulator of neutrophils (40), and STSS-associated lung injury is characterized by massive infiltration of neutrophils (32, 39). Neutrophils are generally considered to be the first line of defense against microbial invasion. Nonetheless, overwhelming neutrophil infiltration appears to constitute a rate-limiting step in the pathophysiology of M1 protein-induced lung damage. For example, systemic depletion of neutrophils abolishes acute lung injury in mice challenged with M1 protein (32, 40). Chemokines are known to be key regulators of leukocyte trafficking at sites of inflammation. CXC chemokines, such as CXCL1 and CXCL2, orchestrate tissue recruitment of neutrophils. CXCR2 (interleukin-8 [IL-8] receptor B) is the high-affinity receptor for CXCL1 and CXCL2 on murine neutrophils (16). Herbold et al. (13) have shown that CXCR2 is critical in mediating pulmonary recruitment of neutrophils in streptococcal infection (13). Moreover, a recent study reported that M1 protein-provoked accumulation of neutrophils in the lung is mediated by CXCR2 (39). Although the role of CXC chemokines appears convincing, the intracellular signaling mechanisms controlling CXC chemokine formation in M1 protein-induced lung injury are not known.
Statins are mainly used to control cholesterol levels in patients with cardiovascular diseases. However, recent studies have suggested that statins might reduce morbidity and mortality in septic patients (17, 29). Moreover, several reports indicate that statin treatment inhibits pulmonary damage (39) and increases survival in murine sepsis (21, 22). Indeed, we have recently shown that simvastatin is a potent inhibitor of M1 protein-triggered neutrophil recruitment and lung damage (40). Convincing data in the literature demonstrate that statins, such as simvastatin, exert pleiotropic anti-inflammatory effects, including attenuated expression of cytokines, chemokines, and adhesion molecules, as well as decreased formation of nitric oxide (10, 34, 36). Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting enzyme in mevalonate formation (3). Mevalonate is a precursor of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are critical in protein isoprenylation, such as farnesylation and geranylgeranylation. Farnesyltransferase catalyzes the attachment of farnesyl pyrophosphate to cysteine residues in small regulatory proteins, such as small G proteins of the Ras-homologous (Rho) family, that are needed for a broad range of cellular functions, including membrane trafficking, cytoskeletal organization, growth, apoptosis, and differentiation (18, 27). Interestingly, a recent study reported that inhibition of farnesyltransferase antagonized T-lymphocyte infiltration in the nervous system (35). However, the potential role of farnesyltransferase in regulating pulmonary recruitment and tissue damage in M1 protein-induced inflammation remains elusive.
Based on the above-described considerations, we hypothesized that farnesyltransferase activity might be involved in streptococcal M1 protein-induced neutrophil infiltration and tissue damage in the lung.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice weighing 20 to 25 g were used for experiments and kept under standard laboratory conditions, maintained on a 12-h/12-h light/dark cycle and fed a laboratory diet and water ad libitum. Animals were anesthetized with 75 mg of ketamine hydrochloride (Hoffman-La Roche, Basel, Switzerland) and 25 mg of xylazine (Janssen Pharmaceutica, Beerse, Belgium) per kg of body weight. All experimental procedures were performed in accordance with the legislation on the protection of animals and were approved by the Regional Ethical Committee for Animal Experimentation at Lund University, Sweden.
Experimental model.
M1 protein was purified from the isogenic mutant MC25 strain (derived from the AP1 Streptococcus pyogenes strain 40/58 from the WHO Collaborating Centre for references and Research on Streptococci, Institute of Hygiene and Epidemiology, Prague, Czech Republic) as described previously (4). Mice were intravenously (i.v.) injected with 15 μg of M1 protein in phosphate-buffered saline (PBS). M1 protein was purified from a mutated Streptococcus pyogenes strain, making the likelihood of endotoxin contamination close to zero. Nevertheless, we also measured the endotoxin content in the M1 protein samples and confirmed that endotoxin levels were below the detection limit. Sham-treated mice received only PBS intravenously. The farnesyltransferase inhibitor, FTI-277 (2.5 or 25 mg/kg; Sigma Chemical Co., St. Louis, MO), or vehicle (PBS) was administered intraperitoneally (i.p.) 10 min prior to M1 protein challenge. FTI-277 was dissolved in sterile distilled water and diluted by PBS just before injection. Animals were reanesthetized 4 h after M1 protein challenge. The left lung was ligated and excised for edema measurement. The right lung was used for collecting bronchoalveolar lavage fluid (BALF) to quantify neutrophils. Then, the lung was excised, one lobe was fixed in formaldehyde for histology, and the remaining lung tissue was snap-frozen in liquid nitrogen and stored at −80°C for later myeloperoxidase (MPO) assay and enzyme-linked immunosorbent assay (ELISA) as described below.
Systemic leukocyte count.
Blood was collected from the tail vein and mixed with Turk's solution (0.2 mg gentian violet in 1 ml glacial acetic acid, 6.25% [vol/vol]) in a 1:20 dilution. Leukocytes were identified as monomorphonuclear leukocytes (MNLs) and polymorphonuclear leukocytes (PMNLs) in a Burker chamber.
Lung edema.
The left lung was excised, washed in PBS, gently dried using a blotting paper, and weighed. The tissue was then dried at 60°C for 72 h and reweighed. The change in the ratio of wet weight to dry weight was used as the indicator of lung edema formation.
MPO activity.
Lung tissue was thawed and homogenized in 1 ml of 0.5% hexadecyltrimethylammonium bromide. Samples were freeze-thawed, after which the MPO activity of the supernatant was determined spectrophotometrically as the MPO-catalyzed change in absorbance in the redox reaction of H2O2 (450 nm, with a 540-nm reference filter, at 25°C) as previously described (1). Values were expressed as MPO units per gram of tissue.
BALF.
Animals were placed supine, and the trachea was exposed by dissection. A catheter was inserted into the trachea. BALF was collected by 5 washes of 1 ml of PBS containing 5 mM EDTA. The numbers of MNLs and PMNLs were counted in a Burker chamber.
ELISA.
The levels of CXCL1 and CXCL2 in lung homogenates were analyzed by using double-antibody Quantikine ELISA kits (R&D Systems Europe, Abingdon, Oxon, United Kingdom) using recombinant murine CXCL1 and CXCL2 as standards. The lower limit of the assay was 0.5 pg/ml.
Flow cytometry.
For analysis of surface molecule expression on circulating neutrophils, blood was collected (1:10 in citric acid-dextrose) 4 h after M1 protein challenge, incubated (10 min, reverse transcriptase) with an anti-CD16/CD32 antibody blocking Fcγ III/II receptors to reduce nonspecific labeling, and then incubated with phycoerythrin (PE)-conjugated anti-Gr-1 antibody (clone RB6-8C5, rat IgG2b; eBioscience, San Diego, CA) and fluorescein isothiocyanate (FITC)-conjugated anti-Mac-1 antibody (clone M1/70, integrin αM chain, rat IgG2b). The mean fluorescence intensity (MFI) was determined by comparison to the fluorescence of an isotype control antibody (FITC-conjugated rat IgG2b). All antibodies were purchased from BD Biosciences Pharmingen, San Jose, CA, unless otherwise indicated. Cells were fixed and erythrocytes were lysed with BD lysis buffer (BD Biosciences), and then neutrophils were recovered following centrifugation. Flow cytometric analysis was performed by first gating the neutrophil population of cells based on forward and side scatter characteristics, after which Mac-1 expression on Gr-1-positive cells was determined in this gate on a FACSCalibur flow cytometer (Becton, Dickinson, Mountain View, CA). A PE-conjugated anti-mouse F4/80 antibody (clone BM8; Biolegend) was used to identify macrophages isolated from the lung (20, 41). Flow cytometric analysis was performed by first gating the macrophage population of cells based on forward and side scatter characteristics, after which the percentage of macrophages in the BALF was determined on F4/80-positive cells in this gate on a FACSCalibur flow cytometer.
Histology.
Lung samples were fixed in 4% formaldehyde phosphate buffer overnight and then dehydrated and embedded in paraffin. Six-micrometer sections were stained with hematoxylin and eosin. Lung injury was quantified in a blinded manner with the adoption of a modified preexisting scoring system as described previously (38), including size of alveolar spaces, thickness of alveolar septas, alveolar fibrin deposition, and neutrophil infiltration graded on a scale of 0 (absent) to 4 (extensive). In each tissue sample, 5 random areas were scored, and the median value was calculated. The histology score was the sum of all four parameters.
In vitro activation of neutrophils.
Blood was collected from healthy animals 1:10 in citric acid-dextrose. Whole blood was incubated with M1 protein (1 μg/ml) with or without FTI-277 (1 or 10 μM) and vehicle at 37°C for 20 min. Cells were stained for flow cytometric analysis of Mac-1 expression on neutrophils (Gr-1+) as described above.
Quantitative RT-PCR.
Mice were challenged with M1 protein for 30 min, and alveolar macrophages were isolated as previously described (41). Total RNA was then isolated from alveolar macrophages by using an RNeasy minikit (Qiagen, West Sussex, United Kingdom) and treated with RNase-free DNase (DNase I; Amersham Pharmacia Biotech, Sollentuna, Sweden) to remove potential genomic DNA contaminants. RNA concentrations were determined by measuring the absorbance at 260 nm. Each cDNA was synthesized by reverse transcription (RT) from 10 μg of total RNA by using the StrataScript First-Strand synthesis system and random hexamer primers (Stratagene-AH diagnostics, Stockholm, Sweden). Real-time PCR was performed using a Brilliant SYBR green quantitative-PCR (qPCR) master mix and MX 3000P detection system (Stratagene). The sequences of the primers for CXCL1, CXCL2, and β-actin were as follows: CXCL1 (forward), 5′-GCT TCC TCG GGC ACT CCA GAC-3′, and CXCL1 (reverse), 5′-TTA GCC TTG CCT TTG TTC AGT AT-3′; CXCL2 (forward), 5′-GCC AAT GAG CTG CGC TGT CAA TGC-3′, and CXCL2 (reverse), 5′-CTT GGG GAC ACC TTT TAG CAT CTT-3′; and β-actin (forward), 5′-ATG TTT GAG ACC TTC AAC ACC-3′, and β-actin (reverse), 5′-TCT CCA GGG AGG AAG AGG AT-3′. Standard PCR curves were generated for each PCR product to establish linearity of the RT-PCR. PCR amplifications were performed in a total reaction mixture volume of 50 μl, containing 25 μl of 2× SYBR green PCR master mix, 2 μl of 0.15 μM each primer, 0.75 μl of reference dye, and 1 μl of cDNA as a template, adjusted up to 50 μl with water. PCRs were started with 10 min at the denaturing temperature of 95°C, followed by a total of 40 cycles of 95°C for 30 s and 55°C for 1 min and then by 1 min of elongation at 72°C. The relative differences in expression between groups were expressed by using cycling time values. Cycling time values for the specific target genes were first normalized with that of β-actin in the same sample, and then relative differences between groups were expressed as the percentage of the control value.
Chemotaxis assay.
Neutrophils isolated from bone marrow by use of Ficoll-Paque were preincubated with FTI-277 (10 μM) for 30 min, and 1.5 × 106 neutrophils were placed in the upper chamber of the transwell inserts (5-μm pore size; Corning Costar, Corning, NY). Inserts were placed in wells containing medium alone (control) or medium plus CXCL2 (100 ng/ml; R&D Systems). After 120 min, inserts were removed, and migrated neutrophils were transferred 1:1 into 0.5% hexadecyltrimethylammonium bromide. Chemotaxis was determined by MPO activity as described above.
Statistics.
Data are presented as mean values ± standard errors of the means (SEM). Statistical evaluations were performed using Kruskal-Wallis one-way analysis of variance on ranks followed by multiple comparisons versus control group (Dunnett's method). A P value of <0.05 was considered significant, and n represents the number of animals.
RESULTS
Pulmonary edema and damage.
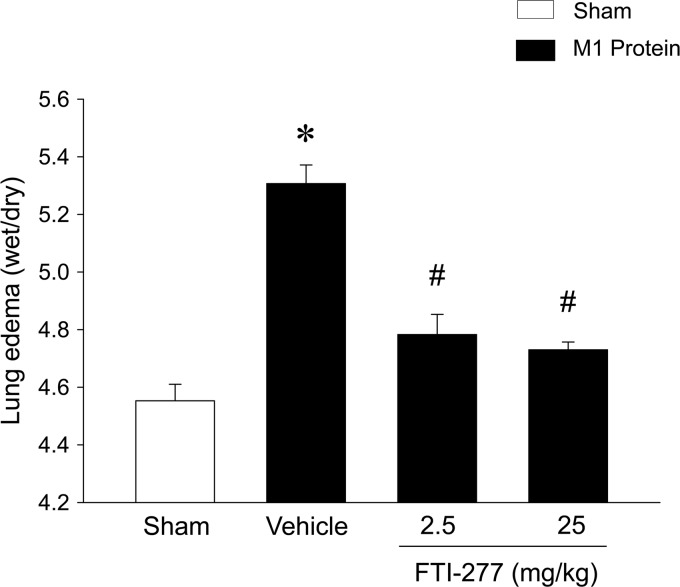
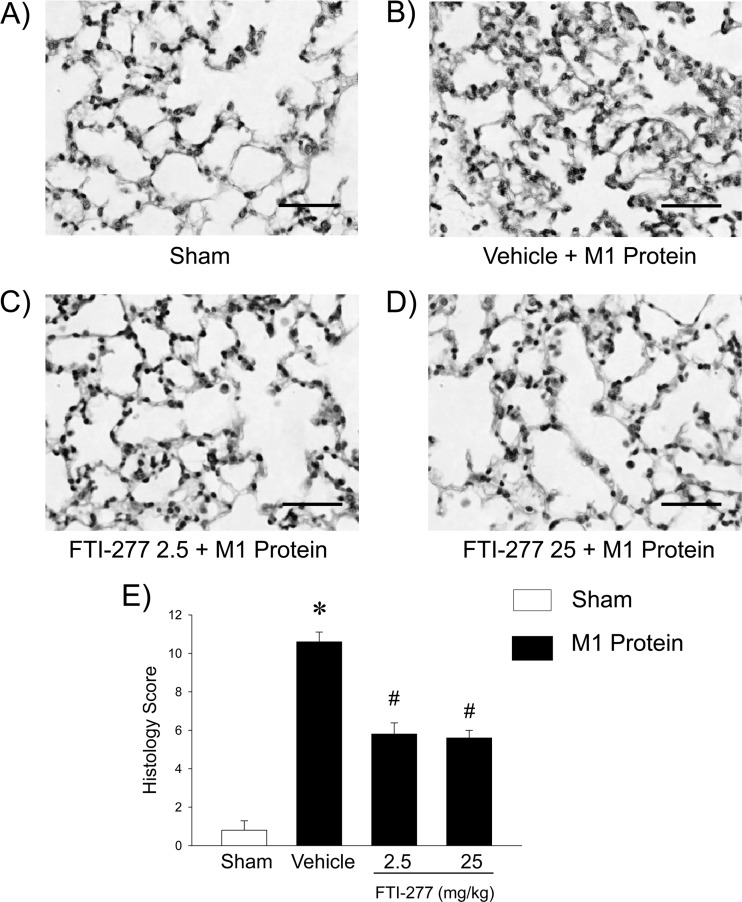
Challenge with M1 protein induced clear-cut lung injury, indicated by the significant increase in lung edema formation (Fig. 1). Thus, the lung wet/dry ratio in M1 protein-treated animals increased from 4.6 ± 0.1 (mean ± SEM) to 5.3 ± 0.1 (Fig. 1). The administration of 25 mg/kg of the farnesyltransferase inhibitor FTI-277 reduced the lung wet/dry ratio to 4.7 ± 0.0 in mice challenged with M1 protein (Fig. 1). Thus, inhibition of farnesyltransferase signaling decreased M1 protein-provoked lung edema by 77%. Moreover, morphological examination revealed normal lung microarchitecture in sham-treated mice (Fig. 2A), whereas M1 protein caused clear-cut destruction of the lung tissue structure, characterized by interstitial edema, capillary congestion, and neutrophil accumulation (Fig. 2B). It was observed that inhibition of farnesyltransferase activity reduced M1 protein-provoked changes in the microarchitecture and neutrophil accumulation in the lung (Fig. 2D). Quantification of the morphological changes revealed that M1 protein increased the lung injury score and that administration of the farnesyltransferase inhibitor significantly decreased the lung injury score in animals challenged with M1 protein (Fig. 2E).
Fig 1.
Edema formation in the lung. Mice were treated with the farnesyltransferase inhibitor FTI-277 (2.5 or 25 mg/kg) or vehicle (PBS) 10 min prior to M1 protein injection. Mice treated with PBS served as sham-treated animals. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment; #, P < 0.05 versus results of vehicle + M1 protein.
Fig 2.
Representative sections of lungs stained with hematoxylin and eosin. (A) Sham-treated animals were treated with PBS only. (B to D) Mice were pretreated with vehicle (PBS) (B) or with 2.5 (C) or 25 (D) mg/kg FTI-277 10 min prior to M1 protein administration. Samples were harvested 4 h after M1 protein challenge. Scale bar indicates 100 μm. (E) Histology score of lung injury. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment; #, P < 0.05 versus results of vehicle + M1 protein.
Neutrophil infiltration.
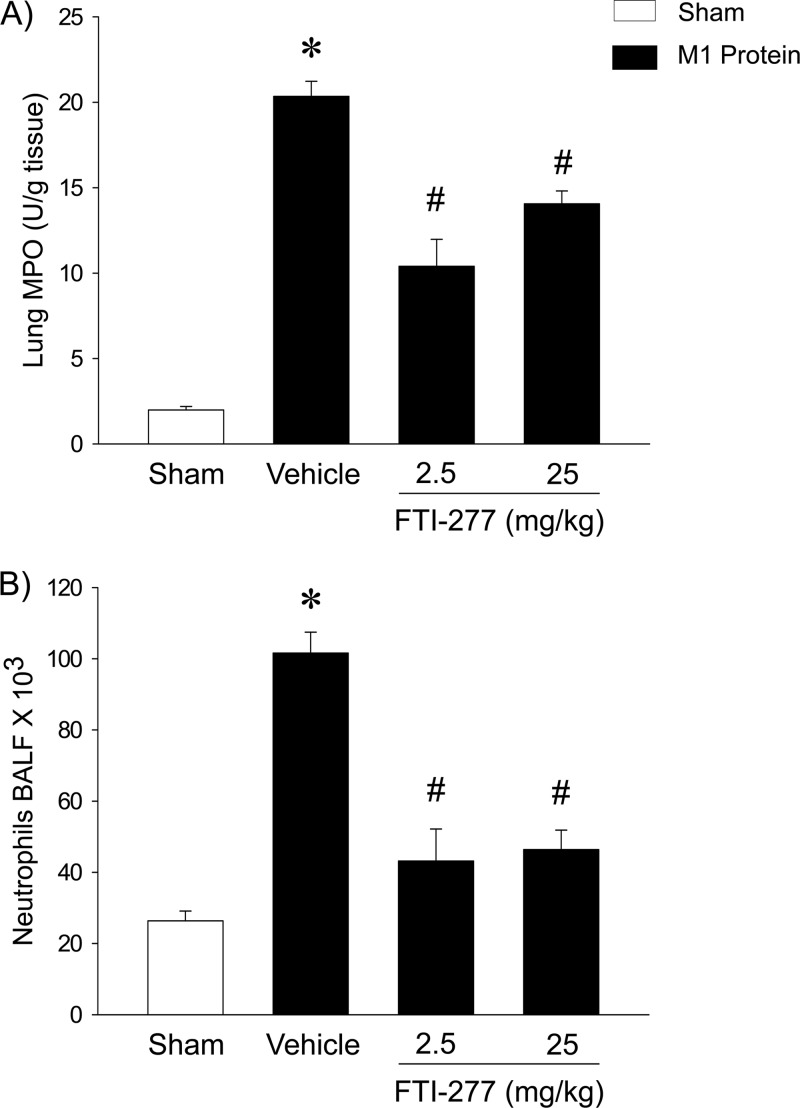
The injection of M1 protein increased lung levels of MPO by more than 10-fold (Fig. 3A). Inhibition of farnesyltransferase signaling reduced the M1 protein-provoked increase in pulmonary MPO activity by 34% (Fig. 3A). Quantification of BALF neutrophils revealed a massive enhancement in the number of alveolar neutrophils 4 h after the administration of M1 protein (Fig. 3B). We observed that treatment with 25 mg/kg of FTI-277 reduced the number of pulmonary neutrophils in the lung from 101.6 × 103 ± 5.9 × 103 to 46.4 × 103 ± 5.5 × 103, corresponding to a 73% reduction, 4 h after M1 protein challenge (Fig. 3B). Moreover, it was found that the administration of M1 protein reduced the number of PMNLs and MNLs in the blood (Table 1). Inhibition of farnesyltransferase signaling significantly reduced this M1 protein-provoked leucopenia (Table 1).
Fig 3.
Farnesyltransferase regulates M1 protein-induced pulmonary infiltration of neutrophils. MPO levels in lung (A) and numbers of neutrophils in BALF (B). Animals were treated with FTI-277 (2.5 or 25 mg/kg) or vehicle (PBS) 10 min prior to M1 protein injection. Samples were harvested 4 h after M1 protein challenge. Mice treated with PBS served as sham-treated animals. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment; #, P < 0.05 versus results of vehicle + M1 protein.
Table 1.
Differential counts of systemic leukocytes in mice treated or not treated with FTI-277 and challenged with M1 proteina
| Treatment | Mean no. (106 cells/ml) of leukocytes ± SEM (n = 5) |
||
|---|---|---|---|
| MNL | PMNL | Total | |
| Sham (PBS) | 4.0 ± 0.2 | 1.3 ± 0.1 | 5.2 ± 0.3 |
| Vehicle + M1 protein | 0.8 ± 0.1b | 0.3 ± 0.0b | 1.1 ± 0.1b |
| FTI-277 (2.5 mg/kg) + M1 protein | 3.9 ± 0.5c | 1.0 ± 0.2c | 5.0 ± 0.5c |
| FTI-277 (25 mg/kg) + M1 protein | 3.4 ± 0.5c | 1.2 ± 0.2c | 4.6 ± 0.6c |
Blood was collected from sham-treated animals receiving only vehicle (PBS) and from mice treated with FTI-277 or vehicle before challenge with M1 protein for 4 h. Monomorphonuclear leukocytes (MNLs) and polymorphonuclear leukocytes (PMNLs) were identified.
P < 0.05 versus results for sham treatment.
P < 0.05 versus results for vehicle + M1 protein.
CXC chemokines in the lung.
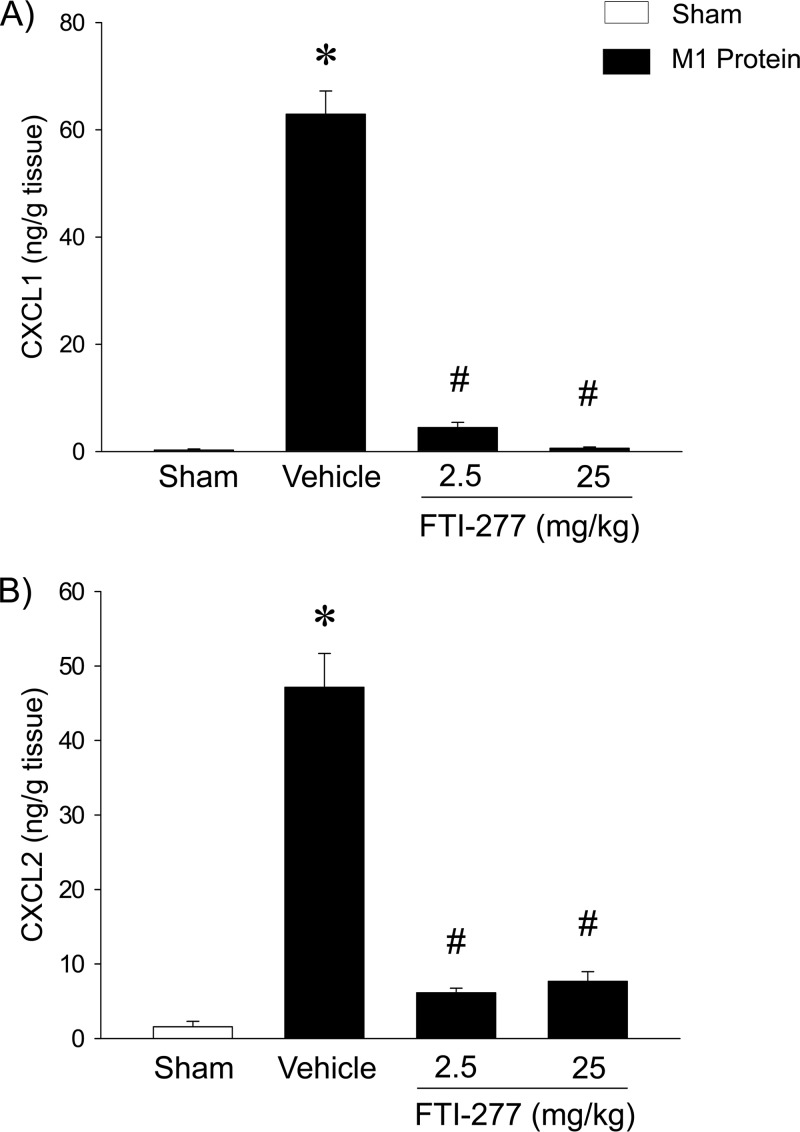
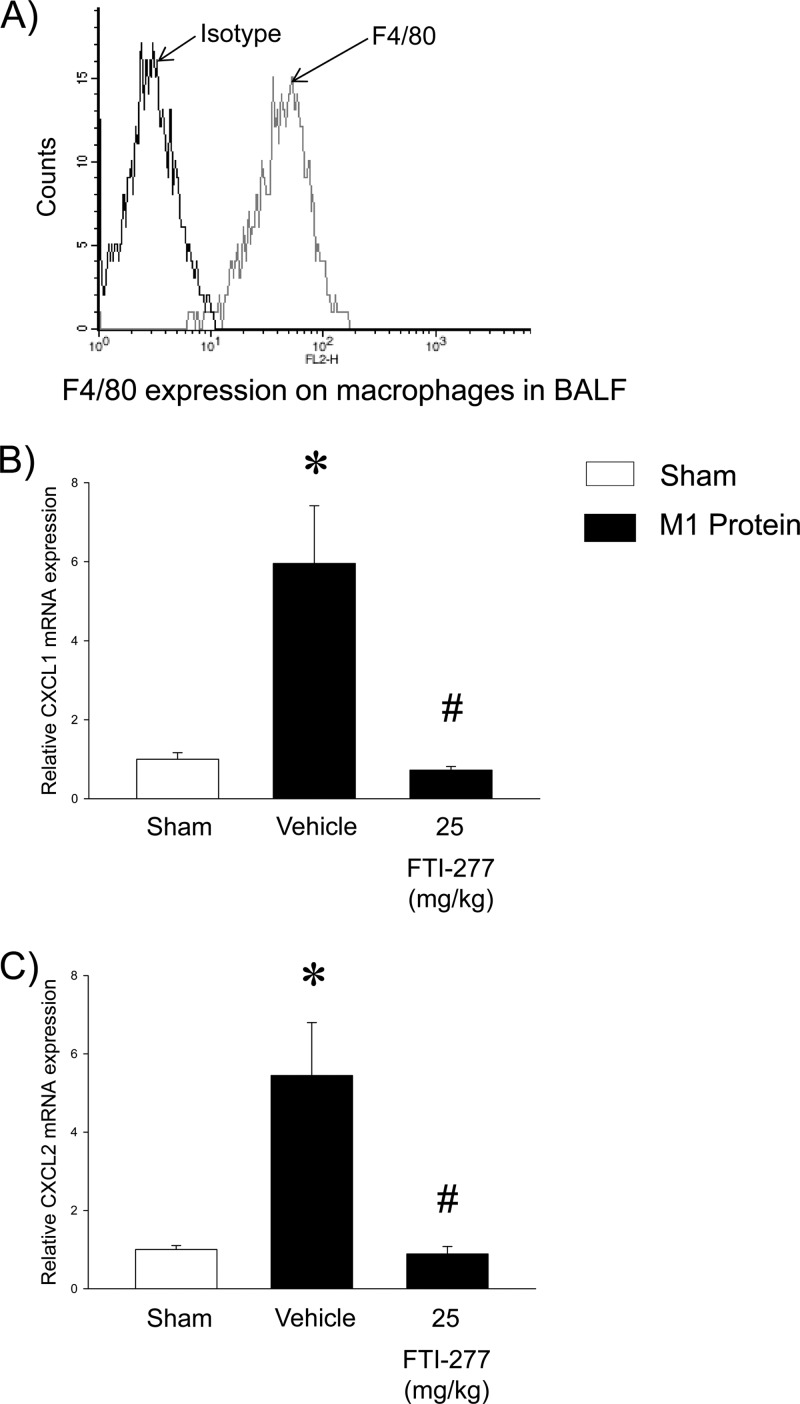
CXC chemokine levels were low in the lungs of control mice (Fig. 4). M1 protein administration markedly increased the pulmonary levels of CXCL1 and CXCL2, by 233-fold and 30-fold, respectively (Fig. 4). Treatment with FTI-277 abolished M1 protein-induced formation of CXCL1 and CXCL2 (Fig. 4). We next isolated alveolar macrophages in BALF, with a purity higher than 94% (Fig. 5A). The gene expression of CXC chemokines was examined in alveolar macrophages isolated from mice challenged with M1 protein and pretreated with FTI-277 or vehicle. We found that M1 protein caused a marked enhancement of mRNA levels of CXCL1 and CXCL2 in alveolar macrophages. Notably, inhibition of farnesyltransferase abolished M1 protein-evoked gene expression of CXCL1 and CXCL2 in alveolar macrophages (Fig. 5B and C).
Fig 4.
Farnesyltransferase regulates CXC chemokine formation in the lung. Animals were treated with FTI-277 (2.5 or 25 mg/kg) or vehicle (PBS) 10 min prior to M1 protein injection. Mice treated with PBS served as sham-treated animals. ELISA was used to quantify the levels of CXCL1 (A) and CXCL2 (B) in the lungs of mice 4 h after M1 protein challenge. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment; #, P < 0.05 versus results of vehicle + M1 protein.
Fig 5.
Farnesyltransferase regulates gene expression of CXC chemokines in alveolar macrophages. (A) F4/80 expression on isolated macrophages from BALF. (B and C) Quantitative RT-PCR was used to determine mRNA levels of CXCL1 (B) and CXCL2 (C) in alveolar macrophages 30 min after M1 protein injection. Levels of CXCL1 and CXCL2 mRNA were normalized to mRNA levels of β-actin. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment; #, P < 0.05 versus results of vehicle + M1 protein.
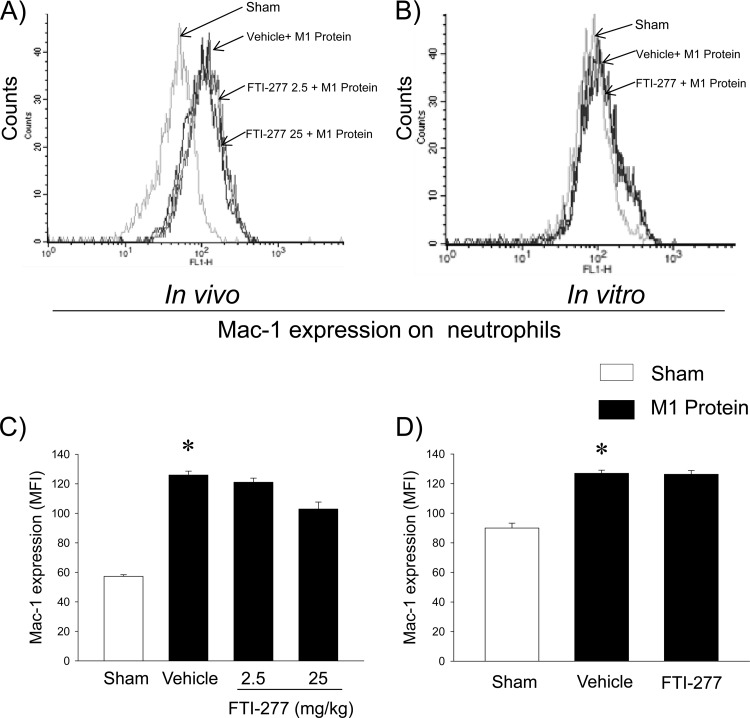
Neutrophil migration and Mac-1 expression.
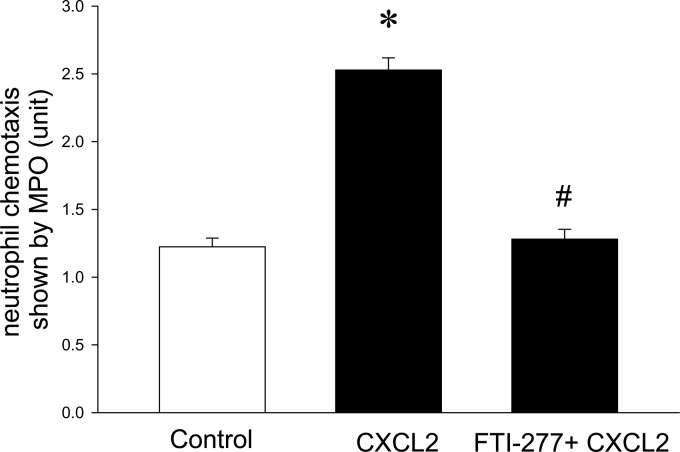
It was observed that 100 ng/ml CXCL2 caused a clear-cut increase in neutrophil migration over a time period of 120 min (Fig. 6). Preincubation of neutrophils with FTI-277 completely inhibited CXCL2-induced neutrophil migration in vitro (Fig. 6). Challenge with M1 protein triggered a significant increase in Mac-1 expression on neutrophils in vivo and in vitro (Fig. 7). However, we observed that inhibition of farnesyltransferase had no significant effect on M1 protein-induced expression of Mac-1 on neutrophils in vitro or in vivo (Fig. 7).
Fig 6.
Farnesyltransferase inhibits neutrophil migration in vitro. Neutrophil migration was determined in response to medium alone (Control) or medium plus CXCL2 (100 ng/ml) with or without preincubation of neutrophils with FTI-277 (10 μM). Data represent means ± SEM (n = 5). *, P < 0.05 versus results for control; #, P < 0.05 versus results with CXCL2.
Fig 7.
Farnesyltransferase does not regulate M1 protein-induced Mac-1 expression on neutrophils. (A and C) Mac-1 expression on neutrophils in animals treated with vehicle (PBS) or FTI-277 (2.5 or 25 mg/kg). Blood samples were obtained 4 h after M1 protein injection. Fluorescence intensity of Mac-1 is shown on the x axis, and cell counts on the y axis. (B and D) Mac-1 expression on neutrophils in vitro. Whole blood was incubated with PBS only or with M1 protein (1 μg/ml) and vehicle (PBS) or FTI-277 (10 μM). Samples were harvested 4 h after M1 protein challenge. Data represent means ± SEM (n = 5). *, P < 0.05 versus results of sham treatment.
DISCUSSION
Management of patients with severe infections and respiratory failure poses a major challenge to clinicians due to the limited therapeutic options. Infections with Streptococcus pyogenes of the M1 serotype can cause STSS and acute lung injury. The present study demonstrates that the administration of the farnesyltransferase inhibitor FTI-277 protects against lung damage triggered by streptococcal M1 protein. The protective effect of FTI-277 was related to a substantial reduction in neutrophil infiltration in the lung. FTI-277 has been widely tested and demonstrated to possess high specificity for farnesyltransferase (19, 33), although one cannot exclude the small possibility of off-target effects at some level. Nonetheless, our data indicate that inhibition of farnesyltransferase abolished CXC chemokine formation in lung macrophages in animals exposed to M1 protein. Our data suggest that farnesyltransferase is an important regulator of pulmonary recruitment of neutrophils and tissue damage in streptococcal infections.
Treatment of patients with STSS is largely restricted to supportive care due to an insufficient understanding of the underlying pathophysiology. Soluble M1 protein derived from the surface of Streptococcus pyogenes is a powerful stimulator of innate immunity, causing activation of neutrophils, monocytes, and platelets (14, 25, 26). We have recently reported that treatment with simvastatin inhibits streptococcal M1 protein-induced lung damage in mice, although the mechanisms remain elusive (38). Statins not only reduce cholesterol levels but, also, the formation of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, constituting components in protein isoprenylation, such as farnesylation and geranylgeranylation (9, 11). Farnesylation regulates several protein functions, including membrane localization, activity, and protein interactions. Accumulating studies have reported that inhibition of farnesyltransferase inhibits proinflammatory activities, such as inhibition of NF-κΒ and Ras activation (23, 28). Here, we show that inhibition of farnesyltransferase protects against M1 protein-provoked lung edema and tissue injury. These findings are in line with two previous reports showing that farnesyltransferase inhibition reduces apoptosis and mortality in endotoxemia and abdominal sepsis (31, 37). Knowing that neutrophil accumulation is considered to be a rate-limiting step in septic lung injury and that activation of circulating neutrophils is a prominent feature in M1 protein-induced lung damage (12, 40), it was of great interest to study pulmonary infiltration of neutrophils. In the present study, we could demonstrate that FTI-277 reduced M1 protein-induced lung activity of MPO, a marker of neutrophil recruitment, suggesting that farnesyltransferase is an important regulator of neutrophil trafficking in streptococcal infections. This reduction in MPO activity is also in line with the inhibition of neutrophil accumulation in the alveolar space in FTI-277-treated mice exposed to M1 protein. Indeed, this is the first study showing that inhibition of farnesyltransferase can inhibit tissue accumulation of neutrophils. These findings might also help to explain the decreased mortality in endotoxemic and septic mice treated with FTI-277 (31, 37). Nonetheless, keeping in mind the close relationship between neutrophil accumulation and lung damage, it may be proposed that the lung-protective effect of FTI-277 might be due to attenuation of neutrophil infiltration.
Leukocyte accumulation at sites of inflammation is a multistep process mediated by specific adhesion molecules. In the lung, several studies have shown that P-selectin glycoprotein ligand-1 (PSGL-1), as well as the β2-integrins LFA-1 and Mac-1, on neutrophils mediate their extravascular accumulation in septic lung injury (1, 6). Notably, one report showed that the tetrapeptide Gly-Pro-Arg-Pro attenuates M1 protein-provoked lung injury, suggesting that β2-integrins may be useful targets in severe streptococcal infections (14). Thus, we next asked whether farnesyltransferase inhibition might change the expression of Mac-1 on neutrophils. As shown before (40), we observed that systemic challenge with M1 protein increased Mac-1 on neutrophils. However, the administration of FTI-277 had no effect on M1 protein-triggered expression of Mac-1 on neutrophils in vivo or in vitro, indicating that Mac-1 is not a protective target of FTI-277 in streptococcal infections. On one hand, we have recently observed that simvastatin had no significant impact on M1 protein-induced expression of Mac-1 on neutrophils in vitro, but on the other hand, simvastatin was found to decrease Mac-1 expression on circulating neutrophils in mice exposed to M1 protein (38). These findings indicate that although simvastatin and FTI-277 exert overlapping anti-inflammatory effects, simvastatin also exerts farnesyltransferase-independent effects in vivo in M1 protein-induced inflammation. Whether such statin-mediated farnesyltransferase-independent effects might be regulated by geranylgeranylation-dependent signaling is not known and should be addressed in future studies. Extravascular navigation of neutrophils is orchestrated by secreted chemokines (7, 30). Neutrophils are particularly responsive to CXC chemokines, comprising CXCL1 and CXCL2, which are murine homologues of human interleukin-8 (42). Convincing lines of evidence have demonstrated clear-cut formation of CXC chemokines in streptococcal infections (8, 15), and we have recently observed that M1 protein triggers significant pulmonary formation of CXCL1 and CXCL2 (40). Thus, we next asked whether farnesyltransferase might regulate M1 protein-induced formation of CXC chemokines in the lung. Indeed, we found that inhibition of farnesyltransferase greatly decreased M1 protein-evoked generation of CXCL1 and CXCL2 in the lung. Interestingly, similar reductions in M1 protein-induced pulmonary formation of CXC chemokines have been observed in mice treated with simvastatin (38). Moreover, we found that M1 protein markedly enhanced CXCL1 and CXCL2 mRNA levels in alveolar macrophages. Notably, the administration of FTI-277 abolished M1 protein-provoked gene expression of CXC chemokines in alveolar macrophages, indicating that farnesyltransferase activity is an important signaling pathway in macrophage production of CXC chemokines in streptococcal infections. Considering a previous study showing that CXC chemokines, via their high-affinity receptor CXCR2, are critical in supporting M1 protein-induced accumulation of neutrophils in the lung (38), our current results suggest that inhibition of CXC chemokine generation helps to explain the regulatory role of farnesyltransferase in M1 protein-induced lung injury.
Our novel findings demonstrate that farnesyltransferase is an important regulator of neutrophil-mediated acute lung injury triggered by streptococcal M1 protein. Moreover, we demonstrate that inhibition of farnesyltransferase abolishes the macrophage-dependent generation of CXC chemokines in response to M1 protein challenge. Thus, we conclude that these farnesyltransferase-dependent mechanisms may, at least in part, help to clarify the beneficial effects exerted by statins and that targeting farnesyltransferase activity might be useful in order to protect against respiratory failure in streptococcal infections.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (2009-4872), Einar och Inga Nilssons stiftelse, Mossfelts stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital, and Lund University.
Songen Zhang, Milladur Rahman, and Su Zhang performed experiments and wrote the manuscript. Bengt Jeppsson, Heiko Herwald, and Henrik Thorlacius supervised the project, designed the experiments, and wrote the manuscript.
The authors have no financial disclosures to declare.
Footnotes
Published ahead of print 4 September 2012
REFERENCES
- 1. Asaduzzaman M, Zhang S, Lavasani S, Wang Y, Thorlacius H. 2008. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock 30:254–259 [DOI] [PubMed] [Google Scholar]
- 2. Baracco GJ, Bisno AL. 1999. Therapeutic approaches to streptococcal toxic shock syndrome. Curr. Infect. Dis. Rep. 1:230–237 [DOI] [PubMed] [Google Scholar]
- 3. Beltowski J, Wojcicka G, Jamroz-Wisniewska A. 2009. Adverse effects of statins: mechanisms and consequences. Curr. Drug Saf. 4:209–228 [DOI] [PubMed] [Google Scholar]
- 4. Collin M, Olsen A. 2000. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol. Microbiol. 36:1306–1318 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding ZM, et al. 1999. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163:5029–5038 [PubMed] [Google Scholar]
- 7. Ebnet K, Vestweber D. 1999. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem. Cell Biol. 112:1–23 [DOI] [PubMed] [Google Scholar]
- 8. Fritzer A, et al. 2009. Chemokine degradation by the Group A streptococcal serine proteinase ScpC can be reconstituted in vitro and requires two separate domains. Biochem. J. 422:533–542 [DOI] [PubMed] [Google Scholar]
- 9. Gazzerro P, et al. 2012. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol. Rev. 64:102–146 [DOI] [PubMed] [Google Scholar]
- 10. Giusti-Paiva A, et al. 2004. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock 21:271–275 [DOI] [PubMed] [Google Scholar]
- 11. Greenwood J, Steinman L, Zamvil SS. 2006. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 6:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grommes J, Soehnlein O. 2011. Contribution of neutrophils to acute lung injury. Mol. Med. 17:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbold W, et al. 2010. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect. Immun. 78:2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herwald H, et al. 2004. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 116:367–379 [DOI] [PubMed] [Google Scholar]
- 15. Hidalgo-Grass C, et al. 2006. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 25:4628–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huber AR, Kunkel SL, Todd RF, III, Weiss SJ. 1991. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254:99–102 [DOI] [PubMed] [Google Scholar]
- 17. Kopterides P, Falagas ME. 2009. Statins for sepsis: a critical and updated review. Clin. Microbiol. Infect. 15:325–334 [DOI] [PubMed] [Google Scholar]
- 18. Lancet JE, Karp JE. 2003. Farnesyltransferase inhibitors in hematologic malignancies: new horizons in therapy. Blood 102:3880–3889 [DOI] [PubMed] [Google Scholar]
- 19. Lerner EC, et al. 1995. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J. Biol. Chem. 270:26802–26806 [DOI] [PubMed] [Google Scholar]
- 20. McGarry MP, Stewart CC. 1991. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J. Leukoc. Biol. 50:471–478 [DOI] [PubMed] [Google Scholar]
- 21. Merx MW, et al. 2005. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 112:117–124 [DOI] [PubMed] [Google Scholar]
- 22. Merx MW, et al. 2004. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation 109:2560–2565 [DOI] [PubMed] [Google Scholar]
- 23. Na HJ, et al. 2004. Inhibition of farnesyltransferase prevents collagen-induced arthritis by down-regulation of inflammatory gene expression through suppression of p21(ras)-dependent NF-kappaB activation. J. Immunol. 173:1276–1283 [DOI] [PubMed] [Google Scholar]
- 24. Page AV, et al. 2011. Systemic dysregulation of angiopoietin-1/2 in streptococcal toxic shock syndrome. Clin. Infect. Dis. 52:e157–e161 [DOI] [PubMed] [Google Scholar]
- 25. Pahlman LI, et al. 2006. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J. Immunol. 177:1221–1228 [DOI] [PubMed] [Google Scholar]
- 26. Pahlman LI, et al. 2008. Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell. Microbiol. 10:404–414 [DOI] [PubMed] [Google Scholar]
- 27. Rowinsky EK, Windle JJ, Von Hoff DD. 1999. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J. Clin. Oncol. 17:3631–3652 [DOI] [PubMed] [Google Scholar]
- 28. Saha B, Nandi D. 2009. Farnesyltransferase inhibitors reduce Ras activation and ameliorate acetaminophen-induced liver injury in mice. Hepatology 50:1547–1557 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt H, et al. 2006. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med. 32:1248–1251 [DOI] [PubMed] [Google Scholar]
- 30. Schramm R, Thorlacius H. 2003. Staphylococcal enterotoxin B-induced acute inflammation is inhibited by dexamethasone: important role of CXC chemokines KC and macrophage inflammatory protein 2. Infect. Immun. 71:2542–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinozaki S, et al. 2010. Farnesyltransferase inhibitor improved survival following endotoxin challenge in mice. Biochem. Biophys. Res. Commun. 391:1459–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soehnlein O, et al. 2008. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur. Respir. J. 32:405–412 [DOI] [PubMed] [Google Scholar]
- 33. Sun J, Qian Y, Hamilton AD, Sebti SM. 1995. Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-Ras mutation and p53 deletion. Cancer Res. 55:4243–4247 [PubMed] [Google Scholar]
- 34. Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. 2007. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect. Dis. 7:358–368 [DOI] [PubMed] [Google Scholar]
- 35. Walters CE, et al. 2002. Inhibition of Rho GTPases with protein prenyltransferase inhibitors prevents leukocyte recruitment to the central nervous system and attenuates clinical signs of disease in an animal model of multiple sclerosis. J. Immunol. 168:4087–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weber C, Erl W, Weber KS, Weber PC. 1997. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J. Am. Coll. Cardiol. 30:1212–1217 [DOI] [PubMed] [Google Scholar]
- 37. Yang W, et al. 2011. Farnesyltransferase inhibitor FTI-277 reduces mortality of septic mice along with improved bacterial clearance. J. Pharmacol. Exp. Ther. 339:832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S, Rahman M, Qi Z, Herwald H, Thorlacius H. 2011. Simvastatin regulates CXC chemokine formation in streptococcal M1 protein-induced neutrophil infiltration in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 300:L930–L939 [DOI] [PubMed] [Google Scholar]
- 39. Zhang S, Rahman M, Qi Z, Thorlacius H. 2011. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J. Leukoc. Biol. 89:735–742 [DOI] [PubMed] [Google Scholar]
- 40. Zhang S, Zhang S, Rahman M, Herwald H, Thorlacius H. 2011. Streptococcal M1 protein-induced lung injury is independent of platelets in mice. Shock 35:86–91 [DOI] [PubMed] [Google Scholar]
- 41. Zhang X, Goncalves R, Mosser DM. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14:14.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang XW, Liu Q, Wang Y, Thorlacius H. 2001. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br. J. Pharmacol. 133:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]