Abstract
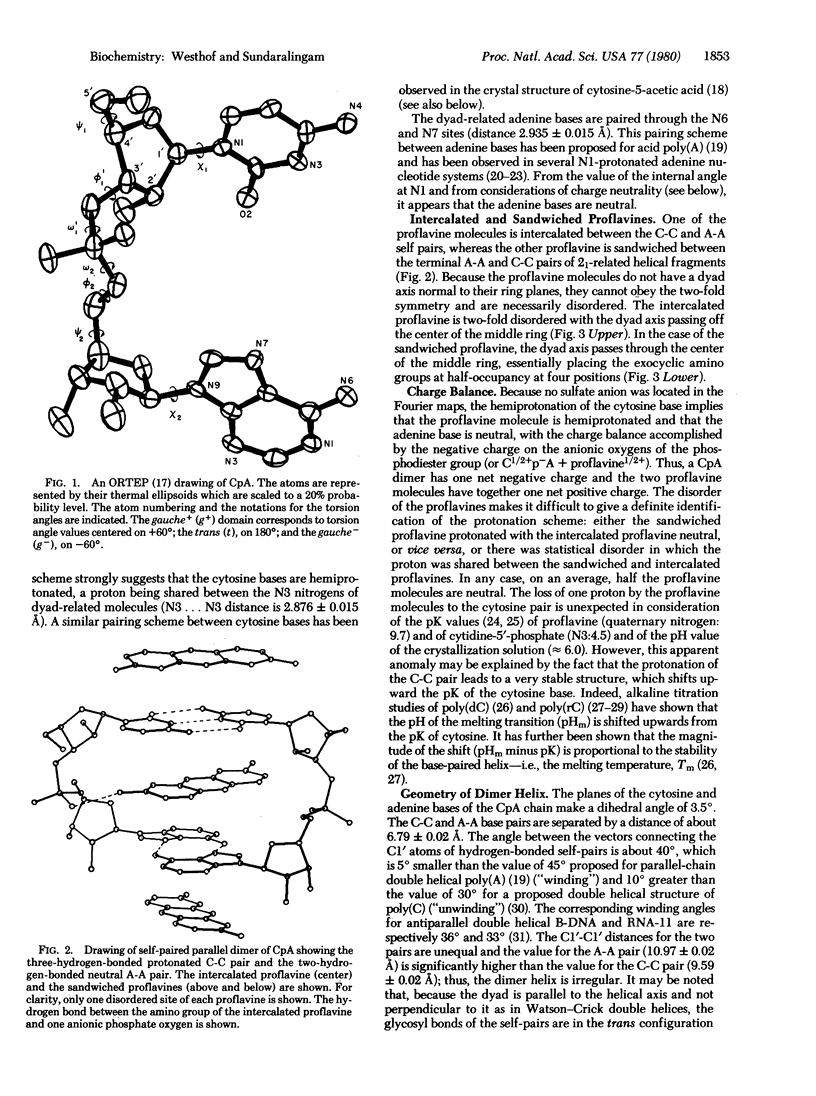
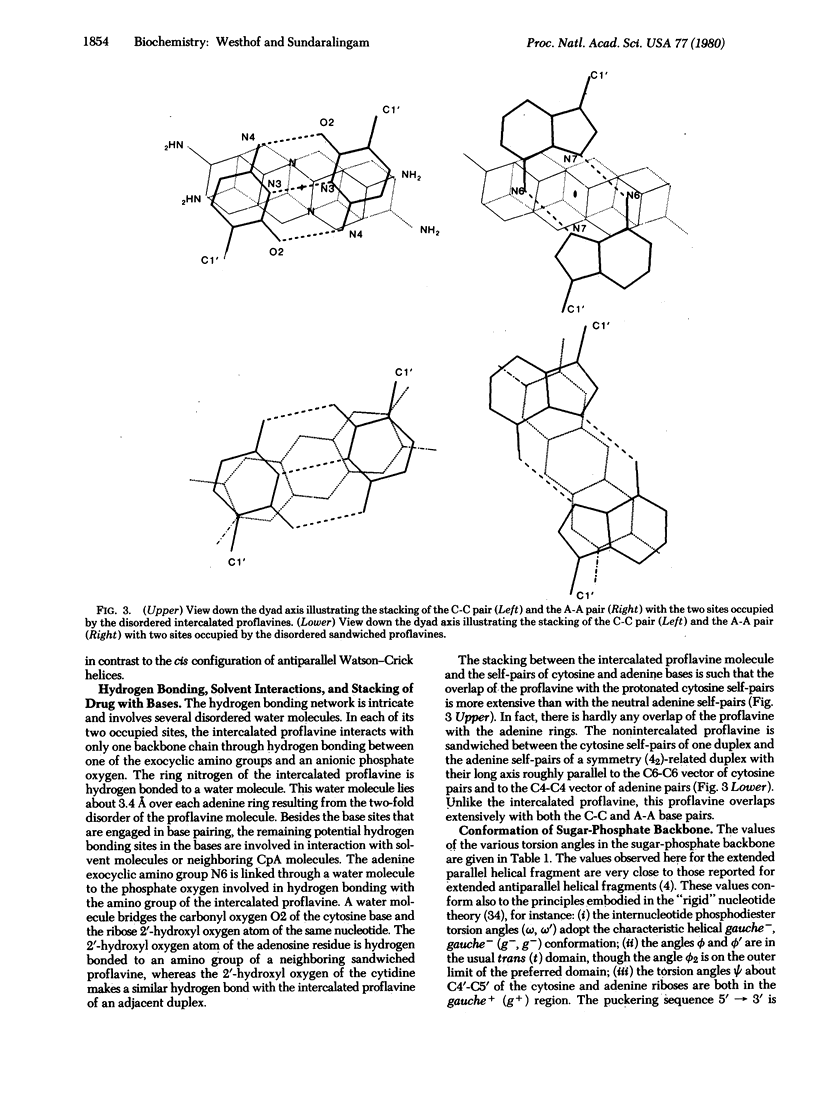
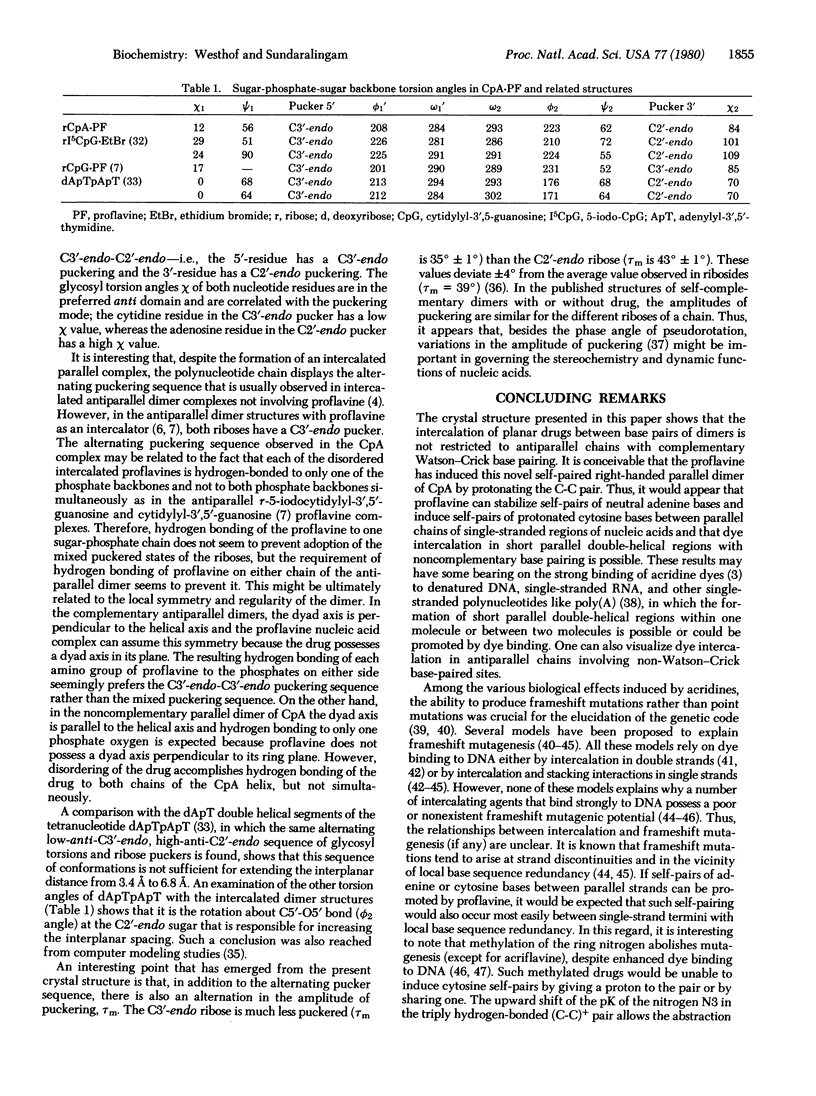
The non-self-complementary dinucleoside monophosphate cytidylyl-3',5'-adenosine (CpA) forms a base-paired parallel-chain dimer with an intercalated proflavine. The dimer complex possesses a right-handed helical twist. The dimer helix has an irregular girth with a neutral adenine-adenine (A-A) pair, hydrogen-bonded through the N6 and N7 sites (C1'...C1' separation of 10.97 A), and a triply hydrogen-bonded protonated cytosine-cytosine (C-C) pair with a proton shared between the base N3 sites (Cl'...Cl' separation of 9.59 A). The torsion angles of the sugar-phosphate backbone are within their most preferred ranges and the sugar puckering sequence (5' leads to 3') is C3'-endo, C2'-endo. There is also a second proflavine molecule sandwiched between CpA dimers on the 21-axis. Both proflavines are necessarily disordered, being on dyad axis, and this suggests possible insights into the dynamics of intercalation of planar drugs. This structure shows that intercalation of planar drugs in nucleic acids may not be restricted to antiparallel complementary Watson-Crick pairing regions and provides additional mechanisms for acridine mutagenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKINRIMISI E. O., SANDER C., TS'O P. O. Properties of helical polycytidylic acid. Biochemistry. 1963 Mar-Apr;2:340–344. doi: 10.1021/bi00902a028. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Berman H. M., Neidle S., Stodola R. K. Drug-nucleic acid interactions: conformational flexibility at the intercalation site. Proc Natl Acad Sci U S A. 1978 Feb;75(2):828–832. doi: 10.1073/pnas.75.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Dourlent M., Hélène C. A quantitative analysis of proflavine binding to polyadenylic acid, polyuridylic acid, and transfer RNA. Eur J Biochem. 1971 Nov 11;23(1):86–95. doi: 10.1111/j.1432-1033.1971.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Finkelstein T., Weinstein I. B. Proflavine binding to transfer ribonucleic acid, synthetic ribonucleic acids, and deoxyribonucleic acid. J Biol Chem. 1967 Sep 10;242(17):3763–3768. [PubMed] [Google Scholar]
- Grosjean H., Wérenne J., Chantrenne H. The binding of proflavine to transfer ribonucleic acid: dependence on secondary structure. Biochim Biophys Acta. 1968 Oct 29;166(3):616–627. doi: 10.1016/0005-2787(68)90368-7. [DOI] [PubMed] [Google Scholar]
- HARTMAN K. A., Jr, RICH A. THE TAUTOMERIC FORM OF HELICAL POLYRIBOCYTIDYLIC ACID. J Am Chem Soc. 1965 May 5;87:2033–2039. doi: 10.1021/ja01087a031. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- INMAN R. B. TRANSITIONS OF DNA HOMOPOLYMERS. J Mol Biol. 1964 Sep;9:624–637. doi: 10.1016/s0022-2836(64)80171-6. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Tsai C. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. II. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium:5-iodocytidylyl (3'-5') guanosine. J Mol Biol. 1977 Aug 15;114(3):317–331. doi: 10.1016/0022-2836(77)90253-4. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., RICH A. Molecular structure of helical polycytidylic acid. Nature. 1963 May 25;198:725–728. doi: 10.1038/198725a0. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle S., Taylor G., Sanderson M. A 1:2 crystalline complex of ApA:proflavine: a model for binding to single-stranded regions in RNA. Nucleic Acids Res. 1978 Nov;5(11):4417–4422. doi: 10.1093/nar/5.11.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Davies D. R. The interaction of acridine dyes with DNA: an x-ray diffraction and optical investigation. J Mol Biol. 1966 May;17(1):57–74. doi: 10.1016/s0022-2836(66)80094-3. [DOI] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- Riva S. C. Interactions of methylated acridines with DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):606–611. doi: 10.1016/0006-291x(66)90442-6. [DOI] [PubMed] [Google Scholar]
- Rubin J., Brennan T., Sundaralingam M. Crystal and molecular structure of a naturally occurring dinucleoside monophosphate. Uridylyl-(3'-5')-adenosine hemihydrate. Conformational "rigidity" of the nucleotide unit and models for polynucleotide chain folding. Biochemistry. 1972 Aug 1;11(16):3112–3128. doi: 10.1021/bi00766a027. [DOI] [PubMed] [Google Scholar]
- Sakore T. D., Jain S. C., Tsai C. C., Sobell H. M. Mutagen-nucleic acid intercalative binding: structure of a 9-aminoacridine: 5-iodocytidylyl(3'-5')guanosine crystalline complex. Proc Natl Acad Sci U S A. 1977 Jan;74(1):188–192. doi: 10.1073/pnas.74.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Jain S. C., Gilbert S. G. Visualization of drug-nucleic acid interactions at atomic resolution. III. Unifying structural concepts in understanding drug-DNA interactions and their broader implications in understanding protein-DNA interactions. J Mol Biol. 1977 Aug 15;114(3):333–365. doi: 10.1016/0022-2836(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Seeman N. C., Kim S. H., Berman H. M. Crystal structure of a naturally occurring dinucleoside phoaphate: uridylyl 3',5'-adenosine phosphate model for RNA chain folding. J Mol Biol. 1972 May 28;66(3):403–421. doi: 10.1016/0022-2836(72)90423-8. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Finkelstein I. H. Proflavine inhibition of protein synthesis. J Biol Chem. 1967 Sep 10;242(17):3757–3762. [PubMed] [Google Scholar]
- Werenne J., Grosjean H., Chantrenne H. Effect of proflavine on the binding of isoleucine to transfer RNA. Biochim Biophys Acta. 1966 Dec 21;129(3):585–593. doi: 10.1016/0005-2787(66)90073-6. [DOI] [PubMed] [Google Scholar]
- Wróbel A., Rabczenko A., Shugar D. Conformation of acid forms of poly C: temperature and ionic strength dependence of protonation of cytidine and cytidine-5'-phosphate. Acta Biochim Pol. 1970;17(4):339–349. [PubMed] [Google Scholar]



